Abstract
Extracellular nucleotides are emerging as important inflammatory mediators. Here, we demonstrate that these molecules mediate LPS-induced neutrophil migration in vitro and in vivo. Apyrase, a nucleotide scavenger, reduced the ability of LPS-stimulated monocytes to recruit neutrophils, as assayed using a modified Boyden chamber. This effect resulted from the inhibition of IL-8 release from monocytes. Furthermore, LPS-induced IL-8 release by monocytes was attenuated significantly by P2Y6 receptor antagonists, RB-2 and MRS2578. Reciprocally, UDP, the selective P2Y6 agonist, induced IL-8 release by monocytes. As for LPS, the media of UDP-stimulated monocytes were chemotactic for neutrophils; IL-8 accounted for ~50% of neutrophil migration induced by the media of LPS- or UDP-treated monocytes in transendothelial migration assays. It is important that in the murine air-pouch model, extracellular nucleotides were instrumental in LPS-induced neutrophil migration. Altogether, these data imply that LPS induces the release of nucleotides from monocytes and that by autocrine stimulation, the latter molecules regulate neutrophil migration caused by Gram-negative bacteria, suggesting a proinflammatory role of extracellular nucleotides in innate immunity.
Keywords: inflammation, monocyte, IL-8, P2 receptor
INTRODUCTION
Extracellular nucleotides induce a variety of physiological responses in various cell types [1, 2]. These effects are mediated by P2 nucleotide receptors, which include ion channel P2X receptors (P2X1–7) and G protein-coupled P2Y receptors (P2Y1,2,4,6,11–14). As P2 receptors are expressed on cells involved in innate immunity, it has been proposed that extracellular nucleotides participate in immune responses [3]. Primary monocytes and HUVEC express the mRNA of P2Y1, P2Y2, P2Y4, and P2Y6 receptors, and polymorphonuclear leukocytes appears to express all P2Y receptor mRNAs except P2Y12 [4–6]. In neutrophils, P2Y2 appeared dominantly expressed at the mRNA level and was also functionally demonstrated at the protein level [5, 7, 8]. P2Y2 and P2Y11 are activated by ATP, P2Y1 and P2Y13 by ADP, P2Y4 by UTP, P2Y6 by UDP, and P2Y14 by UDP glucose [1]. The stimulation of P2 receptors and therefore, biological responses triggered by nucleotides are modulated/terminated by ectonucleotidases such as nucleoside triphosphate diphosphohydrolases (NTPDases), which hydrolyze γ and β phosphate residues of nucleotides [9, 10].
LPS is a cell-wall component of gram-negative bacteria and a potent activator of innate immunity [11]. Cellular responses to LPS are mediated essentially by TLR-4 and involve the release of various soluble inflammatory mediators, which subsequently recruit neutrophils at sites of bacterial infection [12]. It is interesting that LPS stimulation of macrophages, microglia, and endothelial cells is accompanied by the release of ATP [13–15]. Extracellular nucleotides can activate cells to release proinflammatory mediators and therefore, initiate inflammatory responses. For instance, the release of IL-1 from LPS-treated microglial cells was effectively prevented by a nucleotide scavenger (apyrase) [14]. Likewise, overexpression of ectonucleotidase NTPDase1 at the surface of endothelial cells increased nucleotide hydrolysis and significantly attenuated LPS-induced IL-1α release [15]. In macrophages, extracellular ATP was required for LPS-induced production of IL-1β and IL-18 [16, 17].
In this study, we demonstrate that extracellular nucleotides mediate LPS-induced neutrophil migration in vitro and in vivo.
MATERIALS AND METHODS
Materials
LPS from Escherichia coli O111:B4, potato apyrase grade VII, nucleotides (ATP, ADP, UTP, and UDP), α,β-methyleneadenosine-5′-diphosphate (α,β-meADP), pyridoxal-phosphate-6-azophenyl-2′, 4′-disulfonate (PPADS), and suramin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Adenosine deaminase (ADA) was provided by Roche Diagnostics (Indianapolis, IN, USA), MRS2578 by Tocris Bioscience (Bristol, UK), and reactive blue 2 (RB-2) by ICN Biochemicals Inc. (Aurora, OH, USA). Human recombinant (hr)IL-8 was purchased from Medicorp (Montreal, Canada), IL-8 neutralizing antibody MAB208 from R&D Systems (Minneapolis, MN, USA), and RPMI-1640 medium from Wisent (St-Bruno, Canada). A matching isotype mouse IgG1 antibody to DNP was used as a control in Boyden chamber transmigration assays.
