Abstract
The concentrations of extracellularly released nucleotides are controlled by metabolism via ecto-nucleotidases, but the precise physiological roles of the ecto-nucleoside triphosphate diphosphohydrolases in the modulation of purinergic receptor signalling are still unclear. Bacterial endotoxin lipopolysaccharide (LPS) treatment (administered intraperitoneally, 2 mg/kg body weight) of rats resulted in no significant changes in the overall ecto-nucleotidase activities of the hippocampus, however, LPS treatment did cause transient changes in the morphology of endothelial cells and pericytes and in the localization pattern of ecto-ATPase activity in rat hippocampus. The transient decrease in NTPDase1 (ecto-nucleoside triphosphate diphosphohydrolase1) activity, located on the luminal side of the endothelial cells, was balanced by increases in ecto-nucleotidase activities in pericytes and at other sites, consistent with an unchanged overall ecto-ATPase activity of the hippocampus.
Since the transient loss of NTPDase1 activity was not accompanied by a loss of NTPDase1 protein, we hypothesize that LPS caused transient alterations in the lipid membranes, since NTPDase1 activity is known to be sensitive to changes in membrane structure via its transmembrane domains. After 2–3 days, the LPS-induced changes in cell morphology and ecto-nucleotidase localization disappeared. We conclude that a low dose of LPS causes transient changes in the localization pattern of ecto-nucleotidases in endothelial cells and pericytes, which, coupled with the observed cellular morphological changes, may indicate modified cellular signalling in the hippocampus.
Keywords: Nucleoside triphosphate diphosphohydrolases (NTPDases), ATP, Purinergic signalling, Lipopolysaccharide (LPS), Hippocampus
1. Introduction
The concentration of extracellular ATP is controlled by several different ecto-nucleotidases working in concert, including the ecto-nucleotide pyrophosphatases/phosphodiesterases (E-NPPs), the ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases), and the ecto-5′-nucleotidase/ CD73 (Atkinson et al., 2006; Baraldi et al., 2004; Bianco et al., 2005; Bigonnesse et al., 2004; Derks and Beaman, 2004; Kittel et al., 2005; Kukulski et al., 2005; Meme et al., 2004; Robson et al., 2005, 2006; Sträter, 2006). Approximately 10 years ago it was demonstrated that the lymphocyte cell marker CD39 (Maliszewski et al., 1994) (now named NTPDase1) is the major ecto-nucleotidase present in the vasculature. This enzyme, found in endothelial (Enjyoji et al., 1999; Kaczmarek et al., 1996; Marcus et al., 1997) and smooth muscle cells (Braun et al., 2000; Kittel et al., 2002; Kordas et al., 2004), is also expressed by microglia (Braun et al., 2000) which release massive amounts of ATP during inflammation or upon stimulation with the bacterial endotoxin lipopolysaccharide (LPS) (Ferrari et al., 1997; Sperlagh et al., 1998). NTPDase2, an ecto-nucleotidase which favors hydrolysis of nucleoside triphosphates over diphosphates (an ecto-ATPase), is the dominant ecto-nucleotidase on neural precursor cells of the dentate gyrus (Wink et al., 2006), non-myelinated Schwann cells, and peripheral glia cells (Braun et al., 2003, 2004). A third cell-surface NTPDase, NTPDase3, appears to be exclusively expressed by neurons in very limited regions of the brain (Belcher et al., 2006). Expression of NTPDase1 on the luminal side and NTPDase2 on the basolateral side of endothelial cells (Kittel et al., 2004b), along with the presence of NTPDase2 in the neighbouring astrocytes and nearby glial cells, and NTPDase3 expression on neuronal cells, may suggest a concerted, functionally linked role for these enzymes in the regulation of extracellular nucleotide concentrations in this part of the brain (Belcher et al., 2006; Kittel et al., 2004b).
Extracellular ATP is an cellular signalling molecule, mediating a variety of biological responses in the nervous system by binding different P2 nucleotide receptors (Ralevic and Burnstock, 1998). Various traumas, viral infections and inflammatory processes, including LPS-triggered inflammation, increase the amount of extracellular ATP released by several types of cells (Derks and Beaman, 2004; Ferrari et al., 1997; Kaczmarek et al., 1996; Maliszewski et al., 1994). Importantly, activation of P2X7 receptors initiates an inflammatory response, via induction of the secretion of IL-1 beta from the endothelium, as well as from macrophages, astrocytes and microglial cells (Derks and Beaman, 2004; Ferrari et al., 1997).
The duration of action of extracellular ATP is controlled by its metabolism, which is catalyzed by ecto-nucleotidases, including the NTPDases. It is known that the nucleotidase activity of the cell-surface, integral membrane protein NTPDases can be modified by structural changes in the cell membrane (Grinthal and Guidotti, 2007; Pizzirani et al., 2007; Wang et al., 1998; Wu et al., 2005), which are known to occur during inflammation (Kittel et al., 1999b, 2005). Although high ecto-ATPase activity and the presence of three members of the NTPDase family of ecto-nucleotidases were previously shown to be expressed in the brain in separate studies (Belcher et al., 2006; Braun et al., 1998, 2004; Kittel, 1994; Kittel and Bácsy, 1994), the interplay between these enzymes, as well as their significance for central nervous system function, are only starting to be appreciated. In addition to the three cell surface NTPDases known to be expressed in brain (NTPDase1-3), there are five other NTPDase enzymes expressed in mammals. However, these five NTPDases do not appear to be of physiological importance in the regulation of P2 receptor signalling in the brain, since NTPDase4-7 are mainly associated with intracellular organelles and NTPDase8 mRNA was not detected in the brain (Atkinson et al., 2006; Bigonnesse et al., 2004). The goal of the present study was to investigate the consequences of LPS-triggered inflammation on ATP hydrolysis in the rat hippocampus, and to determine changes in the expression and localization of ecto-nucleotidases which modulate purinergic signalling in the hippocampus.
