Abstract
Background:
Nifuroxazide is well known and often used anti-diarrhoeal medicine which has been pushed back from routine practice in recent years and often replaced with probiotics. Even probiotics are accepted and placed in some therapeutic guidelines for diarrhoea treatment, there are no enough evidence for its effectiveness and no comparative efficacy data with nifuroxazide in treatment of acute diarrhea.
Patients and Methods:
In open, prospective observational study, the efficacy and safety of nifuroxazide were compared with a probiotic containing lactic acid bacteria in the treatment of acute diarrhoea. A total number of 169 adult patients were included in this study, who administered nifuroxazide in the dose of 200 mg/4 times a day, while they took preparation containing lactic acid bacteria (1,2 x 107 live lyophilised lactic-acid bacteria) three times a day for three days.
Results:
Mean time to last unformed stool (TLUS) in a group which was treated with nifuroxazide was two days, while it took five days for the stool normalisation in the group using probiotic (p=0.0001).
Conclusions:
Orally administered nifuroxazide has demonstrated better efficiency as compared to probiotic in treating acute diarrhoea, and both medicines have shown the same safety and tolerance in this study.
Keywords: Acute diarrhea in adults, Nifuroxazide, Probiotics
1. INTRODUCTION
Gastrointestinal infections pose a big health and clinical problem throughout the world. In adult patients suffering from acute diarrhoea (duration of diarrhoea <1 week) enteropathogens are discovered in up to 65%–80% cases.1, 2
In Western countries, an average person will probably face one or two episodes of gastrointestinal infections every year. It can rarely be a severe infection and it usually does not require medical care. In the United Kingdom, Campylobacter, as well as types of non-typhoid Salmonellae are the most common causative agents of bacterial gastroenteritis. Gastroenteritis induced by viruses, such as rotavirus, adenovirus, small ball-shaped structured viruses, calioviruses and astroviruses are also frequent. In developing countries, the incidence of gastrointestinal infections is at least twice higher and the number of pathogenic types is much greater. The treatment with wide-spectrum antibiotics affects the intestinal flora by creating conditions for superinfections with microorganisms that might cause the diarrhoea. Clostridium difficile is a microorganism, most frequently found as a causative agent of the diarrhoeas related to treatment with antibiotics. The diarrhoeas related to C. difficile are more common in patients already having severe basic illness and in the elderly persons.
The incidence of acute diarrhoeas in Bosnia and Herzegovina in one year is relatively high and this infective illness has for many years been found on the third place according to the frequency of infectious diseases, immediately after influenza and varicella. Around 6000 patients on average, suffering from acute diarrhoea and asking for medical help, are registered in Bosnia and Herzegovina on annual basis.
The treatment of acute diarrhoea is today mainly associated with the usage of symptomatic therapy in the form of rehydration and adding minerals, and antibiotics as well as appropriate supportive therapies are used only in severe cases. In some circumstances, rehydration and diets cannot result in a rapid symptoms relief, and out of that reason, as well as for the purpose of reducing absence from work or everyday obligations, an effective antibiotics treatment may change the duration of the diarrhoea from five days into one day.3
The therapy with antibiotics with poor absorption is justified as such usage has almost no systemic effect on the organism and we thusly expect better efficacy with greater safety at the same time, due to a lower number of adverse effects, toxicity and drug interactions. Additionally, unlike systemically absorbed oral antibiotics, the poorly absorbed antibiotics have a special target action in the intestine and they do not induce antimicrobial action on the bacterial flora outside the intestine.3
Several poorly absorbable antibiotics including aztreonam, bicozamycin, nifuroxazide and rifaximine were investigated in controlled trials in humans suffering from traveller’s diarrhoea.4, 5, 6, 7, 8
Probiotics are nowadays highly recommended as a therapy for diseases occuring as a result of disrupting the physiological microbiota in humans, but strong and scientifically based claims of their efficacy are still missing.
