Abstract
Stimulation of receptors for either ATP or adenosine leads to physiologic changes in retinal pigment epithelial (RPE) cells that may influence their relationship with the adjacent photoreceptors. The ectoenzyme nucleoside-triphosphate diphosphohy-drolase-1 (NTPDase1) catalyzes the dual dephosphorylation of ATP and ADP to AMP. Although NTPDase1 can consequently control the balance between ATP and adenosine, it is unclear how its expression and activity are regulated. Classic negative feedback theory predicts an increase in enzyme activity in response to enhanced exposure to substrate. This study asked whether exposure to ATP increases NTPDase1 activity in RPE cells. Although levels of NTPDase1 mRNA and protein in cultured human ARPE-19 cells were generally low under control conditions, exposure to slowly hydrolyzable ATPγS led to a time-dependent increase in NTPDase1 mRNA that was accompanied by a rise in levels of the functional 78-kDa protein. Neither NTPDase2 nor NTPDase3 mRNA message was elevated by ATPγS. The ATPase activity of cells increased in parallel, indicating the up-regulation of NTPDase1 was functionally relevant. The up-regulation of NTPDase1 protein was partially blocked by P2Y1 receptor inhibitors MRS2179 (N 6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate) and MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine 3′,5′-bisphosphate] and increased by P2Y1 receptor agonist MRS2365 [(N)-methanocarba-2MeSADP]. In conclusion, prolonged exposure to extracellular ATPγS increased NTPDase1 message and protein levels and increased ecto-ATPase activity. This up-regulation reflects a feedback circuit, mediated at least in part by the P2Y1 receptor, to regulate levels of extracellular purines in subretinal space. NTPDase1 levels may thus serve as an index for increased extracellular ATP levels under certain pathologic conditions, although other mechanisms could also contribute.
Extracellular ATP and adenosine act at distinct receptors to mediate discrete actions in many tissues (Bucheimer and Linden, 2004). The two transmitters can act as a balanced pair, with diverse effects resulting from their activation of different receptors (Zhang et al., 2006b). The corresponding effects on cell physiology are influenced by the relative availability of each purine. P2 receptors for ATP are typically stimulated by ATP released from cells via physiologic mechanisms (Schwiebert, 2001; Lazarowski et al., 2003), whereas the adenosine capable of stimulating P1 receptors is frequently produced from the consecutive dephosphorylation of ATP (Dubyak and el-Moatassim, 1993). The extracellular enzymes that mediate this dephosphorylation can dramatically alter responsiveness by simultaneously removing ATP and producing adenosine. It follows that regulation of these enzymes offers great potential to alter the balance of purines and coordinate their effects.
The retinal pigment epithelium (RPE) lies adjacent to the photoreceptors in the posterior eye. Short- and long-term communication between the two cell types is necessary for optimal visual function (Strauss, 2005). Adenosine and ATP can both modulate this interaction by stimulating P1 and P2 receptors, respectively (Mitchell and Reigada, 2007). For example, activation of the P2Y2 receptor for ATP on the RPE apical membrane elevates Ca2+ and enhances fluid movement across the tissue, thus helping to keep photoreceptors in place (Peterson et al., 1997; Maminishkis et al., 2002). Stimulation of an A2 receptor for adenosine decreases the phagocytosis of photoreceptor outer segments by the RPE (Gregory et al., 1994).
Levels of purine agonists in the subretinal space between the RPE and photoreceptors are regulated by various mechanisms. The RPE itself releases ATP into the subretinal space in response to N-methyl-D-aspartic acid receptor activation, chemical ischemia, cell swelling, and other stimuli (Mitchell, 2001; Reigada and Mitchell, 2005; Reigada et al., 2006a; Mitchell and Reigada, 2007). Multiple nucleotidases capable of converting the released ATP into adenosine have been identified. Ecto-5′-nucleotidase (CD73), which converts AMP to adenosine, was recently localized to the apical membrane of RPE cells, with enzyme activity decreased following stimulation of the α1 epinephrine receptor (Reigada et al., 2006b). RPE cells are also capable of degrading extracellular ATP, with the cells expressing multiple ATPase enzymes. Message for eNPP1, eNPP2, and eNPP3, as well as NTPDase2 and NTPDase3, was clearly detected in cultured human ARPE-19 cells (Reigada et al., 2005). However, message for NTPDase1 was detected only occasionally, with most trials failing to pick up any PCR product.
The variable presence of NTPDase1 suggested that expression of the enzyme in RPE cells was regulated. Exposure to ATP has been shown to increase ectonucleotidase activity (Wiendl et al., 1998), although the specific identity of the contributing enzyme(s) has not been determined. Because NTPDase1 uses ATP as a primary substrate and because the levels of ATP bathing cells can be modified by a variety of environmental and experimental conditions (Grygorczyk and Hanrahan, 1997), we asked whether the presence of ATP in the bath was responsible for the appearance of the enzyme. Because the dephosphorylation of ATP by ectonucleotidases would severely limit its half-life, ATPγS was used to prolong availability of the nucleotide because the replacement of oxygen with sulfur on the terminal phosphate greatly slows the hydrolysis of ATPγS with respect to ATP. Changes in NTPDase1 levels were examined on molecular, protein, and functional levels and demonstrate physiological regulation of the enzyme.
Materials and Methods
Cell Culture and Treatment Regime
The human ARPE-19 cell line was obtained from the American Type Culture Collection (Manassas, VA) and grown in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F-12 medium with 3 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 mg/ml fungizone (all from Invitrogen, Carlsbad, CA), and 10% fetal bovine serum (HyClone Laboratories, Inc., Logan, UT) as described previously (Reigada et al., 2005). Cells were grown for a total of 14 to 16 days, with confluence reached after 5 to 8 days. Cells grown for less time gave less consistent results. Cells were maintained for indicated times in growth medium containing 100 μM ATPγS or P2Y1 agonists. For the 48-h preincubation time, the medium was replaced with fresh ATPγS solution after 24 h. Solution was replaced with growth medium alone at the same time points in controls. P2 inhibitors were added 10 min before ATPγS.