2. Experimental procedures
All animal procedures were carried out in accordance with the guidelines of the Institutional Ethical Committee for Experimental Animals. Every effort was made to minimize the number of animals used and their suffering.
2.1. Materials
Chemicals were purchased from Sigma Chemical (St. Louis, MO) unless otherwise indicated.
2.1.1. Antibodies
A rabbit polyclonal antibody against rat NTPDase1 (rN1-6L, I4) was generated by a cDNA immunization technique. The properties and specificity of that antibody have recently been reported (Fausther et al., 2007). Antisera against the C-terminal peptide sequence of the rat NTPDase2 (CLRQVRSAKSPGAL-oh) were generated in Dr. Kirley’s laboratory, and behaved in a similar fashion to antisera raised against the C-terminal peptide sequence of the human NTPDase2 reported previously (Hicks-Berger and Kirley, 2000).
2.2. Methods
2.2.1. LPS treatment
Male Wistar rats (200–230 g, bred in the local animal house) were injected intraperitoneally with LPS (2 mg/kg body weight). Five rats were used for each time point (2 h, 1 day, 2 days, 3 days, and 4 days after LPS treatment).
Rats were anaesthetized with CO2 and decapitated. The hippocampus from each animal was excised, part used for Western blot experiments, and part used for HPLC analyses.
2.2.2. Western blot analysis
Tissue blocks of rat hippocampi for Western blot analysis were kept at −80 °C until used. The tissue samples were placed in liquid nitrogen, ground to powder and homogenized in PBS. The protein concentration of each sample was measured using the Lowry method (Lowry et al., 1951) and adjusted with SDS-PAGE sample buffer to 2 mg/ml. Forty micrograms of each protein sample was separated by means of gradient SDS-PAGE (8–12% acrylamide) and transferred to nitrocellulose filters (Amersham Life Science Inc., Germany). The filters were blocked with 3% BSA (dissolved in 0.1% Tween-PBS), and probed overnight with either polyclonal NTPDase1 antibody (rN1-6L, I4) at a dilution of 1:1000, or polyclonal NTPDase2 antibody at a dilution of 1:2000. Other details of immunoblotting and the ECL detection method were performed as indicated by the manufacturer (Amersham Life Science Inc., Germany).
2.2.3. High-performance liquid chromatography (HPLC) analysis of ATP metabolism
Freshly removed hippocampi were immediately placed into ice-cold Krebs solution oxygenated with 95% O2 and 5% CO2. Four hundred micrometer thick slices were cut transversely with a McIlwain tissue chopper and incubated in 3 ml of Krebs solution at 37 °C, saturated with 95% O2 + 5% CO2. Subsequently, 20 μM ATP was added to the bath and aliquots of 70 μl were taken out 2.5, 5, 10, 15, 20, 25, 30 and 60 min. The concentrations of ATP, ADP, AMP, adenosine, and inosine in the aliquots were measured by high-performance liquid chromatography combined with ultraviolet detection (HPLC-UV) according to the method described earlier (Kittel et al., 2005). The identification and quantification of different purines was based on the retention times and peak areas of known amounts of standards, and was carried out by the Agilent ChemStation program. A linear correlation between the peak area and the injected amount was observed for all the nucleotides. The actual concentrations of ATP, ADP, AMP adenosine, and inosine were expressed in μM units.
2.2.4. Preparation of tissue sections for immuno- and enzyme-histochemical staining
Animals were anaesthetized with ketamine (60 mg/kg i.p.) and transcardially perfused briefly with a 0.9% NaCl solution. Perfusion fixation was performed with cold Zamboni fixative (4% paraformaldehyde, 0.5% glutaraldehyde (Taab Equipment Ltd., Aldermaston, Berkshire, England), containing 15% saturated picric acid in 0.1 M phosphate buffer, pH 7.4). The brain was removed and post-fixed for 1 h in 4% paraformaldehyde. Coronal 40 μm sections of the desired area containing hippocampus were cut using a vibrating microtome (VT1000S; Leica Microsystems, Milton Keynes, UK). Tissue sections were separated into two groups. Sections for immunohistochemistry were washed and kept in 0.1 M PBS, and other sections for enzyme histochemistry were washed in PBS and subsequently thoroughly rinsed several times in 0.07 M Tris–maleate buffer (pH 7.4) to remove all traces of phosphate.