Nifuroxazide in treatment of acute diarrhoeaa
The antimicrobial and antiparasitic properties of nifuroxazide, as well as other nitrofuran derivatives, probably originate from nitro (NO2) group that has a very expressive electro-magnetic force. Local inertness and impossibility for diffusion into organic systems and tissues makes nifuroxazide unique as compared to other nitrofuran derivatives, because any systemic action of this antidiarrhoeic is missing. The following gram-positive cocci have proven to be especially susceptible to this preparation: Streptococcus pyogenes, Staphylococcus pyogenes, as well as the following Gram-negative enterobacteria: E. coli, Salmonellae and Shigellae. In this trial, we used drug Enterofuryl manufactured by Bosnalijek dd, Bosnia and Herzegovina, in the form of capsules, which contain 200 mg nifuroxazide. The patients administered Enterofuryl four times daily in the period of three days. Nifuroxazide is effective therapy for acute diarrhea syndrome and it is often empirically indicated for use prior to results of stool cultures.
The role of probiotics in treatment of acute diarrhoea
Lactic acid bacteria are normally present in the small and large intestine, where they maintain acidic-alkaline balance, which is necessary for normal function of digestive enzymes. In case of insufficient amount of lactic acid bacteria, the environment becomes more alkaline, what disables the action of digestive enzymes and enables excessive multiplication of the harmful bacteria. The balance of intestinal flora is thusly disrupted, what results in various digestive difficulties. In this trial, we used hard capsules containing 1,2 x 107 live lyophilised lactic acid bacteria (Lactobacillus acidophilus, Bifidobacterium infantis v. Liberorum, Enterococcus faecium). It is indicated for usage as supportive therapy in treatment of acute diarrhoea as supportive treatment.
Very often probiotics are the only therapy used in treatment of acute diarrhea or therapy that is used as replacement therapy instead of Enterofuryl and without objective evidence based medicine for it.
We conducted the first comparative study with two most often empirically used antidiarrheal therapy, nifuroxazide and probiotics, as per author’s knowledge.
2. AIMS
The goals of our trial were to evaluate the efficacy and safety of using nifuroxazide as compared to preparation containing lactic acid bacteria in treating acute diarrhoeas in adult patients.
3. PATIENTS AND METHODS
This trial was designed as multi-centre, parallel-group, open and prospective. Inclusions criteria were: Patients suffering from acute diarrhoea or “traveller’s diarrhoea” (≥ 3 non-formed stools in last 24 hours and disease duration of ≤ 72 hours), one of the signs or symptoms of the intestinal infection must be present (nausea, vomiting impulse, vomiting, abdominal spasms, tenesma, dysentery and urgent impulse for defecation), age between 18 and 65 (age is estimated based on a calendar year), being able to independently make decisions. In the very beginning of the trial, if the patients meet the inclusion criteria and after signing the informed consent, with random allocation method, the subjects will be divided in two groups, both experimental, nifuroxazide group (Group N) or probiotic group (Group M) over the period of three days (72 hours), depending on:
Duration of diarrhoea (number of hours from the first diarrhoea until the beginning of the treatment)
Severity of diarrhoea (number of stools)
Age.
After signing the Informed Consent, the subjects adhered to the recommendations for their diet with intake of an appropriate amount of liquid. The health condition of the patients was evaluated prior to their inclusion into the trial and therapeutic effects were monitored in the period of seven days on three control check-ups performed by an infectious diseases specialist. The subjects recorded in their logbooks the number and consistency of their stools every day, and they monitored and recorded symptoms which may be associated with the acute diarrhoea (stomach pain, stomach cramps, high temperature, vomiting, dehydration). Therapy duration in both groups is 3 days and there were two control examinations (after 3 and 7 days of treatment).
Statistical analysis
Statistical analyses were performed using MedCalc for Windows, version 12.6.1.0 (MedCalc Software, Mariakerke, Belgium). In statistical methods of this trial, we used chi-squared test, D’Agostino-Pearson test for checked normality of continuous data distribution and based on the distribution of results, Student T test or Mann-Whitney test, the statistical significance was defined as p<0.05.
4. RESULTS
The trial was performed in three cities in Bosnia and Herzegovina (Sarajevo, Tuzla and Zenica), on two clinics for infectious diseases and in eight out-patient clinics for family medicine. Out of the total number of 169 patients that were randomly divided, 85 patients belonged to ‘N’ Group, and 84 patients belonged to ‘M’ Group. In ‘N’ Group, 81 patients finished the study, while 78 of them finished the study in ‘M’ Group. The average age of patients in ‘N’ Group was 43.68 (SD 13.33) years, while it was 41.28 (SD 13.87) in ‘M’ Group. There were 38 (47%) men in ‘N’ Group, while there were 24 (31%) men in ‘M’ Group. The average duration of the diarrhoea in ‘N’ Group was 35.56 (SD 21.01) hours, while it was 31.32 (SD 21.95) hours in ‘M’ Group (Table 4).
