Abstract
Introduction:
Tonsillopharyngitis (sore throat) is a common disease mainly related to the seasonal common cold. To relieve unpleasant symptoms and discomfort of acute tonsillopharyngitis associated with common cold, patients usually take some non-prescription drugs.
The aim:
The primary aim of this study was to assess subjective determinations of the efficacy and the safety/tolerability of an oral spray comprising a combination of lysozyme chloride and cetylpyridinium chloride in those patients.
Material and methods:
The study involved 1727 patients with tonsillopharyngitis associated with common cold and treated with the studied drug, in the period from December 2014 through March 2015.
Results:
In total, 95% of patients rated the studied drug to be well, very well and excellently effective. In 32% of patients, the symptoms were relieved 10 minutes after the application of the spray. Significant correlations were found between the two subjective assessments of the drug efficiency with the total of 74.11% (95% CI: 73.41, 77.47%) of patients who said that the feeling of pain in the throat completely disappeared after the drug administration, evaluated the impact/effect of the drug was very good or good (Pearson Chi Square=391.401, p<0.001). The effectiveness was significantly better in patients with up to two episodes of common cold a year (Pearson Chi Square=6.101; p=0.014). The studied drug was rated to be well, very well and excellently tolerated by 97% of patients.
Conclusion:
According to patients’ subjective assessment, the combination of lysozyme chloride and cetylpyridinium chloride in a formulation of spray can quickly, efficiently and safely resolve the symptoms of acute tonsillopharyngitis associated with common cold.
Keywords: tonsillopharyngitis, lysozyme, cetylpyridinium, oral spray, efficacy, safety/tolerability
1. INTRODUCTION
Tonsillopharyngitis (a sore throat) is a common disease mainly related to the seasonal common cold. Although tonsillopharyngitis usually resolves on its own, without complications in adults, it is important to relieve symptoms which most commonly affect the quality of life and daily activities. Most often, cough can make breathing difficult, leading to difficulties during sleeping or performing physical exercises (1). Significant influence of common cold on lifestyle habits was reported in 52% of 2505 patients in the USA as follows: their productivity was reduced by an average of 26.4%; 44.5% were absent from work or school for at least 1-2 days; 93% had difficulties in their sleep, and the majority of respondents complained of nasal congestion and cough (2). Another four-region online questionnaire survey, conducted in four European countries, showed the sore throat to be a major discomfort for the patients with common cold (3). To relieve unpleasant symptoms and discomfort of tonsillopharyngitis and to improve the quality of life, those patients usually take symptomatic treatment, most often a variety of non-prescription drugs. Different products containing pain relievers, topical anaesthetics, antiseptics or antimicrobics are available on the market. Those drugs are mainly used on the principle of self-medication, where a decision on the choice of treatment is made by the patients alone or in consultation with pharmacists.
Lysozyme is enzybiotic, i.e. enzyme hydrolase with antibiotic activity. Alexander Fleming discovered it in 1922. He noticed that the nasal mucus of a patient with cold inhibits the growth of bacteria on the agar substrate (4, 5). In human organism, immune system cells which are present in tears, urine, breast milk, saliva, liver, cartilage and skin produce lysozyme. It is the first line of defense against bacteria. Its concentration in saliva ranges from 2 to 60 µg/ml (6). However, the best studied and most used is the lysozyme from egg white (7). Its antibacterial activity is related to its ability to increase the permeability of the bacterial cell wall acting via 1.4-β-N-acetyl-muramidase, which breaks the glyosidic bond between the C1 atom of N-acetyl-muramic acid and C4 atom of N-acetyl-glucosamine of the peptidoglycan layer of the bacterial cell wall (8, 9). Besides its well-known effect on bacterial peptidoglycans, lysozyme is effective against bacteria through other, non-enzymatic mechanisms such as the activation of bacterial autolytic enzymes (so-called autolysins), aggregation of bacteria as well as the destabilization of the bacterial cytoplasmic membrane resulting in the removal of divalent ions. Some studies indicate its non-enzymatic mechanisms to be most important part of its antibacterial activity (6).