2.2.5. Immunohistochemical staining for NTPDase1
After the fixative was washed out, the rat brain coronal sections were incubated in blocking solution (PBS containing 5% normal goat serum and 1 mg/ml bovine serum albumin) for 1 h at 22 °C. Incubation in the solution of the polyclonal NTPDase1 antibody (rN1-6L, I4 at 1:500 dilution), was performed overnight at 4 °C. Following three, 10 min washes in PBS, the sections were incubated with biotinylated secondary antibody for 2 h. The staining was performed with Vectastain ABC Elite kit (Vector Laboratories; Burlingame, CA) using 3,3-diaminobenzidine (DAB) as the chromogen, according to the manufacturer’s instructions. After washing thoroughly with distilled water, sections were postfixed in 1% OsO4, dehydrated in ethanol, stained with 2% uranyl acetate in 70% ethanol for 1 h, and embedded in Taab 812 (Taab Equipment Ltd.). Negative control experiments were performed using the same protocol but substituting pre-immune serum for the primary antibody. Ultrathin sections were cut using a Leica UCT ultramicrotome (Leica Microsystems, Milton Keynes, UK) and examined using a Hitachi 7100 transmission electron microscope (Hitachi; Tokyo, Japan).
2.2.6. Enzyme histochemistry for detection of ecto-ATPase activity
A cerium precipitation method was used for electron microscopic investigation of ecto-ATPase activity (Kittel et al., 1999a). Briefly, thoroughly washed sections were incubated in a medium containing 1 mM ATP as substrate, 3 mM CeCl3 (precipitating agent for the liberated phosphate), 1 mM levamisole (inhibitor of alkaline phosphatases; Amersham, Poole, UK), 1 mM ouabain (Na+, K+-ATPase inhibitor; Merck, Darmstadt, Germany), 50 μM αβ-methylene ADP (5′-nucleotidase inhibitor) and 5 mM KCl in 70 mM Tris–maleate buffer (pH 7.4) for 30 min at room temperature. Incubation was followed by three rinses in Tris–maleate buffer and washing with distilled water. The tissue blocks were then postfixed, dehydrated and treated and embedded into Taab 812 resin for ultrathin sectioning and microscopic examination as described above. Control reactions were performed without adding the ATP substrate.
3. Results
3.1. HPLC analysis of ATP metabolism
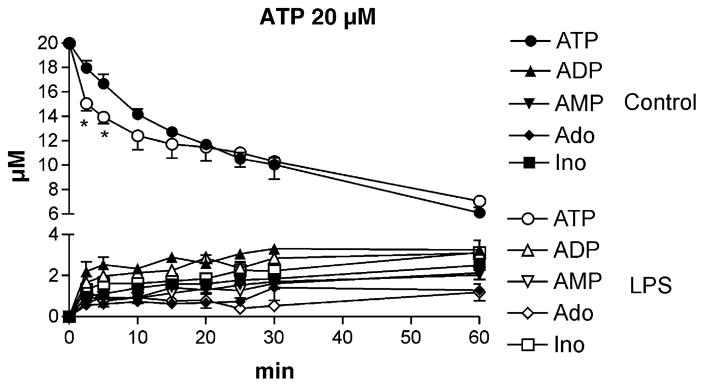
The extracellular degradation of ATP was examined in control and LPS treated animals following the addition of 20 μM ATP to hippocampal tissue slices. As shown in Fig. 1, ATP is hydrolyzed by control brain slices to ADP, AMP, adenosine, and inosine, consistent with previous results (Cunha et al., 1998) and with the presence of ecto-NTPDase/ecto-NPPase, ecto-5′nucleotidase, and adenosine deaminase enzymes, respectively. ATP metabolism was investigated in hippocampal slices 2 h, and 1, 2, and 3 days following LPS treatment. Only the sample analyzed 1 day after LPS treatment showed significant differences in nucleotide metabolism from the control (Fig. 1). After 1 day of LPS treatment the degradation of ATP was more rapid in the first 20 min after addition. However, there were no statistically significant differences between the rates of formation of ADP, AMP, adenosine and inosine in the hippocampi of control and LPS treated animals (Fig. 1).
Fig. 1.
Extracellular metabolism of ATP in rat hippocampal slices under control conditions and 1 day after LPS treatment. Hippocampal slices were incubated in 3 ml Krebs solution in the presence of 20 μM ATP. Seventy microliters of aliquots were taken 2.5, 5, 10, 15, 20, 25, 30 and 60 min after the addition of ATP. Nucleotides (ATP, ADP, and AMP) and nucleosides (Ado, adenosine; Ino, inosine) in the aliquots were determined by the HPLC-UV method and expressed in μM units, as a function of time. Filled symbols represent results from control slices (control, n = 4), whereas open symbols represent data from LPS treated animals (LPS, n = 5).
3.2. Results of enzyme and immunohistochemical staining for detection of hippocampal ecto-ATPase activities and Western blot analysis
The main advantage of this method is that it is able to demonstrate changes in cellular and sub-cellular localization patterns of the activity, which measurements of homogenized tissues and protein extracts are not able to resolve. Inhibitors were used in the incubation medium to inhibit other nucleotidases (e.g., levamisole to inhibit alkaline phosphatase and ouabain to inhibit (Na, K)-ATPase), in order to obtain the most reliable results. In addition, according to our previous experiments, Ca-pump activity is sensitive to aldehyde fixation, so no inhibitor of that ATPase was needed. Also, from previous experiments it was observed that results using ATP and ADP as substrates to localize ecto-ATPase activities in the CNS were essentially the same, which is why only ATP, and not also ADP, was used as substrate in the current study.