RT-PCR
Total RNA was extracted from ARPE-19 cells using the TRIzol reagent (Invitrogen). Oligo(dT)s were used to reverse transcribe 2 μg of total RNA at 42°C for 50 min using SuperScript II RT (Invitrogen). Increasing concentrations of cDNA were used for semiquantitative RT-PCR. β-Actin was selected as an endogenous internal standard gene. The primers used were designed to human NTPDase1 (sense, 5′-CTACCCCTTTGACTTCCAGG-3′; antisense, 5′-GCACACTGG-GAGTAAGGGC-3′; Macvector Program; Oxford Molecular Group/Accelrys, Burlington, MA; Reigada et al., 2005), NTPDase2 (sense, 5′-TGGAGGCAGCCGCAGTGAATGT-3′; antisense, GGAGGCGAAG-AGCAGCAGGAGGAC; Wood et al., 2002), NTPDase3 (sense, 5′-AGC-CTGGTCTCTTGGCTACA-3′; antisense, 5′-ACCCCAGGCTGACTC-TAAGCA-3′; Primer3 software, http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), and β-actin (sense, 5′-GGACTTCGAGCAA-GAGATGG-3′; antisense, 5′-AGCACTGTGTTGGCGTACAG-3′; Primer3). The predicted amplification products were 558, 300, 271, and 244 bp for the NTPDase1, NTPDase2, NTPDase3, and actin primer pairs, respectively. The amplification conditions were 1 min at 95°C, 1 min at 58°C, and 1 min at 72°C. This cycle was repeated 33 to 35 times for the NTPDases and 26 times for β-actin because initial trials determined that the PCR products for the later increased linearly between 23 and 33 cycles. In both cases, final extension was carried out at 72°C for 10 min. To control for genomic contamination, parallel PCR reactions were performed using cDNA that had not been reverse transcribed. The reaction products were separated on a 1% agarose gel including 10 ng/ml ethidium bromide and photographed using an ultraviolet transilluminator (Fisher Scientific, Suwanee, GA). Bands were scanned (Hewlett Packard Scanjet 3570c; Hewlett Packard Corp., Palo Alto, CA) and quantified with ImagePro Plus software (Media Cybernetics, Silver Spring, MD). The lack of increase in message for NTPDase2 and NTPDase3 was seen in two independent samples.
Real-Time PCR
Real-time PCR experiments were carried out in a Stratagene Mx3000P system (Stratagene Corp., Cedar Creek, TX). Total RNA was isolated as described above. Each PCR reaction contained 1 μl of RT product (cDNA samples were first diluted 1:10), 0.15 μM of each primer, 10 μl of 2× Brilliant SYBR Green QPCR Master Mix, and 300 nM of reference dye (all Stratagene Corp.). After a 95°C denaturation for 10 min, the reactions underwent 40 cycles of 95°C for 30 s, 58°C for 1 min, and 72°C for 1 min. New primers were chosen to give a smaller product appropriate for real-time PCR; primers for human NTPDase1 amplification were 5′-CAGGGACCCATGCTTTCATCC-3′ and 5′-GCTGGAATGGAA-GAGTCATCTCA-3′ (Primer bank, http://pga.mgh.harvard.edu/cgi-bin/primerbank/search.cgi; 104 bp), and for β-actin, they were 5′-CTCCTCCTGAGCGCAAGTACTC-3′ and 5′-TCGTCATACTCC-TGCTTGCTGAT-3′ (101 bp). Again, specificity for the NTPDase1 primers was confirmed using BLAST analysis. Three replicates of each reaction were performed, and each run included a no-template control. The CT was determined when the level of fluorescence from accumulating amplicons crossed a threshold assigned during the period of linear growth. Relative starting mRNA template concentrations of each sample were calculated using the comparative ΔΔCT method for relative quantification as done previously (Zhang et al., 2006a). In brief, CT values for the β-actin gene were subtracted from that of the NTPDase1 gene for samples treated with control (ΔCTcont) or ATPγS (ΔCTATPγS) solutions. The mean corrected difference of CT between control and ATPγS-treated cells was defined as ΔCTATPγS − ΔCTcont (mean ΔΔCT). Additional replicates were performed using the ABI 7500 (Applied Biosystems, Foster City, CA) shared by the Vision Research Core of the University of Pennsylvania using the same primers and analogous protocols.
Western Blots
ARPE-19 cells were washed twice with cold Dulbecco’s phosphate-buffered saline (Invitrogen), and lysed in buffer containing 50 mM Tris-HCl, 150 mM NaCl, protease inhibitor cocktail (Complete; Roche Diagnostics, Indianapolis, IN), 1% Triton X-100, 0.1% SDS, and 10% glycerol. The samples were sonicated and cleared by centrifugation (10,000g) for 30 min at 4°C. The protein concentrates were determined using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL). Homogenate containing 60 μg of protein was separated using conventional SDS-polyacrylamide gel electrophoresis under nonreducing conditions and transferred to a polyvinylidene difluoride membrane. Nonspecific binding was blocked with 5% nonfat dried milk for 1 h at 25°C. Blots were then incubated with a mouse monoclonal antibody against human NTPDase1, BU61 (1 μg/ml; Ancell Corp., Bayport, MN), overnight at 4°C. This was followed by incubation with anti-mouse IgG conjugated with horseradish peroxidase (1:5000 dilution; Amersham Biosciences Corp., Arlington Heights, IL) at 25°C for 1 h and developed by chemiluminescence detection (ECL detection system; Amersham Biosciences Corp.). ImagePro Plus software was used to quantify the intensity of the specific bands as above.
For membrane purified extracts, cells were homogenized in 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, and protease inhibitor cocktail. Nuclei and cell debris were removed from the homogenate by centrifugation at 10,000g for 15 min at 4°C. The resulting supernatant was centrifuged at 40,000g for 35 min at 4°C. The membrane pellet was solubilized in buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 0.5% Triton X-100, protease inhibitor cocktail) for a minimum of 1 h at 4°C.
