Abstract
Promising targeted treatments and immunotherapy strategies in oncology and advancements in our understanding of molecular pathways that underpin cancer development have reignited interest in the tumor-associated antigen Folate Receptor alpha (FRα). FRα is a glycosylphosphatidylinositol (GPI)-anchored membrane protein. Its overexpression in tumors such as ovarian, breast and lung cancers, low and restricted distribution in normal tissues, alongside emerging insights into tumor-promoting functions and association of expression with patient prognosis, together render FRα an attractive therapeutic target. In this review, we summarize the role of FRα in cancer development, we consider FRα as a potential diagnostic and prognostic tool, and we discuss different targeted treatment approaches with a specific focus on monoclonal antibodies. Renewed attention to FRα may point to novel individualized treatment approaches to improve the clinical management of patient groups that do not adequately benefit from current conventional therapies.
Keywords: folate receptor alpha, cancer, biomarker, monoclonal antibodies, immunotherapy
INTRODUCTION
Water soluble vitamin B9 can occur as ‘folate’ (enriched in dark leafy vegetables) and as ‘folic acid’ (a synthetic folate compound used as a vitamin supplement). A sufficient intake of folate is needed in rapidly proliferating cells for the one-carbon metabolic reaction and DNA biosynthesis, repair and methylation [1]. Dysregulated folate metabolism has been associated with embryonic developmental disorders, cardiovascular disease and brain defects [2–4]. Folate is transported across the cellular membrane in three ways. The main route of uptake is through the reduced folate carrier (RFC), which is ubiquitously distributed and aids the uptake of dietary folate [5]. The second route is through the proton-coupled folate transporter (PCFT), which utilizes the transmembrane proton gradient to mediate folate transport into the cells [6]. Finally, folate can be transported by folate receptors, of which there are four glycopolypeptide members (FRα, FRβ, FRγ and FRδ), with molecular weights ranging from 38 to 45 kDa [7]. The alpha isoform, Folate Receptor α (FRα), also known as FOLR1 or folate binding protein (FBP), is a glycosylphosphatidylinositol (GPI)-anchored membrane protein with high affinity for binding and coordinating transport of the active form of folate, 5-methyltetrahydrofolate (5-MTF) [8, 9].
FRα has been reported to be overexpressed in solid tumors such as ovarian, lung and breast carcinomas [10–12]. On the other hand, the distribution of FRα in normal human tissues is restricted to low level expression in the apical surfaces of some organs such as the kidney, lung and choroid plexus [9]. Studies have demonstrated that overexpression of FRα may render a growth advantage for cancer cells through mechanisms both relating to, as well as being independent of, folate uptake [13, 14].
Here, we review FRα as a folate carrier and a tumor-associated signalling molecule in relation to its potential as a target, and as a diagnostic and prognostic tool, in oncology. We describe examples of past and present treatment approaches and promising therapeutic avenues, with specific focus on monoclonal antibodies for ovarian, lung and breast cancer, as examples of FRα-positive malignancies.
FRα-MEDIATED INTERNALIZATION OF FOLATES AND REGULATION OF CANCER SIGNALING
Traditionally FRα is described as a transporter to internalize folate. Folate trafficking via FRα is thought to occur by a non-classical lipid raft-mediated endocytosis pathway, namely potocytosis, which does not involve clathrin-coated pits. This pathway is associated with caveolae vesicles [15]. Folate binds specifically to FRα creating a receptor-ligand complex; then through invagination and budding off, intracellular vesicles are formed. Once internalized, the vesicles uncoat and single vesicles join together forming early endosomes, which undergo acidification and subsequent fusion with lysosomes to release folates for the one-carbon metabolic reaction [16, 17].
FRα overexpression in different solid tumors can potentially contribute to cancer development in different ways. A number of studies have suggested parallel roles of FRα in both cell growth regulation and signaling functions. Boshnjaku et al. reported that following folate uptake and internalization, FRα can then translocate to the nucleus and act as a transcription factor, binding to cis-regulatory elements. Through this mechanism, FRα may directly regulate the expression of key developmental genes in cancer cells [18]. Ovarian cancer cells transfected with a single-chain intrabody targeting FRα showed reduced cell surface expression and subsequent impaired tumor cell proliferation, reduced colony formation, and dysregulated adhesion; together signs of reversing tumor cell transformed phenotype [19]. Furthermore, folate uptake can promote cancer cell proliferation, migration and loss of adhesion through downregulation of the cell-cell adhesion molecule, E-cadherin, promoting cellular motility and metastasis. In concordance, it was also reported that the absence of E-cadherin expression correlated with decreased patient survival in ovarian carcinomas [20]. FRα knockdown in ovarian carcinoma cell lines could inhibit folate-mediated cell proliferation and suppress an invasive phenotype [14]. FRα has also been demonstrated to inhibit caveolin-1, thereby supporting anchorage-independent growth and proliferation of tumor cells and promoting cancer progression [21, 22].
More recently, FRα has been demonstrated to contribute to cancer malignancy by acting as a signaling molecule. Similarly to other GPI family proteins, FRα is thought to initiate intracellular regulatory signaling networks upon binding with folate. FRα overexpression has been reported to be associated with increased STAT3 signaling [23]. Hansen et al. demonstrated that folate binding to FRα could induce STAT3 activation via a GP130 co-receptor-mediated JAK-dependent process [24]. Moreover, phosphorylated LYN tyrosine kinase was found in anti-FR mAb precipitates of FRα-expressing tumor cell lysates [25]. These findings suggest the receptor has the potential to form macromolecular complexes in which FRα can trigger intracellular signaling. The basal subtype of breast cancers have been observed to express high levels of LYN, which has been reported to regulate the phosphorylation of a non-receptor tyrosine kinase, PEAK1, to promote ERK and STAT3 activation, as well as to support cellular transition to a mesenchymal phenotype, increasing cell motility and invasion [26]. Furthermore, FRα overexpression is frequently reported to be expressed in metastatic foci and recurrent tumors [27], even in microenvironments with limited folate availability.
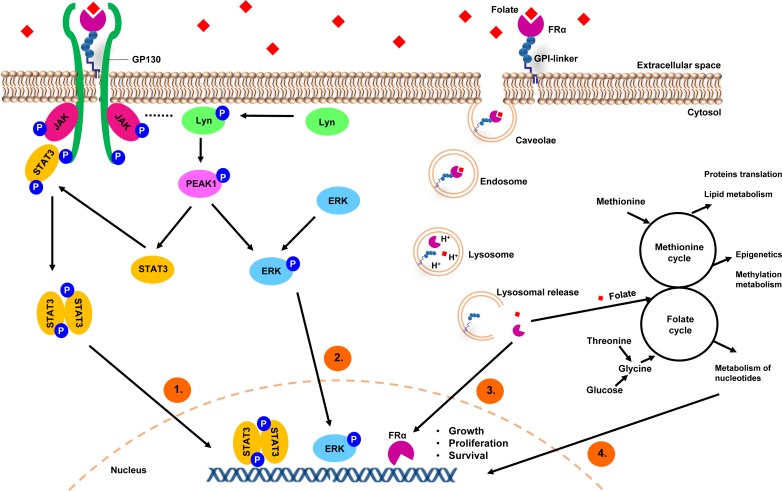
Together, these studies strongly suggest that FRα may function not only as a folate transporter, but may also confer signaling and growth advantages on malignant cells (as depicted in Figure 1) [28].
Figure 1. A model depicting FRα-mediated internalization of folates and regulation of cancer signaling.
1) Folate binding to FRα could induce STAT3 activation via a GP130 co-receptor mediated JAK-dependent process. 2) FRα may form macromolecular complexes with LYN tyrosine kinase, which has been reported to regulate the phosphorylation of PEAK1 to promote ERK and STAT3 activation. 3) GPI-anchored FRα is internalized in caveolae vesicles and forms early endosomes, which undergo acidification and subsequent fusion with lysosomes to release FRα and folate. FRα is then translocated to the nucleus and acts directly as a transcription factor. 4) FRα acts as a folate transporter; a sufficient intake of folate is needed in rapidly proliferating cells for the one-carbon metabolic reaction and DNA biosynthesis, repair and methylation.
FRα: A THERAPEUTIC TARGET, DIAGNOSTIC AND/OR PROGNOSTIC TOOL FOR THE MANAGEMENT OF SOLID TUMORS
FRα expression in non-malignant tissues
FRα expression has been examined by several methods including immunohistochemistry using monoclonal antibodies, folate ligand binding assays, measurement of mRNA levels by qPCR, and by flow cytometry [8, 29–31]. Expression of FRα in normal tissues is restricted to the luminal surface of the kidney, intestine, lung, retina, placenta and choroid plexus [30, 32, 33]. Importantly, in all normal tissues except the kidneys, the receptor is confined to the apical surface of the epithelium that is out of direct contact with folate and any folate receptor-targeting agents in the circulation [34, 35]. The FRα expressed in the kidneys functions as a salvage receptor to retrieve and prevent loss of folate in the urine. However, folate is not retained in the kidneys, and as such no lethal toxicities have been observed in rodent or human subjects treated with FRα-targeting agents [35, 36].
FRα expression in epithelial tumors
Cancers found to overexpress FRα are often of epithelial origin, including cancers of the ovary, breast, pleura, lung, cervix, endometrium, kidney, bladder and brain [29, 37]. FOLR1 mRNA expression levels for various types of cancer have been studied (expression levels in cell line panels summarized in Figure 2) [38].
Figure 2. FOLR1 gene expression levels in cancer models.
A summary of gene expression data (generated from the Cancer Cell Line Encyclopaedia) showing the levels of mRNA expression for the FOLR1 gene on a logarithmic scale in various cancer cell types. (parentheses show number of cell lines per tumor type)
Ovarian cancer
In ovarian cancer, FRα is overexpressed in 80% of epithelial ovarian cancers (EOCs) and expression has been shown to significantly correlate with histological grade and stage [10, 14]. The expression of FRα is considered a marker of tumor aggressiveness, and although there is conflicting data when all ovarian carcinoma histotypes are considered, elevated FRα expression is associated with lower disease-free interval (DFI) and poor overall survival (OS) in patients with disease of serous origin [27, 39].
FRα is also thought to facilitate resistance to chemotherapy in ovarian carcinoma patients, with higher tissue FRα expression associated with lower response rate to chemotherapeutic agents [28, 39, 40]. Nevertheless, a number of studies have shown that FRα tumor surface expression does not differ between paired samples before and after chemotherapy. This suggests that chemotherapeutic agents do not affect antigen expression and FRα-positivity can be used to detect recurrent disease [41, 42] and strengthen the rationale for the potential utility of FRα-targeting therapeutics for both newly diagnosed and recurrent disease.
Lung cancer
Non-small cell lung cancer (NSCLC) accounts for 80% of lung cancers. Despite advances in surgical techniques, radiotherapeutic approaches and chemotherapy regimens, and the development of novel molecular targeted agents, the prognosis is poor with a 5-year all-stage survival rate of 21% [11]. Both common subtypes of NSCLC, adenocarcinoma and squamous cell carcinomas (SCCs), to a lesser degree, have been reported to express high levels of FRα [43–46]. The level of FRα expression by tumor cells was shown to be associated with improved OS in patients with resected adenocarcinomas [46]. Similarly, in another study, higher FRα expression levels were observed in well-differentiated, earlier stage lung tumors compared to more poorly-differentiated tumors from advanced stage adenocarcinoma patients, and higher FOLR1 gene expression levels correlated with significantly higher 3-year disease free survival (DFS) and OS rates in this cohort as well [44]. Most recently, the validity of FRα-targeting therapies, has been strengthened by observed concordance of high FRα levels between biopsies, primary tumors and metastases in these patients [11].
Overexpression of FRα has also been detected in up to 72% of patients with another uncommon and aggressive form of thoracic cancer, pleural mesothelioma [47, 48]. However, more studies are required to evaluate the diagnostic and prognostic significance of tumor FRα expression in mesothelioma.
Breast cancer
A number of studies have reported overexpression of FRα in breast cancers [12, 49]. Most recently, high FRα tissue expression was observed in 74% of estrogen receptor (ER)/progesterone receptor (PR)-negative breast cancers [13, 50–52]. Steroid hormones mediate physiologically normal FRα regulation; in particular, oestrogen has been found to downregulate the expression of FRα. This has been demonstrated in studies that report a negative correlation between tumors that express ER and those which express FRα [13, 52]. Supporting this notion, drugs such as tamoxifen, which bind and inhibit ER function, can also cause a rise in FRα expression [53]. Triple negative breast cancer (TNBC), which is characterized by the lack of ER, PR and human epidermal growth factor receptor 2 (HER2), represent 10-15% of all breast carcinomas. Despite representing a minority of breast cancer cases, this particular subtype accounts for a disproportionate number of cases of metastatic disease and deaths, due to its more aggressive natural history and lack of available targeted therapies [54, 55]. High FRα tissue expression was observed in 80% of TNBCs, making it an attractive therapeutic target in this breast cancer type [51].