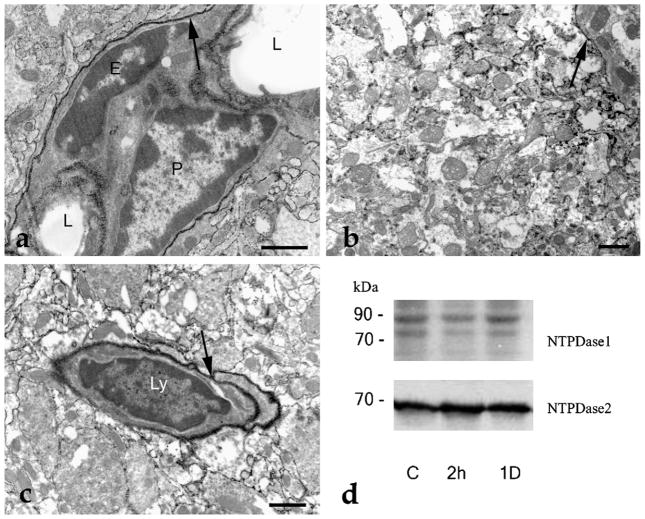
A cursory examination of the histochemical results suggested that the localization pattern of ATPase activity did not change significantly following LPS administration, either after 2 h or longer time periods (1–2 days) in neuronal and glial cells (Fig. 2a–c). The most intense staining was observed on the cell membranes of endothelial cells, pericytes (Fig. 2a) and circulating lymphocytes (Fig. 2c). In addition, Western blot analysis did not demonstrate changes in the overall expression of NTPDase1 or NTPDase2 (Fig. 2d). However, in agreement with the literature, we did observe increased expression of IL-1 beta 2 h after LPS administration (Fernandez-Martinez et al., 2004), and a subsequent decrease of its expression to the control level 1 day after LPS treatment (data not shown).
Fig. 2.
Ecto-ATPase activity in rat hippocampal tissue sections and Western results for NTPDase1 and NTPDase2. (a) Ecto-ATPase activity in rat CA3 hippocampal region 2 h after LPS administration. The ATPase activity is visualized by the black cerium phosphate precipitate resulting from ATP hydrolysis. The most intense staining (arrow) is found on the cell membrane of the pericyte (P) and endothelial (E) cells, but the cell membranes of astrocytes, dendrites, and non-myelinated axons also exhibit ATPase activity. L: lumen of the capillary. (b) General hippocampal ecto-ATPase staining. Cell membranes, regardless the cell type (glia or nerve cells) show strong ecto-ATPase activity (arrow) 1 day after LPS administration. (c) Localization pattern of the ecto-ATPase activity is unchanged on the second day after LPS treatment. A lymphocyte (Ly) is shown having very high ecto-ATPase activity (arrow). (d) Western blot analysis of hippocampal tissue. Compared with the control (C) there is no apparent change in the expression of NTPDase1 and NTPDase2 either 2 h or 1 day following LPS treatment. Scale bars: 2 μm.
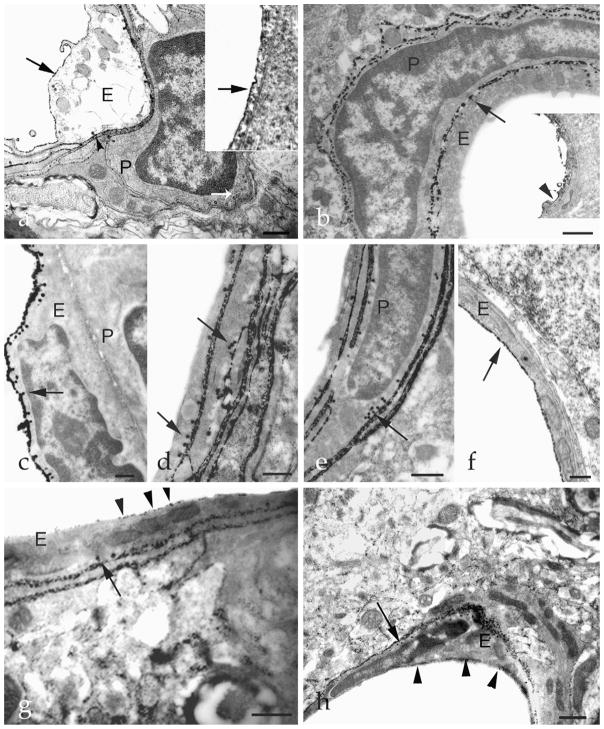
More thorough examination of the enzyme histochemical staining revealed morphological changes and alterations in the localization of ecto-ATPase activity in the endothelial cells and pericytes following LPS treatment (Fig. 3). Generally, caveolae of non-activated endothelial cells are located on the basolateral side of the cell, and previous enzyme histochemical/immunohistochemical staining demonstrated that these caveolae contain NTPDase1 protein and activity (Kittel et al., 1999a; Koziak et al., 2000). This is also observed in the present study, as is evident in Fig. 3a. Immunostaining also demonstrates the presence of NTPDase1 on the luminal side of the cell. Ecto-ATPase activity of neighbouring pericytes is visibly less intense and caveolae are not stained, indicating little ecto-ATPase activity in these locations. However, 2 h after LPS treatment, ecto-ATPase activity on the luminal side of the endothelial cell has disappeared (although the NTPDase1 enzyme is still present as demonstrated by immunostaining), while ecto-ATPase activity has remained high on the basolateral side and in the caveolae, as well as in the cell membranes of the pericytes and their caveolae (Fig. 3b). The most interesting and surprising result was observed 2 days after the treatment. At this time, ecto-ATPase activities of the endothelial cells and their caveolae were still high, but the sub-cellular localization had changed (Fig. 3c–f). Now, stronger ATPase activity was evident on the luminal side of the endothelial cells (with (c) or without (f) caveolae), and the basolateral side possessed much weaker ecto-ATPase activity. In addition, in the neighbouring cells and pericytes, no activity was observable on the luminal side, but high activity was evident on the basolateral side (d and e). All these changes disappeared on the third day following the LPS challenge, resulting in a pattern of staining similar to that observed in control sections (Fig. 3g and h).
