Abstract
Purpose
This pilot prospective study sought to determine whether dynamic contrast enhanced MRI (DCE-MRI) could be used as a clinical imaging biomarker of tissue toxicity from whole brain radiotherapy (WBRT).
Method
14 patients who received WBRT were imaged using dynamic contrast enhanced DCE-MRI prior to and at 8-weeks, 16-weeks and 24-weeks after the initiation of WBRT. Twelve of the patients were also enrolled in the RTOG 0614 trial, which randomized patients to the use of placebo or memantine. After the unblinding of the treatments received by RTOG 0614 patients, DCE-MRI measures of tumor tissue and normal appearing white matter (NAWM) vascular permeability (Initial Area Under the Curve (AUC) Blood Adjusted) was analyzed. Cognitive, quality-of-life (QOL) assessment and blood samples were collected according to the patient's ability to tolerate the exams. Circulating endothelial cells (CEC) were measured using flow cytometry.
Results
Following WBRT, there was an increasing trend in the vascular permeability of tumors (p=0.09) and NAWM (p=0.06) with time. Memantine significantly (p=0.01) reduced NAWM AUC changes following radiotherapy. Patients on memantine retained (COWA p= 0.03) better cognitive functions than those on placebo. No association was observed between the level of CEC and DCE-MRI changes, time from radiotherapy or memantine use.
Conclusions
DCE-MRI can detect vascular damage secondary to WBRT. Our data suggests that memantine reduces WBRT-induced brain vasculature damages.
Keywords: MRI, biomarker, imaging, radiotherapy, memantine
INTRODUCTION
The incidence of brain metastasis in cancer patients has been 30-40% [1–3]. This figure may rise as novel therapeutic agents improve the systemic control of cancers outside of the central nervous system. The brain represents a watershed area for tumor metastasis to seed and colonize as the normal blood-brain-barrier (BBB) prevents systemic agents from reaching tumor cells. Radiotherapy currently remains as the main treatment option for patients with brain metastases [4].
Despite the increasing use of radiosurgery to treat brain metastases, patients with brain metastases often receive whole brain irradiation (WBRT) for widespread metastases, post-radiosurgery progression or leptomeningeal seeding [4]. WBRT may control the progression of brain metastases and lengthen lifespan, but it may induce cognitive deficits. As radiation may induce neuronal N-methy-D-aspartate (NMDA) receptor stimulation and excitotoxicity, inhibition of the NMDA receptor using a competitive antagonist, memantine may reduce WBRT-induced cognitive decline. The Radiation Therapy Oncology Group (RTOG) 0614 study randomized patients treated with WBRT to receive concurrent placebo or memantine [5]. The RTOG 0614 study demonstrated that patients who received memantine maintained better cognitive functions than patients who had placebo [5].
In vivo murine experiments suggested that the side-effects of radiotherapy to normal brain tissue is secondary to the induction of global vascular damages in the form of devascularization, gliosis, demyelination and white matter necrosis [6]. A preliminary clinical study suggested that Dynamic Contrast-Enhanced MRI (DCE-MRI) could detect increased permeability of the BBB and blood-tumor-barrier at the completion of 60Gy of fractioned radiotherapy for the treatment of glioma [7]. Similarly, it has been suggested that DCE-MRI could be used to assess the efficacy of focused ultrasound in disrupting the BBB [8].
The current pilot study aims at determining whether DCE-MRI is able to detect and measure changes in vascular permeability during the first 6 months following WBRT (37.5 Gy in 15 fractions) in patients with brain metastasis, and whether vascular changes in normal appearing white matter (NAWM) are associated with neurocognitive function and/or memantine use.
RESULTS
Our patient population was composed of a heterogeneous group of patients with different cancer histology and who had received various local treatments prior to WBRT (see Table 1). Most patients had lung (n = 7) or breast cancers (n = 4). Twelve patients had unresected tumor(s) present in the brain at the time of WBRT. The median overall survival from WBRT initiation was 9.75 (0-33.6) months. Seven patients received memantine, while 7 patients received either placebo or neither.
Table 1. Characteristics of the study patients.
