Abstract
Human immunodeficiency virus type 1 (HIV-1) carries a variety of host proteins in addition to virus-encoded structural proteins, both in its envelope and inside the viral particle. Previous studies have reported that the HIV-1 life-cycle is affected by such virus-associated host cell surface proteins. The nucleoside triphosphate diphosphohydrolase-1 (NTPDase1), also known as CD39, is a plasma membrane-bound ectoenzyme that hydrolyzes extracellular ATP and ADP to AMP. It has been shown that CD39 inhibits platelet function, and is thus a critical thromboregulatory molecule. We demonstrate here that host-derived CD39 is acquired by both laboratory-adapted and clinical variants of HIV-1 produced in cellular reservoirs of the virus. Moreover, purified CD39-bearing virions, but not isogenic viruses lacking CD39, display strong ATPase and ADPase activities. It is of particular interest that virions bearing this cellular enzyme can inhibit ADP-induced platelet aggregation, an effect blocked by an NTPDase inhibitor. On the basis of published and the present data on the functionality of human cellular proteins embedded within HIV-1, it can be proposed that these proteins might contribute to some of the immunologic deficiencies seen in infected individuals.
Keywords: HIV-1, cell surface molecules, CD39, ATPase, thromboregulation
Introduction
Human immunodeficiency virus type 1 (HIV-1) incorporates a variety of host proteins during its formation.1–3 Because this enveloped virus is released by budding through cell membranes, a vast array of cell surface components, including major histocompatibility complex class I and II,4–7 adhesion molecules,8,9 complement regulatory proteins,10 and costimulatory molecules,11,12 have been found inserted in mature virions. In addition, intracellular proteins are incorporated within HIV-1 particles. These include actin cytoskeletal proteins,13 cyclophilin A,14,15 ubiquitin,16 chromatin proteins,17 as well as signaling molecules such as ERK2 mitogen-activated protein kinase,18,19 and cAMP-dependent protein kinase A.20 Although the precise involvement of these cellular constituents in HIV-1 pathogenesis is still unclear, a number of host-encoded proteins have been shown to affect the viral life-cycle. For example, the adhesion molecule ICAM-1 and the costimulatory molecules CD28, CD80 and CD86 enhance HIV-1 infectivity by facilitating virus binding and entry into target cells that express their cognate ligands.8,9,11,12,21,22 Other factors of host origin have been demonstrated to play a pivotal role in HIV-1 replication. For example, the presence of an active catalytic subunit of cAMP-dependent protein kinase Awithin HIV-1 is required for viral infectivity, possibly because of its ability to catalyze phosphorylation of the viral p24 capsid protein.20 Hence, analyses aimed at assessing the function of host-encoded molecules that are found inserted within nascent viruses are needed to define whether this phenomenon might eventually affect disease progression.
Nucleoside triphosphate diphosphohydrolase-1 (NTPDase1; EC 3.6.1.5) is a plasma membrane enzyme that hydrolyses extracellular ATP and ADP to AMP in the presence of divalent cations (usually Ca2+ or Mg2+).23,24 This ectoenzyme, described also as vascular ATP diphosphohydrolase, ATPDase, ecto-apyrase, ecto-ATPase, ecto-ADPase and nucleotide phosphohydrolase, was shown to be identical with the previously described B-cell activation marker CD39.25–27 It is noteworthy that this enzyme was first identified in zymogen granules of the exocrine pancreas, a membrane involved in exocytosis, a classical fusion process.28 CD39 is a highly glycosylated, integral membrane acidic protein of 70–100 kDa that carries two transmembrane regions and six putative N-glycosylation sites.29,30 Its large extracellular domain contains the active site, and it has been well established that CD39 exerts its activity on the outer face of the plasma membrane and is not active intracellularly. Moreover, its N-glycosylation state seems essential for its surface localization and enzymatic activity.31 The two transmembrane domains are also critical for its activity through dynamic motions enabling crosstalk with the active site.32 Furthermore, the enzyme was shown to undergo palmitoylation at the N-terminal cytoplasmic domain, a modification that targets it to caveolae.33,34 The CD39 molecule is expressed in quiescent endothelial cells, activated B cells, natural killer (NK) cells and subsets of T cells, as well as in macrophages and dendritic cells.35–43
Initially described as a modulator of homotypic adhesion in B cells,42,44 CD39 is now recognized as the dominant vascular NTPDase and a critical thromboregulatory molecule.25,27,45 It is actually the major ectonucleotidase responsible for the hydrolysis of nucleotides in the blood.25,46 CD39 converts ATP and ADP into AMP following their activation-induced release from, for example, platelet-storage granules. The latter product is in turn hydrolysed to adenosine by the ecto-5′-nucleotidase CD73.47 While the substrates of CD39 induce proinflammatory effets, control the vascular tone and trigger platelet recruitment and activation, adenosine exhibits also anti-thrombotic and anti-inflammatory properties. Thus, through modulation of extracellular concentrations of ATP, ADP and adenosine, CD39 is involved in the maintenance of blood fluidity and flow. It has been documented clearly that CD39 modulates platelet aggregation both in vitro and in vivo.25,27,45,48,49 In vivo experiments with cd39−/− mice, transgenic mice and over-expression of the enzyme by an adenovirus vector have confirmed the key thromboregulatory function of this enzyme.45,50–54 Another putative role of CD39 is related to its ability to induce homotypic adhesion of B lymphocytes by mechanisms both dependent and independent of leukocyte function antigen-1 (LFA-1).42,44
CD39 is expressed in physiologic cellular reservoirs of HIV-1 such as CD4+ T lymphocytes, macrophages and dendritic cells.40,42,55 Moreover, a recent study has reported an enhanced CD39 expression and NTPDase activity in lymphocytes isolated from HIV-1-infected patients.56 These observations prompted us to explore the possible incorporation of this enzyme in newly formed virions. Our results show that laboratory-adapted and clinical isolates of HIV-1 incorporate host-derived CD39 in their envelope where it maintains its natural functions. Altogether, these observations suggest that cellular enzymes, such as the NTPDase1/CD39, can remain functional when inserted within nascent HIV-1 and potentially modulate the course of the disease.
Results
Host-derived CD39 is incorporated within newly-formed HIV-1 particles
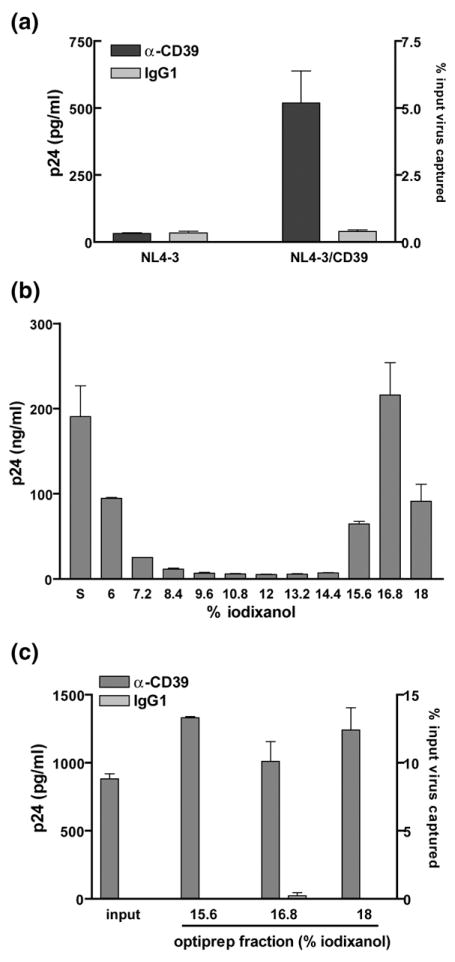
We used our previously described transient transfection-and-expression system to determine whether CD39 is acquired by mature HIV-1 particles.8,9 To this end, we transiently cotransfected 293T cells with an infectious molecular clone of HIV-1 (pNL4-3) and a mammalian expression vector coding for the human CD39 protein (pCDNA3-CD39). Flow cytometric studies indicated that co-transfection resulted in expression of CD39 in 293T cells, which do not constitutively express this cell surface constituent (data not shown). Virions produced in such CD39-expressing 293T cells are captured by magnetic beads coated with an anti-CD39 antibody but not with an isotype-matched irrelevant control antibody (Figure 1(a)), suggesting an acquisition of host CD39 by HIV-1. To confirm that the CD39 molecule was associated with virus particles, CD39-positive virions were separated from contaminating microvesicles using an Opti-prep velocity gradient centrifugation (Figure 1(b)). Data from a capture assay indicate that such purified HIV-1 particles carry host-derived CD39 molecules (Figure 1(c)). These data demonstrate that this ectoenzyme is incorporated into HIV-1 particles produced in CD39-expressing cells.