Measurement of ATP Hydrolysis
ARPE-19 cells were cultured in 96-well white plates with clear bottoms (Corning Inc., Corning, NY) to confluence. Cells were washed, and 90 μl of isotonic buffer containing 1 μM ATP was added to the wells immediately before recording began. Isotonic buffer contained 105 mM NaCl, 5 mM KCl, 6 mM HEPES acid, 4 mM NaHEPES, 5 mM NaHCO3, 60 mM mannitol, 5 mM glucose, 0.5 mM MgCl2, and 1.3 mM CaCl2, at pH 7.4. The plate was placed into a luminometer (Luminoskan Ascent; Labsystems, Franklin, MA), and 10 μl of luciferin-luciferase solution was injected into each well using the internal injector as described previously (Reigada and Mitchell, 2005). Luminescence levels were measured every 60 s for 3 h, with an integration time of 100 ms for each well.
Materials and Analysis
MRS2500 and MRS2365 were obtained from Tocris Bioscience (Ellisville, MO). Information concerning the structure and synthesis of these compounds is available at MRS2500 (Kim et al., 2003) and MRS2365 (Chhatriwala et al., 2004). All other materials were from Sigma Chemical Corp. (St. Louis, MO) unless otherwise noted. Statistical analysis was performed using unpaired Student’s t test or an analysis of variance test with appropriate post hoc test. Time constants (τ) of ATP hydrolysis were calculated by fitting each individual record to an exponential decay curve, y = ae(−bx), using Sigmaplot Software (Systat Software Inc., Richmond, CA), with τ = 1/b.
Results
Up-Regulation of NTPDase1 Message
Exposure of ARPE-19 cells to 100 μM ATPγS for 48 h enhanced expression of NTPDase1 message in three separate trials using standard PCR, as defined by the presence of a bright band at the expected 558 bp in cells treated with ATPγS and its absence in control cells. To strengthen the analysis, the amount of PCR-amplified product was analyzed as a function of starting cDNA. The densitometric values of the NTPDase1 amplification products increased linearly with the amount of reverse-transcribed cDNA added (Fig. 1, A and B). In contrast, no bands were detected using any concentration of cDNA obtained from cells grown in control medium. Similar results were found using message extracted from three independent sets of cells in the presence and absence to 100 μM ATPγS. To ensure that this increase in message after exposure to ATPγS was specific for NTPDase1, the analysis was repeated using primers for β-actin as an internal control. The amount of β-actin product was the same for ATPγS-treated and control cells regardless of the amount of starting cDNA (Fig. 1, C and D). No increase in message for either NTPDase2 or NTPDase3 was found in cells exposed to 100 μM ATPγS for 48 h, even though NTPDase1 was substantially elevated in the same samples (data not shown). In all cases, no product was detected when reverse transcriptase was omitted from the reaction.
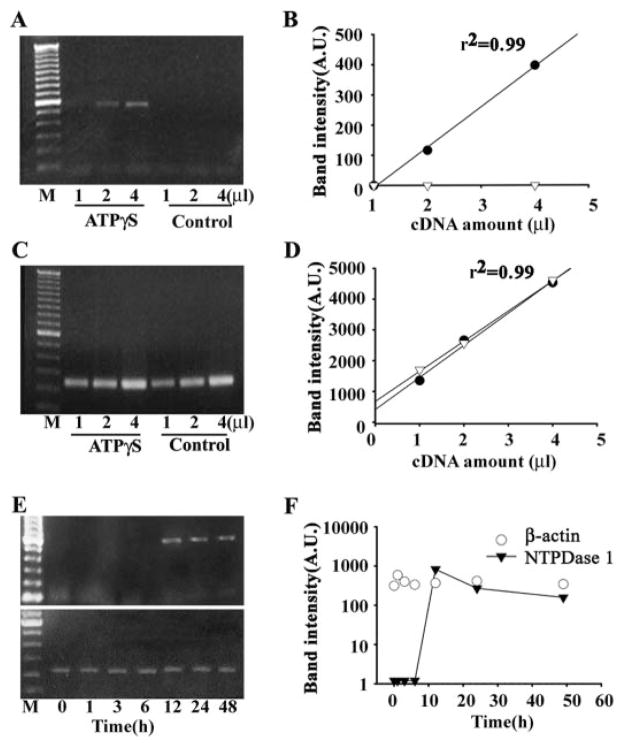
Fig. 1.
RT-PCR for NTPDase1 from human ARPE-19 cells. A, PCR with a primer pair specific for NTPDase1 generated a 558-bp band in cDNA from cells treated with ATPγS for 48 h but not in untreated control cells. The band intensity increased with the starting volume of cDNA. B, densitometric values of the RT-PCR amplified NTPDase1 products increased linearly with an increasing amount of cDNA in cells exposed to 100 μM ATPγS (black circles). No product was detected from cells in control medium (white triangles). A first order linear regression is fit to the data. Similar increases in NTPDase1 message were found in three independent sets of cells exposed to ATPγS for 48 h, and in all cases, densitometric values increased with the amount of cDNA. C, PCR with β-actin primer pair generated single 244-bp bands in both control and ATPγS-treated cells. D, densitometric values of β-actin products increased linearly in both ATPγS-treated (black circles) and control cells (white triangles). Data are fit with a single order regression. E and F, time course of up-regulation of NTPDase1 mRNA. Cells were exposed to ATPγS continuously for the time indicated. Message for NTPDase1 was first detected after 12-h exposure to ATPγS, whereas levels of β-actin remained constant throughout. NTPDase1 data points are connected with a line. A similar time course was found in three independent trials.
The time course of up-regulation was examined by collecting RNA from cells exposed to ATPγS for 0, 1, 3, 6, 12, 24, and 48 h. PCR product was first detected in material obtained after 12-h exposure (Fig. 1, E and F). In contrast, levels of β-actin did not vary much with the duration of exposure. Similar results were obtained in three independent sets of cells exposed to ATPγS for the indicated durations. In the set shown, levels of message were highest at 12 h and declined slightly afterward but still remained >100-fold greater than control after 48 h of treatment. In one trial, levels at 12 h were 80% of the maximum found at 24 h, whereas in the third set, levels at 12 h were 20% of those found at 24 h. Although message was first detected in the 12-h sample in all cases, this variation in relative amount suggests that the greatest increase in message occurs near the 12-h point.