Fig. 3.
Alterations in the localization of ecto-ATPase activity in endothelial cells and pericytes after LPS treatment. (a) Control tissue. Both luminal and basolateral membranes of the endothelial cell (E) are stained with cerium phosphate deposit demonstrating ecto-ATPase activity (arrow). Also, some caveolae (arrowhead) show ecto-ATPase activity. A neighbouring pericyte (P) shows less intense ecto-ATPase activity in its cell membrane and caveolae (white arrow). Insert: immunostaining reveals NTPDase1 expression on the luminal side of an endothelial cell (arrow). (b) Ecto-ATPase activity localization 2 h after LPS treatment. High activity is observed on the basolateral membrane of the endothelial cell (E) and on some caveolae (arrow), but the luminal membrane is not stained. A large amount of staining in the membrane and caveolae of adjacent pericyte (P) is evident. Insert: immunoreactivity for NTPDase1 (arrowhead) is on the luminal side of the endothelial cell, and is identical to the control sample. (c–f) Differential localization patterns of ecto-ATPase activity in endothelial cells and pericytes 2 days after LPS treatment. Endothelial cells (E) exhibit strong activity in their cell membranes and caveolae on their luminal side (arrow, c) or with little or no activity on their luminal side but instead in their basolateral membranes and caveolae (arrow, d and e). The ecto-ATPase activity of the adjacent cells, including pericytes (P) is high (arrow, e). An endothelial cell (E) with high ecto-ATPase activity on its apical membrane (arrow) without caveolae was also observed (f). When strong activity is present on the luminal side, the basolateral membrane generally shows weak staining (c and f). (g and h) Three days after LPS treatment the localization of ecto-ATPase activity in the endothelial cells (E) reverts to that that seen in the control tissue. Caveolae located on the basolateral side (arrows) are again heavily stained, while staining on the luminal side decreased to levels observed in control samples. Arrowheads denote punctate staining on the luminal surface of endothelial cells. E: endothelial cell and P: pericyte. Scale bars: 1 μm.
4. Discussion
Despite the fact that it has been known for decades that the concentrations of extracellularly released nucleotides are controlled by ecto-nucleotidases, the precise physiological roles of the NTPDases in the modulation of purinergic receptor signalling is still unclear. Pathological conditions, including inflammation and ischemia, increase the extracellular release of ATP. As several research groups have described, extracellular ATP present during these pathological processes stimulates P2X7 purinoceptors, which induces the secretion of IL-1 beta from macrophages, activated microglial cells, and endothelial cells (Imai et al., 2000; Moller et al., 2000; Nguyen et al., 1998; Suzuki et al., 2004). IL-1 beta then initiates an inflammatory response (Derks and Beaman, 2004). In agreement with these previous studies, we also found increased IL-1 beta protein expression 2 h after LPS treatment (data not shown). However, increased expression of NTPDase1 or 2 was not observed, although changes in the localization pattern of ecto-ATPase activity and changes in the morphology of endothelial cells were evident. We previously reported an increased number of caveolae in the capillary endothelial cells as a consequence of LPS treatment (Kittel, 1999). Our present work demonstrates that a single, low dose of LPS dramatically, but transiently, decreases the enzyme activity of NTPDase1 present on the luminal side of the endothelial cells. At the same time, other nucleotidase activities, presumably due partly to NTPDase2, increase, maintaining the overall ecto-ATPase activity. However, after 2–3 days these transient changes in cell morphology and pattern of ecto-ATPase activity are no longer evident.
Western blot experiments did not demonstrate any significant differences in the overall expression levels of NTPDase1 or 2, and HPLC analyses of nucleosides and nucleotides did not show significant changes ecto-nucleotidase activities of the control and treated hippocampi, with the lone exception of a slightly faster rate of ATP hydrolysis in LPS samples 1 day after treatment (Fig. 1). Since the extracellular decomposition of ATP measured by HPLC assay reflects all nucleotidase activities present in the hippocampal tissue block, and the majority of the tissue blocks are neuronal and glial cells, it is not surprising that overall ecto-ATPase activity does not reflect subtle changes in the expression pattern of endothelial ecto-ATPase activity.