Figure 1.
CD39 is incorporated in HIV-1. (a) Isogenic viral particles differing only in the absence or the presence of host-derived CD39 were produced in 293T cells by cotransfection of pNL4-3 and the empty pCDNA3 control vector (NL4-3) or the CD39 expression vector (NL4-3/ CD39). A similar amount of each viral preparation was subjected to a viral capture assay using magnetic beads coated with an anti-CD39 antibody or an isotype-matched irrelevant control antibody (IgG1). The amount of captured virus was estimated by measuring the p24 content. The results shown are the mean±standard deviation of triplicate samples and are representative of three separate experiments. (b) NL4-3/CD39 viral particles were purified by velocity gradient ultracentrifugation in 6%–18% iodixanol (Optiprep) and each fraction was analyzed for the p24 content (S: Samples before being subjected to the Optiprep velocity gradient centrifugation step). (c) Unpurified samples (input) and Optiprep fractions containing purified virions were submitted to a virus capture test (i.e. 15.6%, 16.8% and 18% fractions).
CD39 palmitoylation is not required for its incorporation within HIV-1
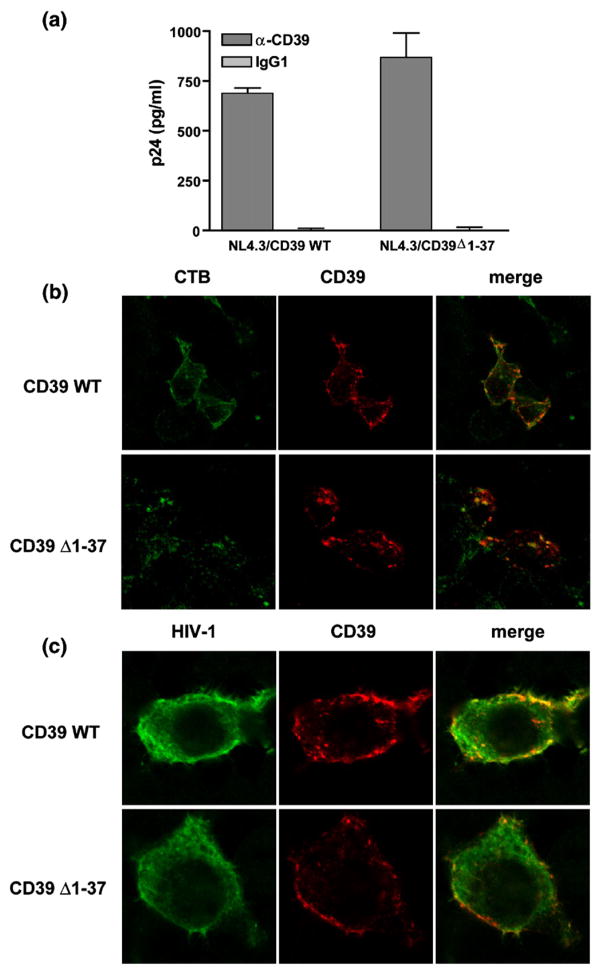
CD39 undergoes palmitoylation at its N-terminal region, and this modification directs the enzyme to specialized forms of lipid rafts called caveolae.34 Since both lipid rafts and the myristoylation of Gag precursor proteins are critical for HIV-1 membrane targeting and assembly, which result in the release of viruses from lipid rafts,57,58 it was tempting to speculate that CD39 is incorporated simply because of its physical presence in the membrane microdomains where virus budding is occurring. To assess the putative involvement of the post-translational modification in CD39 incorporation within HIV-1, we used a truncated form of the enzyme lacking the N-terminal intracytoplasmic region, i.e. CD39Δ1-37. This mutant is not subject to palmitoylation and appears to be absent from the plasmalemmal microdomains where the wild type enzyme is concentrated.34 Cotransfection of a wild-type or truncated CD39 expression vector along with pNL4-3 resulted in a high level of expression of membrane CD39 in 293T cells (data not shown). Moreover, virions produced upon transfection with both CD39 vectors were captured with a similar efficiency (Figure 2(a)). These data demonstrate that palmitoylation is not required for CD39 incorporation in HIV-1 particles. Interestingly, results from confocal microscopy revealed that both wild-type and mutant forms of CD39 partially colocalize with lipid rafts (Figure 2(b)). Therefore, it is not surprising to find that both forms of CD39 are concentrated in regions where HIV-1 proteins are located (Figure 2(c)).
Figure 2.
CD39 palmitoylation is not required for its incorporation in HIV-1. (a) Isogenic viral particles were produced in 293T cells by cotransfection of pNL4-3 and an expression vector coding for either wild type or N-truncated CD39 (CD39Δ1-37). A similar amount of each viral preparation was subjected to a viral capture assay using magnetic beads coated with an anti-CD39 antibody or an isotype-matched irrelevant control antibody (IgG1). The amount of captured virus was estimated by measuring the p24 content. The results shown are the mean ± standard deviation of triplicate samples and are representative of two separate experiments. (b) Cells were transfected with wild-type CD39 or CD39Δ1-37 vector and stained with FITC-conjugated cholera toxin B (CTB) and anti-CD39 to visualize lipid rafts and CD39, respectively. (c) Cells were cotransfected with pNL4-3 and wild-type CD39 or CD39Δ1-37 vector before staining with human anti-HIV-1 and anti-CD39. Cells were imaged with a scanning confocal microscope. The images shown represent the middle stack slice.
Virion-associated CD39 possesses ADPase and ATPase activities
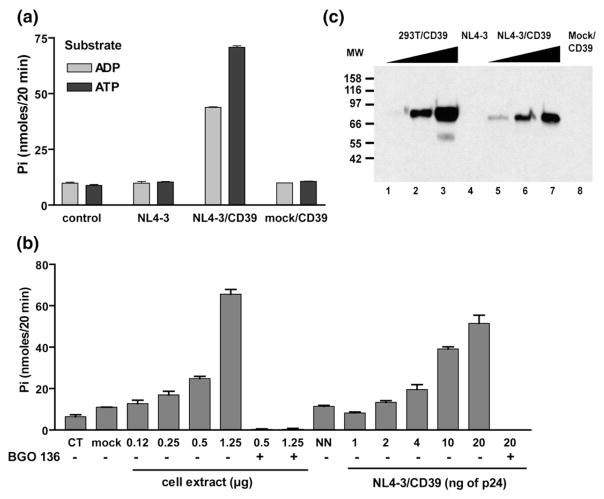
We assessed whether CD39 is still enzymatically active once found embedded within the virus envelope. Isogenic viruses differing only by the absence or the presence of host CD39 were produced in 293T cells and purified to remove the phosphate that could interfere with the enzymatic assay. A high level of enzymatic activity was observed with CD39-bearing viruses when using both ATP and ADP as substrates, whereas no such activity was detected in virions lacking CD39 (Figure 3(a)). A control consisting of the supernatant from CD39-expressing 293T cells subjected to the purification procedures used for viral stocks (called mock/CD39) was devoid of any activity, indicating that the activity associated with NL4-3 particles bearing host CD39 was not due to contaminating microvesicles or membrane debris. A dose-dependent augmentation in ADPase and ATPase activities was detected when cell extracts from CD39-expressing 293T cells (used as a positive control) and increasing concentrations of CD39-bearing viruses were tested (Figure 3(b)). The complete suppression of activity induced by the NTPDase inhibitor BG0 136 confirmed that the enzyme exerting the observed virion-associated NTPDase activity is indeed CD39. Moreover, the observed enzymatic activity was correlated to the amount of CD39 protein detected by Western blotting in both virus and cell extracts (Figure 3(c)). Taken together, these data demonstrate that CD39, once inserted within HIV-1 envelope, displays an NTPDase activity that is similar to that of its cellular counterpart.
Figure 3.
Virus-anchored CD39 is enzymatically active. (a) Isogenic virions either lacking or bearing CD39 were assayed for NTPDase activity using ATP or ADP (0.4 mM) as a substrate. The data shown represent the amounts of inorganic phosphate released in 20 min at 37 °C. A first control reaction was performed without the enzyme (control) and a second control was carried out with cell-free supernatants from CD39-expressing 293T cells (mock/CD39). (b) Increasing amounts of CD39-expressing 293T cell extracts or CD39-bearing viruses were assayed for ADPase activity in the presence of ADP (0.4 mM) (CT, control reaction; mock, cell extract from parental 293T cells; and NN, viruses that lack host CD39). The inhibitor BG0 136 (10 mM final concentration) was added where indicated. (c) Detection of CD39 using Western blot analyses. Lanes 1 to 3, extracts of CD39-expressing 293T cells (1.25 μg, 2.5 μg and 5 μg); lane 4, NL4-3 (80 ng of p24); lanes 5–7, NL4-3/CD39 (40 ng, 90 ng and 170 ng of p24); and lane 8, cell-free supernatant from CD39-expressing 293T cells. The results shown in (a) and (b) are the mean±standard deviation of triplicate samples and are representative of three separate experiments.