FRα expression by breast carcinomas, including TNBCs, is significantly associated with high histologic grade and advanced stage, and high proliferative activity as determined by expression of Ki-67. Overexpression of FRα was also significantly associated with poorer disease-free survival [49, 52]. In addition, correlation of high FRα expression between primary tumors and local and distant metastases has been observed, suggesting that most metastatic disease could potentially be treated with anti-FRα therapies when the primary tumor shows overexpression.
Overall, these studies, across a number of cancer cell types, suggest that targeting this tumor-associated antigen may offer clinical benefit to patients with unmet need for novel therapeutic options. The low and restricted distribution of FRα in normal tissues, alongside the presence of other folate transporters, which can process folates, may suggest that targeting FRα would not restrict all folate uptake, and may not affect normal cell survival or proliferation nor impair normal tissue homeostasis. Overexpression of FRα in the blood-accessible basal and lateral membranes of epithelial carcinomas, and suggested contributions to tumor cell growth and proliferation, together point to targeting FRα as an attractive and potentially non-toxic treatment approach.
Soluble FRα
Membrane-associated FRα can also be released by proteolytic cleavage with membrane-associated protease and GPI-specific phospholipases [9, 56]. FRα is detected as a soluble form in the sera of some cancer patients by a number of techniques, including microfiltration, immunoblotting, electrochemiluminescence, and ELISA [23, 57–59]. Soluble FRα (sFRα) is reported to be low or undetected in normal human sera [23, 58]. A number of studies of the serum from patients with both serous and non-serous ovarian carcinomas have demonstrated elevated levels of sFRα, compared to healthy individuals. Importantly for the potential use of sFRα as a diagnostic biomarker, elevated levels of sFRα were observed even in early stage I/II disease [23, 59]. Furthermore, levels of sFRα have been reported to correlate with FIGO stage, histological types and tumor grade [57, 59]. Most recently, in a cohort of 221 patients, the prognostic significance of sFRα was supported by findings that patients with lower sFRα levels had significantly longer progression-free survival (PFS) than those with high serum levels. A trend in increasing sFRα and tumor expression levels measured by immunohistochemistry was also reported, suggesting that sFRα may offer a minimally-invasive alternative to predict local tumor FRα positivity and may present a surrogate marker for FRα tumor expression [57].
Studies are still needed to examine sFRα levels in the serum of patients with breast and lung cancers in order to evaluate its potential as a diagnostic and prognostic biomarker across cancer types and in those individuals where other clinically-used serum biomarkers are absent or unhelpful in monitoring or predicting disease progression. In addition, studies are required to evaluate the potential inhibitory impact of high sFRα levels on the efficacy of FRα-targeting therapies.
CLINICAL APPLICATION OF FRα-BASED AGENTS
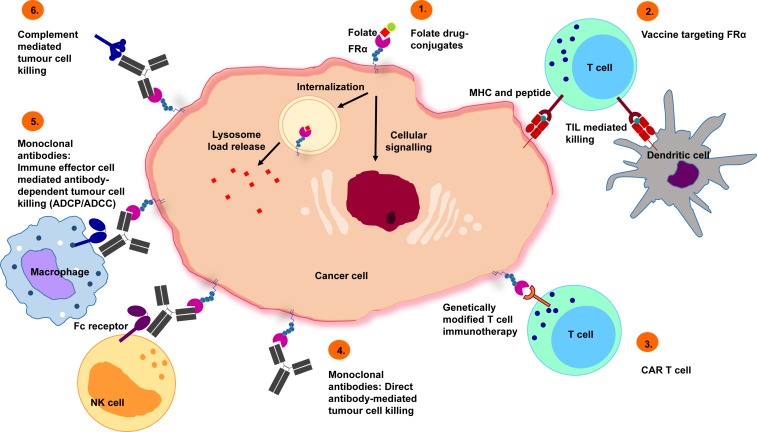
A range of FRα-targeting approaches, including folic acid derivatives, folate drug-conjugates and small molecules, vaccines, T-cell therapies and monoclonal antibodies, have been developed for clinical application for both imaging and therapeutic purposes (summarized in Figure 3).
Figure 3. Potential treatment approaches targeting FRα.
1) Folate drug-conjugates: chemotherapeutic agents, such as vintafolide, have been conjugated to folate for FRα targeting. 2) Vaccines targeting FRα: autologous dendritic cells engineered with FRα mRNA commence an anti-FRα immune response, mediated by T-cells. 3) Chimeric antigen receptor (CAR) T cells: CAR T cells recognizing FRα trigger tumor cell killing. 4) Monoclonal antibodies (direct effects): Specific recognition of FRα lead to inhibition of downstream signaling events that cause tumor cell death. 5) Monoclonal antibodies (immune effector cell engagement): antibodies link FRα-expressing tumor cells with immune effector cells that bear Fc receptors, potentiate effector cell activation and target-neutralizing functions by engendering antibody-dependent effector cell-mediated cytotoxicity (ADCC), phagocytosis (ADCP) or 6) complement-dependent cytotoxicity (CDC) activation.
FRα-targeted imaging
Although there is evidence to suggest that FRα-positive tumors can be detected by radiolabelled antibodies [60, 61], the use of antibodies as diagnostic or prognostic tools may be limited. Poor contrasts between tumor and non-target tissue are often achieved following prolonged circulation times and slow uptake by tumors [62].
Imaging of radiolabelled derivatives of folic acid may offer greater tissue selectivity. Folic acid derivatives conjugated to a variety of metal chelates have allowed non-invasive detection of FRα-positive tumors in both animal models and patients [62]. Of these probes, the most extensively studied is 111In-DPTA-folate, which demonstrated good on-target to off-tumor contrast in mouse models, leading to a phase I/II trial. Patients with newly diagnosed or suspected recurrent ovarian cancer were imaged by single-proton emission computed tomography (SPECT) using this agent. Newly diagnosed malignancies were detected at a sensitivity of 100% (n = 7), although detection of recurrent disease was more problematic. Overall, this agent appeared to be safe, and possibly an effective diagnostic tool, although this evaluation was limited by the small sample size of 33 patients [63]. Another folic acid peptide derivative (EC20) conjugated with 99mTechnetium (99mTc-EC20), which is less costly and has a shorter half-life than 111In-DPTA-folate, has also demonstrated promising qualities in preclinical models of ovarian cancer [64]. Furthermore, this agent has demonstrated superior FRα affinity and clearance from the blood and kidneys, and has been used to image > 200 patients to date, without any imaging-related adverse events reported [36].
By contrast, using macromolecular dendrimer polychelates conjugated to folic acid in magnetic resonance imaging (MRI) showed improved tumor to non-target contrast in mouse models of ovarian cancer, with specific accumulation in the tumor. This is thought to be due to a reduction in microcapillary permeation by the large probe molecule, and thus a greater relative accumulation in FRα-positive tumors [65]. A more recent study demonstrated the clinical promise of FRα-specific ultrasmall superparamagnetic iron oxides in MRI of a rat model of breast cancer. This agent was specifically retained in FRα-positive breast tumors and may also be useful as a non-invasive tool to diagnose and discriminate FRα-positivity [66].
Agents comprising folic acid conjugated to fluorescent dyes have been evaluated. These agents provide good contrast between malignant and healthy tissues in animal models. However, due to the optical qualities of the dyes, surgical opening of the cancer site was required to obtain images. In future it may be possible to use such agents in cutaneous or subcutaneous cancers, or to guide endoscopic or open surgery for cancer [36].
Overall, these imaging agents may allow clinical detection of FRα-positive tumors, which may aid diagnosis and disease surveillance. However, improvements in sensitivity and future studies to evaluate prognostic utility are needed to validate this approach as a clinical tool.
Etarfolatide
Etarfolatide is a 99mTc-based imaging agent, also known as FolateScan, which has been the subject of many studies in patients with ovarian, kidney, lung cancer and other refractory solid tumors (NCT01686256, NCT01684098) [67]. The purpose of etarfolatide is use as a companion agent with vintafolide, to enable pre-selection of patients with tumors expressing FRα. The administration of folic acid prior to etarfolatide infusion was shown to improve SPECT images, and a phase I clinical study was performed to investigate the safety and pharmacokinetics of etarfolatide up to 7 days following folic acid injection [68]. Etarfolatide has since been used to evaluate the tumor FRα expression of individuals in a number of trials (NCT00511485, NCT01577654 and NCT01999738).
Folate drug-conjugates
The first folate-conjugated cytotoxic agent to be evaluated in tumor therapy was a maytansinoid conjugate [69]. Since then, a series of chemotherapy agents has been conjugated to folate for FRα targeting, with varying degrees of success. Key clinical trials are summarized in Table 1 [70].
Table 1. Key clinical trials of FRα-drug conjugate therapeutics.
| Drug Name | Alternative Name(s) | Tumor Type | Trial Design | Efficacy Outcome | Safety Outcome | Imaging Outcome | Reference/Trial Number |
|---|---|---|---|---|---|---|---|
| Vintafolide |
|
Platinum-resistant ovarian cancer | Randomized phase II trial of Vintafolide + PLD vs. PLD alone in platinum-resistant ovarian cancer (PRECEDENT) | Vintafolide demonstrated significantly improved clinical activity compared with PLD alone. Median PFS was 5.0 and 2.7 months for vintafolide + PLD and PLD-alone arms, respectively (P = 0 .031). Greatest benefit observed in patients with 100% FR+ lesions, with median PFS 5.5 vs. 1.5 months for PLD alone (P = 0.013) | Vintafolide + PLD was well tolerated. Frequency of leukopenia, neutropenia, abdominal pain, and peripheral sensory neuropathy was higher in vintafolide + PLD vs. PLD arm | N/A | [77] NCT00722592 |
| FR+ platinum-resistant ovarian cancer | Phase III study of vintafolide + PLD vs. PLD alone in patients with FR+ platinum-resistant ovarian cancer (PROCEED). | At a prespecified interim data analysis, cessation of the study was recommended because vintafolide + PLD vs. PLD alone did not meet the prespecified criteria for PFS | No safety concerns were detailed | N/A | [28] NCT01170650 | ||
| FR+ NSCLC | Randomized, open-label, phase II trial of vintafolide as second-line treatment vs. vintafolide + docetaxel vs. docetaxel alone in patients with FR+ NSCLC (TARGET) | Preliminary data for vintafolide + docetaxel showed improvement across all efficacy endpoints vs. docetaxel alone. The best improvement was observed in the predefined adenocarcinoma patient subgroup | The safety profile was manageable and consistent with the AEs observed with both therapies | N/A | [75, 76] NCT01577654 | ||
| EC0225 |
|
Solid tumors | Phase I study of EC0225 in patients with solid tumors (refractory or metastatic) | Disease stabilization (≥ 4 months) was observed in 26/63 patients | EC0225 was well -tolerated at doses ≤2.3 mg/m2. Most frequent AEs included anemia, constipation, leukopenia, fatigue. G3 hypotension and G4 neutropenia occurred in 1 patient each at the highest dose tested (2.88mg/m2) defining the MTD | N/A | [79 NCT00441870 |
| Epofolate |
|
Solid tumors | Phase I study of epothilone folate (BMS-753493) in patients with advanced solid tumors | Best overall response was SD in 19% patients. Median duration of response was 85 days. No correlation between FR status and response. Interim analysis indicated that the benefit risk profile of BMS-753493 was not favorable and did not support further investigation of this agent | The MTD was reached. DLTs included ALT and AST elevations, diarrhoea, nausea, fatigue, oesophagitis and mucosal inflammation | N/A | [80, 81] NCT00546247 |
| EC0489 | Folate–desacetylvinblastine hydrazide with modified linker | Solid tumors | Phase I study of EC0489 in patients with refractory or metastatic tumors | Not reported | Patients treated at 2.5mg/m2 experienced toxicities characteristic of vinca alkaloids (e.g., mild neuropathy), but not significant constipation nor gastrointestinal-related toxicity | N/A | [82] NCT00852189 |
| EC1456 | Folate–tubulysin | TNBC, advanced NSCLC and ovarian cancer | Phase I dose-escalation study of EC1456 (Part A) including a study of efficacy in patients with TNBC, advanced NSCLC and ovarian cancer treated at the MTD (Part B) | Data awaited | Data awaited | N/A | NCT01999738 |
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DLT, dose-limiting toxicity; G, CTCAE grade; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; SD, stable disease; TNBC, triple negative breast cancer. Clinical trial number (NCT) available from: http://clinicaltrials.gov.
