Abstract
Introduction:
To the reduction of bone density and osteoporosis in postmenopausal women contribute elevated lipid parameters and Body Mass Index (BMI).
Goal:
The goal of our study was to determine the correlation between lipid parameters, BMI and osteoporosis in postmenopausal women.
Material and methods:
The study was carried out by matched type between experimental group and controls. The experimental group consisted of 100 females at postmenopausal age, in which by the DEXA method was diagnosed osteoporosis at the Department of Endocrinology, Diabetes and Metabolic Diseases, University Medical Center of RS during 2015-2016, while the control group consisted of 100 females in a postmenopausal age but without diagnosed osteoporosis. The groups were matched by age (± 2 years). To all participants of the study were carried out biochemical analysis of blood, or the analysis of the lipid profile that included total cholesterol, LDL cholesterol, triglycerides (TG) and HDL cholesterol, and was determined the values of BMI and waist circumference (WC).
Results:
Analysis of the data of our research shows that by the univariate logistic regression the values of lipid parameters total cholesterol (p=0.000), LDL (p=0.005) and TG (p=0.033) were significantly associated with osteoporosis, while in multivariate logistic model only total cholesterol (p= 0.018) was found as an independent risk factor for osteoporosis in postmenopausal women. BMI values were not statistically significantly associated with osteoporosis (p=0.727).
Conclusion:
On the decrease in bone mineral density and osteoporosis in postmenopausal women influence many risk factors whose identification has the aim to develop more effective prevention of this disease in the elderly.
Keywords: osteoporosis, menopause, lipid profile, BMI
1. INTRODUCTION
Osteoporosis is a disease which affect the population of so-called “third age”, or the elderly, while it more affect females as a result of the fact that they have a 30% lower bone mass than men, and that among them there is a rapid process of losing bone mass after entering the menopause and the occurrence of ovarian insufficiency (1, 2). The emergence of this disease involves number of factors (genetic and environmental factors), as well as other pathological entities that can lead to rapid loss of bone mass, while recent studies indicate that there is no association between low bone mineral density (osteopenia, osteoporosis) and hyperlipidemia in obese people and that this correlation was probably the result of increased mechanical pressure on the bone, especially in postmenopausal women (3-8), or the direct effects of lipids on the function of bone cells (9).
2. GOAL
The goal of our study was to determine whether in postmenopausal women there is a correlation between lipid parameters, BMI and osteoporosis.
3. MATERIAL AND METHODS
Experimental group consisted of 100 females in a postmenopausal age (at least two years after the last menstrual cycle) who have a newly diagnosed osteoporosis in the Cabinet for Osteodensitometry (Department of Endocrinology, Diabetes and Metabolic Diseases, University Medical Center of RS) by determining bone mineral density, DEXA method at the lumbar spine (L2-L4) and hip, and the upper part of the femur. The control group consisted of 100 females in a postmenopausal age which after determination of bone mineral density, DEXA method, has not been diagnosed with osteoporosis.
Exclusion criteria were malignant disease, diabetes, disease of the thyroid, parathyroid and adrenal glands, chronic renal failure, inflammatory arthritis, the use of statins in the treatment of dyslipidemia, corticosteroids, hormones and diuretics for more than three months, secondary osteoporosis due to endocrine diseases, diseases of the gastrointestinal tract (Crohn’s disease, malabsorption), peptic ulcer surgery, chronic liver disease and osteoporosis induced by medications.
All the examinees were undergoing biochemical analysis of blood. Samples were taken from a peripheral vein after 12 hours of fasting and were immediately centrifuged at four degrees Celsius (4° C). Plasma is used to analyze the lipid profile (total cholesterol, LDL cholesterol, TG, HDL cholesterol). To all respondents was also determined anthropometric status, determination of BMI and waist circumference (WC).
4. RESULTS
From the standpoint of demographic data, the median age of respondents in experimental group amounted to 64, in the control group 63, which is not a statistically significant difference. In terms of education level in both groups dominated women with primary or secondary education (in a group of cases 78% and in the control, group 84%), without statistically significant differences between groups.
