Abstract
Xeroderma pigmentosum (XP) variant patients show the clinical characteristics of the disease, with increased frequencies of skin cancer, but their cells have a normal, or nearly normal, rate of nucleotide excision repair of UV-induced DNA damage and are only slightly more sensitive than normal cells to the cytotoxic effect of UV radiation. However, they are significantly more sensitive to its mutagenic effect. To examine the mechanisms responsible for this hypermutability, we transfected an XP variant cell line with a UV-irradiated (at 254 nm) shuttle vector carrying the supF gene as a target for mutations, allowed replication of the plasmid, determined the frequency and spectrum of mutations induced, and compared the results with those obtained previously when irradiated plasmids carrying the same target gene replicated in a normal cell line [Bredberg, A., Kraemer, K. H. & Seidman, M. M. (1986) Proc. Natl. Acad. Sci. USA 83, 8273-8277]. The frequency of mutants increased linearly with dose, but with a slope 5 times steeper than that seen with normal cells. Sequence analysis of the supF gene showed that 52 of 53 independent mutants generated in the XP variant cells contained base substitutions, with 62 of 64 of the substitutions involving a dipyrimidine. Twenty-eight percent of the mutations involved A.T base pairs, with the majority found at position 136, the middle of a run of three A.T base pairs. (In the normal cells, this value was only 11%.) If the rate of excision of lesions from supF in the two cell lines is equal, our data suggest that XP variant cells are less likely than normal cells to incorporate dAMP opposite bases involved in photo-products. If such incorporation also occurs during replication of chromosomal DNA, this could account for the hypermutability of XP variant cells with UV irradiation.
Full text
PDF
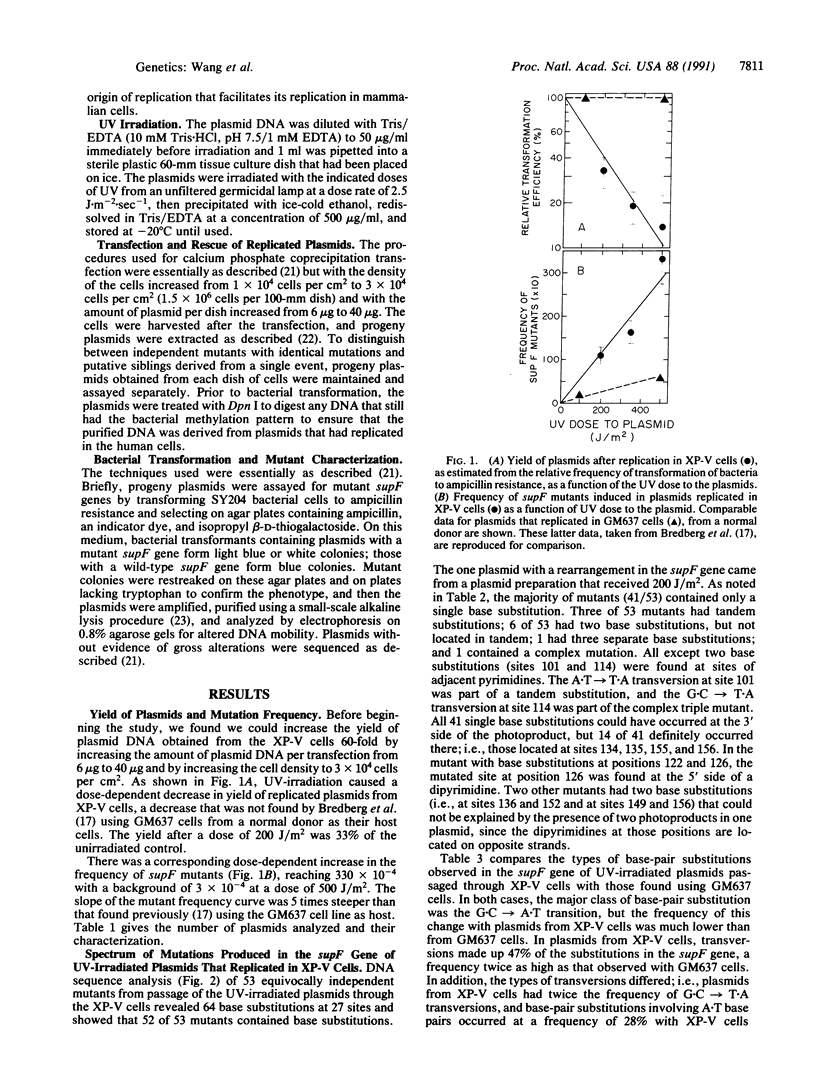
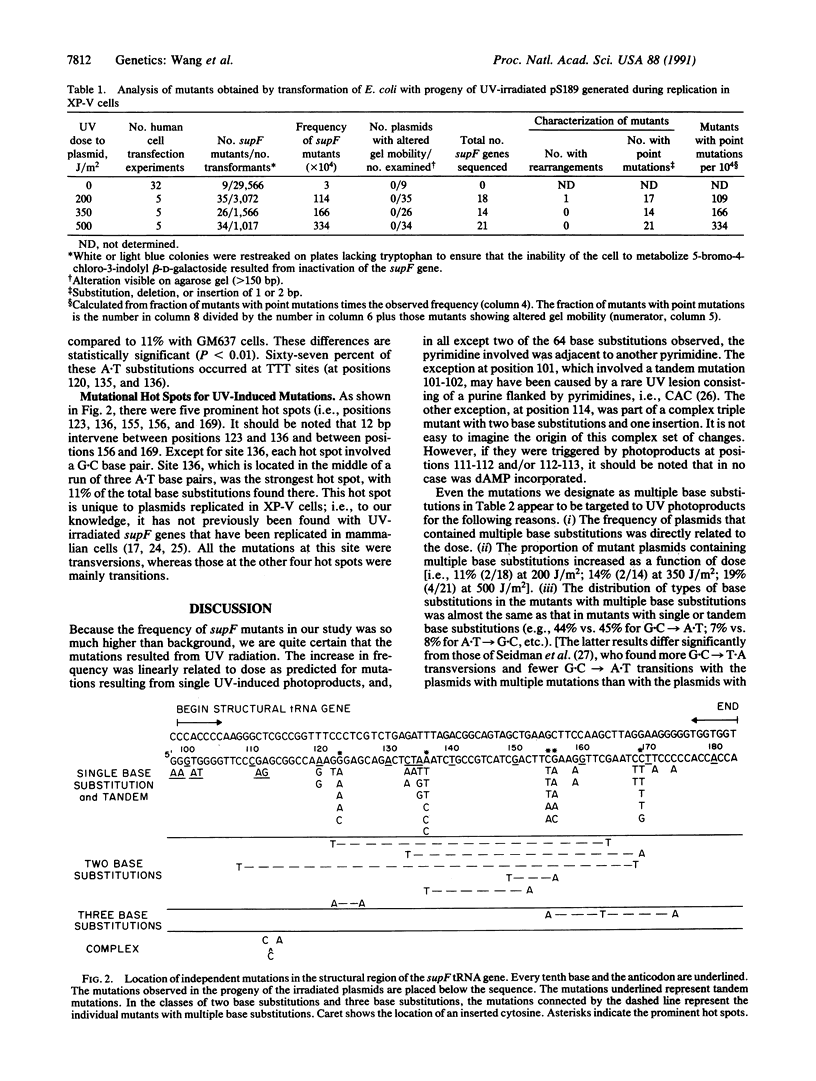


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. C., Kaufmann W. K., Brylawski B. P., Cordeiro-Stone M. Defective postreplication repair in xeroderma pigmentosum variant fibroblasts. Cancer Res. 1990 May 1;50(9):2593–2598. [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Park S. D. Xeroderma pigmentosum variants have a slow recovery of DNA synthesis after irradiation with ultraviolet light. Biochim Biophys Acta. 1979 Aug 29;564(1):122–131. doi: 