Vintafolide and other folate conjugates
Vintafolide, or EC145, is a folate conjugate of desacetylvin-blastinemonohydrazide (DAVLBH), a derivative of the microtubule destabilizing agent vinblastine [71]. Following receptor-mediated endocytosis, the self-immolative disulphide-linker system enables the release of DAVLBH into the tumor cell endosome, leading to inhibition of cell division and induction of cell death [72, 73]. The safety of vintafolide was first assessed in a phase I clinical study in various solid tumors [74], while multiple phase II and III clinical trials have been conducted in lung and ovarian cancer (NCT00507741, NCT01577654, NCT00722592, NCT00511485 and NCT01170650) [69].
One clinical study, EC-FV-03 (NCT00511485), consisted of a single-arm phase II trial that assessed vintafolide as a single agent in patients with lung adenocarcinoma, who had previously been treated with at least two chemotherapy regimens. Analysis of survival outcomes demonstrated superior median PFS in patients with high FRα tumor expression compared with those with low expression. The median OS also showed a trend towards improvement [75], although the non-randomized study design precluded drawing any firm efficacy conclusion given the likely prognostic value of FR-alpha expression. The randomized, open-label, phase II TARGET, study (NCT01577654), has also recruited patients with FRα-positive non-small cell lung cancer. Preliminary results for vintafolide combined with docetaxel suggest improvement across all efficacy endpoints over docetaxel as single agent in the control arm [76].
Among the multiple clinical studies of vintafolide conducted on ovarian cancer patients, of particular interest is the open-label phase II PRECEDENT study (NCT00722592) [77]. In this trial, patients who had received more than two previous chemotherapeutics were randomized to receive vintafolide and PEGylated liposomal doxorubicin (PLD) or PLD alone until progression or death. Median PFS, compared to PLD treatment alone, was most superior in individuals with higher FRα expression. This was the first combination therapy to suggest a significant prolongation of PFS over standard therapy in platinum-resistant ovarian cancer patients [28]. The fact that FRα-negative patients (based on etarfolatide imaging) did not benefit from vintafolide and PLD combination therapy, whereas patients with highly FRα-positive tumors did, supported the strategy of using an imaging agent to identify the FRα-positive patient population for FRα-targeted therapy. However, the subsequent phase III PROCEED trial, designed to further evaluate the clinical efficacy of vintafolide combined with PLD (NCT01170650) was discontinued at an interim analysis because the experimental arm did not meet the pre-specified primary outcome for PFS improvement required [78].
Following vintafolide, several additional folate-conjugated agents have been studied. EC0225, a folate conjugated to both a vinca alkaloid and mitomycin, was evaluated in a phase I trial which determined the maximum tolerated dose (MTD) (NCT00441870) [79]. Results are awaited from a phase I/II trial (NCT00546247) of BMS-753493 (Epofolate), a folate conjugate of epothilone A, which is a microtubule stabilizing agent [80, 81]. EC0489, an analogue of vintafolide, has been shown to have reduced hepatic clearance [82] and its MTD was evaluated in a phase I trial of metastatic tumors (NCT00852189). EC1456, a folic acid-tubulysin small-molecule drug conjugate, is currently undergoing phase I trial in patients with advanced NSCLC, ovarian cancer and TNBC (NCT01999738). IMGN853, an anti-FRα mAb conjugated with the maytansinoid, DM4, is now being evaluated in a number of phase I clinical trials to evaluate its safety and pharmacokinetics/pharmacodynamics alone (NCT01609556), and in combination with chemotherapy and bevacizumab (NCT02606305), in patients with EOC and other FRα-positive tumors. Furthermore, a phase II trial, to compare the efficacy of IMGN853 to standard chemotherapies, is recruiting patients with FRα-positive tumors (NCT02631876). Folate-conjugated carboplatin was not considered a promising therapy due to neutralization by folate receptor-mediated endocytosis [83], but several other microtubule poisons have shown moderate promise in in vitro and in vivo models [84, 85].
Unlike anti-FRα antibodies, folate conjugates target both FRα and the functional form of FRβ. This could potentially enable the targeting of tumors that are low in FRα but high in FRβ, including tumors that are infiltrated by large numbers of tumor-associated macrophages (TAMs), known to express FRβ. Targeting pro-tumoral TAMs with FRβ-specific agents is a widening avenue of research discussed in detail in other publications [86–89].
The bi-specificity of folate drug-conjugates might reduce the selectivity of these drugs for tumor cells, but may potentially expand the range of targets to include elements of the tumor microenvironment. A potential disadvantage of folate-conjugated drug delivery is that binding is competitively-inhibited by excess serum free folate. A further potential issue is the exposure of FRα expressed in the apical membrane of the proximal renal tubules following filtration of low molecular weight (LMW) folate conjugates [90]. While this has not adversely affected clinical translation of folate conjugates, it may affect the clinical application of LMW folate conjugate-based radiopharmaceuticals for targeted radiotherapy.
Anti-FRα small molecule drugs
Conventional anti-folate drugs, such as pemetrexed and methotrexate, are often carried by the high capacity RFC, which is ubiquitously expressed on normal and tumor cells, leading to non-specific activity and associated reductions in patient tolerability. Therefore, anti-folate thymidylate synthase (TS) and glycinamide ribonucleotide formyl transferase (GARFTase) inhibitors, with negligible affinity for RFC and high-affinity for FRα, have been developed. These anti-FRα small molecules are thought to be more tumor-targeted, due to the restricted expression of FRα on normal tissue on apical membranes away from the bloodstream [91, 92]. Examples of anti-FRα TS inhibitors include CB300638 and ONX-0801. Pre-clinical studies of these agents have demonstrated anti-tumor efficacy in FRα-expressing tumors, and reduced transportation through RFC, which is expected to confer reduced patient toxicity [91, 93, 94]. A limitation for anti-FRα small molecules may be the level of FRα expression by target tumor cells, which needs to be sufficient to transport adequate amounts of the drug into the cell in order to inhibit TS and cause cell death. Much of the pre-clinical studies have used cell lines and therefore future work will be required to determine whether the expression levels of FRα are sufficient for functional effects against patient tumors [95]. A phase I trial of ONX-0801 is now recruiting patients with solid tumors, expected to have high FRα expression, such as EOC patients, in order to evaluate the safety and efficacy of two dosing schedules (NCT02360345).
Vaccines targeting FRα
Increased immunity to FRα has been reported in patients with FRα-expressing ovarian cancer in comparison to healthy controls, suggesting that this may be a target for cell-based and peptide immunotherapies [23]. Key clinical trials are summarized in Table 2 [70].
Table 2. Clinical trials of FRα-targeting vaccines and CAR T cells.
| Drug Name | Alternative Name(s) | Tumor Type | Trial Design | Efficacy Outcome | Safety Outcome | Imaging Outcome | Reference/Trial Number |
|---|---|---|---|---|---|---|---|
| Vaccines | |||||||
| Folate Immune | EC90/GPI-0100/EC17 | Renal cell carcinoma | Phase I study of Folate Immune (EC90 vaccine administered with GPI-0100 adjuvant followed by EC17) in patients with renal cell carcinoma | 1/28 (4%) patients had a PR, 15/28 (54%) had SD | Two DLTs seen (G4 anaphylaxis and G3 pancreatitis). Mild - mod injection site reactions were most common AE during vaccination phase. Transient hypersensitivity reactions were most common during treatment phase | N/A | [97] NCT00329368 |
| Folate Immune | EC90/GPI-0100/EC17 | Renal cell carcinoma | Phase I study of Folate Immune (EC90 vaccine administered with GPI-0100 adjuvant followed by EC17) in patients with progressive metastatic renal cell carcinoma | Trial terminated due to poor patient accrual | N/A | NCT00485563 | |
| Autologous dendritic cells engineered with FRα mRNA | / | FRα+ serous papillary ovarian carcinoma | Single patient report of a patient with serous papillary ovarian carcinoma at second relapse (platinum-resistant) who received a vaccination regimen with autologous dendritic cells engineered with mRNA encoded FRα | CT before treatment and 3 months after the last vaccination (13 months total) demonstrated a PR. CA-125 greatly reduced 4 weeks after the first vaccination and were still at baseline at 11 months after completion of vaccination | Not reported | N/A | [96] |
| CAR T Cells | |||||||
| CAR-T cells specific to FRα | / | Ovarian cancer | Phase I study of adoptive transfer of FRα redirected autologous T cells, either with high-dose IL-2 (cohort 1), or followed by immunization with allogeneic peripheral blood mononuclear cells (cohort 2), for recurrent ovarian cancer | No reduction in tumor burden was seen in any patient | 5/8 (63%) patients in cohort 1 experienced a G3 – 4 AE | Lack of specific localization of T cells to tumor was observed by tracking 111In-labeled adoptively transferred T cells | [133] |
AE, adverse event; DLT, dose-limiting toxicity; G, CTCAE grade; PR, partial response; SD, stable disease. Clinical trial number (NCT) available from: http://clinicaltrials.gov.
A case report of a single patient with ovarian carcinoma, vaccinated with autologous dendritic cells that were engineered with mRNA-encoded FRα, demonstrated a vaccine-induced T cell reactivity, resulting in more than 50% of tumor regression, as well as a reduction of tumor markers levels, suggesting this strategy may hold promise as a cancer treatment [96]. A FRα-targeted hapten immunotherapeutic regimen known as ‘Folate Immune’, which combines folate-targeted vaccine EC90, together with an adjuvant, GPI-0100, and a folate-hapten conjugate EC17, was designed to convert poorly immunogenic tumors to highly immunogenic tumors. Patients first underwent vaccination with EC90 and GPI-0100 adjuvant to stimulate production of specific antibodies, and were then treated with EC17, which is thought to bridge antibodies with FRα-expressing tumor cells and trigger antibody-dependent cellular cytotoxicity and/or phagocytosis (ADCC/ADCP). A phase I study confirmed that this regimen was well tolerated in 33 patients, with mild to moderate injection site reactions being the most common adverse effects reported [97]. A phase II trial was then initiated in 2012to evaluate this therapeutic strategy in patients with metastatic renal cell carcinoma, but has since been terminated due to low patient accrual (NCT00485563).
Oncolytic virus therapy
Virotherapy is a treatment approach using wild type or genetically engineered viruses [98]. An attenuated measles virus has been evaluated pre-clinically as FRα targeting treatment for ovarian cancer. The anti-tumor activity of oncolytic measles virus highly specific for FRα (MV-αFR) was tested in a xenograft model of ovarian cancer. Treatment of human ovarian cancer-bearing mice with MV-αFR resulted in significant inhibition of tumor growth compared to controls and 50% of mice showed complete regression of tumors. These findings suggested the merit of clinically testing the oncolytic virotherapy approach in FRα-expressing tumors [99].
Monoclonal antibodies
Monoclonal antibodies (mAbs) can target tumor-associated antigens, such as FRα, on the surface of tumor cells. They can mediate specific anti-tumor activity either by blocking cell signaling or by eliciting immune-mediated cell killing by engaging effector cells or complement. Examples of clinical trials of monoclonal antibodies directed against FRα are summarized in Table 3 [70].