| Pt # | Primary disease | Age at time of WBRT | Number of brain lesions | Volume of tumor analyzed (cc) | Time between WBRT and prior surgery or SRS brain treatment | OS from WBRT (months) | Use of Pla, Mem or Neither |
|---|---|---|---|---|---|---|---|
| 1 | Breast | 34 | 1 | 1.73 | Sx: 4 weeks | 20.5 | Pla |
| 2 | Breast | 53 | 4 | 0.78 | N/A | 33.6 | Pla |
| 3 | Lung | 71 | 2 | 0.62 | Sx: 6 weeks RS: 4 weeks |
23.4 | Neither |
| 4 | Breast | 51 | 1 | 1.58 | Sx: 6 weeks RS: 4 weeks |
18.6 | Mem |
| 5 | Colon | 66 | 4 | 0.48 | RS: 4 weeks | 4.0 | Mem |
| 6 | Lung | 65 | >10 | 0.94 | N/A | 5.6 | Mem |
| 7 | Lung | 66 | 4 | 13.06 | N/A | 10.9 | Mem |
| 8 | Melanoma | 65 | 3 | 0.53 | RS: 5 weeks | 4.9 | Mem |
| 9 | Thryoid | 57 | 2 | 1.78 | RS: 59 weeks | 21 | Neither |
| 10 | Lung | 67 | 6 | 0.60 | N/A | 7.2 | Plac |
| 11 | Lung | 44 | 1 | 3.70 | RS: 2 weeks | 0 | Mem |
| 12 | Lung | 78 | >10 | 0.62 | N/A | 5.9 | Plac |
| 13 | Lung | 63 | 2 | 1.37 | Sx: 5 weeks RS: 2 weeks |
28.5 | Mem |
| 14 | Breast | 57 | 2 | 0.65 | RS: 44 weeks | 8.6 | Plac |
WBRT: Whole brain radiotherapy; Sx: Surgery; RS: Radiosurgery; N/A: no surgery or RS; OS: Overall Survival; Pla: Placebo; Mem: Memantine
Compared to Ktrans (Figure 1), AUC measurements of tumor and NAWM provided more consistent (Ktrans: 12% failed model fitting; AUC: no missing data) and reproducible values. Mean coefficients of variation of Ktrans and AUC measurements were similar in tumors (ANOVA p = 0.673). In NAWM, the mean coefficients of variation of AUC was significantly less (ANOVA p = 0.012) than for Ktrans. As both measurements are recommended endpoints for the assessments and reporting of MRI oncology trials [9], we proceeded to use AUC for subsequent analysis.
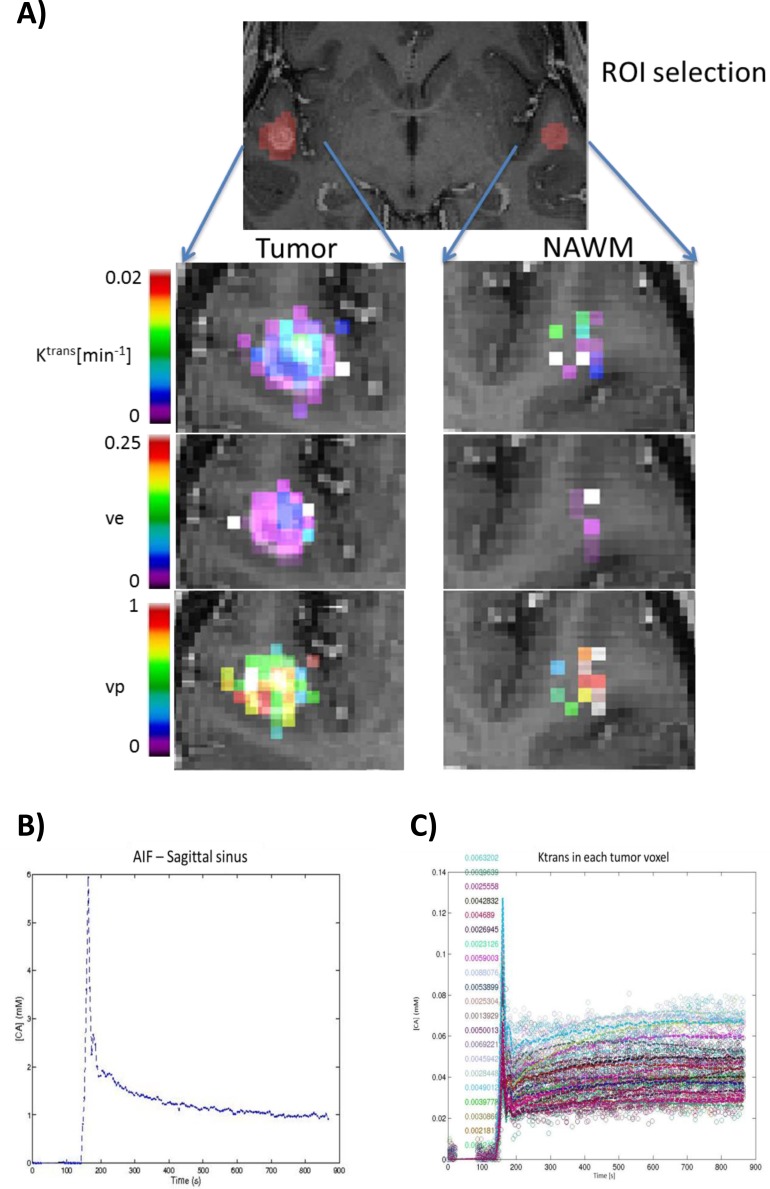
Figure 1. Sample parametric maps and time course of a tumor.
Using the Tofts and Kermode modeling of DCE-MRI data, A. parametric maps of Ktrans, ve and vp were obtained for Tumor and normal appearing white matter (NAWM). Note that when the fit did not converge (Ra2 < 50%), all the parameters are set to 0. This was the case for many voxels within the region of interest (ROI) defined as NAWM. Ktrans: transfer constant; ve: extravascular extracellular space (EES) fractional volume; vp: blood plasma volume. A sample time course of the constrast agent concentration [CA] within B. the sagittal sinus (AIF) and C. each Tumor voxel from Figure 1A.