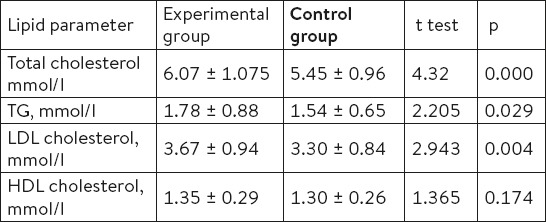
Analyzing the average values of the lipid profile (total cholesterol, LDL cholesterol, TG, HDL-cholesterol) in our study, we see that the same in the experimental group of patients was classified as a so-called borderline high category as compared to the control group where the values of these parameters were within the normal range. Average values of total cholesterol in the experimental group were 6.07 mmol/l with an average deviation of ±1.075 in the control group 5.45 mmol/l (± 0.96). If we observe other parameters of lipid profiles and their average values, we can see that there is a statistically significant difference between experimental group and controls with the exception of HDL cholesterol (Table 1).
Table 1.
Average values of lipid parameters of the experimental and control group
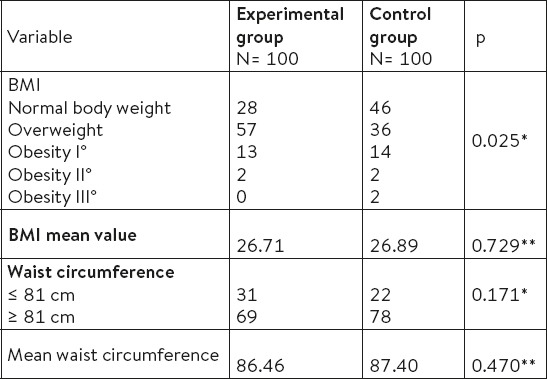
A statistically significant difference between experimental group and controls was found in terms of categorizing weight χ2=11.157, p=0.025. In the control group, there was significantly more women with normal weight (46%) than in the experimental group (28%). In the experimental group the highest percentage of women (57%) are overweight 25 to 30 kg/m2. A statistically significant difference in the values of waist circumference is not found between the groups χ2=1.64, p=0.171. The highest percentage of women in both groups had WC > 81 cm (Table 2).
Table 2.
Relationship of BMI and WC in women of experimental and the control group. * χ2 test ** t- test
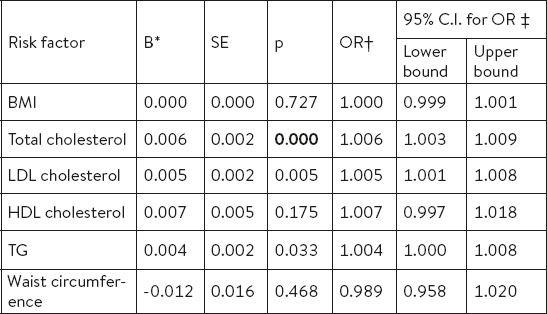
Univariate logistic regression model, for all tested parameters in our study (total cholesterol, LDL cholesterol, TG, HDL cholesterol, BMI and WC) showed that the statistically significantly the osteoporosis in postmenopausal women is linked with: total cholesterol (p=0.000), LDL cholesterol (p=0.005), triglycerides (p=0.033), while the value of BMI (p=0.727) was not a significant factor for osteoporosis (Table 3).
Table 3.
Risk factors for osteoporosis identified by univariate logistic regression. * coefficient; † odds ratio; ‡ confidence interval
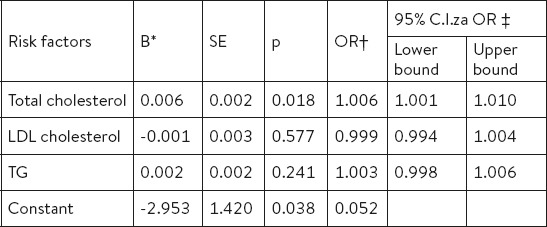
The examined factors that are statistically significant in the model of univariate logistic regression were entered into the model of multivariate regression, the results of which show that elevated levels of total cholesterol are independent risk factor for osteoporosis in postmenopausal women (p=0.018), while the other parameters of lipid profile were not relevant for osteoporosis development (Table 4).