The transient loss of NTPDase1 activity (but not protein) may be explained by transient alterations in the membrane structure due to the LPS-evoked inflammation, since NTPDase1 activity is sensitive to changes in its transmembrane domains and to changes in the properties of the membranes in which they are embedded (Grinthal and Guidotti, 2007; Wang et al., 1998), and LPS induces inflammation which leads to alterations of membrane structure (Kittel et al., 1999b, 2005).
The disappearance of luminal ecto-ATPase activity and the observed morphological changes, namely “translocation” of “message center” caveolae (Anderson, 1993; Anderson and Jacobson, 2002; Schwencke et al., 2006) from the luminal to the basolateral side of endothelial cells, raises the possibility of other LPS-induced changes. Both NTPDase1 and the P2Y1 ADP-purinoreceptor have been demonstrated to be present in caveolae of endothelial cells (Kittel et al., 2004a). The observed changes in the localization pattern of caveolae may support the assertion that LPS treatment alters signalling between cells, since the ecto-ATPase activity of the caveolae of pericytes increases following treatment. It has been suggested that NTPDase2 may be a novel marker for pericytes (Sevigny et al., 2002). Its presence in microvascular pericytes (and in the basolateral surface of endothelium, as well as the adventitia of muscularized vessels) has been demonstrated by previous studies (Kittel et al., 2004b). However, the increase in ecto-ATPase activity in pericytes in response to LPS treatment is difficult to explain since NTPDase2, unlike NTPDase1, is not palmitoylated and does not enter lipid rafts or caveolae (Atkinson et al., 2006; Wink et al., 2006). Therefore, what is the explanation for the increased ecto-ATPase activity in the caveolae of pericytes? Unfortunately, because the NTPDase2 antibody used in this study was unsuitable for immunohistochemistry (and no others are available), direct measurement of NTPDase2 protein could not be used to answer this question. The possibility that palmitoylation is not required for targeting NTPDase2 to the caveolae of pericytes seems unlikely. However, a recent study demonstrated the physiological role of alternatively spliced NTPDase2 in the regulation of extracellular nucleotide concentrations in a range of organ systems (Wang et al., 2005). Those authors found that this alternatively spliced variant possesses several intracellular phosphorylation sites for casein kinase 2 (CK2) on its unique, extended C-terminus. Since it is known that CK2 can be activated by LPS (Xagorari et al., 2002) and participates in inflammatory processes and in nociceptive signal transmission (Kweon et al., 2006; Li et al., 2005; Parhar et al., 2007; Yamada et al., 2005), upregulated phosphorylation via CK2 may play a role in the modulation of activity of alternatively spliced NTPDase2. In addition, an alternatively spliced variant of NTPDase2 has been demonstrated in the brain (Vlajkovic et al., 1999). In light of these data, it may be hypothesized that LPS could induce the expression of alternatively spliced NTPDase2, which could traffic the nucleotidase to different sub-cellular locations.
Changes in the dynamic interactions known to occur between endothelial cells and pericytes (Pardridge, 2002) can have serious pathological consequences, including lethal cardiovascular defects, tumor angiogenesis, stroke, and triggering of the CADASIL dementia syndrome (Armulik et al., 2005). Our observations, namely the changes in the localization patterns of ecto-ATPase activities in endothelial cells and the increased activity of a nucleotidase in the caveolae of pericytes after LPS treatment, may have biological relevance, since these enzymes can modify signalling between endothelial cells and pericytes, and are likely to influence neighbouring cells in the hippocampus as well.
Acknowledgments
This study was supported by grants from the Hungarian Medical Research Council (ETT 480/2003) to A. Kittel, the Hungarian Research and Development Fund (NKFP1A/002/2004) to Beáta Sperlágh, and by National Institutes of Health grants HL59915 and HL72882 to T. Kirley. J. Sévigny was the recipient of a New Investigator award from the Canadian Institutes of Health Research (CIHR). The work of Dr. Nándor Müllner (Semmelweis University, Budapest, Hungary) with Western blot experiments and the technical assistance of Ms. Judit Őszi and Ms. Éva Szénássy (Institute of Experimental Medicine, Budapest, Hungary) were greatly appreciated.