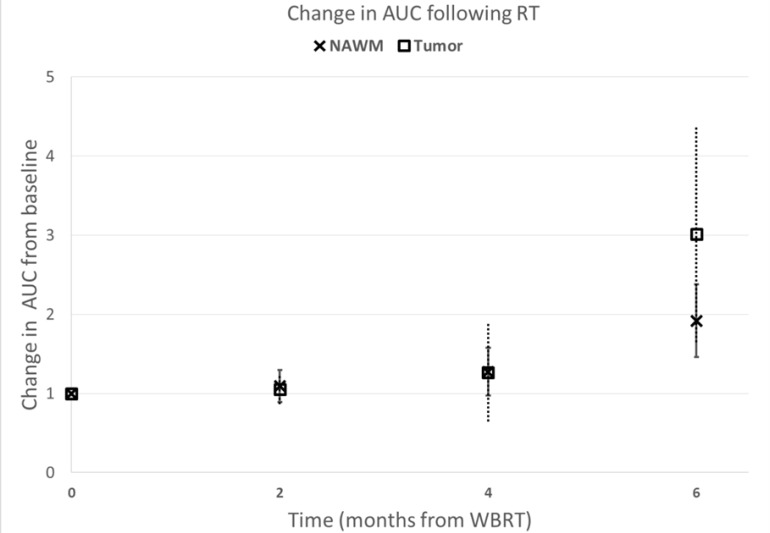
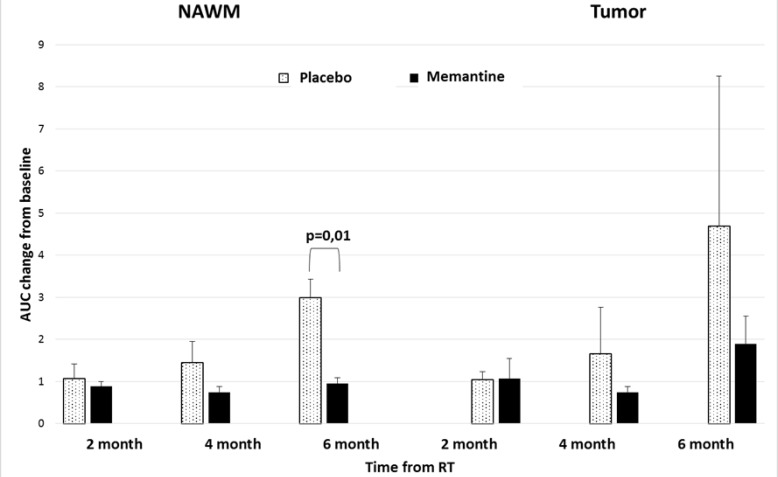
We observed a trend to suggest increased AUC of tumors (p = 0.09) and NAWM (p = 0.06) 6 months following WBRT initiation (Figure 2). We found that patients receiving memantine had significantly (p = 0.01) less NAWM AUC changes following radiotherapy than those who received placebo (Figure 3). When the 12 RTOG 0614 patients were analyzed separately, the memantine arm maintained a trend (p = 0.03) in the reduction in NAWM AUC changes following radiation as compared to the placebo arm.
Figure 2. Tumor and normal tissue vascular permeability changes following brain irradiation.
Contrast uptake following brain irradiation. Increase in contrast uptake (Area Under the uptake Curve (AUC)) of normal appearing white matter (NAWM) and tumor following whole brain irradiation (WBRT). Data were normalized to the AUC at baseline, prior to beginning WBRT. Error bars represent the standard error of the means.
Figure 3. Tissue contrast uptake after irradiation in relation to memantine use.
Contrast uptake (AUC) of normal appearing white matter (NAWM) and tumor in patients on placebo and memantine. NAWM of patients receiving memantine have reduced AUC changes following radiotherapy (p = 0.01) in comparison to patients receiving placebo. Error bars represent the standard error of the means.
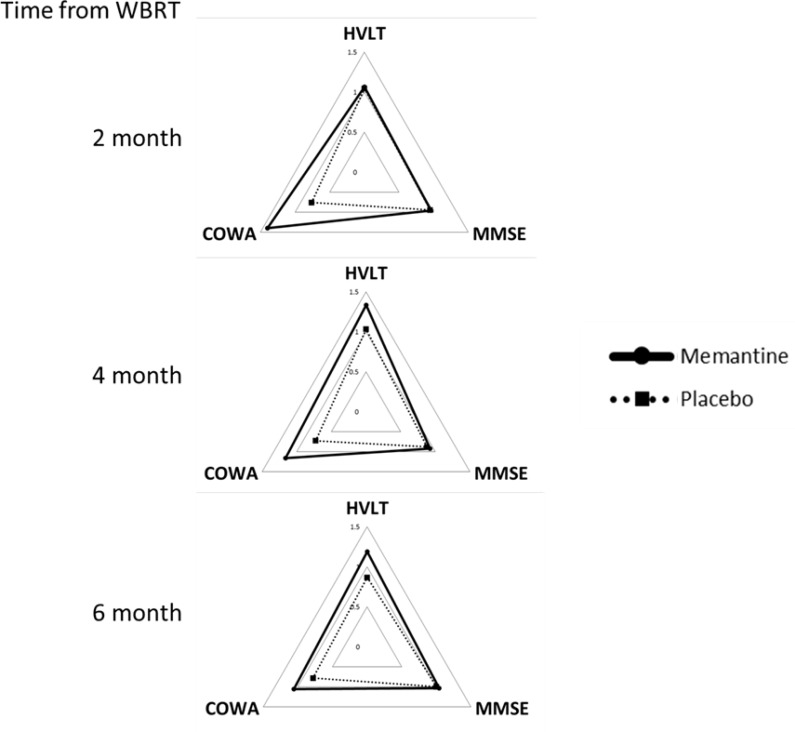
Consistent with the overall results from RTOG 0614, patients on memantine retained better cognitive functions (COWA p = 0.03) than those on placebo (Figure 4). HVLT (p = 0.10) measures were trending towards improvements in the memantine group as well. The cognitive results and their statistical significance remained the same when the analyses were repeated using RTOG 0614 patients only. QOL data were not compared as only 4 patients who had sequential MRIs completed more than 1 QOL questionnaire. Using blood samples collected prior to each MRIs, we explored the association between CEC and DCE-MRI AUC changes (Supplementary Figure 2). No association was observed between the level of CEC and DCE-MRI changes, time from radiotherapy or memantine use.