10.1016/0005-2787(79)90193-x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Park S. D. Xeroderma pigmentosum variants have a slow recovery of DNA synthesis after irradiation with ultraviolet light. Biochim Biophys Acta. 1979 Aug 29;564(1):122–131. doi: 10.1016/0005-2787(79)90193-x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Xeroderma pigmentosum: variants with normal DNA repair and normal sensitivity to ultraviolet light. J Invest Dermatol. 1972 Mar;58(3):124–128. doi: 10.1111/1523-1747.ep12538913. [DOI] [PubMed] [Google Scholar]
- Francis A. A., Regan J. D. Detection and repair of a UV-induced photosensitive lesion in the DNA of human cells. Mutat Res. 1986 May;165(3):151–157. doi: 10.1016/0167-8817(86)90049-0. [DOI] [PubMed] [Google Scholar]
- Gelfand R. A., Barrett E. J. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987 Jul;80(1):1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD B. D., TESSMAN I. IDENTIFICATION OF THE ALTERED BASES IN MUTATED SINGLE-STRANDED DNA. 3. MUTAGENESIS BY ULTRAVIOLET LIGHT. J Mol Biol. 1964 Aug;9:372–375. doi: 10.1016/s0022-2836(64)80214-x. [DOI] [PubMed] [Google Scholar]
- Hansson J., Keyse S. M., Lindahl T., Wood R. D. DNA excision repair in cell extracts from human cell lines exhibiting hypersensitivity to DNA-damaging agents. Cancer Res. 1991 Jul 1;51(13):3384–3390. [PubMed] [Google Scholar]
- Hauser J., Seidman M. M., Sidur K., Dixon K. Sequence specificity of point mutations induced during passage of a UV-irradiated shuttle vector plasmid in monkey cells. Mol Cell Biol. 1986 Jan;6(1):277–285. doi: 10.1128/mcb.6.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Cleaver J. E. Mechanisms of inhibition of DNA replication by ultraviolet light in normal human and xeroderma pigmentosum fibroblasts. J Mol Biol. 1981 Jun 25;149(2):171–187. doi: 10.1016/0022-2836(81)90297-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Cleaver J. E. Mechanisms of inhibition of DNA replication by ultraviolet light in normal human and xeroderma pigmentosum fibroblasts. J Mol Biol. 1981 Jun 25;149(2):171–187. doi: 10.1016/0022-2836(81)90297-7. [DOI] [PubMed] [Google Scholar]