Lysozime is usually used to improve the antimicrobial capacity of saliva. Although lysozyme is active mainly against gram-positive bacteria with the exception of some strains of Staphylococcus aureus and Enterococcus faecalis (10), its activity against gram-negative bacteria can be facilitated by cationic peptides in saliva. Those cationic peptides are earlier shown to be able to stimulate permeability of the outer peptidoglycan cell layer of gram-negative bacteria. In addition to beneficial antibacterial activity, lysozyme shows antiviral (e.g. HIV, Herpes simplex, Herpes zoster), anti-fungal (e.g. Candida albicans), anti-inflammatory, anti-tumour and immunomodulatory activities (5, 11-13). Although it has been increasingly used over the last few decades, this active substance it is still under-explored.
In the pharmaceutical spray formulation, lysozyme chloride is combined with cetylpyridinium chloride (CPH), which is chemically synthesized, amphiphilic, quaternary substance with a mild anaesthetic and antimicrobic efficacy (3, 14-18). Unlike lysozyme, CPH penetrates the bacterial cell membrane, causes the lysis of cellular components, metabolic disorder, inhibition of cell growth, leading eventually to the bacterial death (14, 15). It is bactericidal against gram-positive and some gram-negative bacteria (19). Food and Drug Administration classified CPH as safe and effective substance (16). A great number of studies is currently examining the effect of CPH in preventing or treating dental plaque and cavities (3, 16-18).
The primary aim of this study was to assess subjective determinations of the efficacy and the safety/tolerability of an oral spray formulation comprising a combination of lysozyme chloride and cetylpyridinium chloride in patients with acute tonsillopharyngitis associated with common cold. In addition, we aimed to investigate the covariates (number of common cold episodes a year) of the assessed effectiveness of the studied spray and the time necessary for the spray to take an effect.
2. MATERIAL AND METHODS
This was an observational, post marketing study that involved 93 study centres in 38 cities in Bosnia and Herzegovina. The study was conducted by community pharmacists in the period from December 2014 through March 2015. After pharmacists had recognized patients with symptoms of acute tonsillopharyngitis associated with common cold who came to pharmacy, asked for an oral antiseptic preparation and bought the combination of lysozyme chloride and cetylpyridinium chloride in a formulation of spray (Lysobact spray®, Bosnalijek, Bosnia and Herzegovina), they explained the aim of the study, the study inclusion and exclusion criteria and provided a specially developed questionnaire. The questionnaire consisted of a total of 36 questions, of which four assessed baseline characteristics while remaining 32 questions assessed the symptoms, efficacy and tolerability of the studied medicine. The first part of the questionnaire, referring to symptoms of acute tonsillopharyngitis, time of their appearance, limitation of everyday activities due to the symptoms, annual frequency of common cold, was filled out by a patient with the pharmacist’s assistance. The second part of the questionnaire, referring to symptoms, a change in the quality and intensity of symptoms, and also to subjectively assess the efficacy and the tolerability of the medicine, was filled in five days after the drug therapy was initiated.
Exclusion criteria were deterioration of the underlying disease, the occurrence of symptoms such as severe tonsillopharyngitis, headache, nausea, vomiting, or high temperature, development of serious adverse effects that require discontinuation of therapy, as well as the use of other oral antiseptics during the study period.
To determine whether the effectiveness of the combination of lysozyme chloride and cetylpyridinium chloride in a formulation of spray is related to the number of common cold episodes a year, patients were divided into two groups, those who had up to two episodes of common cold a year, and those who had more than two episodes of common cold a year.
After data collection, the data are analyzed using MS Excel (Microsoft Excel 2010) and the IBM SPSS Statistics software (Statistical Package for Social Sciences, SPSS Inc, Chicago, Illinois, USA) version 20.0. Besides the descriptive statistics, inferential statistics, i.e. Pearson chi-square test was used was used to determine the relationship between categorical variables. P-values less than 0.05 were considered statistically significant.