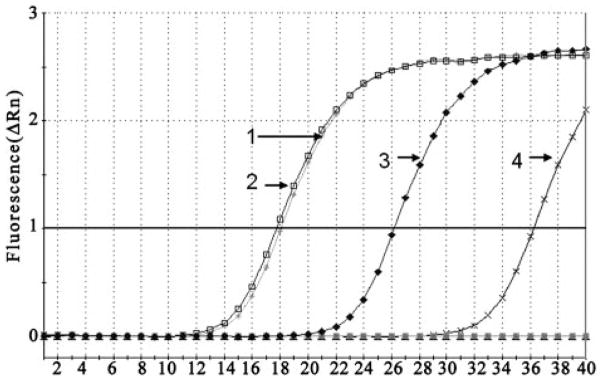
The semiquantitative PCR above suggested that exposure of RPE cells to ATPγS led to an increase in mRNA for NTP-Dase1. More quantitative evidence for this transcriptional regulation was sought using real-time PCR. Real-time PCR was performed with cDNA isolated from cells exposed to 100 μM ATPγS and control medium for 48 h. Message for NTP-Dase1 was detected at a much lower number of amplification cycles in cells treated with ATPγS than with control medium, indicating a larger amount of starting message (Fig. 2). Treatment with ATPγS had no effect on detection of β-actin message. When adjusted for the amount of β-actin, message from ATPγS-treated cells crossed the threshold after 8.42 cycles, whereas that from control cells crossed at 18.17 cycles, giving a mean ΔΔCT of 9.75. There was no observable amplification without template. A similar increase was seen using cells from three independent trials.
Fig. 2.
Quantitative PCR analysis of NTPDase1 expression in RPE cells. Amplification of NTPDase1 in RPE cells exposed to control or 100 μM ATPγS medium for 48 h was performed with SYBR Green real-time PCR. cDNA samples were diluted 1/10, and all reactions were performed in triplicate. The CT of housekeeping gene β-actin was similar for both control and ATPγS-treated cells (lines 1 and 2, respectively), whereas CT for the NTPDase1 message was attained after considerably fewer amplification cycles in ATPγS-treated cells (line 3) compared with untreated cells (line 4). No signal was detected from the no-template control (bottom line). Similar increases were found using message from three independent trials, each performed in triplicate. The figure indicates the mean of such triplicate reactions from one particular trial.
Up-Regulation of NTPDase1 Protein
The relative expression of NTPDase1 was examined using Western blotting techniques to determine whether the increase in transcription led to a corresponding increase in protein. Protein from whole-cell extracts was run and probed with the NTPDase1 monoclonal anti-human antibody BU61. Although nothing was detected from cells under control conditions, protein from cells exposed to ATPγS for 48 h showed a clear, single band of 78 kDa (Fig. 3A). This size band corresponds to the functional monomeric glycosylated form of NTPDase1 (Sévigny et al., 1997). Similar increases were found in three independent trials after 48-h treatment with 100 μM ATPγS. The 78-kDa band was also detected in a fraction specifically enriched in membrane proteins (Fig. 3B), suggesting that the increase could have functional implications.
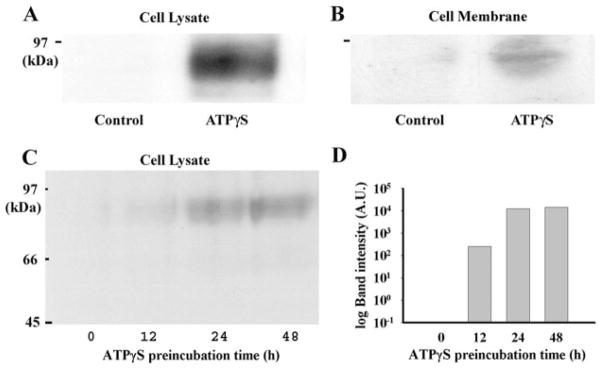
Fig. 3.
Western blot analysis for NTPDase1 protein levels. A, immunoblotting with antibody BU61 demonstrates that incubation of cells with 100 μM ATPγS for 48 h led to detectable 78-kDa bands in protein from whole-cell lysates. B, bands (78 kDa) were also detected in protein purified from cell membranes after incubation with 100 μM ATPγS for 48 h. C, incubation of cells with 100 μM ATPγS for 12, 24, and 48 h led to detectable 78-kDa bands on immunoblots of increasing intensity from cell lysate material. D, quantification of staining intensity indicates that the largest increase in NTPDase1 protein occurred after 12 to 24 h of exposure to ATPγS. A similar increase in NTPDase1 protein levels after 24 h in ATPγS was found in 14 trials.
Further analysis was performed to determine the time course of the increase in protein. Exposure to ATPγS for 12, 24, and 48 h led to a corresponding increase in band intensity (Fig. 3C). Densitometric quantification of the blot supported this observation and indicated the biggest jump in protein occurred between 12 and 24 h after exposure to ATPγS began (Fig. 3D). Because levels of protein were similar for 24- and 48-h exposure, subsequent experiments were performed at 24 h. In total, band intensity increased in 14 preparations after 24-h exposure to 100 μM ATPγS. Band intensity was usually below the limits of detection in material from cells not exposed to ATPγS. Faint bands were found in protein from untreated control cells in 3 of the 14 preparations. The presence of NTPDase1 protein in some controls but not others is consistent with the occasional presence of NTPDase1 found previously (Reigada et al., 2005).