Table 3. Clinical trials of FRα-targeting monoclonal antibodies.
| Drug Name | Alternative Name(s) | Tumor Type | Trial Design | Efficacy Outcome | Safety Outcome | Imaging Outcome | Reference/Trial Number |
|---|---|---|---|---|---|---|---|
| Farletuzumab | MORab003 | Platinum-resistant EOC | Phase I dose escalation study of weekly farletuzumab | Following 1 treatment cycle (4 weeks): No objective responses. SD in 36% patients and CA-125 reduction in 16% | No DLTs. MTD not reached | Radiolabeled tracer studies conducted in 3 patients showed significant tumor uptake | [105] NCT00428766 |
| Platinum-sensitive EOC | Phase II study of farletuzumab as single agent or in combination with a platinum and taxane in platinum-sensitive, recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer | Farletuzumab alone: 0% normalised CA125, 30% SD as best response, 70% PD. Farletuzumab + chemotherapy: 80.9% normalised CA125. ORR 75%. In 21%, the second progression-free interval was longer than the first | Farletuzumab well-tolerated as single agent, without additive toxicity when administered with chemotherapy. SAEs in 37% patients – 9% considered related to farletuzumab | N/A | [107] NCT00318370 | ||
| Platinum-sensitive EOC | Phase Ib safety study of farletuzumab, carboplatin and PLD in patients with platinum-sensitive EOC at first or second relapse | CR in one patient (7%), PRs in 10 patients (67%), SD in 4 patients (27%). | Combination well-tolerated - no farletuzumab-related G3-4 adverse events. | N/A | [106] NCT01004380 | ||
| Platinum-sensitive EOC | Phase III double-blind placebo-controlled study of weekly farletuzumab + carboplatin/taxane in platinum-sensitive ovarian cancer at first relapse. | Median PFS 9.0 (placebo), 9.5 (farletuzumab 1.25 mg/kg), and 9.7 (farletuzumab 2.5 mg/kg) months with no statistically significant difference between arms | Most common AEs across arms were those known to be associated with chemotherapy. | N/A | [28] NCT00849667 | ||
| Platinum-sensitive recurrent EOC with a low CA125 | Phase III global multicenter double-blind randomized placebo-controlled trial of farletuzumab + platinum and a taxane or PLD in patients with first relapse of platinum-sensitive ovarian cancer and a low CA125 | Data awaited | Data awaited | N/A | NCT02289950 | ||
| Platinum-resistant EOC | Phase III double-blind placebo-controlled study of weekly farletuzumab + paclitaxel in platinum-resistant ovarian cancer at relapse | Predefined criteria for trial continuation were not met. | Trial terminated | N/A | NCT00738699 | ||
| FRα+ TNBC with low levels of CA125 | Phase II trial of farletuzumab in FRα+ TNBC with low levels of CA125 | Data awaited | Data awaited | N/A | [78] | ||
| NSCLC (adenoCa) | Phase II randomized, placebo-controlled, multicenter study of a platinum containing doublet +/− farletuzumab in stage IV adenocarcinoma of the lung | Data awaited | Data awaited | N/A | NCT01218516 | ||
| MOv18 IgG | / | Ovarian carcinoma | Phase I single infusion dose-escalating study of cMOv18 IgG in patients with primary, residual or recurrent ovarian cancer | Efficacy outcomes not reported | No DLT observed. At doses of 350mg all patients experienced G2 side effects, including fever, headache, and nausea/vomiting | N/A | [112] |
| 131I-cMOv18 IgG1 | / | Ovarian carcinoma | Kinetics and tissue distribution of 131I-cMOv18 IgG1 in patients with ovarian carcinoma | Not reported | Not reported | Good localisation in ovarian carcinoma tissue | [33] |
| Ovarian carcinoma | Phase I study of i.p. radioimmunotherapy with 131I-mMOv18 IgG1 in patients with ovarian cancer with minimal residual disease | 5/16 patients had a CR, 6/16 patients had SD and 5/16 patients had PD | Minimal toxicities observed. One patient mild and transient bone marrow suppression | N/A | [111] | ||
| Ovarian carcinoma | Phase I study of i.p. and i.v. radioimmunotherapy with 131I-cMOv18 IgG1 in patients with suspected ovarian cancer scheduled to undergo exploratory laparotomy | Efficacy outcomes not reported | No normal organ toxicity | Tumor uptake in ovarian cancer tissue 3.4% - 12.3% ID/kg for i.p. and 3.6% - 5.4% ID/kg for i.v. administration | [113] | ||
| 125I-, 123I-, 131I-, cMOv18 IgG1 | / | Ovarian carcinoma | Study of simultaneous i.v. and i.p injection of radiolabeled c-MOv18 (using different radionuclides) in patients with ovarian cancer to determine the optimum way to deliver radiolabeled cMOv18 | Not reported | No AEs reported | No significant differences found in tumor:normal tissue and tumor:blood ratios for both i.v. and i.p routes. Tumor uptake varied between patients and within same patient | [61] |
| MOv18 IgE | / | FRα+ advanced tumors | Phase I: First in human study of MOv18 IgE in patients with FRα+ advanced cancer | Data awaited | Data awaited | N/A | NCT02546921 |
AE, adverse event; cMOv18, chimeric MOv18; CR, complete response; DLT, dose-limiting toxicity; EOC, epithelial ovarian cancer; G, CTCAE grade; i.p., intraperitoneal; i.v., intravenous; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; ORR, overall response rate; PD, progressive disease; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; PR, partial response; SAE, serious adverse event; SD, stable disease; TNBC, triple negative breast cancer. Clinical trial numbers (NCT) available from: http://clinicaltrials.gov.
Farletuzumab
A mAb that has been widely studied in ovarian and lung cancers is farletuzumab, (MORab003). It is a fully humanized IgG1 antibody specific for FRα. Farletuzumab does not prevent binding of folate to its receptors, nor does it inhibit the transport of folate into the cell via the receptor. Instead, in vitro studies have demonstrated that farletuzumab exhibits activity against FRα-expressing tumor cells by a number of mechanisms. These include tumor cell killing by antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), sustained tumor cell autophagy, and inhibition of Lyn kinase substrate phosphorylation [100–104].
Farletuzumab was first given as monotherapy in a phase I trial in 25 patients with platinum-resistant ovarian cancer (NCT00428766). No dose-limiting toxicities (DLTs) were observed, and dose escalation was continued to a maximum 400 mg/m2 dose [105]. Safety of farletuzumab, in combination with carboplatin and liposomal doxorubicin, was then demonstrated in a phase Ib study in patients with platinum-sensitive ovarian cancer (NCT01004380) [106]. In a subsequent phase II study, patients with the same disease were given farletuzumab combined with carboplatin and a taxane, followed by farletuzumab maintenance therapy (NCT00318370). Response rates comparing favorably with historical controls were observed. Overall in these trials, farletuzumab was well tolerated as a single-agent, and did not seem to confer additive toxicity when combined with chemotherapy [107].
Following these promising results, a large phase III trial in patients with platinum-sensitive recurrent ovarian cancer was carried out (NCT00849667). In this trial, farletuzumab, in combination with carboplatin and a taxane, was compared to carboplatin/taxane treatment alone. The primary endpoint of improved PFS was not met, however subsequent analysis suggested an improved PFS in some patient subgroups given higher doses, and in those with lower CA125 levels [28]. Farletuzumab in combination with paclitaxel was also compared to paclitaxel alone in patients with platinum-resistant ovarian cancer in a second phase III study (NCT00738699), but this trial was discontinued early for futility.
Given that patients with high FRα-expressing tumors may derive greater benefit from farletuzumab therapy than those with low expression in tumors, future trials may involve the stratification of patients based on their tumor FRα expression. In fact, the manufacturer of farletuzumab has announced the development of a diagnostic assay to better identify patients with high FRα expression [108]. Furthermore, a phase II study comparing farletuzumab, in combination with carboplatin and paclitaxel, or with carboplatin and liposomal doxorubcin, is currently recruiting patients with platinum-sensitive ovarian cancer and low CA125 (NCT02289950). Another phase II trial will be conducted in patients with FRα-positive TNBC who have low serum levels of CA125 [109].
The clinical efficacy of farletuzumab, in combination with platinum-containing chemotherapy has also been evaluated in non-small-cell lung cancer. This phase II study (NCT01218516) enrolled 130 patients with stage IV adenocarcinoma of the lung and has now completed, although results are yet to be published.
MOv18 IgG1
The MOv18 IgG1 murine monoclonal antibody was generated by immunization of mice with a surgical specimen of human ovarian carcinoma [32]. The FRα-specific variable regions of the resultant antibody were cloned, and the murine γ1-heavy chains and κ-light chains were subsequently replaced with their human equivalents to engineer a chimeric version of MOv18 IgG [110]. Previous clinical studies of MOv18 IgG (either murine or chimeric) administered to ovarian cancer patients have suggested therapeutic benefit with no overt toxicity.
The first clinical administration of MOv18 IgG1 was as a radiolabeled murine antibody (131I-MOv18 IgG1) in 1991. The main aim of this study was to investigate the feasibility of radioimmunoscintigraphy (RIS, the administration of a radiolabelled antibody against a tumor surface marker, for the purpose of imaging the tumor and any metastases). Another objective was to evaluate biodistribution of MOv18 IgG1 for further therapeutic applications [60]. A total of 30 patients with ovarian carcinoma were given 131I-MOv18 IgG1 either intravenously (i.v.) (n = 20) or intraperitoneally (i.p.) (n = 10). High tumor uptake, a good tumor to background ratio and low non-specific uptake in non-affected organs were observed, with i.p. administration superior to i.v. administration. These findings suggested that MOv18 IgG1 represented a promising mAb for RIS in ovarian cancer.
Subsequently the same 131I-radiolabelled murine antibody was administered as i.p. radioimmunotherapy to 16 ovarian cancer patients with minimal residual disease [111]. Efficacy was observed, with 5 complete responses and 5 patients demonstrating stable disease. In addition the toxicity was reported to be low with only one patient showing mild and transient bone marrow suppression. However, human anti-mouse antibody (HAMA) production was demonstrated in 94% of patients.
In order to reduce HAMA, a chimeric MOv18 IgG1 was generated, and radiolabeled with either 131I or 125I, before administration to 24 patients with ovarian carcinoma [33]. Six days after injection, the antibody was demonstrated to localize well in ovarian carcinoma tissue with a mean tumor to normal tissue ratio of 6.7, indicating a prolonged accumulation in the tumor relative to normal tissues. In view of these positive findings, the safety of a single i.v. infusion of increasing doses (5-75mg) of chimeric MOv18 IgG1 was subsequently evaluated in a phase I study of 15 ovarian carcinoma patients [112]. Administration of MOv18 IgG1 was demonstrated to be safe with no significant changes in hematological, biochemical, or urine profiles detected. However, at doses of 50 mg and above all patients experienced minor (World Health Organization, WHO grade 2) side effects, including fever, headache, and nausea/vomiting. Interestingly, no human anti-chimeric antibody (HACA) response was detected up to 12 weeks post injection. These findings suggested that targeting of FRα with chimeric MOv18 IgG1 was safe and that this mAb represented a promising therapeutic for the treatment of ovarian carcinoma.
Since ovarian cancer is mainly limited to the peritoneal cavity, locoregional delivery of therapeutics can be an option. Therefore the influence of the route of administration (i.v. or i.p.) of radiolabeled chimeric MOv18 IgG1 was investigated in two studies [61, 113]. In the first study, 131I-MOv18 IgG1 was administered to 12 patients with ovarian cancer. Scintigraphic images after i.p. administration showed better accumulation in ovarian cancer lesions compared with after i.v. administration. Furthermore, there was no normal organ toxicity. This study concluded that the i.p. route of administration was safe and seemed to be preferable to i.v. administration [113]. In the second study 15 patients received chimeric MOv18IgG1 labeled with 131I, 125I and 123I, either via the i.p. or i.v. route. No adverse events and no HACA response were reported. Furthermore, in contrast to the previous study, no advantage could be demonstrated for the i.p. route of administration with respect to tumor uptake; however, it was suggested that it may be the preferred route with respect to bone marrow toxicity since the area under the curve (AUC) was significantly lower for the i.p. versus the i.v. route [61].
In summary, numerous clinical studies conducted with murine or chimeric MOv18 IgG1 to date suggest that targeting the tumor antigen FRα with a therapeutic mAb is safe, with minimal toxicities observed across all studies. However, some studies have suggested superior tumor targeting when the antibody was administered locoregionally to the tumor, perhaps indicating relatively poor tumor penetrance of MOv18 IgG1 when administered via the i.v. route. This suggests the possible benefit of undertaking antibody engineering strategies, including re-engineering approaches or changing the isotype of the antibody, with the aim of improving bioactivity, potency or tumor penetrance aimed at enhancing antibody efficacy.
MOv19 and derivatives
The mAb MOv19 was selected from the same fusion from which MOv18 was derived. MOv19 and LK26 (the murine mAb from which the fully humanized Farletuzumab was derived) recognize the same or overlapping epitopes on FRα, but independent from the epitope recognized by MOv18, as detected by competition assay in Biacore analysis and ELISA [32, 114].
Chimeric versions of MOv18 and MOv19 IgG antibodies, similarly to Farletuzumab, mediate both ADCC [115] and low levels of CDC [116] in vitro. However, when the two chimeric mAbs were mixed, a significant increase in tumor cell killing was observed of up to 50%. This value increased to 70% after neutralization of CD46 and CD59 (membrane C regulatory molecules) without an appreciable change of ADCC. These results suggest that complement can contribute to the killing of ovarian carcinoma cells induced by the mixture of cMOv18 and cMOv19.