Table 1.
Characteristics of subjects involved into the trial

Table 2.
Clinical symptoms before the randomisation. *–used chi-squared test for evaluating the significance of differences in frequencies of the clinical symptoms.

Table 3.
Clinical symptoms at first control check-up. *- used chi-squared test for evaluating the significance of differences in the frequencies of clinical symptoms.
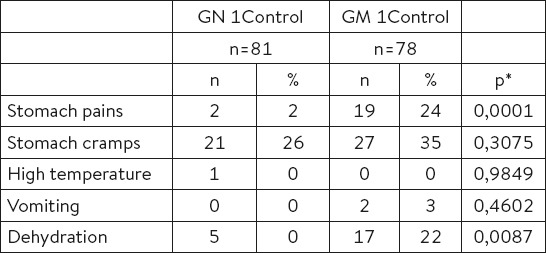
Table 4.
Clinical symptoms. *- used Mann-Whitney test (independent samples)
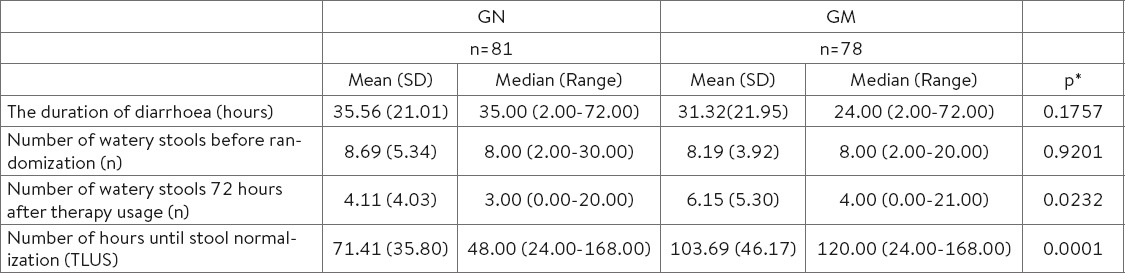
Prior to inclusion into the study, there were 8.69 (SD 5.33) watery stools in ‘N’ Group, as compared to 8.19 (SD 3.91) watery stools in ‘M’ Group (Table 4).
The clinical symptoms (pains and cramps in the stomach, high body temperature, vomiting and dehydration) were analysed before the randomisation and no statistically significant differences between the groups have been reported (Table 2).
At first control check-up (72 hours from the beginning of therapy usage), a statistically significant difference in the number of stools has been reported, ‘N’ Group Md=3 (range: 0.00-20.00) and ‘M’ Group Md=4 (range: 0.00-20.00), p=0.0232 (Table 4.).
The analysis of the clinical symptoms at first check-up has shown a significant difference in the number of patients who complained of the stomach pains [‘N’ Group = 2 (2%) vs ‘M’ Group = 19 (24%), p=0.0001] and dehydration [‘N’ Group =2 (2%) vs ‘M’ Group =19 (24%), p=0.0001] [‘N’ Group =5 (6%) vs ‘M’ Group =17 (22%), p=0.0087] (Table 3).
At the second control check-up, the differences have been reported in the number of stools in the period of 72 hours [‘N’ Group M=0.259 (SD 0.948) vs ‘M’ Group M=2.282 (SD 3.759), p<0.0001]. The differences in clinical symptoms were significant.
The analysis of the number of hours until stool normalisation after the randomisation (TLUS) has shown a significant difference between ‘N’ Group (Md=48.000, 95% CI 48.000-72.000 hours) as compared to ‘M’ Group (Md=120.000, 95% CI 96.000-120.000 hours; p=0.0001) (Table 4.).
Therapy safety
During the research we followed the safety of the therapy in all patients who used at least one dose of the researched drugs. We paid special attention to the following adverse effects: headache, constipation, flatulence, gag and the feeling of nausea. There were no suspected adverse effects of the therapy observed that could be associated with the used drugs.
5. DISCUSSION
The information from the earlier clinical research mostly encompass the research from the studies performed with the aim of assessing the efficiency and safety of drugs in the patients who suffered due to traveler’s diarrhea. Lately in the available literature we encounter less and less studies which followed the patients suffering from common acute diarrheas.