Abbreviations
- NTPDase
ecto-nucleoside triphosphate diphosphohydrolase
- LPS
lipopolysaccharide
- ATP
adenosine triphosphate
- HPLC
high-performance liquid chromatography
References
- Anderson RG. Caveolae: where incoming and outgoing messengers meet. Proc Nat Acad Sci USA. 1993;90:10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Atkinson B, Dwyer K, Enjyoji K, Robson SC. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential as therapeutic targets. Blood Cells Mol Dis. 2006;36:217–222. doi: 10.1016/j.bcmd.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Di Virgilio F, Romagnoli R. Agonists and antagonists acting at P2X7 receptor. Curr Top Med Chem. 2004;4:1707–1717. doi: 10.2174/1568026043387223. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Zsarnovszky A, Crawford PA, Hemani H, Spurling L, Kirley TL. Immunolocalization of ecto-nucleoside triphosphate diphosphohydrolase 3 in rat brain: implications for modulation of multiple homeostatic systems including feeding and sleep-wake behaviors. Neuroscience. 2006;137:1331–1346. doi: 10.1016/j.neuroscience.2005.08.086. [DOI] [PubMed] [Google Scholar]
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- Bigonnesse F, Levesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJ, Sevigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. doi: 10.1021/bi0362222. [DOI] [PubMed] [Google Scholar]
- Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci. 1998;18:4891–4900. doi: 10.1523/JNEUROSCI.18-13-04891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Robson SC, Enjyoji K, Guckelberger O, Hammer K, Di Virgilio F, Zimmermann H. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain [In Process Citation] Eur J Neurosci. 2000;12:4357–4366. [PubMed] [Google Scholar]
- Braun N, Sevigny J, Mishra SK, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Robson SC, Hammer K, Hanani M, Zimmermann H. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia. 2004;45:124–132. doi: 10.1002/glia.10309. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Sebastiao AM, Ribeiro JA. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks R, Beaman K. Regeneration and tolerance factor modulates the effect of adenosine triphosphate-induced interleukin 1 beta secretion in human macrophages. Hum Immunol. 2004;65:676–682. doi: 10.1016/j.humimm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, II, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- Fausther M, Lecka J, Kukulski F, Lévesque SA, Pelletier J, Zimmermann H, Dranoff JA, Sévigny J. Cloning, purification and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol. 2007;292:G785–G795. doi: 10.1152/ajpgi.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Martinez E, Morales-Rios MS, Perez-Alvarez V, Muriel P. Immunomodulatory effects of thalidomide analogs on LPS-induced plasma and hepatic cytokines in the rat. Biochem Pharmacol. 2004;68:1321–1329. doi: 10.1016/j.bcp.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinthal A, Guidotti G. Bilayer mechanical properties regulate the transmembrane helix mobility and enzymatic state of CD39. Biochemistry. 2007;46:279–290. doi: 10.1021/bi061052p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks-Berger CA, Kirley TL. Expression and characterization of human ecto-ATPase and chimeras with CD39 ectoapyrase. IUBMB Life. 2000;50:43–50. doi: 10.1080/15216540050176584. [DOI] [PubMed] [Google Scholar]
- Imai M, Goepfert C, Kaczmarek E, Robson SC. CD39 modulates IL-1 release from activated endothelial cells. Biochem Biophys Res Commun. 2000;270:272–278. doi: 10.1006/bbrc.2000.2410. [DOI] [PubMed] [Google Scholar]
- Kaczmarek E, Koziak K, Sévigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39 vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- Kittel A. Distribution of Ca-ATPases in the medial habenula in mouse. Scanning Microsc. 1994;8:337–342. [PubMed] [Google Scholar]
- Kittel A. Lipopolysaccharide treatment modifies pH- and cation-dependent ecto-ATPase activity of endothelial cells. J Histochem Cytochem. 1999;47:393–400. doi: 10.1177/002215549904700313. [DOI] [PubMed] [Google Scholar]
- Kittel A, Bácsy E. Presynaptic ecto- and postsynaptic endo-calcium-adenosine-triphosphatases in synaptosomes: doubts about biochemical interpretation of localization. Int J Dev Neurosci. 1994;12:207–211. doi: 10.1016/0736-5748(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Kittel A, Kaczmarek E, Sevigny J, Lengyel K, Csizmadia E, Robson SC. CD39 as a caveolar-associated ectonucleotidase. Biochem Biophys Res Commun. 1999a;262:596–599. doi: 10.1006/bbrc.1999.1254. [DOI] [PubMed] [Google Scholar]
- Kittel A, Kalmár B, Madarász E. Effects of LPS on ecto-ATPase (NTPDase) activity and phagocytosis of cultured astrocytes. In: Vanduffel L, Lemmens R, editors. Proceedings of the Second International Workshop on Ecto-ATPases and Related Ecto-nucleotidases. Shaker Publishing B.V; Maastricht, The Netherlands: 1999b. pp. 158–166. [Google Scholar]
- Kittel A, Garrido M, Varga G. Localization of NTPDase1/CD39 in normal and transformed human pancreas. J Histochem Cytochem. 2002;50:549–556. doi: 10.1177/002215540205000412. [DOI] [PubMed] [Google Scholar]
- Kittel A, Csapo ZS, Csizmadia E, Jackson SW, Robson SC. Co-localization of P2Y1 receptor and NTPDase1/CD39 within caveolae in human placenta. Eur J Histochem. 2004a;48:253–259. [PubMed] [Google Scholar]
- Kittel A, Pelletier J, Bigonnesse F, Guckelberger O, Kordas K, Braun N, Robson SC, Sevigny J. Localization of nucleoside triphosphate diphosphohydrolase-1 (NTPDase1) and NTPDase2 in Pancreas and salivary gland. J Histochem Cytochem. 2004b;52:861–871. doi: 10.1369/jhc.3A6167.2004. [DOI] [PubMed] [Google Scholar]