Figure 4. Neurocognitive functions following brain irradiation.
Neurocognitive functions following brain irradiation. Neurocognitive functions (HVTL, MMSE, and COWA) of patients at 2, 4 and 6 months from receiving whole brain radiotherapy (WBRT) with memantine (solid) or placebo (dotted). Data were normalized to individual patients' baseline values. Higher values represent better quality of life or neurocognitive functions; values > 1 represents an improvement from baseline.
DISCUSSION
In comparison to previous trials that examined the ability of DCE-MRI to detect BBB permeability changes following high-dose radiotherapy targeted at tumors, the current pilot study demonstrated that modest dose WBRT increases the vascular permeability in normal brain tissue (mean NAWM AUC increase from baseline: 1.9 fold; p = 0.06). Using an α/β = 2Gy for late glial cell toxicities [10], the mean normalized 2Gy equivalent biologically effective dose (nBED 2/2) of 37.5Gy given in 15 fractions is nBED2/2 = 42.2Gy. Our observation corroborated a previous publication that suggested that neurovascular permeability was increased following a threshold dose of 20Gy given in 30 fractions (nBED2/2 = 13.35Gy) [7]. Although the authors also observed a recovery in the vascular permeability of the brain parenchyma 6 months after radiotherapy, our placebo patients did not show any recovery in their NAWM AUC within the first 6 months after WBRT (Figure 3), suggesting a potential dose related effect to brain vasculature damage and recovery. Similarly, tumor contrast uptake increased over the first 6 months from WBRT.
The detrimental effect of brain radiotherapy is well documented, particularly in studies involving the treatment of childhood cancers [11]. In childhood cancer survivors, the neurocognitive morbidity of radiotherapy is associated with higher radiation dose, larger volume of irradiation, and younger age at the time of treatment. In adult patients, multiple studies examined the consequences of prophylactic cranial irradiation to patient reported QOL. Using EORTC QOL questionnaires, Slotman et al. and Le Pechoux et al. did not detect significant differences in self-reported cognitive functions between patients who received 25Gy/10 fractions (nBED2/2 = 28.13Gy) vs. observation [12] or 25Gy/10 fractions vs. 36Gy/18-24 fractions [13] (nBED2/2 = 31.5-36Gy), respectively. However, when outcomes were evaluated using standardized neurocognitive tests, Welzel et al. documented acute cognitive dysfunctions soon after WBRT is initiated that remained significant 2-4 weeks after the completion of WBRT [14]. Wolfson et al. similarly found radiation dose and age dependence in the development of neurocognitive defects [15]. Finally, Sun et al. found that in comparison with patients who were observed, patients who received prophylactic cranial irradiation (30Gy in 15 fractions; nBED2/2 = 30Gy) developed significant declines in immediate and delayed recall beginning at 3 months post-WBRT, with mild and incomplete recovery in the following months [16].
The mechanism by which radiation induces brain injury is likely secondary to injuries to multiple cell types, including vascular [17] and parenchymal cells such as neuronal and glial cells [6]. Radiation may induce vascular damage, which leads to ischemia that subsequently results in NMDA receptor stimulation and excitotoxicity. Memantine is a NMDA receptor competitive antagonist that is approved for the treatment of Alzheimer's disease and has shown mixed efficacies in the treatment of vascular dementia. The RTOG 0614 study demonstrated that starting at 8 weeks from WBRT, patients on memantine had less cognitive decline than the placebo cohort [5]. In our study, we observed that patients on memantine were also protected from radiation induced NAWM vasculature permeabilization (Figures 3–4). Our findings were in accordance with another preliminary study that suggested that DCE-MRI could be an imaging biomarker of changes in neurovasculature permeability and increased permeability following radiotherapy potentially predicted for the development of neurocognitive dysfunction following radiotherapy [7]. Similarly, DCE-MRI may be used to detect subtle BBB permeability changes in early neurodegenerative diseases such as Alzheimer's disease [18–20]. Furthermore, a pre-clinical study suggested that memantine reduced brain edema, BBB permeability, infarct volume and neurological deficits when it is given 15 min following middle cerebral artery occlusion in rats [21]. These prior studies corroborate with our results that suggested that memantine not only protected neurons from radiation-induced excitotoxicity, but might also protect the cerebral vasculature from radiation damages.