There are no medical evidence that has directly compare the efficacy of nifuroxazide and probiotics in treatment of acute diarrhea.
The agents of these infections are usually the same and in a great number of cases the agent of diarrhea is not discovered. The earlier research often used the method of assessing the therapy efficiency thorugh time analysis up to stool normalization (time to last unformed stool – TLUS) and in our research we have used this analysis with the aim of comparing the results with other research.
Bouree et al. (1989) published the results of a research in which in a double blind placebo controlled research they researched the efficiency of nifuroxazide in the treatment of acute diarrhea in adults. The authors included in the research 88 adults with acute diarrhea (defined as a condition with three liquid stools per day) who used either 800 mg nifuroxazide per day (divided in two doses) or placebo over 5 days. The research was conducted in France in five hospital centers. The average duration of diarrhea in the nifuroxazide treated group was 2.09 days whereas in the placebo group the diarrhea duration was 3.26 days (p<0.004). The number of daily stools decreased more rapidly and the mucus dissapeared faster in the group which used nifuroxazide compared to the group which used placebo. The treatment tolerabilty was excellent and not a single adverse effect was recorded. The authors concluded that nifuroxazide proved efficient in the treatment of acute diarrhea and that it can be prescribed from the very appearance of difficulties and without waiting for the coproculture results which often last long or are negative.9
Not all probiotics show the same efficiency and Mcfarland has demonstrated it in an articulate article about probiotics and diarrhea in which he stated that 300 RTC about probiotics had been published out of which 19% was about diarrhea treatment and prevention in pediatrics, 16% about prevention of diarrheas caused by antibiotic use and 6% about traveler’s diarrhea prevention.
The author further states based on RCT results that L.casei DN-114001, L.rhamnosus GG and S.boulardii have shown a very good efficiency (>3RCT with >100 subjects) in the prevention and treatment of diarrheas in pediatrics while L.acidophilus has not shown a significant efficiency in RCT or no RCT results have been published for the same indication. Similar results were also obtained in the treatment of traveler’s diarrheas in RCT. In our research we have used a combined probiotic which in its composition had a combination of lyophilized lactic acid bacteria (Lactobacillus acidophilus, Bifidobacterium infantis v. Liberorum, Enterococcus faecium) and the use of this preparation has not shown the expected efficiency.10
Lactobacillus rhamnosus GG (LGG) and Saccharomyces boulardii have proven to be most effective, reducing the duration of illness for one day. There were four meta-analysis considering antibiotics induced diarrhea which have shown that several factors influence the effectiveness of probiotics among which are: age, type of antibiotic, the general condition of the patient. Also the most efficient LGG and S. boulardii13. The study which examined the effectiveness of Lactobacillus rhamnosus GG (LGG) in the prevention of antibiotic-induced diarrhea in children and adults, showed the risk reduction for diarrhea development from 22,4 to 12.3% compared to the placebo group. However, after performing the stratification on children and adults age groups, there is statistically significant difference in pediatric population compared to adults where probiotics shown efficacy in subgroup of patients treated with antibiotics in eradication therapy of Helicobacter pylori. This study once again confirms the insufficiency of research results in adult population.14,15
In a research conducted with rifaximine (non-absorbing antibiotic) on 380 volunteers the mean time (Md) until stool normalization (TLUS) of 32.5 (95%Cl 28.4-43-6.) up to 32.9 hours (95%Cl 28.4-44.0) was recorded in the groups of patients who used different daily doses of rifaximine (600 mg/daily and 1200 mg/daily) and in the same research the time until stool normalization was 60 hours (95%CI 48.4-92.0)5 with the use of placebo.
In a randomized double blind research conducted on 187 patients the authors researched the efficiency of rifaximine (800 mg/daily) compared to ciprofloxacine (1000 mg/daily) and the recorded mean time until stool normalization (TLUS) in the group which received rifaximine was 25.7 hours (95%CI 20.9-38.0) whereas in the group which received ciproflaxacin the recorded time was 25.0 hours (95%CI 18.5-35.2).6
In a reseach conducted by DuPont et al. (1998) the efficiency of rifaximin (600 mg/daily) was compared in relation to to thrimetoprim-sulphometoxazole (320/1600 mg/daily). 35 patients were included in this research, the recorded mean time until stool normalization (TLUS) in the group that received rifaximin was 26.3 hours, whereas the recorded time in the second group was 47.0 hours.11
Similar results were obtained by the authors in a research performed on 399 patients-travelers who traveled to Mexico, Guatemala and India. The efficiency of rifaximin (600 mg/daily over three days) was compared to that of ciprofloxacin (1000 mg/daily for two days + placebo one day) and placebo (three days). In the group which used rifaxamin mean time until stool normalization was 32.0 hours, whereas in the group which used ciprofloxacin it was 28.8 hours and 65.5 hours in the placebo group.