Several different derivatives of MOv19 have been evaluated in different therapeutic applications. Completely human Fab fragments against FRα were produced by using phage display and among them one, named C4, exhibited good specificity [117]. The human C4 in scFv format, after adequate optimization, resulted in CAR redirection [118].
By applying epitope imprinting selection [119], a method that enables isolation of antibodies with the same specificity of a pre-existing antibody, a human Fab (AFRA5), recognizing a FRα epitope overlapping with that of MOv19, was identified [120]. After optimization of the lead reagent, a chemical dimer, named AFRA-DFM5.3, was considered suitable for further in vivo preclinical evaluation in the perspective of a clinical use. In fact, an antibody fragment, in a dimer format that stabilizes binding as soon as the antigen-antibody complex is formed on the target tumor, might be the reagent of choice for i.p. radioimmunotherapy of ovarian cancer because of its relatively small size, which should favor tumor penetration and fast clearance. Due to its fast and high tumor uptake, 131I-AFRA-DFM5.3 resulted in more than 50% of treated animals cured in a preclinical intraperitoneal model [121]. The human origin of AFRA-DFM5.3 and its efficacy when delivered locoregionally as an 131I reagent, together with evidence of the feasibility and acceptable toxicity profile of ovarian cancer treatment with anti-FR mAbs, could provide the basis for rational design of new therapeutic modalities.
MOv18 IgE
MOv18 IgE is an anti-FRα chimeric IgE antibody, engineered from the variable heavy and light chain regions of MOv18 IgG1 in order to investigate the hypothesis that IgE antibodies may offer advantages as immunotherapeutic agents against cancer compared to their IgG counterparts [122]. The rationale for using IgE antibodies against cancer stems from the unique properties of this class, which make it a critical contributor to allergic and parasitic immune responses. These properties include the exceptionally high affinity of IgE to its Fc receptors (FcεRI and CD23), the lack of an inhibitory IgE receptor, and the significantly longer half-life of IgE in tissues compared to IgG1, which results in improved local retention of IgE. IgE antibodies are thus able to confer potent immune responses by activating tumor-resident immune effector cells [123].
To date, a number of in vivo models have been used to evaluate the efficacy of MOv18 IgE. Firstly, immunodeficient (SCID) mice challenged subcutaneously (s.c.) with FRα-expressing human ovarian carcinoma cells, were treated with human peripheral blood lymphocytes (PBLs) and MOv18 IgE or IgG1 antibodies i.v. [122]. Secondly, a xenograft mouse model was set-up using patient-derived FRα-expressing human ovarian carcinoma, and mice were given human peripheral blood mononuclear cells (PBMCs) i.p. with MOv18 IgE or IgG1 antibodies [124–126]. MOv18 IgE afforded superior anti-tumor efficacy and animal survival, compared with its IgG1 counterpart, in both models.
These studies also provided evidence that MOv18 IgE efficacy may be mediated via anti-tumor effector cell functions in vivo: addition of PBMCs was required for the observed protective effect and this efficacy was ablated upon depletion of monocytes; monocytic infiltration was observed in tumor sections from mice treated with MOv18 IgE; and large areas of tumor necrosis were detected following MOv18 IgE treatment [122, 126]. MOv18 IgE-mediated tumor cell killing by human monocytes and eosinophils was also confirmed in vitro by ADCC/ADCP [126, 127]. Emerging evidence point to the capacity of IgE antibodies to activate host immune effector cells against cancer and support its anti-tumor efficacy [128–131]. This agent is now being translated for clinical testing in FRα-expressing carcinomas.
T cell activating strategies
The possibility to combine the antibody specificity with the potency of T cell weapons to treat tumors, independently from the T cell receptor (TcR)-defined specificity, and expression of human leukocyte antigen (HLA) on cancer cells, has been explored and specific reagents for T cell retargeting were developed. Among different approaches, the most promising are the use of bi-specific antibodies (BsAbs), T-cell engineering to create chimeric antigen receptors (CARs), as well as combinations with immune checkpoint inhibitors.
Adoptive transfers of ex vivo T cells armed with the BsAb OC/TR (MOv18 x anti-CD3) [132] or of CAR-engineered T cells recognizing FRα [133] (Table 2) were among the first clinical attempts with these therapeutic tools.
In a phase I/II study, among the 28 ovarian cancer patients treated intraperitoneally with OC/TR-armed activated T cells and IL-2, response to treatment could be assessed in 26 patients by explorative laparotomy. The overall intraperitoneal response rate was 27%. The complete responses seen in three patients lasted 26 months in one patient, 23 months in the second, and 18 months in the third [132]. On the contrary the outcome of the phase I clinical trial with a FRα-specific CAR T cell treatment was discouraging with no response observed in any patient and the presence of significant toxicities [133]. It is noteworthy that both trials have been conducted using the anti-FRα murine monoclonal antibody MOv18 (see further discussion of this antibody below) and that in the OC/TR trial almost all the patients developed a HAMA response that precluded further treatment [134] and in the CAR trial an inhibitory factor developed in the serum of three of six patients tested over the period of treatment, which significantly reduced the ability of gene-modified T cells to respond against FRα positive tumor cells [133].
Even if no clinical results are at present available with these anti-FRα tools, advances in protein engineering and increased knowledge in T cell biology have enabled the rise of both BsAbs and CARs from inefficient first generation reagents to promising molecules for cancer treatment. In particular, first generation CARs failed because of poor T cell expansion and adverse effects; new second and third generation CARs have solved at least in part these problems and good pre-clinical in vivo results, i.e. tumor regression, longer persistence in circulation and better localization towards the tumor, have been obtained with MOv19-based CAR constructs containing the co-stimulatory motif of CD137 or CD27 [118, 135].
Another approach, which has recently seen increased interest, is the use of antibodies targeting immune cell checkpoints that negatively regulate anti-tumor immunity [136]. Ipilimumab targets cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), which is involved in an alternative interaction between T cells and antigen-presenting cells (APCs) that inhibits T cell effector functions. Similarly, nivolumab and pembrolizumab target the interaction between programmed cell death receptor 1 (PD-1), which is expressed on activated effector cells such as T cell, B cells and other myeloid cells, and its ligand PD-L1, which is expressed on tumor cells and APCs. Treatment with these immune checkpoint-targeting antibodies prevents the attenuation of T cell activation and thereby enhances anti-tumor immunity. Ipilimumab and nivolumab have shown significant efficacy in patients with metastatic melanoma and are now widely approved for treatment of this solid tumor. Clinical trials in patients with other tumor types, including ovarian, lung and breast, are now underway and demonstrating promising results [137–140]. Thus, the combination of checkpoint inhibitors and FRα-targeting strategies may well lead to greater therapeutic success.
CONCLUSIONS
Insights into tumor expression and distribution of FRα and its emerging roles in cancer growth and metastasis are now focusing renewed interest on this tumor-associated antigen as a potential target and tumor marker for solid tumours such as ovarian, lung and basal breast cancers. There is an unmet need in these malignancies, associated with particularly poor prognosis, for further treatment options as well as biomarkers (predictive and prognostic). Future focus on FRα offers real potential for the development of targeted cancer therapies. Past and ongoing clinical evaluations of FRα-targeted therapies, such as vintafolide and farletuzumab, allow for cautious optimism, but suggest that both improved patient selection and optimised modes of action are still needed.
Low levels of FRα expression in most normal tissues predict a low probability of significant toxicity for these agents, supported by clinical experience so far with treatment strategies targeted using folate and antibodies. FRα expression in tumor may serve as both a predictive marker to guide patient selection for such strategies, and also as a potential prognostic indicator. Furthermore, serum detection of circulating soluble antigen levels might also serve as an effective diagnostic or prognostic marker, and potentially an indicator of treatment response. These possible uses still require further study in FRα-expressing malignant indications.
A range of novel immunotherapeutic modalities, including vaccines, oncolytic viruses, monoclonal antibodies and adoptive T-cell strategies, with novel mechanisms of action, are in preclinical or clinical studies. Perhaps those agents with enhanced immune activating properties, more resistant to tumor-associated immune suppressive mechanisms, may in future become effective strategies against FRα-expressing tumors.
Acknowledgments
The authors acknowledge support by Breast Cancer Now (147) - the UK's largest breast cancer charity - created by the merger of Breast Cancer Campaign and Breakthrough Breast Cancer; Cancer Research UK (C30122/A11527; C30122/A15774); the Medical Research Council (MR/L023091/1); the Academy of Medical Sciences; CR UK//NIHR in England/DoH for Scotland, Wales and Northern Ireland Experimental Cancer Medicine Centre (C10355/A15587); CR UK/EPSRC/MRC/NIHR KCL/UCL Comprehensive Cancer Imaging Centre (C1519/A10331). The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors are solely responsible for the decision to publish, and preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gueant JL, Namour F, Gueant-Rodriguez RM, Daval JL. Folate and fetal programming: a play in epigenomics? Trends Endocrinol Metab. 2013;24:279–289. doi: 10.1016/j.tem.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Kerek R, Geoffroy A, Bison A, Martin N, Akchiche N, Pourie G, Helle D, Gueant JL, Bossenmeyer-Pourie C, Daval JL. Early methyl donor deficiency may induce persistent brain defects by reducing Stat3 signaling targeted by miR-124. Cell Death Dis. 2013;4:e755. doi: 10.1038/cddis.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75–81. doi: 10.1007/s10545-010-9177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm. 2003;66:403–456. doi: 10.1016/s0083-6729(03)01012-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr. 2011;31:177–201. doi: 10.1146/annurev-nutr-072610-145133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol. 2015;26:2034–2043. doi: 10.1093/annonc/mdv250. [DOI] [PubMed] [Google Scholar]
- 8.Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26:141–152. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- 9.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119:243–250. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 10.Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–198. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Shi H, Guo J, Li C, Wang Z. A current review of folate receptor alpha as a potential tumor target in non-small-cell lung cancer. Drug Des Devel Ther. 2015;9:4989–4996. doi: 10.2147/DDDT.S90670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boogerd LS, Boonstra MC, Beck AJ, Charehbili A, Hoogstins CE, Prevoo HA, Singhal S, Low PS, van de Velde CJ, Vahrmeijer AL. Concordance of folate receptor-alpha expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients. Oncotarget. 2016 doi: 10.18632/oncotarget.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'shannessy DJ, Somers EB, Maltzman J, Smale R, Fu YS. Folate receptor alpha (FRA) expression in breast cancer: identification of a new molecular subtype and association with triple negative disease. Springerplus. 2012;1:22. doi: 10.1186/2193-1801-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siu MK, Kong DS, Chan HY, Wong ES, Ip PP, Jiang L, Ngan HY, Le XF, Cheung AN. Paradoxical impact of two folate receptors, FRalpha and RFC, in ovarian cancer: effect on cell proliferation, invasion and clinical outcome. PLoS One. 2012;7:e47201. doi: 10.1371/journal.pone.0047201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritter TE, Fajardo O, Matsue H, Anderson RG, Lacey SW. Folate receptors targeted to clathrin-coated pits cannot regulate vitamin uptake. Proc Natl Acad Sci U S A. 1995;92:3824–3828. doi: 10.1073/pnas.92.9.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothberg KG, Ying YS, Kolhouse JF, Kamen BA, Anderson RG. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990;110:637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabharanjak S, Mayor S. Folate receptor endocytosis and trafficking. Adv Drug Deliv Rev. 2004;56:1099–1109. doi: 10.1016/j.addr.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Boshnjaku V, Shim KW, Tsurubuchi T, Ichi S, Szany EV, Xi G, Mania-Farnell B, McLone DG, Tomita T, Mayanil CS. Nuclear localization of folate receptor alpha: a new role as a transcription factor. Sci Rep. 2012;2:980. doi: 10.1038/srep00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figini M, Ferri R, Mezzanzanica D, Bagnoli M, Luison E, Miotti S, Canevari S. Reversion of transformed phenotype in ovarian cancer cells by intracellular expression of anti folate receptor antibodies. Gene Ther. 2003;10:1018–1025. doi: 10.1038/sj.gt.3301962. [DOI] [PubMed] [Google Scholar]