Up-Regulation of ATP Hydrolysis
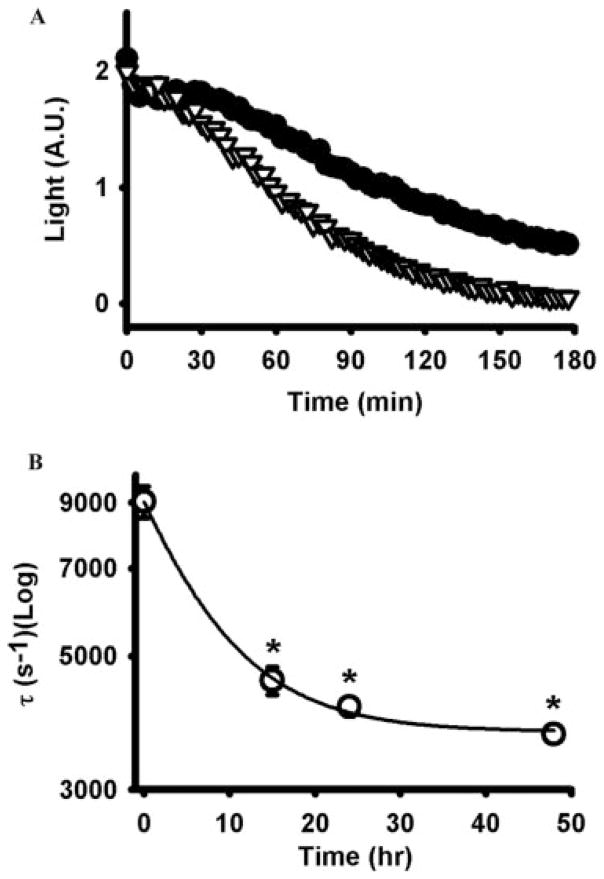
Functional analysis of extracellular ATP hydrolysis was performed to determine whether the increases in NTPDase1 mRNA and protein levels were accompanied by an increase in biochemical activity. The time course of ATP dephosphorylation was monitored after injection of 1 μM hydrolyzable ATP into the bath because this concentration is below that previously demonstrated to trigger an ATP-mediated ATP release in these cells (Reigada et al., 2005). Although extracellular ATP was hydrolyzed by control cells, this enzymatic activity was substantially enhanced in cells treated with 100 μM ATPγS (Fig. 4A). Levels of ATP bathing control cells dropped to 23.9 ± 2.9% of the initial values after 3 h of enzymatic reaction, but this fell to 2.1 ± 0.5% in cells exposed to ATPγS for 48 h. Exposure to ATPγS for intermediate times had intermediate effects on hydrolysis, with 6.9 ± 0.9 and 4.3 ± 0.4% ATP remaining after 15 and 24 h, respectively. The speed of decay was also enhanced, with the time constant for hydrolysis falling with increased exposure to ATPγS (Fig. 4B). The majority of the increase in ATPase activity occurred in the first 24 h, in agreement with the increase in protein and message (Fig. 3B). In total, the mean time constant fell from 5387 ± 435 to 3515 ± 250 s−1 after 48-h preincubation with ATPγS (n = 45–50 wells from three independent trials, p < 0.0002). The closeness of the initial luminescence values indicated that residual ATP from the ATPγS pretreatment was not affecting the functional assay. In one set of experiments, ATPγS treatment failed to enhance activity, although mRNA for NTPDase1 was already elevated in untreated controls and did not increase further with ATPγS treatment.
Fig. 4.
Incubation with ATPγS increased ecto-ATPase activity. A, cells exposed to 100 μM ATPγS for 48 h (gray triangles) hydrolyzed 1 μM ATP more rapidly than untreated control cells (black circles). Intermediate preincubations of 15 and 24 h led to intermediate increases in hydrolysis but are not shown here for reasons of clarity. Results show the mean of five to eight wells from a trial representative of three independent experiments. Light levels represent the photons given off with the luciferin/luciferase reaction, an index of the level of ATP present in the bath [in arbitrary units (A.U.)]. B, time constant of decay decreased exponentially with preincubation time. *, p < 0.05 versus no preincubation, n = 5 to 8. In A, error bars are smaller than symbols.
P2Y1 Receptor and Up-Regulation of NTPDase1
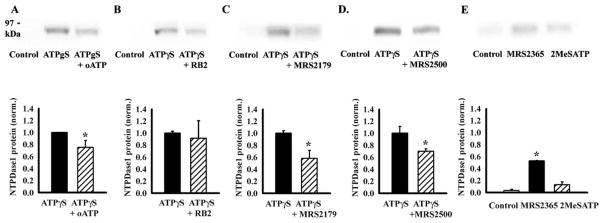
We hypothesized that stimulation of P2 receptors present on these cells might initiate the changes that led to increased transcription of NTPDase1 message and elevated levels of protein. The ability of P2 antagonists to inhibit this up-regulation was quantified from the 78-kDa band on Western blots because this provided post-translational information specifically about the functional form of NTPDase1. Two relatively nonspecific antagonists were initially tested. Antagonists oATP (100 μM) and RB2 (50 μM) were added to cells 10 min before ATPγS, and proteins were extracted 24 h later. Levels of NTPDase1 were decreased in all four trials with oATP, with a mean decrease of 25 ± 11% (Fig. 5A). The effect of RB2 was more variable, with levels decreasing by over 90% in one trial but increasing by 250% in another, giving a nonsignificant change overall (Fig. 5B).
Fig. 5.
Involvement of P2Y1 receptors in up-regulation of NTPDase1. P2 antagonists were given to cells 10 min before 100 μM ATPγS, and the 78-kDa band was detected with antibody BU61 from protein extracted from whole-cell lysates 24 h later. A, nonspecific P2 antagonist oATP (100 μM) decreased the net up-regulation of NTPDase1 by 25%. The upper trace is representative of four independent trials, with the mean ± S.E. given in the bars below. B, effect of RB2 (50 μM) was variable with some trials like that illustrated above indicating a drastic reduction, although the mean decrease was not significant (n = 8). C, P2Y1 receptor antagonist MRS2179 (100 μM) led to a clear decrease in band intensity (n = 7). D, a second P2Y1 antagonist, MRS2500 (10 nM), also decreased band intensity (n = 4). E, P2Y1 agonist MRS2365 (100 nM) significantly increased NTPDase1 levels compared with control after 24-h exposure, whereas the rise with 100 μM 2MeSATP was not significant (n = 2–3). *, p < 0.05 versus ATPγS alone (A–D) or versus control (E).