LPS and apyrase were reconstituted in an endotoxin-free saline (Sigma Chemical Co.). Nucleotides, α,β-meADP and P2 receptor antagonists (RB-2, PPADS, and suramin) were dissolved in tissue-culture, endotoxin-free water purchased from Sigma Chemical Co. Prior to cell stimulation, LPS was sonicated for 10 min in a water bath sonicator. Before use, ADA was dialyzed against sterile 0.9% NaCl and 10 mM Hepes, pH 7.4, in a Slide-A-Lyser® cassette (MWCO 3500, Pierce, Rockford, IL, USA) to remove ammonium sulfate present in the commercial preparation that induces calcium mobilization in cells. MRS2578 was dissolved in DMSO at the concentration of 0.1 M and diluted further with RPMI 1640 plus 5% FBS to the working concentration of 10 μM, which therefore contained 0.01% DMSO.
Isolation of monocytes and neutrophils
Human monocytes and neutrophils were isolated as described originally [18] with some modifications. Briefly, venous blood of healthy volunteers was collected on isocitrate anticoagulant solution and centrifuged (250 g, 10 min, 24°C), and the resulting platelet-rich plasma was discarded. Leukocytes were obtained following erythrocyte sedimentation in 2% Dextran T-500 and centrifuged (525 g, 20 min, 24°C) through a 10-ml Ficoll-Paque cushion (Wisent). Neutrophils were recovered from the pellet and PBMC from the interface. Neutrophils were subjected to a 15-s hypotonic lysis to remove contaminating erythrocytes, whereas monocytes were purified further from PBMC by depleting contaminating lymphocytes. To this end, PBMC were mixed with an equal volume of HBSS containing Ca2+, seeded on a 5-cm Petri dish, and allowed to adhere for 10 min at 37°C. Nonadherent lymphocytes were discarded by aspiration. Adherent monocytes were washed four times with PBS and subsequently detached by 10 min incubation with 0.02% EDTA in PBS at 37°C. Neutrophils and monocytes purified in such a manner were centrifuged (1000 g, 5 min, 24°C), resuspended in culture medium (RPMI 1640 with 5% FBS), and stimulated as indicated below to obtain the conditioned media.
Monocytes were also purified from PBMC by MACS® magnetic cell separation following labeling with CD14 MicroBeads (Auto MACS, Miltenyi Biotec, Auburn, CA, USA), according to the protocol provided by the manufacturer.
HUVEC cultures
HUVEC (Cambrex Bio Science, Walkersville, MD, USA) were cultured in a complete EGM Bulletkit® medium (Cambrex Bio Science). Cells were used for experiments between passage four and five.
Conditioned media
Monocytes and HUVEC (1×106), as well as neutrophils (10×106), were incubated in the presence of LPS (0.1 μg/ml), with or without apyrase (2 U/ml), or 100 μM UDP, unless specified otherwise. For neutrophils, these stimulations were done in the presence or absence of ADA (2 U/ml) to discriminate an effect of endogenous extracellular adenosine. Two units of enzyme completely degraded 100 μM substrate (ATP for apyrase and adenosine for ADA) in 1 ml in 5 min, as determined by HPLC. Monocyte and neutrophil stimulation was performed for 5 h in a 5-ml sterile tube and HUVEC in a 24-well plate at 37°C in a humid atmosphere containing 5% CO2. Cell samples were then centrifuged (1000 g, 10 min, 24°C), and the resulted supernatants (conditioned media) were used for neutrophil transmigration assays and/or IL-8 quantification. For transmigration assays, the supernatants of LPS-treated monocytes were passed through a 1-ml polymyxin B column to remove the endotoxin (Pierce). Chromatography was carried out at gravity flow following the manufacturer’s instruction. To improve LPS extraction, the flow was stopped for 30 min once the monocyte medium had immersed the matrix. In selected supernatants, UDP was removed by a 30-min incubation with apyrase.
Neutrophil in vitro transmigration assay
Neutrophil transmigration assay was carried out in a Boyden chamber system [19] with some modifications. Briefly, cell culture inserts (3 μm pore size) were used to form dual compartments in a Falcon™ 24-well culture plate (Becton Dickinson, Franklin Lakes, NJ, USA). The polyethylene membrane filters (6.4 mm diameter) of the inserts were coated successively with 1% (w/v) gelatine (overnight; Sigma Chemical Co.), 0.006% (v/v) stabilized human fibronectin (2 h; Biomedical Technologies, Stoughton, MA, USA), and 15 × 104 HUVEC (2 days; Cambrex Bio Science). When the endothelial cells were confluent, the conditioned media of treated monocytes (0.7 ml) were loaded to the lower compartment of the Boyden chamber and freshly isolated human neutrophils (1×106 cells in 0.2 ml 5% FBS in RPMI 1640) on the HUVEC monolayer. In each assay, positive and negative controls for neutrophil migration were performed using 4 ng/ml hrIL-8 diluted in monocyte diluent (5% FBS in RPMI 1640) or the monocyte diluent alone, respectively. Neutrophils were allowed to migrate for 3 h at 37°C and 5% CO2. The cells that had crossed the HUVEC layer were collected from the lower compartment and counted with a hema-cytometer.