CD39-bearing HIV-1 particles inhibit ADP-induced platelet aggregation
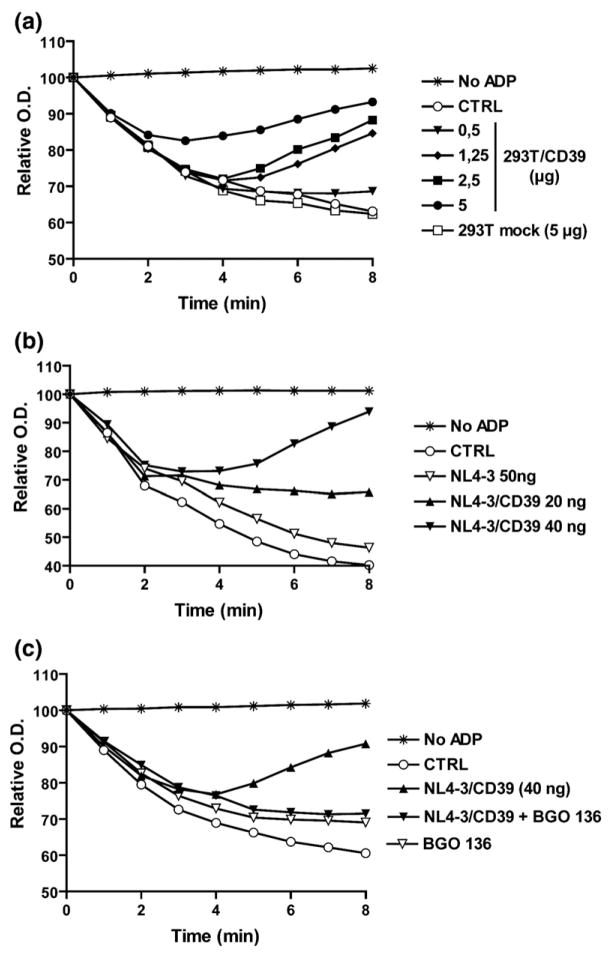
Extracellular ADP interacts with purinergic type 2 (P2) receptors, and is a potent agonist for platelet recruitment, adhesion and aggregation. CD39, by converting ADP to AMP, which is further degraded to the anti-thrombotic and anti-inflammatory mediator adenosine, is a powerful inhibitor of platelet aggregation, hence a critical thromboregulatory molecule. We therefore investigated whether CD39-bearing viruses might display such an antithrombotic activity. In vitro ADP-induced platelet aggregation in platelet-rich plasma was inhibited by CD39 protein extracts in a dose-dependent fashion, whereas control extracts from mock-transfected 293T cells had no such effect (Figure 4(a)). Under similar experimental conditions, CD39-bearing virions, but not isogenic viruses lacking CD39, inhibited ADP-induced platelet aggregation (Figure 4(b)). It should be noted that there was no difference in the ability of viruses lacking or bearing host-derived CD39 to bind platelets as monitored by binding assays (data not shown). To make sure that this inhibition of aggregation was a consequence of the breakdown of ADP by CD39, the NTPDase inhibitor BG0 136 was used. In the presence of BG0 136, virions bearing CD39 were no longer able to inhibit ADP-induced platelet aggregation (Figure 4(c)). Moreover, when a 50-fold excess of ADP was used to trigger aggregation, no inhibition could be achieved when using CD39-bearing viruses (data not shown), confirming that the ADPase activity of CD39 is responsible for the observed anti-thrombotic activity. Similar observations were made when testing R5-tropic NL4.3-balenv viruses (data not shown), suggesting that this phenomenon is not specific to a given HIV-1 strain and is not influenced by the co-receptor usage.
Figure 4.
Virus-associated CD39 inhibits ADP-induced platelet aggregation. Platelet-rich plasma (PRP) was incubated alone (CTRL) or (a) with the indicated amounts of 293T cell extracts or (b) and (c) virus preparations for 5 min at 37 °C. Aggregation was triggered by the addition of ADP (10 μM final concentration), and recorded by measuring absorbance for 8 min at room temperature. Where indicated, the NTPDase1 inhibitor BG0 136 (10 mM final concentration) was added during the incubation.
CD39 is present and active in clinical isolates of HIV-1 expanded in natural cellular reservoirs
The data described above were obtained with isogenic viral particles that differ only by the absence or the presence of CD39 in their envelope. Although these viruses are very useful tools for specifically analyzing incorporation and functionality of a given host cell membrane component, they do not adequately represent the complexity of in vivo conditions where several host-derived molecules are found associated with emerging viruses. To determine if the previous findings hold true for viruses produced in more natural cellular reservoirs, we analyzed the insertion and activity of CD39 in clinical variants of HIV-1 amplified in primary human cells.
Two field isolates of HIV-1, i.e. 92HT599 (X4-tropic) and 92US657 (R5-tropic), were used to infect ex vivo expanded peripheral blood mononuclear cells (PBMCs) and human lymphoid tissue histocultures. Virus-associated CD39 was detected in each virus preparation tested at levels similar to those observed in viruses produced in CD39-transfected cells (Figure 5). Viral preparations displaying high levels of CD39 incorporation were chosen for further analyses. An NTPDase assay performed with 92HT599 and 92US657 clinical isolates expanded in lymphoid tissue revealed the presence of a high level of enzymatic activity associated with both viral preparations (Figure 6(a)). The strong inhibition of the enzymatic reaction observed in the presence of the BG0 136 inhibitor suggests that the incorporated CD39 is responsible for this activity. We next determined if these viral particles could display antithrombotic potential. When added to an ADP-triggered platelet aggregation assay, both 92HT599 and 92US657 virus stocks induced a partial but reproducible inhibition of platelet aggregation (Figure 6(b), upper panel). This inhibition was reduced strongly in the presence of BG0 136, indicating that CD39 is involved in the observed effect (Figure 6(b), lower panel). Furthermore, similarly treated supernatants from mock-infected tonsils did not display any significant inhibitory activity. These data indicate that viruses expanded in natural reservoirs can incorporate CD39 molecules that retain their enzymatic and biological functions.
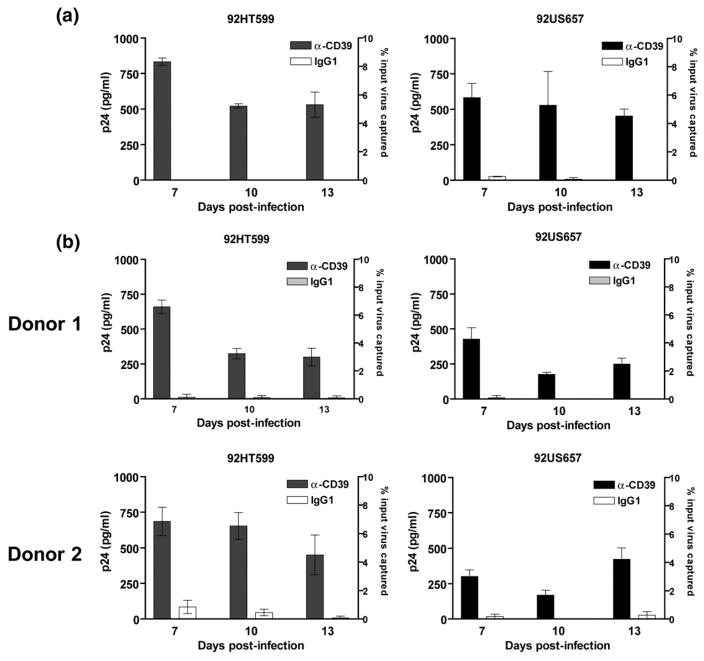
Figure 5.
CD39 is incorporated into HIV-1 clinical isolates expanded in PBMCs and human lymphoid tissue. (a) PBMCs and (b) tonsil tissues were inoculated with HIV-1 clinical isolates 92HT599 (X4-tropic) or 92US657 (R5-tropic). Virus preparations were concentrated and purified from cell-free culture supernatants harvested at seven days, ten days and 13 days post infection. A similar amount of each virus stock, standardized in terms of p24 (i.e. 2 ng of p24), was subjected to the virus capture test using streptavidin-coated beads tagged with biotinylated anti-CD39 or isotype-matched irrelevant (IgG1) antibodies. The amounts of precipitated viruses were estimated with an ELISA specific for the major core viral p24 protein. The data shown are the mean±standard deviation of triplicate samples.