The effect of oATP, and perhaps RB2, suggested some type of P2 receptor involvement. The contribution from the P2Y1 receptor in particular was pursued for several reasons. Although oATP has traditionally been used as a P2X7 receptor inhibitor and is now recognized to have actions as a general P2X blocker, it also has distinct effects on the P2Y1 receptor (Beigi et al., 2003). Previous analysis has indicated the presence of the P2Y1 receptor in these cells on a molecular and functional level (Fries et al., 2004; Reigada et al., 2005), and the recent availability of specific pharmacologic tools for the P2Y1 receptor has enabled a more definitive analysis. Addition of the P2Y1 antagonist MRS2179 to cells 10 min before ATPγS inhibited the response in seven of seven trials. In total, the expression of NTPDase1 in cells treated with MRS2179 (100 μM) was reduced by 58% compared with cells treated with ATPγS alone (Fig. 5C). Although MRS2179 is 3 orders of magnitude more selective for the P2Y1 receptor than the P2Y2, P2Y4, P2Y6, and P2Y11 receptors (Boyer et al., 1998; von Kügelgen, 2006), the compound is not particularly stable (Baurand and Gachet, 2003; Hechler et al., 2006) and requires relatively high levels for effective block over extended periods. In contrast, the recently available inhibitor MRS2500 is substantially more stable and effective in the low-nanomolar range, with no binding or action at P2Y2, P2Y12, or avian P2Y receptors (Kim et al., 2003; Hechler et al., 2006; Houston et al., 2006). Treatment of cells with 10 nM MRS2500 reduced the amount of NTPDase1 by over 30% (Fig. 5D).
The block by both MRS2179 and MRS2500 implicated the P2Y1 receptor, but relative ineffectiveness of ATP at the P2Y1 receptor combined with the potential for contamination of commercially obtained ATPγS with other nucleotide products, led us to seek additional confirmation with a more specific agonist (von Kuügelgen, 2006). The agonist MRS2365 is highly specific for the P2Y1 receptor; it was reported to have no activity at the P2Y12 receptor and very little at the P2Y13 receptor, while stimulating the P2Y1 receptor with an EC50 in the low nanomolar range (Chhatriwala et al., 2004). At 10 nM, MRS2365 significantly increased NTPDase1 levels 14-fold over control (n = 5). Increasing MRS2365 to 100 nM raised NTPDase1 levels 25-fold over control (Fig. 5E), although the up-regulation by MRS2365 was not additive with that of ATPγS. The increase by 2MeSATP (100 μM) was not significant, perhaps due to the ability of NTPDase1 to hydrolyze this agonist (Picher et al., 1996).
Discussion
This study shows that prolonged exposure to ATPγS increased transcription of NTPDase1 in human ARPE-19 cells. This increase in mRNA was accompanied by an increase in NTPDase1 protein and in the rate of extracellular ATP hydrolysis. This response was mediated, at least in part, by the P2Y1 receptor and may reflect the need for cells to maintain low levels of extracellular nucleotides.
The E-NTPDase family is composed of eight members, and four of them, NTPDase1, 2, 3, and 8, are dominant ectonucleotidases that dephosphorylate extracellular nucleotides (Bigonnesse et al., 2004). NTPDase1 catalyzes the dual dephosphorylation of ATP and ADP to AMP plus inorganic phosphorus (Kaczmarek et al., 1996; Robson et al., 2006). NTPDase1 is an acidic glycoprotein with a molecular mass of 78 kDa that contains two transmembrane regions and several potential glycosylation sites (Sévigny et al., 1997). A truncated 54-kDa band is occasionally observed, corresponding to a C-terminal portion created by proteolytic digestion of the larger 78-kDa form (Sévigny et al., 1995; Schulte am Esch et al., 1999; Lemmens et al., 2000). The detection of a 78-kDa band with the monoclonal antibody BU61 corresponds to the active monomeric form of the enzyme and is consistent with an increase in the ATPase activity of RPE cells after treatment with ATPγS.
The ability of MRS2179 and MRS2500 to inhibit the up-regulation of NTPDase1 by ATPγS, combined with the increase induced by MRS2365, strongly implicates the P2Y1 receptor in the control of enzyme levels. The shared utilization of both tri- and diphosphate adenines makes the P2Y1 receptor well matched to regulate NTPDase1. ADP is considerably more effective at the P2Y1 receptor than ATP (von Kügelgen, 2006), whereas ADP is hydrolyzed more effectively by NTPDase1 than other members of the E-NTPDase family (Kaczmarek et al., 1996). Stimulation of the P2Y1 receptor is also affected by the expression of NTPDase1 (Alvarado-Castillo et al., 2005). A contribution from other P2 receptors in the up-regulation of NTPDase1 is possible because relatively high levels of MRS2179 and MRS2500 inhibited only half the response to ATPγS, whereas a maximally effective concentration of MRS2365 increased NTPDase1 levels to only half that of ATPγS (Chhatriwala et al., 2004). However, instability of MRS2365 over the course of 24 h may have led to a submaximal response. The second messenger pathways linking receptor stimulation with transcriptional control are presently unknown, but stimulation of the P2Y1 receptor in myotubes can up-regulate expression of acetylcholine esterase through a pathway involving intracellular Ca2+, protein kinase C, and the transcription factor Elk-1 (Choi et al., 2003), whereas activation of the P2Y1 receptor in ARPE-19 cells increases intracellular Ca2+ (Reigada et al., 2005). Whether this rise in Ca2+ is necessary for the up-regulation of NTPDase1 remains to be determined.
It is possible that other members of the E-NTPDase family, or those of the eNPP family, are also up-regulated after treatment with ATPγS. Ogilvie and colleagues found previous exposure to ATP altered the degradation of numerous nucleotides (Wiendl et al., 1998). However, the consistently high expression of some of these other enzymes in ARPE-19 cells under unstimulated conditions predicts a smaller relative change (Reigada et al., 2005), perhaps explaining why neither NTPDase2 nor NTPDase3 were up-regulated by ATPγS in our hands. Because ATPγS is hydrolyzed slowly, it is also possible that some of the effects are initiated by ADP. We feel that these results may open up an exciting area of investigation into how different nucleotide species stimulate purine receptors to alter expression of nucleotidase enzymes.