- 20.Darai E, Scoazec JY, Walker-Combrouze F, Mlika-Cabanne N, Feldmann G, Madelenat P, Potet F. Expression of cadherins in benign, borderline, and malignant ovarian epithelial tumors: a clinicopathologic study of 60 cases. Hum Pathol. 1997;28:922–928. doi: 10.1016/s0046-8177(97)90007-1. [DOI] [PubMed] [Google Scholar]
- 21.Bagnoli M, Canevari S, Figini M, Mezzanzanica D, Raspagliesi F, Tomassetti A, Miotti S. A step further in understanding the biology of the folate receptor in ovarian carcinoma. Gynecol Oncol. 2003;88:S140–144. doi: 10.1006/gyno.2002.6705. [DOI] [PubMed] [Google Scholar]
- 22.Cerezo A, Guadamillas MC, Goetz JG, Sanchez-Perales S, Klein E, Assoian RK, del Pozo MA. The absence of caveolin-1 increases proliferation and anchorage- independent growth by a Rac-dependent, Erk-independent mechanism. Mol Cell Biol. 2009;29:5046–5059. doi: 10.1128/MCB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basal E, Eghbali-Fatourechi GZ, Kalli KR, Hartmann LC, Goodman KM, Goode EL, Kamen BA, Low PS, Knutson KL. Functional folate receptor alpha is elevated in the blood of ovarian cancer patients. PLoS One. 2009;4:e6292. doi: 10.1371/journal.pone.0006292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen MF, Greibe E, Skovbjerg S, Rohde S, Kristensen AC, Jensen TR, Stentoft C, Kjaer KH, Kronborg CS, Martensen PM. Folic acid mediates activation of the pro-oncogene STAT3 via the Folate Receptor alpha. Cell Signal. 2015;27:1356–1368. doi: 10.1016/j.cellsig.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Miotti S, Bagnoli M, Tomassetti A, Colnaghi MI, Canevari S. Interaction of folate receptor with signaling molecules lyn and G(alpha)(i-3) in detergent-resistant complexes from the ovary carcinoma cell line IGROV1. J Cell Sci. 2000;113(Pt 2):349–357. doi: 10.1242/jcs.113.2.349. [DOI] [PubMed] [Google Scholar]
- 26.Croucher DR, Hochgrafe F, Zhang L, Liu L, Lyons RJ, Rickwood D, Tactacan CM, Browne BC, Ali N, Chan H, Shearer R, Gallego-Ortega D, Saunders DN, Swarbrick A, Daly RJ. Involvement of Lyn and the atypical kinase SgK269/PEAK1 in a basal breast cancer signaling pathway. Cancer Res. 2013;73:1969–1980. doi: 10.1158/0008-5472.CAN-12-1472. [DOI] [PubMed] [Google Scholar]
- 27.Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, Hartmann LC. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108:619–626. doi: 10.1016/j.ygyno.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergote IB, Marth C, Coleman RL. Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 2015;34:41–52. doi: 10.1007/s10555-014-9539-8. [DOI] [PubMed] [Google Scholar]
- 29.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994;73:2432–2443. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.Forster MD, Ormerod MG, Agarwal R, Kaye SB, Jackman AL. Flow cytometric method for determining folate receptor expression on ovarian carcinoma cells. Cytometry A. 2007;71:945–950. doi: 10.1002/cyto.a.20456. [DOI] [PubMed] [Google Scholar]
- 32.Miotti S, Canevari S, Menard S, Mezzanzanica D, Porro G, Pupa SM, Regazzoni M, Tagliabue E, Colnaghi MI. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987;39:297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- 33.Buist MR, Kenemans P, den Hollander W, Vermorken JB, Molthoff CJ, Burger CW, Helmerhorst TJ, Baak JP, Roos JC. Kinetics and tissue distribution of the radiolabeled chimeric monoclonal antibody MOv18 IgG and F(ab')2 fragments in ovarian carcinoma patients. Cancer Res. 1993;53:5413–5418. [PubMed] [Google Scholar]
- 34.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13:256–262. doi: 10.1016/j.cbpa.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Sega EI, Low PS. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev. 2008;27:655–664. doi: 10.1007/s10555-008-9155-6. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Low PS. Immunotherapy of folate receptor-expressing tumors: review of recent advances and future prospects. J Control Release. 2003;91:17–29. doi: 10.1016/s0168-3659(03)00215-3. [DOI] [PubMed] [Google Scholar]
- 38.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YL, Chang MC, Huang CY, Chiang YC, Lin HW, Chen CA, Hsieh CY, Cheng WF. Serous ovarian carcinoma patients with high alpha-folate receptor had reducing survival and cytotoxic chemo-response. Mol Oncol. 2012;6:360–369. doi: 10.1016/j.molonc.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coward JI, Middleton K, Murphy F. New perspectives on targeted therapy in ovarian cancer. Int J Womens Health. 2015;7:189–203. doi: 10.2147/IJWH.S52379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Despierre E, Lambrechts S, Leunen K, Berteloot P, Neven P, Amant F, O'shannessy DJ, Somers EB, Vergote I. Folate receptor alpha (FRA) expression remains unchanged in epithelial ovarian and endometrial cancer after chemotherapy. Gynecol Oncol. 2013;130:192–199. doi: 10.1016/j.ygyno.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Crane LM, Arts HJ, van Oosten M, Low PS, van der Zee AG, van Dam GM, Bart J. The effect of chemotherapy on expression of folate receptor-alpha in ovarian cancer. Cell Oncol (Dordr) 2012;35:9–18. doi: 10.1007/s13402-011-0052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cagle PT, Zhai QJ, Murphy L, Low PS. Folate receptor in adenocarcinoma and squamous cell carcinoma of the lung: potential target for folate-linked therapeutic agents. Arch Pathol Lab Med. 2013;137:241–244. doi: 10.5858/arpa.2012-0176-OA. [DOI] [PubMed] [Google Scholar]
- 44.Iwakiri S, Sonobe M, Nagai S, Hirata T, Wada H, Miyahara R. Expression status of folate receptor alpha is significantly correlated with prognosis in non-small-cell lung cancers. Ann Surg Oncol. 2008;15:889–899. doi: 10.1245/s10434-007-9755-3. [DOI] [PubMed] [Google Scholar]
- 45.Nunez MI, Behrens C, Woods DM, Lin H, Suraokar M, Kadara H, Hofstetter W, Kalhor N, Lee JJ, Franklin W, Stewart DJ, Wistuba II. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J Thorac Oncol. 2012;7:833–840. doi: 10.1097/JTO.0b013e31824de09c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'shannessy DJ, Yu G, Smale R, Fu YS, Singhal S, Thiel RP, Somers EB, Vachani A. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3:414–425. doi: 10.18632/oncotarget.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bueno R, Appasani K, Mercer H, Lester S, Sugarbaker D. The alpha folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;121:225–233. doi: 10.1067/mtc.2001.111176. [DOI] [PubMed] [Google Scholar]
- 48.Nutt JE, Razak AR, O'Toole K, Black F, Quinn AE, Calvert AH, Plummer ER, Lunec J. The role of folate receptor alpha (FRalpha) in the response of malignant pleural mesothelioma to pemetrexed-containing chemotherapy. Br J Cancer. 2010;102:553–560. doi: 10.1038/sj.bjc.6605501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartmann LC, Keeney GL, Lingle WL, Christianson TJ, Varghese B, Hillman D, Oberg AL, Low PS. Folate receptor overexpression is associated with poor outcome in breast cancer. Int J Cancer. 2007;121:938–942. doi: 10.1002/ijc.22811. [DOI] [PubMed] [Google Scholar]
- 50.Necela BM, Crozier JA, Andorfer CA, Lewis-Tuffin L, Kachergus JM, Geiger XJ, Kalari KR, Serie DJ, Sun Z, Moreno-Aspitia A, O'shannessy DJ, Maltzman JD, McCullough AE, Pockaj BA, Cunliffe HE, Ballman KV, et al. Correction: Folate receptor-alpha (FOLR1) expression and function in triple negative tumors. PLoS One. 2015;10:e0127133. doi: 10.1371/journal.pone.0127133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Necela BM, Crozier JA, Andorfer CA, Lewis-Tuffin L, Kachergus JM, Geiger XJ, Kalari KR, Serie DJ, Sun Z, Moreno-Aspitia A, O'shannessy DJ, Maltzman JD, McCullough AE, Pockaj BA, Cunliffe HE, Ballman KV, et al. Folate receptor-alpha (FOLR1) expression and function in triple negative tumors. PLoS One. 2015;10:e0122209. doi: 10.1371/journal.pone.0122209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Wang J, Tacha DE, Li P, Bremer RE, Chen H, Wei B, Xiao X, Da J, Skinner K, Hicks DG, Bu H, Tang P. Folate receptor alpha associated with triple-negative breast cancer and poor prognosis. Arch Pathol Lab Med. 2014;138:890–895. doi: 10.5858/arpa.2013-0309-OA. [DOI] [PubMed] [Google Scholar]
- 53.Kelley KM, Rowan BG, Ratnam M. Modulation of the folate receptor alpha gene by the estrogen receptor: mechanism and implications in tumor targeting. Cancer Res. 2003;63:2820–2828. [PubMed] [Google Scholar]
- 54.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9(Suppl 2):S73–81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carey LA. Directed therapy of subtypes of triple-negative breast cancer. Oncologist. 2010;15(Suppl 5):49–56. doi: 10.1634/theoncologist.2010-S5-49. [DOI] [PubMed] [Google Scholar]
- 56.Brown D, Waneck GL. Glycosyl-phosphatidylinositol-anchored membrane proteins. J Am Soc Nephrol. 1992;3:895–906. doi: 10.1681/ASN.V34895. [DOI] [PubMed] [Google Scholar]
- 57.Kurosaki A, Hasegawa K, Kato T, Abe K, Hanaoka T, Miyara A, O'shannessy DJ, Somers EB, Yasuda M, Sekino T, Fujiwara K. Serum folate receptor alpha as a biomarker for ovarian cancer: Implications for diagnosis, prognosis and predicting its local tumor expression. Int J Cancer. 2016;138:1994–2002. doi: 10.1002/ijc.29937. [DOI] [PubMed] [Google Scholar]
- 58.Mantovani LT, Miotti S, Menard S, Canevari S, Raspagliesi F, Bottini C, Bottero F, Colnaghi MI. Folate binding protein distribution in normal tissues and biological fluids from ovarian carcinoma patients as detected by the monoclonal antibodies MOv18 and MOv19. Eur J Cancer. 1994;30A:363–369. doi: 10.1016/0959-8049(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 59.O'shannessy DJ, Somers EB, Palmer LM, Thiel RP, Oberoi P, Heath R, Marcucci L. Serum folate receptor alpha, mesothelin and megakaryocyte potentiating factor in ovarian cancer: association to disease stage and grade and comparison to CA125 and HE4. J Ovarian Res. 2013;6:29. doi: 10.1186/1757-2215-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crippa F, Buraggi GL, Di Re E, Gasparini M, Seregni E, Canevari S, Gadina M, Presti M, Marini A, Seccamani E. Radioimmunoscintigraphy of ovarian cancer with the MOv18 monoclonal antibody. Eur J Cancer. 1991;27:724–729. doi: 10.1016/0277-5379(91)90174-c. [DOI] [PubMed] [Google Scholar]
- 61.van Zanten-Przybysz I, Molthoff CF, Roos JC, Verheijen RH, van Hof A, Buist MR, Prinssen HM, den Hollander W, Kenemans P. Influence of the route of administration on targeting of ovarian cancer with the chimeric monoclonal antibody MOv18: i.v. vs. i.p. Int J Cancer. 2001;92:106–114. [PubMed] [Google Scholar]
- 62.Ke CY, Mathias CJ, Green MA. Folate-receptor-targeted radionuclide imaging agents. Adv Drug Deliv Rev. 2004;56:1143–1160. doi: 10.1016/j.addr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Siegel BA, Dehdashti F, Mutch DG, Podoloff DA, Wendt R, Sutton GP, Burt RW, Ellis PR, Mathias CJ, Green MA, Gershenson DM. Evaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: initial clinical results. J Nucl Med. 2003;44:700–707. [PubMed] [Google Scholar]
- 64.Leamon CP, Parker MA, Vlahov IR, Xu LC, Reddy JA, Vetzel M, Douglas N. Synthesis and biological evaluation of EC20: a new folate-derived, (99m)Tc-based radiopharmaceutical. Bioconjug Chem. 2002;13:1200–1210. doi: 10.1021/bc0200430. [DOI] [PubMed] [Google Scholar]