If we compare the results of our research through analyses of the number of hours until stool normalization after randomization (TLUS), there was a significant difference between the ‘N’ Group (Md=48.000, 95%CI 48.0-72.0 hours) compared to the ‘M’ Group (Md=120.000, 95%CI 96.0-120.0 hours; p=0.0001). The obtained results differ little from earlier research of the treatment of acute diarrhea and traveler’s diarrhea and nifuroxazide has shown similar efficiency as rifaximin and ciprofloxacin.
Adverse effects which are recorded in other research such as headache, constipation, flatulence, rectal tenesmus, vertigo, nausea have not been recorded in any patient in our research which further confirms present information on the therapy safety.
Significant differences were recorded in total diarrhea duration (up to full stool normalization). This analysis confirms the success of nifuroxazide treatment and shows that nifuroxazide leads faster to ultimate healing.
We have followed clinical symptoms which usually accompany acute diarrhea and gastroenteritis (stomach pains, stomach cramps, increased temperature, vomiting and dehydration) in both groups during the research. Better and faster efficiency after nifuroxazide use compared to the combination with lactic acid bacteria is very important in daily practice.
An analysis of differences of the success of the used treatments between groups has shown a significantly greater difference in the therapy success in the group of subjects who received nifuroxazide (already after 48 hours of drug use) compared to the group of patients which received the drug containing lactic acid bacteria [Group N=46 (57%) vs Group M=18 (23%)].
The chosen assessment for therapy efficiency was the stool number and consistency and not microbiological analysis which has confirmed that the empirical use of nifuorxazide is justified in acute diarrhea.
Due to the significantly more studies that have examined the application of probiotics in pediatric population, and to the fact that studies on investigating probiotics in adult age have shown conflicting results, the field of research of probiotics’ efficacy in acute diarrheal syndrome remain open for additional investigation. Therefore, our study is a significant step in this specific field.
Research limitation
We could not do the research as a double blind randomized study due to technological limitations in drug design. Nifuroxazide capsules are standard capsules containing yellow granules whereas lactic acid bacillus capsules are hard and white and it is hard to make identical products with completely different ingredients.
6. CONCLUSIONS
On the basis of the conducted research and statistical processing of the results and particularly important parameters such as time until stool formation (TLUS) and the number of watery stools during monitoring period, we can conclude that nifuroxazide has shown a significantly better efficiency compared to the combination of lactic acid bacteria in the treatment of acute diarrhea and probiotics cannot be a substitute therapy for nifuroxazide but only an additional therapy with nifuroxazide.
Footnotes
• Ethical issue: The study was approved by the Ethics Committees of Research Centers (Ethics Council of Cantonal Hospital Zenica and Ethics Committee of University Clinical Center Tuzla) and by the CEO of the The Public Institution Health Centre of Sarajevo Canton.