As shown in Supplementary Figure 3 and 4, the genes (GRIN1, GRIN2A, GRIN2B, GRIN2C, and GRIN2D) and protein of NMDA receptors subunits are widely expressed in different normal tissues and cells, including endothelial cells. Preclinical studies suggested that stimulation of cerebral endothelial cell NMDA receptors could induce vasodilation of brain arteries or excitotoxicity and breakdown of the BBB [22]. Therefore, the neuroprotective effects of memantine might be secondary to the combined reduction in neuronal and endothelial cell deaths from irradiation.
In our study, there was no significant (p = 0.20) difference in overall survival of patients on memantine or placebo. There was no significant correlation between the vascular permeability of tumors or NAWM at any time point with overall survival. The majority (78.6%) of our patients had lung or breast cancers, which is in keeping with the patient population in RTOG 0614 (84.6% lung and breast cancers). Results from the RTOG 0614 [5] trial demonstrated that patients who received memantine (n = 256) or placebo (n = 252) had similar median progression free survival (4.7 vs. 5.5 months; p = 0.27) and overall survival (6.7 vs. 7.8 months; p = 0.28). There was also no additional toxicity observed among patients who received memantine vs. placebo. Therefore, use of memantine and its neurovascular protective effect did not seem to affect tumor control, patient survival or toxicities.
Due to the small number of patients in this pilot study, our results need to be confirmed using a larger cohort of patients. Also, data from this small patient cohort did not allow us to determine whether surgery and/or radiosurgery influenced the vascular permeability of tumors. Our imaging methodologies could be modified to study the radiotherapy effects to specific brain structures, such as the hippocampus by increasing the spatial resolution of the DCE-MRI acquisition at this structure and by using a power injector. As the gene and protein expressions of different NMDA receptors vary between brain structures (Supplementary Figure 3 and 4), the benefit of memantine may differ between tissues and cell types [23, 24]. Furthermore, as many long-term toxicities secondary to radiation therapy are related to the de-vascularization of normal tissues, the use of memantine as an adjunct to radiotherapy may reduce extra-cerebral endothelial cell injuries and the rate of chronic radiation side-effects in a broad range of patients.
In conclusion, DCE-MRI represents an established functional imaging method employed in evaluating vascular permeability changes in tissues and tumors. Radiation therapy induces vasculature damages and many long-term toxicities are related to the de-vascularization of normal tissues. Our pilot study suggests that DCE-MRI could detect BBB vascular damages secondary to WBRT and could be used as a biomarker of neurocognitive dysfunction. Our study also suggested that memantine reduced WBRT damages to neurons and endothelial cells. Future studies should aim at confirming the usefulness of DCE-MRI as a clinical imaging biomarker of tissue toxicity from radiotherapy. The use of memantine as a potential radioprotector for vascular tissues may be explored to reduce radiation induced side-effects.
PATIENTS AND METHODS
Patients
Fourteen adult patients with a pathologically proven diagnosis of solid malignancy within 5 years of registration and with brain metastases visible on contrast-enhanced MRI were recruited to the study. One patient died before WBRT was started. Eligibility criteria included a Karnofsky performance status of ≥ 70, stable systemic disease in the 3 months prior to study entry, serum creatinine ≤ 3 mg/dL, creatinine clearance ≥ 30 mL/min, total bilirubin ≤ 2.5 mg/dL, blood urea nitrogen (BUN) ≤ 20 mg/dL, Mini Mental State Exam (MMSE) score ≥ 18 within 28 days of study entry, no current alcohol or drug abuse, no chronic use of benzodiazepines, and no severe active comorbidity. Patients could have received prior therapy for brain metastasis, including radiosurgery and surgical resection (but no prior cranial external beam radiotherapy). Patients receiving systemic therapy were eligible if such therapy was given > 14 days prior to study entry, and they could not receive chemotherapy for at least 14 days after completing radiotherapy. Table 1 summarizes the patients' characteristics. The study was approved by the McGill University Health Centre Research Ethics Board. All patients provided written informed consent.
Treatment
All 12 patients concurrently randomized on RTOG 0614 were treated with WBRT consisting of 37.5 Gy in 15 fractions, delivered using 2 parallel-opposed fields with or without subfields. Of the 2 patients not on RTOG 0614, patient #3 received 30 Gy in 12 fractions and patient #10 received 37.5 Gy in 15 fractions, delivered using 2 parallel-opposed fields with or without subfields (Table 1). Patients enrolled in the RTOG 0614 trial were randomly assigned to received placebo (n = 5) or memantine (n = 7) at the initiation of WBRT. Patient on memantine received 5mg on week 1, increasing by 5mg every week until the target dose of 10mg twice daily, and was then maintained at this dose for a total of 24 weeks [5]. Patients not on the RTOG0614 did not receive placebo or memantine; for the purpose of analysis, data from these patients were combined with those who received placebo. Following the completion and publication of final data from the RTOG 0614 study, we obtained permission from the RTOG to unblind the treatment (placebo or memantine) that patients received during and after WBRT. One of the 14 patients died prior to receiving the first fraction of WBRT.
Imaging
Patients underwent MRI's prior to WBRT (within 28 days from start of WBRT), then at 8-weeks, 16-weeks and 24-weeks after the initiation of WBRT.