Previous examination of the ectonucleotidases in RPE cells found levels of NTPDase1 were variable, with mRNA for the enzyme detected only occasionally (Reigada et al., 2005). Although it is not possible to examine the reasons for this variability in retrospect, changes in the levels of extracellular ATP may have contributed and release of ATP is exquisitely sensitive to handling (Grygorczyk and Hanrahan, 1997). Conditions of cell growth may also have modified expression because initial investigations indicate that enzyme expression was more reliably regulated when cells were grown for 2 weeks as opposed to the 1 week used in earlier studies. Even with prolonged growth, however, NTPDase1 was occasionally present in control cells, indicating additional yet unknown parameters may influence expression. The dynamic regulation of NTPDase1 levels does emphasize caution when interpreting evidence for the presence or absence of the enzyme in either cultured or fresh cells.
Cells would not be exposed to ATPγS in vivo, of course, but rather to a sustained supply of fresh, excess ATP released into the extracellular space. Because increased protein and ATP hydrolysis was observed after 12 h, this feedback system may have implications for the physiological processes regulated by the light/dark cycle in subretinal space. Glutamate is released by photoreceptors in the dark, and stimulation of an N-methyl-D-aspartic acid receptor for glutamate on the apical surface of the RPE leads to a rapid increase in the levels of extracellular ATP (Mitchell, 2001; Reigada and Mitchell, 2005; Reigada et al., 2006a). The present findings suggest this increased ATP in subretinal space would up-regulate NTPDase1 levels 12 h later, thus altering the dynamics of purinergic signaling in the light.
The causal link between ATPγS and NTPDase1 up-regulation above supports the role of excess ATP in several pathologic conditions. For example, changes in nucleotidase activity have been observed after epileptic seizures (Bonan et al., 2000) and global cerebral ischemia (Schetinger et al., 1994), and NTPDase1 regulation at the transcriptional level has been observed in response to transient forebrain ischemia (Braun et al., 1998) and excess noise in the cochlea (Vlajkovic et al., 2004). The rise in the enzyme was often assumed to occur in response to elevated extracellular ATP, but as far as we are aware, this has not been directly shown until now. Although identification of the specific enzyme involved in these cases is needed, these observations, and those in epidermoid carcinoma cells (Wiendl et al., 1998), make it unlikely that up-regulation of NTPDase1 is restricted to RPE cells.
In addition to confirming a trigger for up-regulation of NTPDase1, the demonstration that ATPγS enhanced NTP-Dase1 levels suggests that the enzyme could provide diagnostic information. Measurements of extracellular ATP are frequently difficult to make in vivo, complicated by high intracellular levels, the release of ATP following physiologic manipulation (Grygorczyk and Hanrahan, 1997), and the restrictive extracellular microenvironment. Levels of NTP-Dase1 might serve as an index for prolonged increases in extracellular ATP concentrations, although contribution of other mechanisms to enzyme levels is, of course, always possible.
Acknowledgments
This study was supported by the National Institutes of Health (Grants EY013434 and EY015537 to C.H.M.) and by National Institutes of Health Core Vision Grant EY001583 to the University of Pennsylvania. J.S. is supported by the Canadian Institutes of Health Research and is also the recipient of a New Investigator award from Canadian Institutes of Health Research.
We thank Richard Stone, Alan Laties, José Boyer, and Mortimer Civan for useful discussions, Alex Laties for technical help, and Kenneth Jacobson for advice on the pharmacology on the P2Y1 receptor.
ABBREVIATIONS
- RPE
retinal pigment epithelium
- eNPP
ectonucleotide pyrophosphatase/phosphodiesterase
- NTPDase
nucleoside-triphosphate diphosphohydrolase
- PCR
polymerase chain reaction
- bp
base pair
- RT
reverse transcriptase
- CT
cycle threshold
- MRS2500
2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine 3′,5′-bisphosphate
- MRS2365
(N)-methanocarba-2MeSADP
- oATP
oxidized ATP
- RB2
Reactive Blue 2
- MRS2179
N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate
Footnotes
A preliminary version of some of these results has been presented in abstract form [Lu W, Reigada D, Sévigny J, and Mitchell CH (2006) ATPγS enhances expression of the ecto-ATPase NTPDase1: relevance to sustained purinergic signaling in RPE cells. Invest Ophthalmol Vis Sci 47:382].
References
- Alvarado-Castillo C, Harden TK, Boyer JL. Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol. 2005;67:114–122. doi: 10.1124/mol.104.006908. [DOI] [PubMed] [Google Scholar]
- Baurand A, Gachet C. The P2Y1 receptor as a target for new antithrombotic drugs: a review of the P2Y1 antagonist MRS-2179. Cardiovasc Drug Rev. 2003;21:67–76. doi: 10.1111/j.1527-3466.2003.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Beigi RD, Kertesy SB, Aquilina G, Dubyak GR. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br J Pharmacol. 2003;140:507–519. doi: 10.1038/sj.bjp.0705470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigonnesse F, Levesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJ, Sevigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. doi: 10.1021/bi0362222. [DOI] [PubMed] [Google Scholar]
- Bonan CD, Walz R, Pereira GS, Worm PV, Battastini AM, Cavalheiro EA, Izquierdo I, Sarkis JJ. Changes in synaptosomal ectonucleotidase activities in two rat models of temporal lobe epilepsy. Epilepsy Res. 2000;39:229–238. doi: 10.1016/s0920-1211(00)00095-4. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci. 1998;18:4891–4900. doi: 10.1523/JNEUROSCI.18-13-04891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheimer RE, Linden J. Purinergic regulation of epithelial transport. J Physiol. 2004;555:311–321. doi: 10.1113/jphysiol.2003.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RC, Siow NL, Cheng AW, Ling KK, Tung EK, Simon J, Barnard EA, Tsim KW. ATP acts via P2Y1 receptors to stimulate acetylcholinesterase and acetylcholine receptor expression: transduction and transcription control. J Neurosci. 2003;23:4445–4456. doi: 10.1523/JNEUROSCI.23-11-04445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Fries JE, Wheeler-Schilling TH, Guenther E, Kohler K. Expression of P2Y1, P2Y2, P2Y4, and P2Y6 receptor subtypes in the rat retina. Invest Ophthalm Vis Sci. 2004;45:3410–3417. doi: 10.1167/iovs.04-0141. [DOI] [PubMed] [Google Scholar]
