Abstract
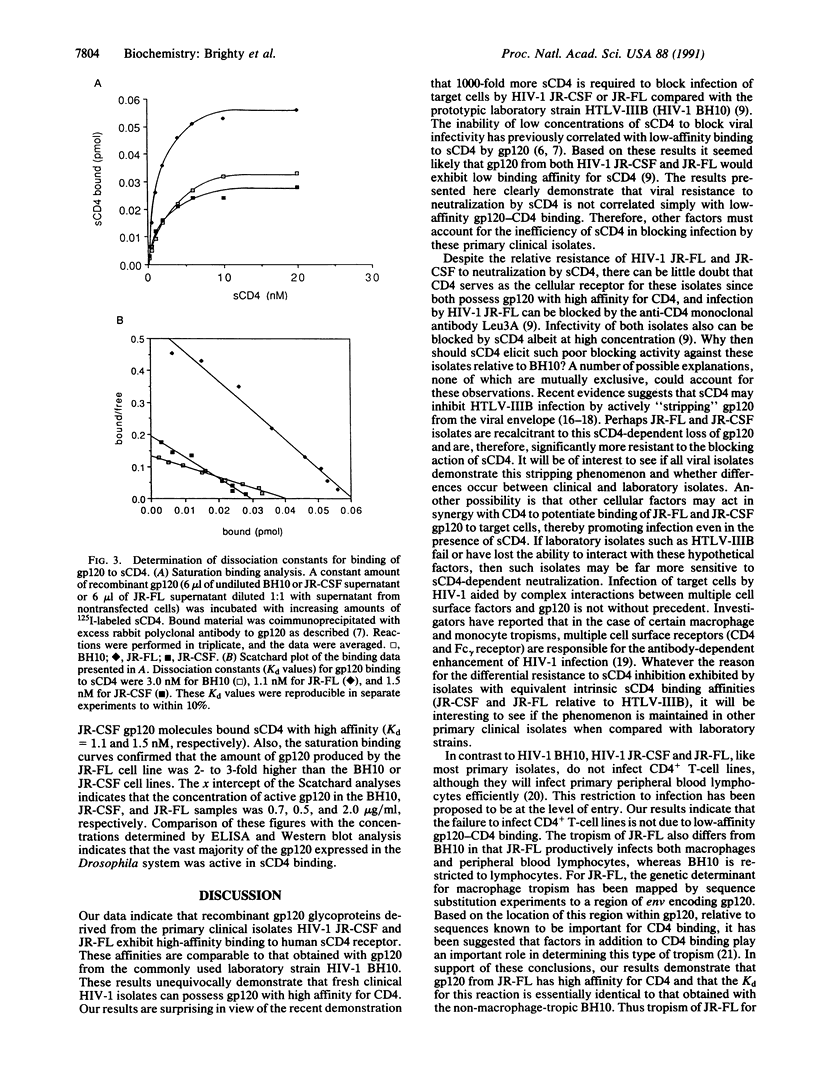
Recent evidence indicates that primary clinical isolates of human immunodeficiency virus type 1 (HIV-1) require significantly more soluble CD4 (sCD4) to block infection than the prototypic laboratory strain HTLV-IIIB. The currently accepted explanation for these observations is that the envelope glycoproteins from primary clinical isolates possess lower affinities for CD4 than laboratory strains. This observation has far reaching implications for the clinical effectiveness of sCD4. To test whether the resistance of clinical isolates to sCD4 neutralization correlates with low-affinity binding to gp120, we have compared gp120 glycoproteins derived from the clinical isolates HIV-1 JR-CSF and JR-FL with those derived from the prototypic strain HIV-1 BH10 in quantitative sCD4 binding studies. Surprisingly, our results demonstrate that gp120 derived from HIV-1 JR-CSF and JR-FL possess sCD4 binding affinities of equal or greater magnitude than gp120 derived from HIV-1 BH10. Thus primary clinical HIV-1 isolates can and do possess gp120 with high affinity for CD4, and sensitivity to neutralization by sCD4 is dependent upon factors other than the intrinsic affinity of gp120 for CD4.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S., Coligan J. E., Barin F., McLane M. F., Sodroski J. G., Rosen C. A., Haseltine W. A., Lee T. H., Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985 May 31;228(4703):1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- Cann A. J., Zack J. A., Go A. S., Arrigo S. J., Koyanagi Y., Green P. L., Koyanagi Y., Pang S., Chen I. S. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J Virol. 1990 Oct;64(10):4735–4742. doi: 10.1128/jvi.64.10.4735-4742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp J. S., Johansen H., Hellmig B., Beck J., Matthews T. J., Delers A., Rosenberg M. Regulated expression allows high level production and secretion of HIV-1 gp120 envelope glycoprotein in Drosophila Schneider cells. Biotechnology (N Y) 1991 Feb;9(2):173–177. doi: 10.1038/nbt0291-173. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Li X. L., Moudgil T., Ho D. D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- DeBlasi A., O'Reilly K., Motulsky H. J. Calculating receptor number from binding experiments using same compound as radioligand and competitor. Trends Pharmacol Sci. 1989 Jun;10(6):227–229. doi: 10.1016/0165-6147(89)90266-6. [DOI] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Bertonis J. M., Meier W., Johnson V. A., Costopoulos D. S., Liu T., Tizard R., Walker B. D., Hirsch M. S., Schooley R. T. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988 Jan 7;331(6151):76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- Hart T. K., Kirsh R., Ellens H., Sweet R. W., Lambert D. M., Petteway S. R., Jr, Leary J., Bugelski P. J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey R. E., Richardson N. E., Kowalski M., Brown N. R., Chang H. C., Siliciano R. F., Dorfman T., Walker B., Sodroski J., Reinherz E. L. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988 Jan 7;331(6151):78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- Ivey-Hoyle M., Culp J. S., Chaikin M. A., Hellmig B. D., Matthews T. J., Sweet R. W., Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen H., van der Straten A., Sweet R., Otto E., Maroni G., Rosenberg M. Regulated expression at high copy number allows production of a growth-inhibitory oncogene product in Drosophila Schneider cells. Genes Dev. 1989 Jun;3(6):882–889. doi: 10.1101/gad.3.6.882. [DOI] [PubMed] [Google Scholar]
- Kirsh R., Hart T. K., Ellens H., Miller J., Petteway S. A., Jr, Lambert D. M., Leary J., Bugelski P. J. Morphometric analysis of recombinant soluble CD4-mediated release of the envelope glycoprotein gp120 from HIV-1. AIDS Res Hum Retroviruses. 1990 Oct;6(10):1209–1212. doi: 10.1089/aid.1990.6.1209. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Moore J. P. Simple methods for monitoring HIV-1 and HIV-2 gp120 binding to soluble CD4 by enzyme-linked immunosorbent assay: HIV-2 has a 25-fold lower affinity than HIV-1 for soluble CD4. AIDS. 1990 Apr;4(4):297–305. doi: 10.1097/00002030-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Takeda A., Sweet R. W., Ennis F. A. Two receptors are required for antibody-dependent enhancement of human immunodeficiency virus type 1 infection: CD4 and Fc gamma R. J Virol. 1990 Nov;64(11):5605–5610. doi: 10.1128/jvi.64.11.5605-5610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]