Timing and MRI sequence acquisition were modified from the methods described by Roberts et al. [25]. All images were acquired on a Siemens 3T Tim Trio with a maximum gradient strength of 40mT/m and a slew rate of 200 mT/m/ms, using a Siemens 32-channel head coil.
Briefly, a sagittal T2-weighted scout scan was obtained to plan a whole brain T1-weigthed MPRAGE (matrix: 256×256×176, 1mm3 isotropic resolution, TR/TE = 2.3s/3.4ms, FA = 90), localize the tumor, and plan the dynamic series. For DCE-MRI, a 3D-FLASH sequence was obtained with the following parameters: TR/TE = 4.4/1.5ms; flip angle: 25°; matrix: 128 × 128, 22 slices; resolution: 2×2×6 mm3; BW = 1775Hz/px,; GRAPPA factor 2. This 3D data set was acquired every 6 secs, starting before intravenous bolus administration of a single dose of contrast material (0.1 mmol/kg Gadovist®, Bayer Inc.) and continuing for the subsequent 15 minutes, for a total of 155 dynamic images. The contrast agent was administered manually as a bolus injection and chased with 30 ml of saline. At the completion of 15 minutes of DCE-MRI, a second, post-contrast MPRAGE, matched to the first acquisition, was acquired. All kinetic images and post-contrast MPRAGE were registered to the initial pre-contrast MPRAGE using the MINC software tools (http://packages.bic.mni.mcgill.ca/tgz). Six-parameter linear registration using mutual information was computed between the first dynamic image and the MPRAGE anatomical. Then each image from the dynamic series was registered to the first image using a 6-parameter linear transformation using cross-correlation. The transformations from these two linear registrations were concatenated and applied to each dynamic image so that all frames were registered to the high resolution anatomical.
3D-FLASH images were acquired for purposes of T1-mapping using the DESPOT1 technique (FA = 2°/10°, 128×128×22 matrix, resolution 2×2×6mm3) [26] (Supplementary Figure 1). The T1-weighted time series was used together with the equilibrium magnetization (M0) map calculated with DESPOT1 to generate quantitative T1 4D volumes and were subsequently converted to concentration using the pre-contrast T1 (T10) maps and contrast agent relaxivity in the plasma (values at 3T, 37°C, pH = 7, in plasma: r1(Gd-BT-DO3A) = 5.0±0.3 mM-1s-1 [27–29]).
All regions of interests (ROI) (tumor, NAWM, sagittal sinus) were manually segmented on the post-contrast MPRAGE by PW. The area of tumor was defined as the contrast enhancing volumes (using the pre and post-injection MPRAGE scans) if the tumor was not resected. If multiple tumors were present, each tumor was segmented as an individual ROI. In the case of resected tumors, any contrast-enhancement surrounding the tumor cavity was contoured. A parenchymal area of the brain contralateral to the tumor, without contrast enhancement on post-injection MPRAGE scans, was considered as NAWM. A segment of the superior sagittal sinus was contoured using contrast-enhanced images (3D-FLASH and MPRAGE sequences) and used for measuring the vascular time course of the contrast agent concentration. Although signals from the sagittal sinus are not arterial, the large size of this vein reduces experimental/analytical partial volume effects. This methodology has been shown to be highly reproducible [30, 31] and a good surrogate for the arterial input function [32, 33].
Concentrations over time were computed using a Matlab (Mathworks, Natick, MA) program developed in-house, and the sagittal sinus ROI was used to define the blood input function. The signal intensity changes (signal intensity after contrast agent administration minus that before contrast agent administration) were calculated for the ROIs for each post-injection time point, and their time courses were used for subsequent kinetic analyses.
Vascular permeability was evaluated using the semi-quantitative Initial Area Under the Curve (AUC) Blood Adjusted method [34, 35], which was calculated as the area under the ROI signal intensity curve from the time of injection to 90 seconds post-injection, divided by the area under the blood concentration curve for the same period of time. This methodology provided simple and robust measurements that did not require model fitting and has been recommended as a primary measure in MRI trials of oncology therapeutics [9]. We also explored the traditional kinetic modeling methods described by Tofts et al [36]. Specifically, a bidirectional 3-compartment model based on the equations of Tofts and Kermode [36], which yielded estimates of fractional tissue blood volume (in milliliters per cubic centimeters), plasma volume, and microvascular permeability, which was expressed as the transendothelial transfer constant, Ktrans, was also evaluated. An example of parametric maps of Ktrans, ve and vp and the corresponding Ktrans of tumor voxels obtained from the Tofts and Kermode modeling are shown in Figure 1.
Quality of life and neurocognitive assessments
At each MRI time point, cognitive, quality-of-life (QOL) assessment and blood samples were collected according to the patient's ability to tolerate the exams. Cognitive assessments were done using the MMSE, Hopkins Verbal Learning Test (HVLT), and Controlled Oral Word Association Test (COWA). QOL assessments were made using validated questionnaires: FACT-G and FACT-Br.
Circulating endothelial cell assessments
Circulating endothelial cells (CEC) were measured as per the protocol described by Duda et al. [37]. Briefly, patient blood was collected prior to MRI imaging using tubes containing EDTA. Fluorescently labelled antibodies were purchased from BD Pharmingen: CD31-FITC, CD34-APC, CD133-PE, CD45-PerCP, Fluorescently labeled isotype-matched IgG1 antibodies, and VEGFR2 (KDR)-PE. Cells from the buffy coat were fixed, labeled using the above listed antibodies, then sorted using the FACSCalibur flow cytometer (Becton Dickinson).