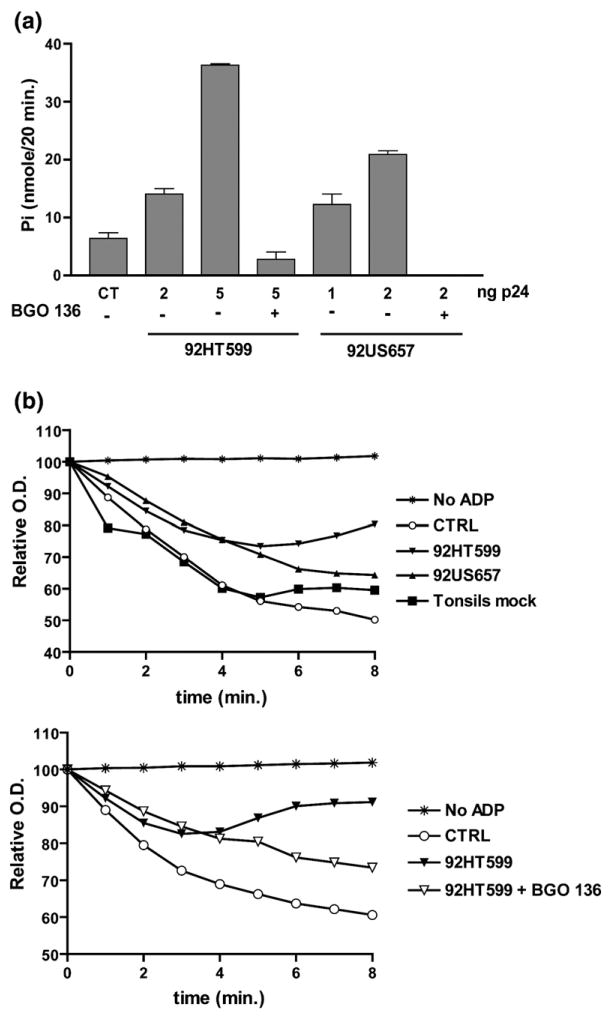
Figure 6.
CD39 incorporated within clinical isolates of HIV-1 possesses enzymatic and biologic activities. (a) Clinical isolates 92HT599 and 92US657 expanded in tonsil histocultures (harvested 13 days post infection) were assayed for ADPase activity in the presence of ADP (0.4 mM final concentration). The data shown are the mean± standard deviation of triplicate samples and are representative of three separate experiments. (b) Aggregation assays were performed in the presence of 92HT599 or 92US657 (6 ng of p24) harvested 13 days post infection (upper panel), or 92HT599 (10 ng of p24) harvested at seven days post infection (lower panel). The NTPDase1 inhibitor BG0 136 (10 mM final concentration) was added where indicated (CTRL, natural ADP-mediated platelet aggregation; tonsils mock, cell-free supernatant from mock-infected tonsil tissue).
Discussion
HIV-1 and other enveloped viruses incorporate a vast array of host proteins in addition to their own structural proteins. Although the implication of such virus-anchored host constituents in viral pathogenesis remains ill defined, it is clear that some of these host cell components have various impacts on HIV-1 life-cycle and virus susceptibility to immune defence. Previous studies have indicated that the inserted host molecules include membrane proteins such as adhesion and costimulatory molecules as well as cytosolic proteins such as cytoskeletal proteins, molecular chaperones, protein kinases and chromatin proteins. Here, we demonstrate the acquisition of the ectoenzyme NTPDase1 or CD39 by HIV-1 and provide evidence that this host cell surface molecule remains enzymatically and biologically active.
The mechanism governing CD39 incorporation into HIV-1 is still unclear. CD39 undergoes palmitoylation at its N-terminal region and this modification directs the enzyme to specialized forms of lipid rafts called caveolae. Our data showing incorporation of a truncated form of CD39 lacking the palmitoylation site indicate that this region is not necessary to lead to acquisition of CD39 by emerging virions. However, although palmitoylation was shown to be crucial for CD39 localization in caveolae,34 it seems to be unnecessary for its localization in lipid rafts. Because HIV-1 particles are known to be released from specialized glycolipid-enriched microdomains commonly called lipid rafts,57,58 our observations support the hypothesis that CD39 is incorporated probably because of its physical presence in the membrane microdomains where virus budding is taking place.
CD39 was found to be incorporated within HIV-1 clinical isolates expanded in both PBMCs and human lymphoid tissue cultured ex vivo. However, whether such CD39-bearing virions originate from CD4+ T lymphocytes or macrophages remains unclear, as this ectoenzyme is expressed in both cell types.42 Previous studies indicate that only 2–3% of T cells express CD39,43 but this percentage increases sharply following T cell activation.42 The CD4-expressing T cells present in both PHA-activated PBMCs and tonsil histocultures are mostly in an activated state, suggesting that CD39-bearing viruses are probably originating from this cell type. Moreover, it has been proposed that an intracellular virus budding process is taking place in macrophages. Since intracellular CD39 has no enzymatic activity,31 it is unlikely that clinical isolates bearing host-derived active CD39 molecules are produced by macrophages. To confirm this hypothesis, the presence of other cell type-specific host cell membrane components found embedded within CD39-bearing virions could be assessed. For example, CD26 and CD36 have been used to differentiate virions produced by lymphocytes from those originating from macrophages.59 We have observed that in PBMCs, the extent of CD39 incorporation in viral particles is dependent on lymphocyte activation level (data not shown), which is in line with the pattern of CD39 expression in such cells.42 In fact, CD39 can be considered as a marker of lymphocyte activation. Hence, conditions of inflammation or immune hyperactivation, such as in the presence of co-infections, could result in an increased incorporation of CD39 in emerging virions. Interestingly, a previous study has demonstrated that a marked increase in HLA-DR-bearing virions is seen in plasma from HIV-1-infected patients experiencing an active tuberculosis compared to patients with no opportunistic infection, and this phenomenon was due to an increase in systemic immune activation.60 It is of interest to note that an enhanced expression of CD39 in lymphocytes originating from HIV-1-positive patients has been reported,56 suggesting that this molecule might be incorporated within HIV-1 under in vivo situations.
We provide evidence that CD39, once embedded in HIV-1, possesses an enzymatic activity that is indiscernible from that seen in the cellular membrane when using both enzymatic and functional assays. Virus-associated CD39 is able to hydrolyze ATP and ADP, and can efficiently inhibit ADP-induced platelet aggregation. The enzymatic activity of CD39 is thought to be controlled by its oligomerization state, being active as a homomultimer.61 Thus, our observations suggest that the natural conformation of CD39 is retained once this enzyme is inserted within the virus envelope.
Other host proteins found embedded within viruses that possess an enzymatic activity are mostly intracellular molecules such as kinases. For example, the mitogen-activated protein kinase ERK-2 is strongly associated with retroviral particles and can phosphorylate cellular and viral substrates.18 Moreover, the phosphorylation of p6gag by ERK-2 appears to be involved in the HIV-1 budding process.19 The catalytic subunit of cAMP-dependent protein kinase Awas also shown to be packaged and to remain enzymatically active within mature HIV-1 particles.20 This virus-associated protein kinase is thought to regulate virus infectivity by catalyzing phosphorylation of the viral capsid p24 protein.20 Very few active ectoenzymes have been identified within the HIV-1 envelope. The complement control proteins CD55 and CD59 are incorporated into HIV-1 particles and have been shown to be active in complement breakdown.15,62,63 Dipeptidyl peptidase IV (CD26) has been found incorporated in lymphocyte-derived HIV-1 particles; however, the enzymatic activity of this molecule once embedded in virions has not been demonstrated.59 To the best of our knowledge, CD39 is the first nucleoside hydrolase found incorporated within HIV-1.
A number of virus-associated membrane and cytosolic host proteins have been demonstrated to influence the life-cycle of HIV-1. No such role was found for CD39, since we could not detect any difference in terms of virus binding and infectivity when comparing isogenic viruses either lacking or bearing CD39, at least under the experimental conditions tested (data not shown). However, it is well known that various cell types release ATP into the extracellular medium in response to activating stimuli, and this extracellular ATP profoundly affects several cellular functions through its interaction with the P2 purinergic receptors.64 It is therefore tempting to speculate that the virus-associated host CD39 might affect indirectly HIV-1 replication due to its well-known ability to hydrolyze ATP and ADP into AMP. For example, a possible CD39-mediated modulatory role in adhesion could be proposed. Although CD39 is not considered as an adhesion molecule per se,65 it is thought to intervene in cell-to-cell adhesion via a regulatory process. More precisely, cell adhesion molecules (CAMs) can be phosphorylated by ectoprotein kinases, which use extracellular ATP as substrate. CD39, by regulating ATP levels, could influence ectokinase(s) activity via substrate concentration and hence, CAM activity.66 We are currently investigating whether virally embedded host CD39 can affect HIV-1 biology in various experimental cell systems due to changes in activity of CAMs that are acquired by virions and/or located onto the target cell surface.