Table 4.
Risk factors for osteoporosis identified by multivariate logistic regression. * coefficient; † odds ratio; ‡ confidence interval
5. DISCUSSION
Osteoporosis is a multifactorial disease that occurs due to the effects of genetic and environmental factors. Today, there are conflicting opinions on the impact of BMI on the quality of the bones. Some studies suggest that in women with lower body mass index there is a greater risk of developing osteoporosis and have osteoporotic fractures of the upper arm, forearm and ankle (10). Also, changes in body weight have an impact on the rate of bone loss in the sense that “skinny” women (BMI <18.5 kg/m2) more rapidly lose bone mass than women with stable body weight (0.8% annually), while women whose body weight increased with the time does not losing bone mass significantly (0.1%) (11, 12). Obesity has for years been considered as a protective factor for the development of the skeleton and osteoporosis, but recent clinical studies indicate that obesity has a negative effect on the quality of the bone despite normal values of bone density measured by densitometry of what is considered to be the result of increased mechanical pressure on the bone (13, 14). A significant percentage of fractures exactly happens in obese women because of the body structure or mechanism of injury (15).
In our study, in more than half of respondents in the experimental group was overweight (57% versus 36% in control group), in contrast to the control group where the highest percentage (46%) had a normal body mass index (BMI). Correlation of BMI with osteoporosis by univariate logistic regression did not show the relationship of this indicator with osteoporosis (p=0.727, OR=1.000, 95% CI=0.999 to 1.001), which is not congruent with the results of other studies (1).
Literature data show that hyperlipidemia may contribute to osteoporosis by its influence on bone resorption and osteoclast viability (16, 17), or to limit the radial lipids permeability of the cortical bone and thus influencing the function of bone cells (9). In a comparative study by Shukl et al was found that postmenopausal women with osteoporosis had significantly increased values of the parameters of the lipid profile (total cholesterol, triglycerides, LDL-cholesterol) in comparison with premenopausal women with osteoporosis (18). Adami and colleagues found no significant association of elevated serum LDL cholesterol, triglycerides and total cholesterol with a decrease in bone mineral density at the hip level for women aged 68-75 years (19). Other studies confirm a reduced bone mineral density in women with elevated total cholesterol (20).
In accordance with the previously presented attitudes and analyzing own research, in our study, the results of univariate logistic regression analysis shows that the total cholesterol (p=0.000, OR=1.006, 95% CI=1.003 to 1.0009), LDL cholesterol (p=0.005, OR=1.005, 95% CI=1.001 to 1.008) and triglycerides (p=0.033, OR=1.004, 95% CI=1.000 to 1.008), significant risk factors for osteoporosis, although the multivariate logistic regression LDL cholesterol and TG did not identified as independent risk factors. Results of multivariate regression analysis showed that the increased value of total cholesterol is a significant independent risk factor for osteoporosis in postmenopausal women (p=0.018, OR=1.006, 95% CI=1.001 to 1.010).
6. CONCLUSION
The results of the conducted study confirm the association between decreased bone mineral density in postmenopausal women and lipid parameters. It follows that the two entities in preventive action should be viewed simultaneously for a more efficient preventive activity that will contribute to a better quality of life for affected individuals.
Footnotes
• Conflict of interest: none declared.
• Author’s contributions: RB and JB performed the examination the patients. RB collected the data, analyzed them and wrote the text. SM assisted in writing the text. All authors have read the text and approved the final manuscript.