Analysis plan
This study was a prospective pilot study with the aim of recruiting up to 15 patients in order to determine the feasibility and ability of DCE-MRI in detecting vascular permeability changes secondary to WBRT. With no prior evidence of DCE-MRI's capacity to detect WBRT-related NAWM neurovascular changes at the time of the trial design, the study was not pre-designed with statistical power estimations. As Cao et al. observed an initial increase in vascular permeability followed by a gradual recovery after the completion of radiotherapy [7], a linear change in vascular permeability over time could not be inferred; hence linear regression and slope analysis might not be appropriate for our data. Therefore, the analysis consisted of comparing the vascular permeability in tumor and NAWM before vs. after WBRT (3 time points within 6 months from the start of WBRT) using the paired student t-test to compare baseline data with 2, 4 and 6 months MRI data. Coefficient of variations of DCE-MRI measurements (Ktrans and AUC) were calculated (Cv = Standard deviation/Mean) and compared using ANOVA. Comparison between placebo and memantine for MRI and neurocognitive results were made using the student t-test at each time point. Changes in CEC levels were analyzed using the spearman correlation test to determine its association with MRI data. All analyses were two-sided and a P-value of < 0.05 is considered as statistically significant. The Bonferroni correction was applied to the change in vascular permeability analyses of 3 different time points (2, 4 and 6 months) such that a P-value of < 0.017 is considered statistically significant.
SUPPLEMENTARY MATERIALS FIGURES
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest to disclose.
GRANT SUPPORT
Funding was provided by the Cedars Cancer Institute H. R. Shibata Cedars Scholarship Award to PW and CM.
Gadovist® was provided in kind by Bayer Inc.
Editorial note
This paper has been accepted based in part on peerreview conducted by another journal and the authors' response and revisions as well as expedited peer-review in Oncotarget.
REFERENCES
- 1.Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016 doi: 10.18632/oncotarget.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin X, DeAngelis LM. Treatment of Brain Metastases. Journal of clinical oncology. 2015;33(30):3475–3484. doi: 10.1200/JCO.2015.60.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM, Kalkanis SN, Whitsett TG, Salhia B, Tran NL, Ryken T, Moore MK, Egan KM, Olson JJ. Current approaches to the treatment of metastatic brain tumours. Nature reviews Clinical oncology. 2014;11(4):203–222. doi: 10.1038/nrclinonc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Choucair A, Fox S, Suh JH, Roberge D, Kavadi V, Bentzen SM, Mehta MP, Watkins-Bruner D, Radiation Therapy Oncology G Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized double-blind placebo-controlled trial. Neuro-oncology. 2013;15(10):1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belka C, Budach W, Kortmann RD, Bamberg M. Radiation induced CNS toxicity--molecular and cellular mechanisms. British journal of cancer. 2001;85(9):1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Tsien CI, Sundgren PC, Nagesh V, Normolle D, Buchtel H, Junck L, Lawrence TS. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for prediction of radiation-induced neurocognitive dysfunction. Clinical cancer research. 2009;15(5):1747–1754. doi: 10.1158/1078-0432.CCR-08-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachos F, Tung YS, Konofagou E. Permeability dependence study of the focused ultrasound-induced blood-brain barrier opening at distinct pressures and microbubble diameters using DCE-MRI. Magnetic resonance in medicine. 2011;66(3):821–830. doi: 10.1002/mrm.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, Jayson GC, Judson IR, Knopp MV, Maxwell RJ, McIntyre D, Padhani AR, Price P, Rathbone R, Rustin GJ, Tofts PS, et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. British journal of cancer. 2005;92(9):1599–1610. doi: 10.1038/sj.bjc.6602550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. The British journal of radiology. 1989;62(740):679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 11.Padovani L, Andre N, Constine LS, Muracciole X. Neurocognitive function after radiotherapy for paediatric brain tumours. Nature reviews Neurology. 2012;8(10):578–588. doi: 10.1038/nrneurol.2012.182. [DOI] [PubMed] [Google Scholar]
- 12.Slotman BJ, Mauer ME, Bottomley A, Faivre-Finn C, Kramer GW, Rankin EM, Snee M, Hatton M, Postmus PE, Collette L, Senan S. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. Journal of clinical oncology. 2009;27(1):78–84. doi: 10.1200/JCO.2008.17.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Pechoux C, Laplanche A, Faivre-Finn C, Ciuleanu T, Wanders R, Lerouge D, Keus R, Hatton M, Videtic GM, Senan S, Wolfson A, Jones R, Arriagada R, Quoix E, Dunant A, Prophylactic Cranial Irradiation Collaborative G Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01 EORTC 22003-08004 RTOG 0212 and IFCT 99-01) Annals of oncology. 2011;22(5):1154–1163. doi: 10.1093/annonc/mdq576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welzel G, Fleckenstein K, Schaefer J, Hermann B, Kraus-Tiefenbacher U, Mai SK, Wenz F. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. International journal of radiation oncology biology physics. 2008;72(5):1311–1318. doi: 10.1016/j.ijrobp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, Werner-Wasik M, Videtic GM, Garces YI, Choy H. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. International journal of radiation oncology biology physics. 2011;81(1):77–84. doi: 10.1016/j.ijrobp.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun A, Bae K, Gore EM, Movsas B, Wong SJ, Meyers CA, Bonner JA, Schild SE, Gaspar LE, Bogart JA, Werner-Wasik M, Choy H. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. Journal of clinical oncology. 2011;29(3):279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiation research. 2010;174(6):865–869. doi: 10.1667/RR1862.1. [DOI] [PubMed] [Google Scholar]