It can be proposed that virus-associated host CD39 can have an indirect effect on virus production and HIV-1 pathogenesis under in vivo situations through regulation of the immune activation status. For example, cytotoxic T lymphocytes exhibit high levels of ecto-ATPase activity that protects them from the lytic effects of high concentrations of extracellular ATP,67 but is necessary also for their cytotoxic activity.68 Hydrolysis of extracellular ATP by ecto-ATPases has been shown to be essential for activation and proliferation of lymphocytes.69 In addition, hydrolysis of extracellular ATP is considered as an essential step in calcium mobilization mediated by T cell receptor and Fc-gamma receptor signalling in cytotoxic T lymphocytes and NK cells.70,71 These observations suggest that ecto-ATPase has an obligatory role in calcium influx and hence in many effector functions of T, B and NK cells. Importantly, this effect is not related to the extracellular levels of ADP and AMP. Since CD39 has not been shown to deliver any intracellular signal and does not act as a receptor, its putative role rather resides in its ability to regulate extracellular ATP, ADP and adenosine levels, or (less likely) in providing inorganic phosphate that could be taken up by the cell, transformed into ATP and used by intracellular kinases. Also, it has been shown that the αβ-T cell receptor ectodomain can be phosphorylated at the cell surface by ectokinases expressed on T lymphocytes, a modification that could serve as a potential mechanism for regulating the αβ-T cell receptor-mediated lymphocyte response.72,73 This process could very well be influenced by the ATPase activity displayed by virus-associated host CD39.
The most studied physiological function of CD39 is its anti-thrombotic potential. CD39 is indeed a major inhibitor of platelet activation and recruitment. In addition, it has been shown to inhibit platelet reactivity induced by ADP, collagen and thrombin.25 We demonstrate here that this activity is retained by CD39 when located on the exterior of virions. Importantly, clinical isolates of HIV-1 that were expanded in a physiological environment (i.e. human lymphoid tissue cultured ex vivo) were able to efficiently inhibit ADP-induced platelet aggregation. In vivo, both thrombosis and thrombocytopenia have been associated with HIV/AIDS.74,75 Thrombocytopenia, a disorder present in 10% of seropositive patients and in one-third of AIDS patients, is characterized by an accelerated immune-mediated platelet destruction and a decreased production and release of platelets from megacaryocytes.74,76 However, severe bleeding is rarely seen in AIDS patients (approximately 8% of thrombocytopenia cases).76 A putative role for CD39 in these rare cases of bleeding, possibly through an inhibition of platelet aggregation by virions displaying a high concentration of host CD39 on their surface, is a possibility that deserves to be investigated.
In conclusion, we have shown that both laboratory-adapted and clinical isolates of HIV-1 originating from physiologic cellular reservoirs such as tonsil explants can incorporate an active NTPDase1/ CD39 in their envelope. Such CD39-bearing viral particles could act as circulating enzymes, which could affect several physiological processes by influencing local levels of ATP, ADP and adenosine. These findings represent additional evidence of the possible role of host-encoded molecules that are acquired by HIV-1 in the pathogenesis of this debilitating disease.
Materials and Methods
Cells and preparation of tonsil tissue blocks
293T are human embryonic kidney cells that express the simian virus 40 (SV40) large T antigen. These cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml of penicillin G, and 100 μg/ml of streptomycin. PBMCs were isolated by a Ficoll-Hypaque gradient from venous blood samples, and seeded in 75 cm2 flasks at 1×106 cells/ml in RPMI 1640 supplemented with 20% FBS, 2 mM glutamine, 100 U/ml of penicillin G and 100 μg/ml of streptomycin. The cells were then stimulated for two days with 1 μg/ml PHA-L (Sigma Chemical Corporation) and 30 U/ml of recombinant IL-2. Human tonsil histocultures were prepared as described.77 Briefly, human tonsil tissues removed during routine tonsillectomy were received within 5 h of excision. The tonsils were washed thoroughly with medium containing antibiotics and then sectioned into 2–3 mm2 blocks. The tissue blocks were placed on top of collagen gel sponges in the culture medium at the air–liquid interface and infected the next day with clinical isolates of HIV-1 (see below).
Vectors and antibodies
pNL4-3 is a full-length infectious molecular clone of HIV-1 (a prototypic X4-tropic strain) and was obtained through the NIH AIDS Repository Reagent Program (Germantown, MD). pNL4.3balenv, kindly provided by Dr R. Pomerantz (Thomas Jefferson University, Philadelphia, PA), is a molecular construct in which the NL4-3 env gene is replaced with that of the R5-tropic Bal strain.78 pCDNA3-CD39 consists of the full-length human CD39 cDNA cloned in the expression vector pCDNA3.25 A truncated form of CD39 lacking the first 37 amino acid residues, i.e. CD39Δ1-37,34 was also used in these studies. Unconjugated and biotin-conjugated mouse monoclonal antibodies against CD39 were purchased from Ancell Immunology Research Products (Bayport, MN).
Production of virus stocks
Isogenic virus particles differing only by the absence or the presence of host-encoded CD39 protein in their outer membrane were produced by calcium phosphate transfection of 293T cells as described.6,8 The HIV-1 B clade clinical isolates 92HT599 (X4-tropic) and 92US657 (R5-tropic) were obtained through the NIH AIDS Repository Reagent Program. For virus production, PBMCs (5×106) were incubated with each viral strain for 2 h at 37 °C in a final volume of 1 ml. Next, cells were washed once with complete RPMI, resuspended at 1×106 cells/ml in the presence of 30 U/ml of recombinant IL-2, and incubated at 37 °C under a 5% CO2 atmosphere. Cells were resuspended this way after each harvest, which took place at seven days, ten days and 13 days post infection. Harvested culture portions were centrifuged, and virus-containing supernatants were stored frozen at −85 °C. Tonsil tissue blocks cultured ex vivo were infected with HIV-1 (same virus preparations as for PBMCs) that was applied to the top of each tissue block (5 μl of virus-containing supernatant containing 1–2 ng of p24) and left at 37 °C under a 5% CO2 atmosphere. Culture supernatants were harvested and frozen at −85 °C at seven days, ten days and 13 post infection. Fresh medium was added to tonsil cultures on days 7 and 10 after virus infection. To eliminate free p24, each unfrozen virus preparation was processed in Centricon® Plus-20 Biomax-100 filter devices (Millipore Corporation) at 4000g, 4 °C, until all the supernatant had been filtered. Then the virus preparation on the membrane was recovered by an additional centrifugation step at 1000g, 4 °C, for 1 min. Virion-containing supernatants were filtered through a 0.22 μm pore size cellulose acetate membrane (Millipore Corporation), and aliquots were frozen at −85 °C. Virus stocks were normalized for virion content using an in-house sensitive double-antibody sandwich ELISA specific for the major core viral p24 protein.21
Optiprep gradients
Virus-containing supernatants from 293T cells were first pelleted by ultracentrifugation at 28,000 rpm for 60 min in a 70Ti rotor. Next, samples were centrifuged in an Optiprep (60% (w/v) iodixanol) velocity gradient, as described.79 In brief, iodixanol gradients were prepared in distilled/deionized water as 11 steps in 1.2% increments ranging from 6–18%. Samples were layered on top of the gradient in an Optiseal tube (Beckman Coulter), and centrifuged using an Optima L-90K apparatus for 75 min at 52,000 rpm in an NVT65 rotor. Gradient fractions were collected and stored frozen at 85 °C.
Virus capture assay
We used our previously described virus precipitation assay based on the capture of HIV-1 particles using immunomagnetic beads.80 In brief, commercially available streptavidin-coated magnetic beads (8.4×106 beads) (Dynal Biotech Inc.) were mixed with 2 μg of biotinylated monoclonal antibodies in a final volume of 1 ml of PBS/ BSA (phosphate-buffered saline plus 10% bovine serum albumin) for 1 h at room temperature on a rocking plate. Immunomagnetic beads were next washed three times in PBS/BSA on a magnet support (Dynal Biotech Inc.) and resuspended in 50 μl of PBS/BSA. HIV-1 (2 ng of p24 for each viral population) was added to the antibodies/beads mixture (50 μl) and the mixture was incubated at 4 °C overnight on a rocking plate. Thereafter, immunomagnetic beads were washed four times in 200 μl of PBS/BSA and finally resuspended in 200 μl of PBS/BSA. Viruses captured on magnetic beads were lysed by adding 50 μl of lysis buffer (BS (pH 7.4), 2.5% (v/v) Triton X-100) and incubated for 30 min at room temperature. Magnetic beads were pelleted with a magnetic plate (Dynal Biotech Inc.) and 125 μl of the cleared supernatants were used to estimate the p24 content.