- Konze-Thomas B., Hazard R. M., Maher V. M., McCormick J. J. Extent of excision repair before DNA synthesis determines the mutagenic but not the lethal effect of UV radiation. Mutat Res. 1982 Jun;94(2):421–434. doi: 10.1016/0027-5107(82)90305-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Paterson M. C., Lohman P. H., de Weerd-Kastelein E. A., Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A. 1975 Jan;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Preston B. D. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Ouellette L. M., Curren R. D., McCormick J. J. Frequency of ultraviolet light-induced mutations is higher in xeroderma pigmentosum variant cells than in normal human cells. Nature. 1976 Jun 17;261(5561):593–595. doi: 10.1038/261593a0. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Brash D. E., Nairn R. S. Rapid repair kinetics of pyrimidine(6-4)pyrimidone photoproducts in human cells are due to excision rather than conformational change. Nucleic Acids Res. 1990 Feb 25;18(4):963–971. doi: 10.1093/nar/18.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. Xeroderma pigmentosum variant cells are not defective in the repair of (6-4) photoproducts. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Aug;52(2):201–205. doi: 10.1080/09553008714551661. [DOI] [PubMed] [Google Scholar]
- Myhr B. C., Turnbull D., DiPaolo J. A. Ultraviolet mutagenesis of normal and xeroderma pigmentosum variant human fibroblasts. Mutat Res. 1979 Sep;62(2):341–353. doi: 10.1016/0027-5107(79)90089-7. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T., Minton K. W. Ultraviolet-induced dimerization of non-adjacent pyrimidines. A potential mechanism for the targeted -1 frameshift mutation. J Mol Biol. 1988 Apr 20;200(4):681–693. doi: 10.1016/0022-2836(88)90480-9. [DOI] [PubMed] [Google Scholar]
- Park S. D., Cleaver J. E. Postreplication repair: questions of its definition and possible alteration in xeroderma pigmentosum cell strains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3927–3931. doi: 10.1073/pnas.76.8.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. D., Rowan L. A., Mendrala A. L., Howell J. N., Maher V. M., McCormick J. J. Xeroderma pigmentosum fibroblasts including cells from XP variants are abnormally sensitive to the mutagenic and cytotoxic action of broad spectrum simulated sunlight. Photochem Photobiol. 1984 Jan;39(1):37–42. doi: 10.1111/j.1751-1097.1984.tb03401.x. [DOI] [PubMed] [Google Scholar]