3. RESULTS
The study involved 1727 patients older than 18 years of age, who, in the period from December 2014 through March 2015, came to the pharmacy and asked for an oral antiseptic preparation because of acute tonsillopharyngitis associated with common cold.
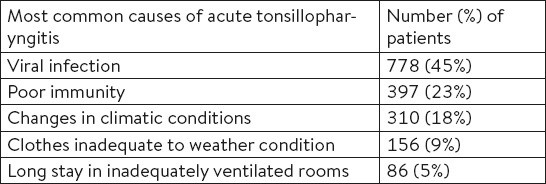
To buy a symptomatic therapy, one-third of patients with tonsillopharyngitis associated with common cold visited a pharmacy in the first two days after the onset of symptoms (Table 1).
Table 1.
The time from the onset of symptoms of tonsillopharyngitis to the patient’s visit to the pharmacy
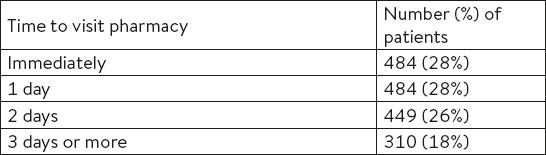
The most common symptom for which the studied drug was used was the pain in the throat or the sore throat (tonsillopharyngitis), and the cough irritation was reported in only 16% of patients. About half of patients had at least two of these symptoms (Table 2).
Table 2.
The most common symptoms for which the combination of lysozyme chloride and cetylpyridinium chloride in a formulation of spray was used
The first effect of the studied drug observed by the largest number of patients (n=965, 56%) was a reduction of the pain in the throat. Since this question could have multiple answers, 88% of patients felt two or more of the above-mentioned effects (reduction in symptoms presented in Table 2) of the studied drug.
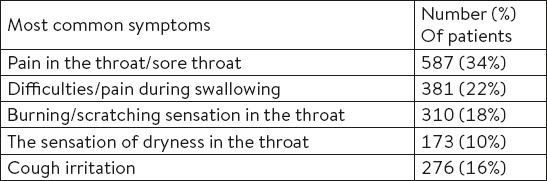
The most commonly reported cause of acute tonsillopharyngitis was viral infection (45%) and the long stay in inadequately ventilated rooms were reported in only 5% of patients (Table 3).
Table 3.
The most common causes of acute tonsillopharyngitis (according to patients’ opinion)
A total of 95% of patients said that the drug was excellently effective, very well or well effective (Table 4).
Table 4.
The number of patients in response to the two subjective assessments of the drug efficiency
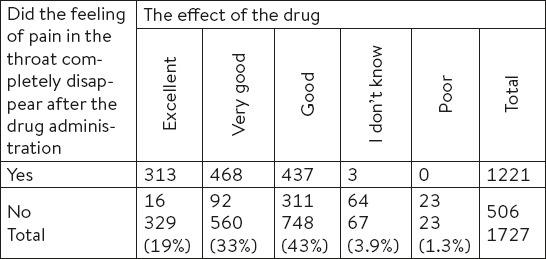
Upon analysis of the time needed for the medicine to take an effect, most of the patients (n =553, 32%) felt the symptoms relief 10 minutes after the drug administration.
A significant relation was found between the two subjective assessments of the drug efficiency (feeling of the pain in the throat and the effect of the drug) (Pearson chi-square = 391.401, p < 0.001) (Table 4). Total of 74.11% (95% 95% CI: 73.41, 77.47%) of patients who said that the feeling of pain in the throat completely disappeared after the drug administration evaluated the impact/effect of the drug as very good or good.