REFERENCES
- 1.Grgurevic A, Gledovic Z, Vujasinovic-Stupar N. Factor associated with postmenopausal osteoporosis:a case-control study of Belgrade women. Women Health. 2010;50:475–90. doi: 10.1080/03630242.2010.507656. [DOI] [PubMed] [Google Scholar]
- 2.The International Classification of Disease 11th. Geneva: World Health Organization; 2012. http://www.who.int/classifications/icd/revision/en/index.html . [Google Scholar]
- 3.Mendosa-Romo MA, Ramirez-Arriola MC, Velasco-Chávez JF. Parity and menarche as risk factors for osteoporosis in postmenopausal women. Ginecol Obstet Mex. 2014;82(2):75–82. [PubMed] [Google Scholar]
- 4.Bijelic R, Balaban M, Milicevic S. Gender Representation of Osteoporosis in Patients with Urolithiasis. Med Arch. 2015;69(5):331–3. doi: 10.5455/medarh.2015.69.331-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciric Z, Stankovic I, Pejcic T, et al. Osteoporosis in Patients with Chronic Obstructive Pulmonaly Disease. Med Arch. 2012;66(6):385–7. doi: 10.5455/medarh.2012.66.385-387. [DOI] [PubMed] [Google Scholar]
- 6.National Osteoporosis Foundation. Clinician’s Guide To Prevention and Treatment of Osteoporosis. Washington, DC, USA: National Osteoporosis Foundation; 2010. [Google Scholar]
- 7.González-Mercado A, Sánchez-López JY, Ibarra B. Risk factors for osteoporosis in postmenopausal women from Guadalajara, Jelisco. Salud Publica Mex. 2013;55(6):627–30. [PubMed] [Google Scholar]
- 8.Pasco A, Brennan SL, Kotowicz M. Morbid obesity in women on the rise:an observational, population- based study. BMC Public Helath. 2013;13:290. doi: 10.1186/1471-2458-13-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poiana C, Radoi V, Carsote M, Bilezikian J. New Clues that May Link Osteoporosis to the Cirkulating Lipid Profile. Bone Res. 2013;1(3):260–6. doi: 10.4248/BR201303004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk:a meta-analysis. Osteoporos Int. 2005;16:1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women:the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 1998;13:1458–67. doi: 10.1359/jbmr.1998.13.9.1458. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd JT, Alley DA, Hawkes WG, et al. Body mass index positively associated with bone mineral density in US older adults. Arch. of Osteop. 2014;9:175. doi: 10.1007/s11657-014-0175-2. [DOI] [PubMed] [Google Scholar]
- 13.Migliaccio S, Greco EA, Fornari R, et al. Skeletal alterations in women affected by obesity. Aging Clin Exp Res. 2013;25(1):35–7. doi: 10.1007/s40520-013-0090-1. [DOI] [PubMed] [Google Scholar]
- 14.Caffareli C, Alessi C, Nuti R, Gonnelli S. Divergent effects of obesity on fragility fractures. Clin Interv Aging. 2014;9:1629–36. doi: 10.2147/CIA.S64625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong T, Opinder S, Tan W, Marshall L. A United Kingdom perceptive on the relationship between body mass index (BMI) and bone health:A cross sectional analisys of data from of Nottingham Fracture Liaison Service. Bone. 2014;59:207–10. doi: 10.1016/j.bone.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Tintut Y, Demer LL. Effects of bioactive lipids and lipoproteins on bone. Trends Endocrinol Metab. 2014;25(2):53–9. doi: 10.1016/j.tem.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohiti-Ardekani J, Soleymani-Salehabadi H, Owlia MB, Mohiti A. Relationships between serum adipocyte hormones (adiponectin, leptin, resistin), bone mineral density and bone matabolic markers in osteoporosis patients. J Bone Miner Metabol. 2014;32(4):400–4. doi: 10.1007/s00774-013-0511-4. [DOI] [PubMed] [Google Scholar]
- 18.Shukla J, Sarkar PD, Bafna A. A comparative study of antioxidant defenses and lipid profile in premenopausl and postmenopausal osteoporotic women. Int J Biol Med Res. 2013;4(2):3196–8. [Google Scholar]
- 19.Adami S, Braga V, Zamboni M, et al. Relationship Between Lipids and Bone mass in 2 Cohorts oh Healthy Women and Men. Calcif Tissue Int. 2004;74(2):136–42. doi: 10.1007/s00223-003-0050-4. [DOI] [PubMed] [Google Scholar]
- 20.Janković D, Janković I, Savić T, Dimić A. Povezanost lipidnog profila i koštane mase kod žena sa hiperholesterolemijom u postmenopauzalnom periodu života. Internist. 2010;2(1):67–72. [Google Scholar]