- Rabkin S. D., Moore P. D., Strauss B. S. In vitro bypass of UV-induced lesions by Escherichia coli DNA polymerase I: specificity of nucleotide incorporation. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1541–1545. doi: 10.1073/pnas.80.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Ryan P. A., Maher V. M., McCormick J. J. Modification of MCDB 110 medium to support prolonged growth and consistent high cloning efficiency of diploid human fibroblasts. Exp Cell Res. 1987 Oct;172(2):318–328. doi: 10.1016/0014-4827(87)90390-9. [DOI] [PubMed] [Google Scholar]
- Santos E., Reddy E. P., Pulciani S., Feldmann R. J., Barbacid M. Spontaneous activation of a human proto-oncogene. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4679–4683. doi: 10.1073/pnas.80.15.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Dasgupta U. B., Summers W. C. Error-prone mutagenesis detected in mammalian cells by a shuttle vector containing the supF gene of Escherichia coli. Mol Cell Biol. 1984 Oct;4(10):2227–2230. doi: 10.1128/mcb.4.10.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Bredberg A., Seetharam S., Kraemer K. H. Multiple point mutations in a shuttle vector propagated in human cells: evidence for an error-prone DNA polymerase activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4944–4948. doi: 10.1073/pnas.84.14.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Dixon K., Razzaque A., Zagursky R. J., Berman M. L. A shuttle vector plasmid for studying carcinogen-induced point mutations in mammalian cells. Gene. 1985;38(1-3):233–237. doi: 10.1016/0378-1119(85)90222-7. [DOI] [PubMed] [Google Scholar]
- Seidman M. The development of transient SV40 based shuttle vectors for mutagenesis studies: problems and solutions. Mutat Res. 1989 Mar-May;220(2-3):55–60. doi: 10.1016/0165-1110(89)90010-9. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Maher V. M., McCormick J. J. Excision repair of UV- or benzo[a]pyrene diol epoxide-induced lesions in xeroderma pigmentosum variant cells is 'error free'. Mutat Res. 1985 Nov;146(3):285–294. doi: 10.1016/0167-8817(85)90070-7. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Kinds of mutations formed when a shuttle vector containing adducts of (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9, 10-tetrahydrobenzo[a]pyrene replicates in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3787–3791. doi: 10.1073/pnas.84.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]
- Zelle B., Lohman P. H. Repair of UV-endonuclease-susceptible sites in the 7 complementation groups of xeroderma pigmentosum A through G. Mutat Res. 1979 Sep;62(2):363–368. doi: 10.1016/0027-5107(79)90091-5. [DOI] [PubMed] [Google Scholar]