The effectiveness of the studied drug (having a feeling that the pain in the throat completely disappeared two days after the drug administration) was compared between the two groups, those who had up to two episodes of common cold a year (n = 1122; 65% of patients), and those who had more than two episodes of common cold a year (n = 605; 35% of patients). The effectiveness has shown to be significantly related to the number of episodes of common cold a year. In the first group, 833 respondents (72.62%) said that the pain in the throat completely disappeared two days after the drug administration, while 388 (66.89%) said the same in the second group (Pearson chi-square = 6.101, p = 0.014).
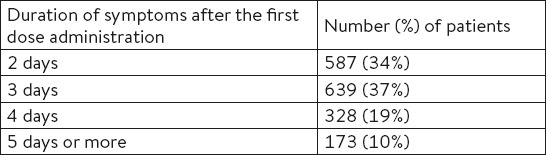
In the majority of the patients, the symptoms did not last longer than three days after the first dose administration, and a smaller number of patients used the studied spray for five days or longer (Table 5).
Table 5.
The duration of symptoms after the first dose administration of the combination of lysozyme chloride and cetylpyridinium chloride in a formulation of spray
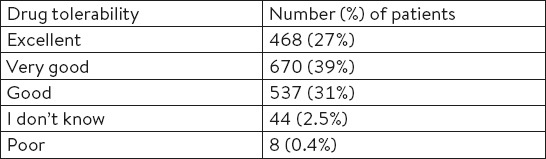
A total of 69.88% (95% CI: 67.72, 72.04%) of patients said that the drug was very well or well tolerated (Table 6).
Table 6.
Drug tolerability assessed by patients
A total of 7 patients (0.004%) reported side effects of the drug. The most commonly reported side effect was skin rash (n = 3, 0.0017%), while the taste disturbance, dry mouth, burning sensation in the mouth and problems with the stomach/digestion occurred in one patient (0.0005%) each.
4. DISCUSSION
Out of 1727 patients included in the study, 95% of them rated the combination of lysozyme chloride and cetylpyridinium chloride in a formulation of spray to be well, very well and excellently effective, with symptoms relief 10 minutes after the application of the spray in 32% of the patients. A significant correlation was found between the two subjective assessments of the drug efficiency; a total of 74.11% of patients who said that the feeling of pain in the throat completely disappeared after the drug administration evaluated that the impact/effect of the drug was very good or good, suggesting the reliability of the answers provided by patients. Furthermore, the effectiveness was significantly better in patients with up to two common cold episodes a year compared to those with the higher number. The studied medicine was rated as well, very well and excellently tolerated by 97% of patients.
In general, the primary concerns of the ordinary patient are related to the relief of symptoms, the recovery and the information related to the disease cause and prognosis. It has been observed that, lately, to relieve symptoms quickly and to avoid their effect on the quality of life and daily activities, patients tend to make the decision on the choice of treatment themselves. This principle of self-medication is becoming more and more popular. Interestingly, a study conducted in Japan showed significantly better improvement of the quality of life of patients affected by the common cold when they resort to self-treatment compared to a standard visit to a doctor for treatment of this condition (20). Patients that resort to self-medication to relieve the symptoms of cold and flu often consult a pharmacist, who by giving advises on appropriate treatment and selection of non-prescription drugs, acts as a “coach for self-medication” (21). This tendency for “self-medication” is also confirmed by the study that reported that the antibiotic prescription was only the 11th ranked reason, out of 13 ones, why patients with the sore throat visit the doctor (22).
In our study, 82% of patients quickly decided (during the first two days) to treat the symptoms of common cold themselves. Those symptoms were sore throat, difficulty in swallowing, burning sensation in the throat and cough irritation.
In addition to its effectiveness, the combination of lysozyme chloride and cetylpyridinium chloride in a formulation of spray was proven to be safe, with adverse effects reported in only 0.0037% (n = 7) patients, of which the skin rash was the most common one and appeared in 3 patients. It has also shown to be well, very well and excellently tolerated by the majority of patients, with only 0.004% (n = 8) of the patients who ranked the drug tolerability as poor. Great tolerability of lysozyme was reported by Kobayashi H et al. who demonstrated that lysozyme may also be used to treat hypersensitivity of pharynx with adverse effect reported in only one patient. This adverse effect ceased immediately upon discontinuing the drug administration (23).