- Kittel A, Kiss AL, Mullner N, Matko I, Sperlagh B. Expression of NTPDase1 and caveolins in human cardiovascular disease. Histochem Cell Biol. 2005;124:51–59. doi: 10.1007/s00418-005-0018-8. [DOI] [PubMed] [Google Scholar]
- Kordas KS, Sperlagh B, Tihanyi T, Topa L, Steward MC, Varga G, Kittel A. ATP and ATPase secretion by exocrine pancreas in rat, guinea pig, and human. Pancreas. 2004;29:53–60. doi: 10.1097/00006676-200407000-00056. [DOI] [PubMed] [Google Scholar]
- Koziak K, Kaczmarek E, Kittel A, Sevigny J, Blusztajn JK, Schulte Am Esch J, II, Imai M, Guckelberger O, Goepfert C, Qawi I, Robson SC. Palmitoylation targets CD39/endothelial ATP diphosphohydrolase to caveolae. J Biol Chem. 2000;275:2057–2062. doi: 10.1074/jbc.275.3.2057. [DOI] [PubMed] [Google Scholar]
- Kukulski F, Lévesque SA, Lavoie ÉG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, TLK, Sévigny J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon SM, Wang B, Rixter D, Lim JH, Koga T, Ishinaga H, Chen LF, Jono H, Xu H, Li JD. Synergistic activation of NF-kappaB by nontypeable H. influenzae and S. pneumoniae is mediated by CK2, IKKbeta-IkappaBalpha, and p38 MAPK. Biochem Biophys Res Commun. 2006;351:368–375. doi: 10.1016/j.bbrc.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shi X, Liang DY, Clark JD. Spinal CK2 regulates nociceptive signaling in models of inflammatory pain. Pain. 2005;115:182–190. doi: 10.1016/j.pain.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maliszewski CR, Delespesse GJ, Schoenborn MA, Armitage RJ, Fan-slow WC, Nakajima T, Baker E, Sutherland GR, Poindexter K, Birks C, Alpert A, Friend D, Gimpel SD, Gayle RB. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J Immunol. 1994;153:3574–3583. [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, Gayle RB, Maliszewski CR. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meme W, Ezan P, Venance L, Glowinski J, Giaume C. ATP-induced inhibition of gap junctional communication is enhanced by interleukin-1 beta treatment in cultured astrocytes. Neuroscience. 2004;126:95–104. doi: 10.1016/j.neuroscience.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Moller T, Kann O, Verkhratsky A, Kettenmann H. Activation of mouse microglial cells affects P2 receptor signaling. Brain Res. 2000;853:49–59. doi: 10.1016/s0006-8993(99)02244-1. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev. 2002;1:131–139. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- Parhar K, Morse J, Salh B. The role of protein kinase CK2 in intestinal epithelial cell inflammatory signaling. Int J Colorectal Dis. 2007;22:601–609. doi: 10.1007/s00384-006-0193-7. [DOI] [PubMed] [Google Scholar]
- Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E, Di Virgilio F. Stimulation of P2 receptors causes release of IL-1{beta}-loaded microvesicles from human dendritic cells. Blood. 2007;19:3856–3869. doi: 10.1182/blood-2005-06-031377. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure, function, relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwencke C, Braun-Dullaeus RC, Wunderlich C, Strasser RH. Caveolae and caveolin in transmembrane signaling: implications for human disease. Cardiovasc Res. 2006;70:42–49. doi: 10.1016/j.cardiores.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Sevigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, Imai M, Zimmermann H, Robson SC. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood. 2002;99:2801–2809. doi: 10.1182/blood.v99.8.2801. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/ P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- Sträter N. Ecto-5′-nucleotidase: structure function relationships. Purinergic Signal. 2006;2:343–350. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlajkovic SM, Housley GD, Greenwood D, Thorne PR. Evidence for alternative splicing of ecto-ATPase associated with termination of purinergic transmission. Brain Res Mol Brain Res. 1999;73:85–92. doi: 10.1016/s0169-328x(99)00244-2. [DOI] [PubMed] [Google Scholar]
- Wang TF, Ou Y, Guidotti G. The transmembrane domains of ectoapyrase (CD39) affect its enzymatic activity and quaternary structure. J Biol Chem. 1998;273:24814–24821. doi: 10.1074/jbc.273.38.24814. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Vlajkovic SM, Housley GD, Braun N, Zimmermann H, Robson SC, Sevigny J, Soeller C, Thorne PR. C-terminal splicing of NTPDase2 provides distinctive catalytic properties, cellular distribution and enzyme regulation. Biochem J. 2005;385:729–736. doi: 10.1042/BJ20040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink MR, Braganhol E, Tamajusuku AS, Lenz G, Zerbini LF, Libermann TA, Sevigny J, Battastini AM, Robson SC. Nucleoside triphosphate diphosphohydrolase-2 (NTPDase2/CD39L1) is the dominant ectonucleotidase expressed by rat astrocytes. Neuroscience. 2006;138:421–432. doi: 10.1016/j.neuroscience.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Choi LE, Guidotti G. N-linked oligosaccharides affect the enzymatic activity of CD39: diverse interactions between seven N-linked glycosylation sites. Mol Biol Cell. 2005;16:1661–1672. doi: 10.1091/mbc.E04-10-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xagorari A, Roussos C, Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br J Pharmacol. 2002;136:1058–1064. doi: 10.1038/sj.bjp.0704803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Katsuma S, Adachi T, Hirasawa A, Shiojima S, Kadowaki T, Okuno Y, Koshimizu TA, Fujii S, Sekiya Y, Miyamoto Y, Tamura M, Yumura W, Nihei H, Kobayashi M, Tsujimoto G. Inhibition of protein kinase CK2 prevents the progression of glomerulonephritis. Proc Natl Acad Sci USA. 2005;102:7736–7741. doi: 10.1073/pnas.0409818102. [DOI] [PMC free article] [PubMed] [Google Scholar]