- Gregory CY, Abrams TA, Hall MO. Stimulation of A2 adenosine receptors inhibits the ingestion of photoreceptor outer segments by retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1994;35:819–825. [PubMed] [Google Scholar]
- Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316:556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK. [32P]2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol. 2006;147:459–467. doi: 10.1038/sj.bjp.0706453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46:4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Lemmens R, Vanduffel L, Kittel A, Beaudoin AR, Benrezzak O, Sevigny J. Distribution, cloning, and characterization of porcine nucleoside triphosphate diphosphohydrolase-1. Eur J Biochem. 2000;267:4106–4114. doi: 10.1046/j.1432-1327.2000.01462.x. [DOI] [PubMed] [Google Scholar]
- Maminishkis A, Jalickee S, Blaug SA, Rymer J, Yerxa BR, Peterson WM, Miller SS. The P2Y2 receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Invest Ophthalmol Vis Sci. 2002;43:3555–3566. [PubMed] [Google Scholar]
- Mitchell CH. Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J Physiol. 2001;534:193–202. doi: 10.1111/j.1469-7793.2001.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Reigada D. Purinergic signalling in the subretinal space: a role in the communication between the retina and the RPE. Purinergic Signal. 2007 doi: 10.1007/s11302-007-9054-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson WM, Meggyesy C, Yu K, Miller SS. Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J Neurosci. 1997;17:2324–2337. doi: 10.1523/JNEUROSCI.17-07-02324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picher M, Sevigny J, D’Orleans-Juste P, Beaudoin AR. Hydrolysis of P2-purinoceptor agonists by a purified ectonucleotidase from the bovine aorta, the ATP-diphosphohydrolase. Biochem Pharmacol. 1996;51:1453–1460. doi: 10.1016/0006-2952(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Reigada D, Lu W, Mitchell CH. Glutamate acts at NMDA receptors on fresh bovine and on cultured human retinal pigment epithelial cells to trigger release of ATP. J Physiol. 2006a;575:707–720. doi: 10.1113/jphysiol.2006.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigada D, Lu W, Zhang X, Friedman C, Pendrak P, McGlinn A, Stone RA, Laties AM, Mitchell CH. Degradation of extracellular ATP by the retinal pigment epithelium. Am J Physiol Cell Physiol. 2005;289:C617–C624. doi: 10.1152/ajpcell.00542.2004. [DOI] [PubMed] [Google Scholar]
- Reigada D, Mitchell CH. Release of ATP from RPE cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol. 2005;288:C132–C140. doi: 10.1152/ajpcell.00201.2004. [DOI] [PubMed] [Google Scholar]
- Reigada D, Zhang X, Crespo A, Nguyen J, Liu J, Pendrak K, Stone RA, Laties AM, Mitchell CH. Stimulation of an α1-adrenergic receptor downregulates ecto-5′ nucleotidase activity on the apical membrane of RPE cells. Purinergic Signal. 2006b;2:499–507. doi: 10.1007/s11302-005-3980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ecto-nucleotidases: structure, function, relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetinger MR, Barcellos CK, Barlem A, Zwestch G, Gubert A, Bertuol C, Arteni N, Dias RD, Sarkis JJ, Netto CA. Activity of synaptosomal ATP diphosphohydrolase from hippocampus of rats tolerant to forebrain ischemia. Braz J Med Biol Res. 1994;27:1123–1128. [PubMed] [Google Scholar]
- Schulte am Esch J, 2nd, Sevigny J, Kaczmarek E, Siegel JB, Imai M, Koziak K, Beaudoin AR, Robson SC. Structural elements and limited proteolysis of CD39 influence ATP diphosphohydrolase activity. Biochemistry. 1999;38:2248–2258. doi: 10.1021/bi982426k. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM. ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol. 2001;28:340–350. doi: 10.1046/j.1440-1681.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- Sévigny J, Cote YP, Beaudoin AR. Purification of pancreas type-I ATP diphosphohydrolase and identification by affinity labelling with the 5′-p-fluorosulphonylbenzoyladenosine ATP analogue. Biochem J. 1995;312:351–356. doi: 10.1042/bj3120351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sévigny J, Levesque FP, Grondin G, Beaudoin AR. Purification of the blood vessel ATP diphosphohydrolase, identification and localisation by immunological techniques. Biochim Biophys Acta. 1997;1334:73–88. doi: 10.1016/s0304-4165(96)00079-7. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Vlajkovic SM, Housley GD, Munoz DJ, Robson SC, Sevigny J, Wang CJ, Thorne PR. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience. 2004;126:763–773. doi: 10.1016/j.neuroscience.2004.04.023. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Wiendl HS, Schneider C, Ogilvie A. Nucleotide metabolizing ectoenzymes are upregulated in A431 cells periodically treated with cytostatic ATP leading to partial resistance without preventing apoptosis. Biochim Biophys Acta. 1998;1404:282–298. doi: 10.1016/s0167-4889(98)00040-8. [DOI] [PubMed] [Google Scholar]
- Wood E, Johan Broekman M, Kirley TL, Diani-Moore S, Tickner M, Drosopoulos JH, Islam N, Park JI, Marcus AJ, Rifkind AB. Cell-type specificity of ectonucleotidase expression and upregulation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Biochem Biophys. 2002;407:49–62. doi: 10.1016/s0003-9861(02)00465-4. [DOI] [PubMed] [Google Scholar]
- Zhang M, Budak MT, Lu W, Khurana TS, Zhang X, Laties AM, Mitchell CH. Identification of the A3 adenosine receptor in rat retinal ganglion cells. Mol Vis. 2006a;12:937–948. [PubMed] [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH. Balance of purines may determine life or death as A3 adenosine receptors prevent loss of retinal ganglion cells following P2X7 receptor stimulation. J Neurochem. 2006b;98:566–575. doi: 10.1111/j.1471-4159.2006.03900.x. [DOI] [PubMed] [Google Scholar]