In an online survey conducted in the period from 2003 to 2004, patients from Great Britain, France, Poland and Malaysia reported most important causes of the sore throat to be either cold and flu (72% cases), or other bacterial and viral infections (46% cases) (24). Similarly, according to 45% of our patients’ opinion, the most common cause of acute tonsillopharyngitis was the viral infection. Although the majority of upper respiratory tract infections spontaneously disappear because of the immune system’s response against the infection (25), it is known that viral tonsillopharyngitis usually lasts four to five days. In our study, in most of the patients treated with the combination of lysozyme chloride and cetylpyridinium chloride in a formulation of spray the symptoms did not last longer than three days after the first dose administration (Table 5). This is consistent with the previous study which confirmed the immunomodulatory effect of lysozyme and its ability to strengthen the local immune response. That study demonstrated that the intake of an external dose of lysozyme can positively influence the therapeutic outcome in the sore throat (26).
In addition to its antimicrobial activity, lysozyme itself is confirmed to be a natural immune modulator (5). Anti-inflammatory activity of lysozyme relates to the neutralization of, in the inflammatory process released, acidic substances. Lysozyme helps wound healing, tissue regression of degenerative and necrotic processes, and express anti-oedematous effect (11, 12). Lysozyme has a positive effect on tissue regeneration after inflammations or surgical interventions. Furthermore, it is believed that the mucosal cells of the palatine tonsils produce lysozyme which becomes a part of the oropharyngeal lining and therefore affects the strengthening of local non-specific natural resistance. In line with this, the slower healing of postoperative wound after tonsillectomy and more pronounced symptoms of inflammation were observed in children with lower lysozyme titre.
The combination of lysozyme and cetylpyridinium has shown to have a synergistic antimicrobial effect. A study conducted by Bienen H and Raus I showed lozenges containing lysozyme and antiseptic agent to be significantly more efficient compared to lozenges comprising an antiseptic agent only (27). Both substances are increasingly used in various pharmaceutical formulations.
For the treatment of symptoms of common cold lysozyme can be found in different pharmaceutical formulations such as mouthwash, throat sprays, and oral gels. In Japan, lysozyme as an active substance can be found in even more pharmaceutical formulations such as tablets (30-90 mg), capsules (30-90 mg), and syrups (0.5-1%) used in the prevention of chronic sinusitis remission and in the treatment of bronchial asthma and bronchitis; ointment (5%) used in the treatment of wounds and various kinds of ulcers (postoperative ulcers, decubitus ulcers, etc.), and eye drops (0.5%) mainly used in the treatment of conjunctivitis. There are also some newer formulations of lysozyme for the healing of the skin wounds as well as in the formulation of antibacterial nanomotors. The harmonization of antibacterial activity of lysozyme with fast-moving nanomotors, along with appropriate fluid dynamics, reinforces the interaction of enzymes with bacteria and prevents the aggregation of dead bacteria (28). However, to understand and to use better its promising therapeutic potential, further studies on lysozyme effects and doses in various indications are needed.
Although the evidence of drug efficacy provided by observational post-marketing studies is not strong enough, those studies can provide satisfactory information on the general perception of drug efficiency in terms of reducing symptoms. Assessment of tolerability and adverse effects in such studies is also satisfactory. In addition, “by patients reported treatment outcomes” are a useful part of patient-centred outcome research and the studies based on the questionnaire with properly designed questions allow the respondents to behave according to their lifestyle and to show the real situation among the population. Symptoms, the level of quality of life related to health or healthcare satisfaction are reported directly by patients without any interpretation by clinicians or other health care providers. Data on morbidity, suffering, especially in patients with chronic diseases as well as insight into the patient’s experience during treatment, treatment options, the practice of treating the disease, treatment results and health policy are also provided (29).