- 65.Konda SD, Aref M, Brechbiel M, Wiener EC. Development of a tumor-targeting MR contrast agent using the high-affinity folate receptor: work in progress. Invest Radiol. 2000;35:50–57. doi: 10.1097/00004424-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Meier R, Henning TD, Boddington S, Tavri S, Arora S, Piontek G, Rudelius M, Corot C, Daldrup-Link HE. Breast cancers: MR imaging of folate-receptor expression with the folate-specific nanoparticle P1133. Radiology. 2010;255:527–535. doi: 10.1148/radiol.10090050. [DOI] [PubMed] [Google Scholar]
- 67.Maurer AH, Elsinga P, Fanti S, Nguyen B, Oyen WJ, Weber WA. Imaging the folate receptor on cancer cells with 99mTc-etarfolatide: properties, clinical use, and future potential of folate receptor imaging. J Nucl Med. 2014;55:701–704. doi: 10.2967/jnumed.113.133074. [DOI] [PubMed] [Google Scholar]
- 68.Yamada Y, Nakatani H, Yanaihara H, Omote M. Phase I clinical trial of 99mTc-etarfolatide, an imaging agent for folate receptor in healthy Japanese adults. Ann Nucl Med. 2015;29:792–798. doi: 10.1007/s12149-015-1006-2. [DOI] [PubMed] [Google Scholar]
- 69.Reddy JA, Westrick E, Santhapuram HK, Howard SJ, Miller ML, Vetzel M, Vlahov I, Chari RV, Goldmacher VS, Leamon CP. Folate receptor-specific antitumor activity of EC131, a folate-maytansinoid conjugate. Cancer Res. 2007;67:6376–6382. doi: 10.1158/0008-5472.CAN-06-3894. [DOI] [PubMed] [Google Scholar]
- 70.ClinicalTrials.gov. US National Library of Medicine, US National Institutes of Health; Bethesda (MD): https://clinicaltrials.gov/ Available from: [Google Scholar]
- 71.Vlahov IR, Santhapuram HK, Kleindl PJ, Howard SJ, Stanford KM, Leamon CP. Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part 1: EC145, a folic acid conjugate of desacetylvinblastine monohydrazide. Bioorg Med Chem Lett. 2006;16:5093–5096. doi: 10.1016/j.bmcl.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 72.Dosio F, Milla P, Cattel L. EC-145, a folate-targeted Vinca alkaloid conjugate for the potential treatment of folate receptor-expressing cancers. Curr Opin Investig Drugs. 2010;11:1424–1433. [PubMed] [Google Scholar]
- 73.Pribble P, Edelman MJ. EC145: a novel targeted agent for adenocarcinoma of the lung. Expert Opin Investig Drugs. 2012;21:755–761. doi: 10.1517/13543784.2012.671294. [DOI] [PubMed] [Google Scholar]
- 74.Lorusso PM, Edelman MJ, Bever SL, Forman KM, Pilat M, Quinn MF, Li J, Heath EI, Malburg LM, Klein PJ, Leamon CP, Messmann RA, Sausville EA. Phase I study of folate conjugate EC145 (Vintafolide) in patients with refractory solid tumors. J Clin Oncol. 2012;30:4011–4016. doi: 10.1200/JCO.2011.41.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edelman MJ, Harb WA, Pal SE, Boccia RV, Kraut MJ, Bonomi P, Conley BA, Rogers JS, Messmann RA, Garon EB. Multicenter trial of EC145 in advanced, folate-receptor positive adenocarcinoma of the lung. J Thorac Oncol. 2012;7:1618–1621. doi: 10.1097/JTO.0b013e318267d051. [DOI] [PubMed] [Google Scholar]
- 76.Hanna N, Juhász E, Cainap C, Gladkov O, Ramlau R, Juan-Vidal O, Lal R, Symanowski J, Perez W, Nguyen B, Harb W. Target: A randomized, phase II trial comparing vintafolide versus vintafolide plus docetaxel, versus docetaxel alone in second line treatment of folate-receptor-positive non-small cell lung cancer (NSCLC) patients. Annals of Oncology. 2014;25:v1–v41. [Google Scholar]
- 77.Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA, Gabrail NY, Depasquale SE, Nowara E, Gilbert L, Gersh RH, Teneriello MG, Harb WA, Konstantinopoulos PA, Penson RT, Symanowski JT, et al. PRECEDENT: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2013;31:4400–4406. doi: 10.1200/JCO.2013.49.7685. [DOI] [PubMed] [Google Scholar]
- 78.Merck Press Release. Merck and endocyte announce independent dsmb recommends vintafolide proceed phase 3 trial be stopped for futility following interim analysis. 2014 http://investor.endocyte.com/releasedetail.cfm?ReleaseID=844838 Available from:
- 79.Sharma S, Sausville EA, LoRusso P, Vogelzang NJ, Samlowski WE, Carter J, Forman K, Bever S, Messmann RA. A phase I study of EC0225 administered weeks 1 and 2 of a 4-week cycle. J Clin Oncol. 2010;28:3082. [Google Scholar]
- 80.Gokhale M, Thakur A, Rinaldi F. Degradation of BMS-753493, a novel epothilone folate conjugate anticancer agent. Drug Dev Ind Pharm. 2013;39:1315–1327. doi: 10.3109/03639045.2012.728226. [DOI] [PubMed] [Google Scholar]
- 81.Peethambaram PP, Hartmann LC, Jonker DJ, de Jonge M, Plummer ER, Martin L, Konner J, Marshall J, Goss GD, Teslenko V, Clemens PL, Cohen LJ, Ahlers CM, Alland L. A phase I pharmacokinetic and safety analysis of epothilone folate (BMS-753493), a folate receptor targeted chemotherapeutic agent in humans with advanced solid tumors. Invest New Drugs. 2015;33:321–331. doi: 10.1007/s10637-014-0171-9. [DOI] [PubMed] [Google Scholar]
- 82.Leamon CP, Reddy JA, Klein PJ, Vlahov IR, Dorton R, Bloomfield A, Nelson M, Westrick E, Parker N, Bruna K, Vetzel M, Gehrke M, Nicoson JS, Messmann RA, LoRusso PM, Sausville EA. Reducing undesirable hepatic clearance of a tumor-targeted vinca alkaloid via novel saccharopeptidic modifications. J Pharmacol Exp Ther. 2011;336:336–343. doi: 10.1124/jpet.110.175109. [DOI] [PubMed] [Google Scholar]
- 83.Aronov O, Horowitz AT, Gabizon A, Gibson D. Folate-targeted PEG as a potential carrier for carboplatin analogs. Synthesis and in vitro studies. Bioconjug Chem. 2003;14:563–574. doi: 10.1021/bc025642l. [DOI] [PubMed] [Google Scholar]
- 84.Lee JW, Lu JY, Low PS, Fuchs PL. Synthesis and evaluation of taxol-folic acid conjugates as targeted antineoplastics. Bioorg Med Chem. 2002;10:2397–2414. doi: 10.1016/s0968-0896(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 85.Reddy JA, Dorton R, Dawson A, Vetzel M, Parker N, Nicoson JS, Westrick E, Klein PJ, Wang Y, Vlahov IR, Leamon CP. In vivo structural activity and optimization studies of folate-tubulysin conjugates. Mol Pharm. 2009;6:1518–1525. doi: 10.1021/mp900086w. [DOI] [PubMed] [Google Scholar]
- 86.Kurahara H, Takao S, Kuwahata T, Nagai T, Ding Q, Maeda K, Shinchi H, Mataki Y, Maemura K, Matsuyama T, Natsugoe S. Clinical significance of folate receptor beta-expressing tumor-associated macrophages in pancreatic cancer. Ann Surg Oncol. 2012;19:2264–2271. doi: 10.1245/s10434-012-2263-0. [DOI] [PubMed] [Google Scholar]
- 87.Nagai T, Tanaka M, Tsuneyoshi Y, Xu B, Michie SA, Hasui K, Hirano H, Arita K, Matsuyama T. Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta. Cancer Immunol Immunother. 2009;58:1577–1586. doi: 10.1007/s00262-009-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 2013;138:93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–446. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 90.Fisher RE, Siegel BA, Edell SL, Oyesiku NM, Morgenstern DE, Messmann RA, Amato RJ. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J Nucl Med. 2008;49:899–906. doi: 10.2967/jnumed.107.049478. [DOI] [PubMed] [Google Scholar]
- 91.Jackman AL, Theti DS, Gibbs DD. Antifolates targeted specifically to the folate receptor. Adv Drug Deliv Rev. 2004;56:1111–1125. doi: 10.1016/j.addr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 92.Wang L, Wallace A, Raghavan S, Deis SM, Wilson MR, Yang S, Polin L, White K, Kushner J, Orr S, George C, O'Connor C, Hou Z, Mitchell-Ryan S, Dann CE, 3rd, Matherly LH, et al. 6-Substituted Pyrrolo [2,3-d]pyrimidine Thienoyl Regioisomers as Targeted Antifolates for Folate Receptor alpha and the Proton-Coupled Folate Transporter in Human Tumors. J Med Chem. 2015;58:6938–6959. doi: 10.1021/acs.jmedchem.5b00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Theti DS, Bavetsias V, Skelton LA, Titley J, Gibbs D, Jansen G, Jackman AL. Selective delivery of CB300638, a cyclopenta [g]quinazoline-based thymidylate synthase inhibitor into human tumor cell lines overexpressing the alpha-isoform of the folate receptor. Cancer Res. 2003;63:3612–3618. [PubMed] [Google Scholar]
- 94.Gibbs DD, Theti DS, Wood N, Green M, Raynaud F, Valenti M, Forster MD, Mitchell F, Bavetsias V, Henderson E, Jackman AL. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to alpha-folate receptor-overexpressing tumors. Cancer Res. 2005;65:11721–11728. doi: 10.1158/0008-5472.CAN-05-2034. [DOI] [PubMed] [Google Scholar]
- 95.Pillai RG, Forster M, Perumal M, Mitchell F, Leyton J, Aibgirhio FI, Golovko O, Jackman AL, Aboagye EO. Imaging pharmacodynamics of the alpha-folate receptor-targeted thymidylate synthase inhibitor BGC 945. Cancer Res. 2008;68:3827–3834. doi: 10.1158/0008-5472.CAN-08-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hernando JJ, Park TW, Fischer HP, Zivanovic O, Braun M, Polcher M, Grunn U, Leutner C, Potzsch B, Kuhn W. Vaccination with dendritic cells transfected with mRNA-encoded folate-receptor-alpha for relapsed metastatic ovarian cancer. Lancet Oncol. 2007;8:451–454. doi: 10.1016/S1470-2045(07)70142-0. [DOI] [PubMed] [Google Scholar]
- 97.Amato RJ, Shetty A, Lu Y, Ellis R, Low PS. A phase I study of folate immune therapy (EC90 vaccine administered with GPI-0100 adjuvant followed by EC17) in patients with renal cell carcinoma. J Immunother. 2013;36:268–275. doi: 10.1097/CJI.0b013e3182917f59. [DOI] [PubMed] [Google Scholar]
- 98.Li S, Tong J, Rahman MM, Shepherd TG, McFadden G. Oncolytic virotherapy for ovarian cancer. Oncolytic Virother. 2012;1:1–21. doi: 10.2147/OV.S31626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hasegawa K, Nakamura T, Harvey M, Ikeda Y, Oberg A, Figini M, Canevari S, Hartmann LC, Peng KW. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12:6170–6178. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 100.Ebel W, Routhier EL, Foley B, Jacob S, McDonough JM, Patel RK, Turchin HA, Chao Q, Kline JB, Old LJ, Phillips MD, Nicolaides NC, Sass PM, Grasso L. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immun. 2007;7:6. [PMC free article] [PubMed] [Google Scholar]
- 101.Kamen BA, Smith AK. Farletuzumab, an anti-folate receptor alpha antibody, does not block binding of folate or anti-folates to receptor nor does it alter the potency of anti-folates in vitro. Cancer Chemother Pharmacol. 2012;70:113–120. doi: 10.1007/s00280-012-1890-2. [DOI] [PubMed] [Google Scholar]
- 102.Thomas A, Maltzman J, Hassan R. Farletuzumab in lung cancer. Lung Cancer. 2013;80:15–18. doi: 10.1016/j.lungcan.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin J, Spidel JL, Maddage CJ, Rybinski KA, Kennedy RP, Krauthauser CL, Park YC, Albone EF, Jacob S, Goserud MT, Martinez BP, Chao Q, Zhou Y, Nicolaides NC, Kline JB, Grasso L. The antitumor activity of the human FOLR1-specific monoclonal antibody, farletuzumab, in an ovarian cancer mouse model is mediated by antibody-dependent cellular cytotoxicity. Cancer Biol Ther. 2013;14:1032–1038. doi: 10.4161/cbt.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wen Y, Graybill WS, Previs RA, Hu W, Ivan C, Mangala LS, Zand B, Nick AM, Jennings NB, Dalton HJ, Sehgal V, Ram P, Lee JS, Vivas-Mejia PE, Coleman RL, Sood AK. Immunotherapy targeting folate receptor induces cell death associated with autophagy in ovarian cancer. Clin Cancer Res. 2015;21:448–459. doi: 10.1158/1078-0432.CCR-14-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, Vander Els N, Phillips MD, Schweizer C, Weil SC, Larson SM, Old LJ. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res. 2010;16:5288–5295. doi: 10.1158/1078-0432.CCR-10-0700. [DOI] [PubMed] [Google Scholar]
- 106.Kim KH, Jelovac D, Armstrong DK, Schwartz B, Weil SC, Schweizer C, Alvarez RD. Phase 1b safety study of farletuzumab, carboplatin and pegylated liposomal doxorubicin in patients with platinum-sensitive epithelial ovarian cancer. Gynecol Oncol. 2016;140:210–214. doi: 10.1016/j.ygyno.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Armstrong DK, White AJ, Weil SC, Phillips M, Coleman RL. Farletuzumab (a monoclonal antibody against folate receptor alpha) in relapsed platinum-sensitive ovarian cancer. Gynecol Oncol. 2013;129:452–458. doi: 10.1016/j.ygyno.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 108.Marchetti C, Palaia I, Giorgini M, De Medici C, Iadarola R, Vertechy L, Domenici L, Di Donato V, Tomao F, Muzii L, Benedetti Panici P. Targeted drug delivery via folate receptors in recurrent ovarian cancer: a review. Onco Targets Ther. 2014;7:1223–1236. doi: 10.2147/OTT.S40947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morphotek®, Inc. Morphotek announces collaboration with mayo clinic to apply its folate receptor alpha diagnostic assays in a phase ii clinical trial in triple-negative breast cancer. 2016 http://www.morphotek.com/news-events/News-Archive/2016-News/Morphotek-Announces-Collaboration-with-Mayo-Clinic.aspx Available from.