REFERENCES
- 1.Svenungsson B, Lagergren A, Ekwall E, Evengård B, Hedlund KO, Kärnell A, Löfdahl S, et al. Enteropathogens in Adult Patients With Diarrhea and Healthy Control Subjects:A 1-Year Prospective Study in a Swedish Clinic for Infectious Diseases. Clin Infect Dis. 2000;30:770–778. doi: 10.1086/313770. doi:10.1086/313770. [DOI] [PubMed] [Google Scholar]
- 2.Steffen R. Epidemiology of Traveler’s Diarrhea. Clin Infect Dis. 2005;41:536–540. doi: 10.1086/432948. doi:10.1086/432948. [DOI] [PubMed] [Google Scholar]
- 3.David N Taylor. Poorly Absorbed Antibiotics for the Treatment of Traveler’s Diarrhea. Clin Infect Dis. 2005;41(8):564–570. doi: 10.1086/432953. doi:10.1086/432953. [DOI] [PubMed] [Google Scholar]
- 4.Ericsson CD, DuPont HL, Sullivan P, Galindo E, Evans DG, Evans DJ. Bicozamycin, A Poorly Absorbable Antibiotic, Effectively Treats Travelers’ Diarrhea. Ann Intern Med. 1983;98(1):20–25. doi: 10.7326/0003-4819-98-1-20. doi:10.7326/0003-4819-98-1-20. [DOI] [PubMed] [Google Scholar]
- 5.Steffen R, Sack DA, Riopel L, Jiang ZD, Stürchler M, Ericsson CD, Lowe B, et al. Therapy of travelers’ diarrhea with rifaximin on various continents. American Journal of Gastroenterology. 2003;98:1073–1078. doi: 10.1111/j.1572-0241.2003.07283.x. doi:10.1111/j.1572-0241.2003.07283.x. [DOI] [PubMed] [Google Scholar]
- 6.DuPont HL, Jiang ZD, Ericsson CD, Adachi JA, Mathewson JJ, DuPont MW, Palazzini E, et al. Rifaximin versus Ciprofloxacin for the Treatment of Traveler’s Diarrhea:A Randomized, Double-Blind Clinical Trial. Clin Infect Dis. 2001;33(11):1807–1815. doi: 10.1086/323814. doi:10.1086/323814. [DOI] [PubMed] [Google Scholar]
- 7.DuPont HL, Ericsson CD, Mathewson JJ, de la Cabada FJ, Conrad DA. Oral Aztreonam, A Poorly Absorbed Yet Effective Therapy for Bacterial Diarrhea in US Travelers to Mexico. JAMA. 1992;267(14):1932–1935. doi:10.1001/jama.1992.03480140058033. [PubMed] [Google Scholar]
- 8.Bourée P1, Kouchner G, Ponti M. Double-blind study of traveller’s diarrhoea using Nifuroxazide. Trans R Soc Trop Med Hyg. 1987;81(5):859. doi: 10.1016/0035-9203(87)90053-8. [DOI] [PubMed] [Google Scholar]
- 9.Bouree P, Chaput JC, Krainik F, Michel H, Trepo C. Double-blind controlled study of the efficacy of nifuroxazide versus placebo in the treatment of acute diarrhea in adults. Gastroenterol Clin Biol. 1989;13:469–272. [PubMed] [Google Scholar]
- 10.McFarland L.V. Probiotics and Diarrhea. Ann Nutr Metab. 2010;57(1):10–11. doi: 10.1159/000309016. doi:10.1159/000309016. [DOI] [PubMed] [Google Scholar]
- 11.DuPont HL, Ericsson CD, Mathewson JJ, Palazzini E, DuPont MW, Jiang ZD, Mosavi A, et al. Rifaximin:A Nonabsorbed Antimicrobial in the Therapy of Travelers’ Diarrhea. Digestion. 1998;59:708–714. doi: 10.1159/000007580. doi:10.1159/000007580. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DN, Bourgeois AL, Ericsson CD, Steffen R, Jiang ZD, Halpern J, Haake R, et al. A Randomized, double-blind, multicentre study of rifaximin compared with placebo and with Ciprofloxacin in the Treatment of Traveler’s Diarrhea. The American journal of tropical medicine and hygiene. 2006;74(6):1060–6. doi: 10.5167/uzh-18632. [PubMed] [Google Scholar]
- 13.Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. Efficacy of probiotics in prevention of acute diarrhoea:a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6(6):374–82. doi: 10.1016/S1473-3099(06)70495-9. doi:10.1016/S1473-3099(06)70495-9. [DOI] [PubMed] [Google Scholar]
- 14.Cottrell J, Koenig K, Perfekt R, Hofmann R For the Loperamide–Simethicone Acute Diarrhoea Study Team. Comparison of Two Forms of Loperamide–Simeticone and a Probiotic Yeast (Saccharomyces boulardii) in the Treatment of Acute Diarrhoea in Adults:A Randomised Non-Inferiority Clinical Trial. Drugs R D. 2015;15(4):363–373. doi: 10.1007/s40268-015-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarino A1, Guandalini S, Lo Vecchio A. Probiotics for Prevention and Treatment of Diarrhea. J Clin Gastroenterol. 2015;49(1):37–45. doi: 10.1097/MCG.0000000000000349. doi:10.1097/MCG.0000000000000349. [DOI] [PubMed] [Google Scholar]