- 18.Barnes SR, Ng TS, Montagne A, Law M, Zlokovic BV, Jacobs RE. Optimal acquisition and modeling parameters for accurate assessment of low K blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magnetic resonance in medicine. 2015 doi: 10.1002/mrm.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer's disease: a case-control MRI study. Psychiatry Res. 2009;171(3):232–241. doi: 10.1016/j.pscychresns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Golob EJ, Su MY. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. Journal of magnetic resonance imaging. 2006;24(3):695–700. doi: 10.1002/jmri.20669. [DOI] [PubMed] [Google Scholar]
- 21.Gorgulu A, Kins T, Cobanoglu S, Unal F, Izgi NI, Yanik B, Kucuk M. Reduction of edema and infarction by Memantine and MK-801 after focal cerebral ischaemia and reperfusion in rat. Acta Neurochir (Wien) 2000;142(11):1287–1292. doi: 10.1007/s007010070027. [DOI] [PubMed] [Google Scholar]
- 22.Sharp CD, Hines I, Houghton J, Warren A, Jackson THt, Jawahar A, Nanda A, Elrod JW, Long A, Chi A, Minagar A, Alexander JS. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. American journal of physiology Heart and circulatory physiology. 2003;285(6):H2592–2598. doi: 10.1152/ajpheart.00520.2003. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Macleod I, Su AI. BioGPS and MyGene info: organizing online gene-centric information. Nucleic acids research. 2013;41(Database issue):D561–565. doi: 10.1093/nar/gks1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Bjorling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nature biotechnology. 2010;28(12):1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 25.Roberts HC, Roberts TP, Brasch RC, Dillon WP. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR American journal of neuroradiology. 2000;21(5):891–899. [PMC free article] [PubMed] [Google Scholar]
- 26.Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med. 2003;49(3):515–526. doi: 10.1002/mrm.10407. [DOI] [PubMed] [Google Scholar]
- 27.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann H-J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investigative Radiology. 2005;40(11):715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 28.Zhu XP, Li KL, Kamaly-Asl ID, Checkley DR, Tessier JJ, Waterton JC, Jackson A. Quantification of endothelial permeability leakage space and blood volume in brain tumors using combined T1 and T2* contrast-enhanced dynamic MR imaging. Journal of magnetic resonance imaging. 2000;11(6):575–585. doi: 10.1002/1522-2586(200006)11:6<575::aid-jmri2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhu XP, Li KL, Waterton J, Tessier JJL, Checkley D, Jones A, Kamaly-Asl ID, Jackson A. 3D T1 Mapping by Means of Fast Field Echo Technique. 1999; 8th ISMRM Annual Meeting; Denver. p. 2143. [Google Scholar]
- 30.Lavini C, Verhoeff JJ. Reproducibility of the gadolinium concentration measurements and of the fitting parameters of the vascular input function in the superior sagittal sinus in a patient population. Magnetic resonance imaging. 2010;28(10):1420–1430. doi: 10.1016/j.mri.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Wu C, Chiang C, Chen C, Chai J. Consistency of permeability measurement using arterial input function and venous output function in DCE-MRI for metastatic brain tumors. 2012; 20th Annual Meeting of ISMRM; Melbourne. p. 3508. [Google Scholar]
- 32.Foottit C, Cron GO, Hogan MJ, Nguyen TB, Cameron I. Determination of the venous output function from MR signal phase: feasibility for quantitative DCE-MRI in human brain. Magnetic resonance in medicine. 2010;63(3):772–781. doi: 10.1002/mrm.22253. [DOI] [PubMed] [Google Scholar]
- 33.Foottit C, Cron GO, Mercier JF, Nguyen TV, Cameron I, Schweitzer ME, Sinclair J, Woulfe J, Hogan MJ, Nguyen TB. Optimizing perfusion imaging of brain tumors: Validation of venous output function used as a surrogate AIF. 2011; 19th Annual Meeting of ISMRM; Montreal. p. 2038. [Google Scholar]
- 34.Evelhoch JL. Key factors in the acquisition of contrast kinetic data for oncology. Journal of magnetic resonance imaging: JMRI. 1999;10(3):254–259. doi: 10.1002/(sici)1522-2586(199909)10:3<254::aid-jmri5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Port RE, Bernstein LJ, Barboriak DP, Xu L, Roberts TP, van Bruggen N. Noncompartmental kinetic analysis of DCE-MRI data from malignant tumors: Application to glioblastoma treated with bevacizumab. Magnetic resonance in medicine. 2010;64(2):408–417. doi: 10.1002/mrm.22399. [DOI] [PubMed] [Google Scholar]
- 36.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. Journal of magnetic resonance imaging. 1999;10(3):223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 37.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nature protocols. 2007;2(4):805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.