NTPDase assay
Membrane-bound NTPDase activity was determined by measurement of the amount of liberated inorganic phosphate hydrolyzed from exogenous ATP or ADP. To eliminate the phosphate present in the culture medium, virus stocks were first concentrated using Centricon®Plus-20 Biomax-100 filter devices (Millipore Corporation), diluted in wash buffer (15 mM Tris–HCl (pH 7.5), 145 mM NaCl) and then pelleted at 15,000g for 90 min at 4 °C. The viral pellet was resuspended in a small volume of reaction buffer (see below). These purified virus stocks were normalized for virion content using the p24 test. Protein extracts that were used as controls were prepared by calcium phosphate transfection of 293T cells with the pcDNA3-CD39 expression vector or the pcDNA3 empty control vector. At 48 h post transfection, cells were washed twice with wash buffer and then stripped using 20 mM Tris–HCl (pH 7.6) 50 mM NaCl. Cells were disrupted by 20 strokes in a glass Dounce homogenizer, cell debris was pelleted by centrifugation at 3000 rpm for 5 min at 4 °C, and the supernatant was assayed for protein concentration by the BCA assay (Pierce) and stored at −85 °C. The enzymatic activity present in protein extracts or viral stocks was measured at 37 °C in 0.5 ml of 80 mM Tris–HCl (pH 7.4) and 5 mM CaCl2.46 Reaction was initiated by adding a substrate (i.e. 0.4 mM ATP or ADP), stopped after 20 min by adding 0.125 ml of malachite green reagent, and the amount of inorganic phosphate released during the hydrolysis of the exogenous nucleotide was determined by measuring absorbance at 610 nm against a standard curve for KH2PO4.81 For assays done with fresh, intact cells, NaCl (145 mM final concentration) was added to the incubation medium to maintain isotonicity. In some assays, 10 mM 1-hydroxynaphtalene-3,6 disulfonic acid (BG0 136) (Sigma-Aldrich) was used as an inhibitor of NTPDase activity.82
Platelet aggregation assay
Platelet-rich plasma (PRP) was prepared from venous blood anticoagulated with 0.1 volume of 3.2% (w/v) sodium citrate by centrifugation at 300g for 15 min at 22 °C. Platelet aggregation measurements were done with a commercially available microtiter plate reader.83 PRP (200 μl) was incubated with cellular extracts or viruses for 5 min at 37 °C in a 96-well flat bottom plate. ADP (10 μM final concentration) was then added to start the aggregation and the plate was immediately placed in the plate reader. The absorbance was measured at room temperature every 60 s for 8 min, with a 30 s shaking (maximal intensity) before each reading. Platelet-poor plasma, obtained by plasma centrifugation at 15,000g for 1 min, was used as a blank for all readings. Aggregation curves were calculated from the mean of triplicate assays.
Western blot analysis
Total 293T cell extracts or concentrated virus preparations were heated at 100 °C for 5 min in sample buffer (62 mM Tris-HCl (pH 6.8), 2% (w/v) SDS, 9% (v/v) glycerol, 0.002% (w/v) bromophenol blue and 1 mM PMSF and loaded onto SDS/10% (w/v) polyacrylamide gels. After electrophoresis, proteins were transferred to Immobilon PVDF membranes (Millipore Corporation, Bedford, MA). Non-specific sites were blocked by incubation in blocking buffer (Tris-buffered saline containing 0.15% (v/v) Tween-20 and 5% (w/v) non-fat dry milk). The CD39 protein was detected using a commercially available anti-CD39 antibody (1:1000 (v/v) in blocking buffer) followed by a horseradish peroxidase-coupled anti-mouse antibody. Signals were revealed using the ECL™ Western blotting detection reagent (Amersham, Piscat-away, NJ).
Confocal microscopy
To determine the location of HIV-1 or CD39 and colocalization of HIV-1 and CD39, 293T cells grown on coverslips and cotransfected with pNL4-3 and a vector coding for wild-type (i.e. pCDNA3-CD39) or truncated CD39 (i.e. pCD39Δ1-37) were incubated with biotin-tagged anti-CD39 antibodies and FITC-conjugated cholera toxin B (CTB) for 30 min on ice followed by incubation with goat anti-CTB to induce raft patching and streptavidin conjugated to Alexa 546 (Molecular Probes, Eugene, OR) for 15 min at 37 °C. For detection of HIV-1, cells were permeabilized for 5 min at 37 °C with 0.025% (w/v) saponin in PBS. The cells were next incubated with pooled human sera from HIV-1-positive patients for 45 min at 37 °C to stain virus proteins, followed by incubation with goat anti-human immunoglobulin secondary antibody conjugated to Alexa 488. After several washes, slides were mounted in SlowFade medium (Molecular Probes, Eugene, OR). Stained cells were visualized by confocal laser scanning microscopy (Fluoview FV300; Olympus, Melville, NY). Digital images were processed with ImageJ (version1.36b). All the images were taken under similar experimental conditions (i.e. exposure time, magnification and intensification).
Acknowledgments
This study was made possible by an operating grant to M.J.T. from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program (grant HOP-14438). This study was performed, in part, by G.M. in partial fulfillment of a PhD degree from the Microbiology-Immunology Program, Faculty of Medicine, Laval University. G. M. holds a CIHR Doctoral Award and J.S. is the recipient of a CIHR New Investigator Award. M.J.T. holds the Tier 1 Canada Research Chair in Human ImmunoRetrovirology.
Abbreviations used
- NTPDase
nucleoside triphosphate diphosphohydrolase
- PBMCs
peripheral blood mononuclear cells
- CAM
cell adhesion molecules
- NK
natural killer
References
- 1.Tremblay MJ, Fortin JF, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1998;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 2.Ott DE. Cellular proteins in HIV virions. Rev Med Virol. 1997;7:167–180. doi: 10.1002/(sici)1099-1654(199709)7:3<167::aid-rmv199>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Ott DE. Potential roles of cellular proteins in HIV-1. Rev Med Virol. 2002;12:359–374. doi: 10.1002/rmv.367. [DOI] [PubMed] [Google Scholar]
- 4.Orentas RJ, Hildreth JE. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 5.Rossio JL, Bess J, Jr, Henderson LE, Cresswell P, Arthur LO. HLA class II on HIV particles is functional in superantigen presentation to human T cells: implications for HIV pathogenesis. AIDS Res Hum Retroviruses. 1995;11:1433–1439. doi: 10.1089/aid.1995.11.1433. [DOI] [PubMed] [Google Scholar]
- 6.Cantin R, Fortin JF, Lamontagne G, Tremblay M. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90:1091–1100. [PubMed] [Google Scholar]
- 7.Cantin R, Martin G, Tremblay MJ. A novel virus capture assay reveals a differential acquisition of host HLA-DR by clinical isolates of human immunodeficiency virus type 1 expanded in primary human cells depending on the nature of producing cells and the donor source. J Gen Virol. 2001;82:2979–2987. doi: 10.1099/0022-1317-82-12-2979. [DOI] [PubMed] [Google Scholar]
- 8.Fortin JF, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin JF, Cantin R, Tremblay MJ. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J Virol. 1998;72:2105–2112. doi: 10.1128/jvi.72.3.2105-2112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montefiori DC, Cornell RJ, Zhou JY, Zhou JT, Hirsch VM, Johnson PR. Complement control proteins, CD46, CD55, and CD59, as common surface constituents of human and simian immunodeficiency viruses and possible targets for vaccine protection. Virology. 1994;205:82–92. doi: 10.1006/viro.1994.1622. [DOI] [PubMed] [Google Scholar]
- 11.Bounou S, Dumais N, Tremblay MJ. Attachment of human immunodeficiency virus-1 (HIV-1) particles bearing host-encoded B7-2 proteins leads to nuclear factor-kappa B- and nuclear factor of activated T cells-dependent activation of HIV-1 long terminal repeat transcription. J Biol Chem. 2001;276:6359–6369. doi: 10.1074/jbc.M002198200. [DOI] [PubMed] [Google Scholar]
- 12.Giguere JF, Paquette JS, Bounou S, Cantin R, Tremblay MJ. New insights into the functionality of a virion-anchored host cell membrane protein: CD28 versus HIV type 1. J Immunol. 2002;169:2762–2771. doi: 10.4049/jimmunol.169.5.2762. [DOI] [PubMed] [Google Scholar]