5. CONCLUSION
According to the patients’ subjective assessment, the combination of lysozyme chloride and cetylpyridinium chloride in a formulation of spray can quickly, efficiently and safely resolve the symptoms of acute tonsillopharyngitis associated with common cold.
Footnotes
• Conflict of interest: The authors declare that they have no conflict of interest.
• Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
REFERENCES
- 1.Hirsch KR. Complications of the common cold. Healthline. 2014. [Accessed 7 Jan 2016]. http://www.healthline.com/health/common-cold-complications .
- 2.Dicpinigaitis PV, Eccles R, Blaiss MS, Wingertzahn MA. Impact of cough and common cold on productivity, absenteeism, and daily life in the United States:ACHOO Survey. Current Medical Research and Opinion. 2015;31:1519–25. doi: 10.1185/03007995.2015.1062355. [DOI] [PubMed] [Google Scholar]
- 3.Addey D, Shephard A. Incidence, causes, severity and treatment of throat discomfort:a four-region online questionnaire survey. BMC Ear, Nose Throat Disord. 2012;12:9. doi: 10.1186/1472-6815-12-9. doi:10.1186/1472-6815-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmfors L. Understanding the dual nature of lysozyme:part villain–part hero. Dissertation, Linkoping University. 2014 [Google Scholar]
- 5.Sava G. Pharmacological aspects and therapeutic applications of lysozymes. EXS. 1996;75:433–49. doi: 10.1007/978-3-0348-9225-4_22. [DOI] [PubMed] [Google Scholar]
- 6.Wang YB, Germaine GR. Effect of Lysozyme on glucose fermentation, cytoplasmic pH, and intracellular potassium concentrations in Streptococcus multans. Infect Immun. 1991;59:638–44. doi: 10.1128/iai.59.2.638-644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villa GT, Veiga-Crespo P. Enzybiotics:antibiotic enzymes as drugs and therapeutics. New York: Wiley; 2010. [Google Scholar]
- 8.Jolles P, Jolles J. What’s new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem. 1984;63:165–89. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- 9.McKenzie HA, White FH., Jr Lysozyme and alpha-lactalbumin:structure, function and interrelationships. Adv Protein Chem. 1991;41:173–315. doi: 10.1016/s0065-3233(08)60198-9. [DOI] [PubMed] [Google Scholar]
- 10.Borysowski J, Gorski A. Enzybiotics and their potential applications in medicine. In: Villa GT, Veiga-Crespo P, editors. Enzybiotics: antibiotic enzymes as drugs and therapeutics. New York: Wiley; 2010. [Google Scholar]
- 11.Vellard M. The enzyme as drug:application of enzymes as pharmaceuticals. Curr Opin Biotechnol. 2003;14:444–50. doi: 10.1016/s0958-1669(03)00092-2. [DOI] [PubMed] [Google Scholar]
- 12.Bethell DR. Generally recognized as safe (GRAS) notification for lysozyme (human) derived from rice. Ventria Bioscience. 2004 [Google Scholar]
- 13.Fabian TK, Hermann P, Beck A, Fejerdy P, Fabian G. Salivary defence proteins:their networked role in innate and acquired oral immunity. Int J Mol Sci. 2012;13:4295–4320. doi: 10.3390/ijms13044295. doi:10.3390/ijms13044295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sreenivasan PK, Haraszthy VI, Zambon JJ. Antimicrobial efficacy of 0.05% cetylpyridinium chloride mouthrinses. Lett Appl Microbiol. 2013;56:14–20. doi: 10.1111/lam.12008. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe E, Tanomaru JMG, Nascimento AP, Matoba-Junior F, Tanomaru-Filho M, Ito IY. Determination of the maximum inhibitory dilution of cetylpyridinium chloride-based mouthwashes against Staphylococcus Aureus:an in vitro study. J Appl Oral Sci. 2008;16:275–9. doi: 10.1590/S1678-77572008000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizwana N. The role of cetylpyridinium chloride mouthwash in the treatment of periodontitis. Int J Pharm Sci Invent. 2013;2:36–7. [Google Scholar]