- 110.Coney LR, Mezzanzanica D, Sanborn D, Casalini P, Colnaghi MI, Zurawski VR., Jr Chimeric murine-human antibodies directed against folate binding receptor are efficient mediators of ovarian carcinoma cell killing. Cancer Res. 1994;54:2448–2455. [PubMed] [Google Scholar]
- 111.Crippa F, Bolis G, Seregni E, Gavoni N, Scarfone G, Ferraris C, Buraggi GL, Bombardieri E. Single-dose intraperitoneal radioimmunotherapy with the murine monoclonal antibody I-131 MOv18: clinical results in patients with minimal residual disease of ovarian cancer. Eur J Cancer. 1995;31A:686–690. doi: 10.1016/0959-8049(94)00454-d. [DOI] [PubMed] [Google Scholar]
- 112.Molthoff CF, Prinssen HM, Kenemans P, van Hof AC, den Hollander W, Verheijen RH. Escalating protein doses of chimeric monoclonal antibody MOv18 immunoglobulin G in ovarian carcinoma patients: a phase I study. Cancer. 1997;80:2712–2720. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2712::aid-cncr50>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 113.Buijs WC, Tibben JG, Boerman OC, Molthoff CF, Massuger LF, Koenders EB, Schijf CP, Siegel JA, Corstens FH. Dosimetric analysis of chimeric monoclonal antibody cMOv18 IgG in ovarian carcinoma patients after intraperitoneal and intravenous administration. Eur J Nucl Med. 1998;25:1552–1561. doi: 10.1007/s002590050335. [DOI] [PubMed] [Google Scholar]
- 114.Casalini P, Mezzanzanica D, Canevari S, Della Torre G, Miotti S, Colnaghi MI, Matzku S. Use of combination of monoclonal antibodies directed against three distinct epitopes of a tumor-associated antigen: analysis of cell binding and internalization. Int J Cancer. 1991;48:284–290. doi: 10.1002/ijc.2910480222. [DOI] [PubMed] [Google Scholar]
- 115.van Zanten-Przybysz I, Molthoff C, Gebbinck JK, von Mensdorff-Pouilly S, Verstraeten R, Kenemans P, Verheijen R. Cellular and humoral responses after multiple injections of unconjugated chimeric monoclonal antibody MOv18 in ovarian cancer patients: a pilot study. J Cancer Res Clin Oncol. 2002;128:484–492. doi: 10.1007/s00432-002-0348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Macor P, Mezzanzanica D, Cossetti C, Alberti P, Figini M, Canevari S, Tedesco F. Complement activated by chimeric anti-folate receptor antibodies is an efficient effector system to control ovarian carcinoma. Cancer Res. 2006;66:3876–3883. doi: 10.1158/0008-5472.CAN-05-3434. [DOI] [PubMed] [Google Scholar]
- 117.Figini M, Obici L, Mezzanzanica D, Griffiths A, Colnaghi MI, Winter G, Canevari S. Panning phage antibody libraries on cells: isolation of human Fab fragments against ovarian carcinoma using guided selection. Cancer Res. 1998;58:991–996. [PubMed] [Google Scholar]
- 118.Schutsky K, Song DG, Lynn R, Smith JB, Poussin M, Figini M, Zhao Y, Powell DJ., Jr Rigorous optimization and validation of potent RNA CAR T cell therapy for the treatment of common epithelial cancers expressing folate receptor. Oncotarget. 2015;6:28911–28928. doi: 10.18632/oncotarget.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Figini M, Marks JD, Winter G, Griffiths AD. In vitro assembly of repertoires of antibody chains on the surface of phage by renaturation. J Mol Biol. 1994;239:68–78. doi: 10.1006/jmbi.1994.1351. [DOI] [PubMed] [Google Scholar]
- 120.Figini M, Martin F, Ferri R, Luison E, Ripamonti E, Zacchetti A, Mortarino M, Di Cioccio V, Maurizi G, Allegretti M, Canevari S. Conversion of murine antibodies to human antibodies and their optimization for ovarian cancer therapy targeted to the folate receptor. Cancer Immunol Immunother. 2009;58:531–546. doi: 10.1007/s00262-008-0575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zacchetti A, Martin F, Luison E, Coliva A, Bombardieri E, Allegretti M, Figini M, Canevari S. Antitumor effects of a human dimeric antibody fragment 131I-AFRA-DFM5. 3 in a mouse model for ovarian cancer. J Nucl Med. 2011;52:1938–1946. doi: 10.2967/jnumed.110.086819. [DOI] [PubMed] [Google Scholar]
- 122.Gould HJ, Mackay GA, Karagiannis SN, O'Toole CM, Marsh PJ, Daniel BE, Coney LR, Zurawski VR, Jr, Joseph M, Capron M, Gilbert M, Murphy GF, Korngold R. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur J Immunol. 1999;29:3527–3537. doi: 10.1002/(SICI)1521-4141(199911)29:11<3527::AID-IMMU3527>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 123.Karagiannis SN, Josephs DH, Karagiannis P, Gilbert AE, Saul L, Rudman SM, Dodev T, Koers A, Blower PJ, Corrigan C, Beavil AJ, Spicer JF, Nestle FO, Gould HJ. Recombinant IgE antibodies for passive immunotherapy of solid tumours: from concept towards clinical application. Cancer Immunol Immunother. 2012;61:1547–1564. doi: 10.1007/s00262-011-1162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karagiannis SN, Bracher MG, Beavil RL, Beavil AJ, Hunt J, McCloskey N, Thompson RG, East N, Burke F, Sutton BJ, Dombrowicz D, Balkwill FR, Gould HJ. Role of IgE receptors in IgE antibody-dependent cytotoxicity and phagocytosis of ovarian tumor cells by human monocytic cells. Cancer Immunol Immunother. 2008;57:247–263. doi: 10.1007/s00262-007-0371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karagiannis SN, Bracher MG, Hunt J, McCloskey N, Beavil RL, Beavil AJ, Fear DJ, Thompson RG, East N, Burke F, Moore RJ, Dombrowicz DD, Balkwill FR, Gould HJ. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol. 2007;179:2832–2843. doi: 10.4049/jimmunol.179.5.2832. [DOI] [PubMed] [Google Scholar]
- 126.Karagiannis SN, Wang Q, East N, Burke F, Riffard S, Bracher MG, Thompson RG, Durham SR, Schwartz LB, Balkwill FR, Gould HJ. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol. 2003;33:1030–1040. doi: 10.1002/eji.200323185. [DOI] [PubMed] [Google Scholar]
- 127.Bracher M, Gould HJ, Sutton BJ, Dombrowicz D, Karagiannis SN. Three-colour flow cytometric method to measure antibody-dependent tumour cell killing by cytotoxicity and phagocytosis. J Immunol Methods. 2007;323:160–171. doi: 10.1016/j.jim.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 128.Saul L, Josephs DH, Cutler K, Bradwell A, Karagiannis P, Selkirk C, Gould HJ, Jones P, Spicer JF, Karagiannis SN. Comparative reactivity of human IgE to cynomolgus monkey and human effector cells and effects on IgE effector cell potency. MAbs. 2014;6:509–522. doi: 10.4161/mabs.27828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rudman SM, Josephs DH, Cambrook H, Karagiannis P, Gilbert AE, Dodev T, Hunt J, Koers A, Montes A, Taams L, Canevari S, Figini M, Blower PJ, Beavil AJ, Nicodemus CF, Corrigan C, et al. Harnessing engineered antibodies of the IgE class to combat malignancy: initial assessment of FcvarepsilonRI-mediated basophil activation by a tumour-specific IgE antibody to evaluate the risk of type I hypersensitivity. Clin Exp Allergy. 2011;41:1400–1413. doi: 10.1111/j.1365-2222.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 130.Josephs DH, Spicer JF, Karagiannis P, Gould HJ, Karagiannis SN. IgE immunotherapy: a novel concept with promise for the treatment of cancer. MAbs. 2014;6:54–72. doi: 10.4161/mabs.27029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Josephs DH, Bax HJ, Karagiannis SN. Tumour-associated macrophage polarisation and re-education with immunotherapy. Front Biosci (Elite Ed) 2015;7:293–308. doi: 10.2741/E735. [DOI] [PubMed] [Google Scholar]
- 132.Canevari S, Stoter G, Arienti F, Bolis G, Colnaghi MI, Di Re EM, Eggermont AM, Goey SH, Gratama JW, Lamers CH, et al. Regression of advanced ovarian carcinoma by intraperitoneal treatment with autologous T lymphocytes retargeted by a bispecific monoclonal antibody. J Natl Cancer Inst. 1995;87:1463–1469. doi: 10.1093/jnci/87.19.1463. [DOI] [PubMed] [Google Scholar]
- 133.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miotti S, Negri DR, Valota O, Calabrese M, Bolhuis RL, Gratama JW, Colnaghi MI, Canevari S. Level of anti-mouse-antibody response induced by bi-specific monoclonal antibody OC/TR in ovarian-carcinoma patients is associated with longer survival. Int J Cancer. 1999;84:62–68. doi: 10.1002/(sici)1097-0215(19990219)84:1<62::aid-ijc12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 135.Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, Figini M, June CH, Coukos G, Powell DJ., Jr In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB) Cancer Res. 2011;71:4617–4627. doi: 10.1158/0008-5472.CAN-11-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ribas A. Releasing the Brakes on Cancer Immunotherapy. N Engl J Med. 2015;373:1490–1492. doi: 10.1056/NEJMp1510079. [DOI] [PubMed] [Google Scholar]
- 137.Hamanishi J, Mandai M, Konishi I. Immune checkpoint inhibition in ovarian cancer. Int Immunol. 2016 doi: 10.1093/intimm/dxw020. [DOI] [PubMed] [Google Scholar]
- 138.Marrone KA, Brahmer JR. Immune Checkpoint Therapy in Non-Small Cell Lung Cancer. Cancer J. 2016;22:81–91. doi: 10.1097/PPO.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 139.Pusztai L, Karn T, Safonov A, Abu-Khalaf MM, Bianchini G. New Strategies in Breast Cancer: Immunotherapy. Clin Cancer Res. 2016;22:2105–2110. doi: 10.1158/1078-0432.CCR-15-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ring KL, Pakish J, Jazaeri AA. Immune Checkpoint Inhibitors in the Treatment of Gynecologic Malignancies. Cancer J. 2016;22:101–107. doi: 10.1097/PPO.0000000000000179. [DOI] [PubMed] [Google Scholar]