- 13.Ott DE, Coren LV, Johnson DG, Kane BP, Sowder RC, 2nd, Kim YD, et al. Actin-binding cellular proteins inside human immunodeficiency virus type 1. Virology. 2000;266:42–51. doi: 10.1006/viro.1999.0075. [DOI] [PubMed] [Google Scholar]
- 14.Franke EK, Yuan HE, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 15.Sokolskaja E, Sayah DM, Luban J. Target cell cyclophilin A modulates human immunodeficiency virus type 1 infectivity. J Virol. 2004;78:12800–12808. doi: 10.1128/JVI.78.23.12800-12808.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott DE, Coren LV, Copeland TD, Kane BP, Johnson DG, Sowder RC, 2nd, et al. Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type 1 and simian immunodeficiency virus and to the p12Gag protein of Moloney murine leukemia virus. J Virol. 1998;72:2962–2968. doi: 10.1128/jvi.72.4.2962-2968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansharamani M, Graham DR, Monie D, Lee KK, Hildreth JE, Siliciano RF, Wilson KL. Barrier-to-autointegration factor BAF binds p55 Gag and matrix and is a host component of human immunodeficiency virus type 1 virions. J Virol. 2003;77:13084–13092. doi: 10.1128/JVI.77.24.13084-13092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cartier C, Deckert M, Grangeasse C, Trauger R, Jensen F, Bernard A, et al. Association of ERK2 mitogen-activated protein kinase with human immunodeficiency virus particles. J Virol. 1997;71:4832–4837. doi: 10.1128/jvi.71.6.4832-4837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemonnot B, Cartier C, Gay B, Rebuffat S, Bardy M, Devaux C, et al. The host cell MAP kinase ERK-2 regulates viral assembly and release by phosphorylating the p6gag protein of HIV-1. J Biol Chem. 2004;279:32426–32434. doi: 10.1074/jbc.M313137200. [DOI] [PubMed] [Google Scholar]
- 20.Cartier C, Hemonnot B, Gay B, Bardy M, Sanchiz C, Devaux C, Briant L. Active cAMP-dependent protein kinase incorporated within highly purified HIV-1 particles is required for viral infectivity and interacts with viral capsid protein. J Biol Chem. 2003;278:35211–35219. doi: 10.1074/jbc.M301257200. [DOI] [PubMed] [Google Scholar]
- 21.Bounou S, Leclerc JE, Tremblay MJ. The presence of host ICAM-1 in laboratory and clinical strains of HIV-1 increases virus infectivity and CD4(+)-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J Virol. 2002;76:1004–1014. doi: 10.1128/JVI.76.3.1004-1014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giguere JF, Bounou S, Paquette JS, Madrenas J, Tremblay MJ. Insertion of host-derived costimulatory molecules CD80 (B7.1) and CD86 (B7.2) into human immunodeficiency virus type 1 affects the virus life cycle. J Virol. 2004;78:6222–6232. doi: 10.1128/JVI.78.12.6222-6232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaudoin AR, Sévigny J, Picher M. ATP diphosphohydrolases, apyrases and nucleotide phosphohydrolases: biochemical properties and functions. In: Lee AG, editor. ATPases. Vol. 5. JAI Press; Greenwich, CT: 1996. pp. 369–401. [Google Scholar]
- 24.Plesner L. Ecto-ATPases: identities and functions. Int Rev Cytol. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- 25.Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 26.Wang TF, Guidotti G. CD39 is an ecto-(Ca2+, Mg2+)-apyrase. J Biol Chem. 1996;271:9898–9901. [PubMed] [Google Scholar]
- 27.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, et al. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeBel D, Poirier GG, Phaneuf S, St-Jean P, Laliberte JF, Beaudoin AR. Characterization and purification of a calcium-sensitive ATP diphosphohydrolase from pig pancreas. J Biol Chem. 1980;255:1227–1233. [PubMed] [Google Scholar]
- 29.Maliszewski CR, Delespesse GJ, Schoenborn MA, Armitage RJ, Fanslow WC, Nakajima T, et al. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J Immunol. 1994;153:3574–3583. [PubMed] [Google Scholar]
- 30.Wang TF, Rosenberg PA, Guidotti G. Characterization of brain ecto-apyrase: evidence for only one ecto-apyrase (CD39) gene. Brain Res Mol Brain Res. 1997;47:295–302. doi: 10.1016/s0169-328x(97)00066-1. [DOI] [PubMed] [Google Scholar]
- 31.Zhong X, Malhotra R, Woodruff R, Guidotti G. Mammalian plasma membrane ecto-nucleoside triphosphate diphosphohydrolase 1, CD39, is not active intracellularly. The N-glycosylation state of CD39 correlates with surface activity and localization. J Biol Chem. 2001;276:41518–41525. doi: 10.1074/jbc.M104415200. [DOI] [PubMed] [Google Scholar]
- 32.Wang TF, Ou Y, Guidotti G. The transmembrane domains of ectoapyrase (CD39) affect its enzymatic activity and quaternary structure. J Biol Chem. 1998;273:24814–24821. doi: 10.1074/jbc.273.38.24814. [DOI] [PubMed] [Google Scholar]
- 33.Kittel A, Kaczmarek E, Sevigny J, Lengyel K, Csizmadia E, Robson SC. CD39 as a caveolar-associated ectonucleotidase. Biochem Biophys Res Commun. 1999;262:596–599. doi: 10.1006/bbrc.1999.1254. [DOI] [PubMed] [Google Scholar]
- 34.Koziak K, Kaczmarek E, Kittel A, Sevigny J, Blusztajn JK, Schulte Am Esch J, 2nd, et al. Palmitoylation targets CD39/endothelial ATP diphosphohydrolase to caveolae. J Biol Chem. 2000;275:2057–2062. doi: 10.1074/jbc.275.3.2057. [DOI] [PubMed] [Google Scholar]
- 35.Koziak K, Sevigny J, Robson SC, Siegel JB, Kaczmarek E. Analysis of CD39/ATP diphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb Haemost. 1999;82:1538–1544. [PubMed] [Google Scholar]
- 36.Sévigny J, Picher M, Grondin G, Beaudoin AR. Purification and immunohistochemical localization of the ATP diphosphohydrolase in bovine lungs. Am J Physiol. 1997;272:L939–L950. doi: 10.1152/ajplung.1997.272.5.L939. [DOI] [PubMed] [Google Scholar]
- 37.Wang TF, Guidotti G. Widespread expression of ecto-apyrase (CD39) in the central nervous system. Brain Res. 1998;790:318–322. doi: 10.1016/s0006-8993(97)01562-x. [DOI] [PubMed] [Google Scholar]
- 38.Beaudoin AR, Sevigny J, Grondin G, Daoud S, Levesque FP. Purification, characterization, and localization of two ATP diphosphohydrolase isoforms in bovine heart. Am J Physiol. 1997;273:H673–H681. doi: 10.1152/ajpheart.1997.273.2.H673. [DOI] [PubMed] [Google Scholar]
- 39.Duensing S, Kirshner H, Atzpodien J. CD39 as a novel marker of in vivo immune activation. Blood. 1994;83:3826–3827. [PubMed] [Google Scholar]
- 40.Gouttefangeas C, Mansur I, Schmid M, Dastot H, Gelin C, Mahouy G, et al. The CD39 molecule defines distinct cytotoxic subsets within alloactivated human CD8-positive cells. Eur J Immunol. 1992;22:2681–2685. doi: 10.1002/eji.1830221031. [DOI] [PubMed] [Google Scholar]
- 41.Clifford EE, Martin KA, Dalal P, Thomas R, Dubyak GR. Stage-specific expression of P2Y receptors, ecto-apyrase, and ecto-5′-nucleotidase in myeloid leukocytes. Am J Physiol. 1997;273:C973–C987. doi: 10.1152/ajpcell.1997.273.3.C973. [DOI] [PubMed] [Google Scholar]
- 42.Kansas GS, Wood GS, Tedder TF. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J Immunol. 1991;146:2235–2244. [PubMed] [Google Scholar]
- 43.Leal DB, Streher CA, Neu TN, Bittencourt FP, Leal CA, da Silva JE, et al. Characterization of NTPDase (NTPDase1; ecto-apyrase; ecto-diphosphohydrolase; CD39; EC 3.6.1.5) activity in human lymphocytes. Biochim Biophys Acta. 2005;1721:9–15. doi: 10.1016/j.bbagen.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Kansas GS, Tedder TF. Transmembrane signals generated through MHC class II, CD19, CD20, CD39, and CD40 antigens induce LFA-1-dependent and independent adhesion in human B cells through a tyrosine kinase-dependent pathway. J Immunol. 1991;147:4094–4102. [PubMed] [Google Scholar]
- 45.Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nature Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 46.Sévigny J, Levesque FP, Grondin G, Beaudoin AR. Purification of the blood vessel ATP diphosphohydrolase, identification and localisation by immunological techniques. Biochim Biophys Acta. 1997;1334:73–88. doi: 10.1016/s0304-4165(96)00079-7. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gayle RB, 3rd, Maliszewski CR, Gimpel SD, Schoenborn MA, Caspary RG, Richards C, et al. Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J Clin Invest. 1998;101:1851–1859. doi: 10.1172/JCI1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcus AJ, Broekman MJ, Drosopoulos JH, Pinsky DJ, Islam N, Maliszewsk CR. Inhibition of platelet recruitment by endothelial cell CD39/ecto-ADPase: significance for occlusive vascular diseases. Ital Heart J. 2001;2:824–830. [PubMed] [Google Scholar]