- 17.Mankodi S, Bauroth K, Witt JJ, et al. A 6-month clinical trial to study the effects of a cetylpyridinium chloride mouthrinse on gingivitis and plaque. Am J Dent. 2005;18:9A–14A. [PubMed] [Google Scholar]
- 18.Garcia-Godoy F, Klukowska MA, Zhang YH, et al. Comparative bioavailability and antimicrobial activity of cetylpyridinium chloride mouthrinses in vitro and in vivo. Am J Dent. 2014;27:185–90. [PubMed] [Google Scholar]
- 19.Versterg PA, Rosema NA, Hoenderdos NL, Slot DE, Van der Weijden GA. The plaque inhibitory effect of CPC mouthrinse in a 3-day plaque accumulation model –a cross-over study. Int J Dent Hyg. 2010;8:269–75. doi: 10.1111/j.1601-5037.2009.00421.x. doi:10.1111/j.1601-5037.2009.00421.x.1–7. [DOI] [PubMed] [Google Scholar]
- 20.Farrer F. Sprays and lozenges for sore throats. S Afr Pharm J. 2011;78:26–31. [Google Scholar]
- 21.Shaku F, Tsutsumi M, Miyazawa A, Takagi H, Maeno T. Self-care behavior when suffering from the common cold and health-related quality of life in individuals attending an annual checkup in Japan:a cross-sectional study. BMC Fam Pract. 2015;16:91. doi: 10.1186/s12875-015-0300-3. doi:10.1186/s12875-015-0300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCallian DJ, Cheigh NH. The Pharmacist’s role in self-care. J Am Pharm Assoc. 2002;42:S40–S41. doi: 10.1331/108658002764653743. doi:10.1331/108658002764653743. [DOI] [PubMed] [Google Scholar]
- 23.Faber MS, Heckenbach K, Velasco E, Eckmanns T. Antibiotics for the common cold:expectations of Germany’s general population. Euro Surveill. 2010;15 pii6119655. [PubMed] [Google Scholar]
- 24.Kobayashi H, Yamamoto K, Zusho H. Comparison of antiphlogistics and Chinese medicines as to therapeutic effects on abnormaln sensation of pharynx. Jibi To Rinsho. 1985;31:569–76. [Google Scholar]
- 25.van Duijn HJ, Kuyvenhoven MM, Schellevis FG, Verheij TJ. Illness behaviour and antibiotic prescription in patients with respiratory tract symptoms. Br J Gen Pract. 2007;57:561–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaoka K, Yoshioka T. Effects of lysozyme chloride on immune responses of patients with uterine cervical cancer. Gan To Kagaku Ryoho. 1983;10:1803–9. [PubMed] [Google Scholar]
- 27.Speijers GJA, van Apeldoom ME. Lysozyme. National Institute of Public Health and Environmental protection laboratory for toxicology Bilthoven, The Netherlands. [Accessed 7 Jan 2016]. http://www.inchem.org/documents/jecfa/jecmono/v30j.e04.htm .
- 28.Bienen H, Raus I. Therapeutic comparison of throat lozenges. MMW Munch Med Wochenschr. 1981;123:745–7. [PubMed] [Google Scholar]
- 29.Kiristi M, Singh VV, Esteban-Fernandez de Avila B, et al. Lysozyme-based antibacterial nanomotors. ACS Nano. 2015;22:9252–9. doi: 10.1021/acsnano.5b04142. doi:10.1021/acsnano.5b04142. [DOI] [PubMed] [Google Scholar]
- 30.Anker SD, Agewall S, Borggrefe M, et al. The importance of patient-reported outcomes:a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J. 2014;35:2001–9. doi: 10.1093/eurheartj/ehu205. doi:10.1093/eurheartj/ehu205. [DOI] [PubMed] [Google Scholar]