- 50.Gangadharan SP, Imai M, Rhynhart KK, Sévigny J, Robson SC, Conte MS. Targeting platelet aggregation: CD39 gene transfer augments nucleoside triphosphate diphosphohydrolase activity in injured rabbit arteries. Surgery. 2001;130:296–303. doi: 10.1067/msy.2001.116032. [DOI] [PubMed] [Google Scholar]
- 51.Guckelberger O, Sun XF, Sévigny J, Imai M, Kaczmarek E, Enjyoji K, et al. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost. 2004;91:576–586. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- 52.Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113:1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imai M, Takigami K, Guckelberger O, Kaczmarek E, Csizmadia E, Bach FH, Robson SC. Recombinant adenoviral mediated CD39 gene transfer prolongs cardiac xenograft survival. Transplantation. 2000;70:864–870. doi: 10.1097/00007890-200009270-00003. [DOI] [PubMed] [Google Scholar]
- 54.Pinsky DJ, Broekman MJ, Peschon JJ, Stocking KL, Fujita T, Ramasamy R, et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J Clin Invest. 2002;109:1031–1040. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizumoto N, Kumamoto T, Robson SC, Sévigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nature Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 56.Leal DB, Streher CA, de Bertoncheli CM, Carli LF, Leal CA, da Silva JE, et al. HIV infection is associated with increased NTPDase activity that correlates with CD39-positive lymphocytes. Biochim Biophys Acta. 2005;1746:129–134. doi: 10.1016/j.bbamcr.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen DH, Hildreth JE. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell SM, Crowe SM, Mak J. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J Clin Virol. 2001;22:217–227. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- 59.Lawn SD, Roberts BD, Griffin GE, Folks TM, Butera ST. Cellular compartments of human immunodeficiency virus type 1 replication in vivo: determination by presence of virion-associated host proteins and impact of opportunistic infection. J Virol. 2000;74:139–145. [PMC free article] [PubMed] [Google Scholar]
- 60.Lawn SD, Butera ST. Incorporation of HLA-DR into the envelope of human immunodeficiency virus type 1 in vivo: correlation with stage of disease and presence of opportunistic infection. J Virol. 2000;74:10256–10259. doi: 10.1128/jvi.74.21.10256-10259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stout JG, Kirley TL. Control of cell membrane ecto-ATPase by oligomerization state: intermolecular cross-linking modulates ATPase activity. Biochemistry. 1996;35:8289–8298. doi: 10.1021/bi960563g. [DOI] [PubMed] [Google Scholar]
- 62.Saifuddin M, Hedayati T, Atkinson JP, Holguin MH, Parker CJ, Spear GT. Human immunodeficiency virus type 1 incorporates both glycosyl phosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J Gen Virol. 1997;78:1907–1911. doi: 10.1099/0022-1317-78-8-1907. [DOI] [PubMed] [Google Scholar]
- 63.Saifuddin M, Parker CJ, Peeples ME, Gorny MK, Zolla-Pazner S, Ghassemi M, et al. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Expt Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barankiewicz J, Cohen A. Extracellular ATP metabolism in B and T lymphocytes. Ann NY Acad Sci. 1990;603:380–393. doi: 10.1111/j.1749-6632.1990.tb37687.x. [DOI] [PubMed] [Google Scholar]
- 65.Stout JG, Brittsan A, Kirley TL. Brain ECTO-Mg-ATPase is not the neural cell adhesion molecule. Biochem Mol Biol Int. 1994;33:1091–1098. [PubMed] [Google Scholar]
- 66.Kannan S. Regulation of E-kinase mediated cell-adhesion molecule(s) function by E-NTPase(s): imminent regulatory inter-dependency for cell adhesion. Med Hypotheses. 2003;61:574–576. doi: 10.1016/s0306-9877(03)00233-0. [DOI] [PubMed] [Google Scholar]
- 67.Filippini A, Taffs RE, Agui T, Sitkovsky MV. Ecto-ATPase activity in cytolytic T-lymphocytes. Protection from the cytolytic effects of extracellular ATP. J Biol Chem. 1990;265:334–340. [PubMed] [Google Scholar]
- 68.Dombrowski KE, Ke Y, Thompson LF, Kapp JA. Antigen recognition by CTL is dependent upon ectoATPase activity. J Immunol. 1995;154:6227–6237. [PubMed] [Google Scholar]
- 69.Dombrowski KE, Ke Y, Brewer KA, Kapp JA. Ecto-ATPase: an activation marker necessary for effector cell function. Immunol Rev. 1998;161:111–118. doi: 10.1111/j.1600-065x.1998.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 70.Dombrowski KE, Cone JC, Bjorndahl JM, Phillips CA. Irreversible inhibition of human natural killer cell natural cytotoxicity by modification of the extracellular membrane by the adenine nucleotide analog 5′-p-(fluorosulfonyl)benzoyl adenosine. Cell Immunol. 1995;160:199–204. doi: 10.1016/0008-8749(95)80028-h. [DOI] [PubMed] [Google Scholar]
- 71.Bajpai A, Brahmi Z. Regulation of resting and IL-2-activated human cytotoxic lymphocytes by exogenous nucleotides: role of IL-2 and ecto-ATPases. Cell Immunol. 1993;148:130–143. doi: 10.1006/cimm.1993.1096. [DOI] [PubMed] [Google Scholar]
- 72.Apasov SG, Smith PT, Jelonek MT, Margulies DH, Sitkovsky MV. Phosphorylation of extracellular domains of T-lymphocyte surface proteins. Constitutive serine and threonine phosphorylation of the T cell antigen receptor ectodomains. J Biol Chem. 1996;271:25677–25683. doi: 10.1074/jbc.271.41.25677. [DOI] [PubMed] [Google Scholar]
- 73.Redegeld FA, Caldwell CC, Sitkovsky MV. Ecto-protein kinases: ecto-domain phosphorylation as a novel target for pharmacological manipulation? Trends Pharmacol Sci. 1999;20:453–459. doi: 10.1016/s0165-6147(99)01399-1. [DOI] [PubMed] [Google Scholar]
- 74.Karpatkin S, Nardi M, Green D. Platelet and coagulation defects associated with HIV-1-infection. Thromb Haemost. 2002;88:389–401. [PubMed] [Google Scholar]
- 75.Saber AA, Aboolian A, LaRaja RD, Baron H, Hanna K. HIV/AIDS and the risk of deep vein thrombosis: a study of 45 patients with lower extremity involvement. Am Surg. 2001;67:645–647. [PubMed] [Google Scholar]
- 76.Scaradavou A. HIV-related thrombocytopenia. Blood Rev. 2002;16:73–76. doi: 10.1054/blre.2001.0188. [DOI] [PubMed] [Google Scholar]
- 77.Glushakova S, Baibakov B, Margolis LB, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nature Med. 1995;1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- 78.Dornadula G, Zhang H, Shetty S, Pomerantz RJ. HIV-1 virions produced from replicating peripheral blood lymphocytes are more infectious than those from nonproliferating macrophages due to higher levels of intravirion reverse transcripts: implications for pathogenesis and transmission. Virology. 1999;253:10–16. doi: 10.1006/viro.1998.9465. [DOI] [PubMed] [Google Scholar]
- 79.Dettenhofer M, Yu XF. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol. 1999;73:1460–1467. doi: 10.1128/jvi.73.2.1460-1467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin G, Tremblay MJ. HLA-DR, ICAM-1, CD40, CD40L, and CD86 are incorporated to a similar degree into clinical human immunodeficiency virus type 1 variants expanded in natural reservoirs such as peripheral blood mononuclear cells and human lymphoid tissue cultured ex vivo. Clin Immunol. 2004;111:275–285. doi: 10.1016/j.clim.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 82.Gendron FP, Benrezzak O, Krugh BW, Kong Q, Weisman GA, Beaudoin AR. Purine signaling and potential new therapeutic approach: possible outcomes of NTPDase inhibition. Curr Drug Targets. 2002;3:229–245. doi: 10.2174/1389450023347713. [DOI] [PubMed] [Google Scholar]
- 83.Fratantoni JC, Poindexter BJ. A new technical approach to platelet aggregation studies. 1990. Measuring platelet aggregation with microplate reader. [DOI] [PubMed] [Google Scholar]