IL-8 ELISA
IL-8 in the conditioned media was measured by Flexia ELISA (Medicorp), following the manufacturer’s instructions. hrIL-8 was used as a standard.
Neutrophil in vivo transmigration assay (murine air pouch)
The animal protection committee of Université Laval (Québec, QC, Canada) approved the experimental protocol. Air pouches were formed on the dorsum of 10- to 12-week-old CD-1 mice (Charles River, St.-Colomban, Canada) by the s.c. injection of 4 ml sterile air on Day 0 and 3 ml on Day 4. On Day 7, samples of 1 ml LPS (0.2 μg), with or without apyrase (2 U), or the diluent (PBS) alone were injected into the air pouches. The mice were killed 4 h later by CO2 asphyxiation. The cells that migrated in the pouches were collected with a 2-ml wash with 5 mM EDTA in sterile PBS and counted with a hemacytometer. Cell subpopulations were distinguished by Diff-Quick staining of cytospins and/or by flow cytometry.
Statistical analysis
Student’s t test was performed using Excel software (Microsoft® Office OneNote™ 2003).
RESULTS
Extracellular nucleotides regulate neutrophil transmigration in vitro
To determine if extracellular nucleotides are involved in neutrophil migration toward the media of LPS-stimulated monocytes, freshly isolated human monocytes were stimulated with LPS in the presence or absence of apyrase, an enzyme that breaks down tri (e.g., ATP, UTP)- and diphosphonucleosides (e.g., ADP, UDP), the natural agonists of P2 receptors. The conditioned media of monocytes were subsequently tested for neutrophil migration in a modified Boyden chamber assay, where neutrophils were allowed to migrate across a membrane coated with a monolayer of endothelial cells (HUVEC). As shown in Figure 1, the media of LPS-stimulated monocytes were more chemotactic than the media where apyrase was added simultaneously with LPS. No decrease in neutrophil migration was observed with heat-inactivated apyrase (95°C, 5 min; Fig. 1).
Fig. 1.
Neutrophil transmigration to the media of LPS-stimulated monocytes is dependent on extracellular nucleotides. Freshly isolated human monocytes (1×106) were stimulated with LPS (0.1 μg/ml) in the absence or presence of apyrase (2 U/ml), as described in Materials and Methods. The supernatants of treated monocytes were passed through a polymyxin B column to remove the endotoxin and were subsequently tested as neutrophil chemoattractants in a Boyden chamber transmigration assay. The media of untreated monocytes designated as “medium” were also tested. Monocyte diluent (RPMI 1640 with 5% FBS), alone or with hrIL-8 (4 ng/ml), was used as negative and positive control, respectively. The media of monocytes incubated with 100 μM UDP were also tested for neutrophil migration. These data represent the mean ± SEM of four independent experiments with cells of eight blood donors. One hundred percent was set for the media of LPS-treated monocytes and depending on experiment, ranged from 0.22 to 0.53 × 106 neutrophils (*, P<0.05). Mϕ, monocyte; Apy, apyrase; b.Apy, boiled (heat-inactivated) apyrase.
These above results suggest that LPS stimulated nucleotide release from monocytes, which in turn, increased the chemotactic potency of their media. In separate experiments, addition of 100 μM ATP, ADP, UTP, or UDP to the monocyte diluent did not increase neutrophil migration (data not shown), suggesting that nucleotides did not activate neutrophil migration directly. We then hypothesized that extracellular nucleotides augment LPS-mediated neutrophil recruitment via an autocrine pathway leading to increased production of IL-8, a potent neutrophil chemoattractant released by LPS-stimulated monocytes [20]. To assess the role of IL-8 in our in vitro model, the media of stimulated monocytes were treated with IL-8 neutralizing antibodies prior to a transmigration assay. As a result of this treatment, the media containing IL-8 neutralizing antibodies recruited significantly fewer neutrophils than the corresponding media of reference (Fig. 2), confirming that IL-8 was a major neutrophil chemoattractant of LPS-activated monocytes. In contrast, the presence of IL-8 neutralizing antibodies did not further reduce neutrophil migration to the media of monocytes stimulated with LPS in the presence of apyrase (Fig. 2). In addition, apyrase inhibited IL-8 secretion significantly in LPS-treated monocytes (Fig. 3A). Heat-inactivated apyrase (95°C, 5 min) did not affect LPS-induced IL-8 release (data not shown). Thus, these results suggest that monocyte IL-8 release induced by LPS is nucleotide-dependent.
Fig. 2.
Nucleotide-dependent neutrophil migration to the media of LPS- and UDP-treated monocytes is a result of IL-8. The monocyte media were prepared as described in Materials and Methods. Before the transmigration assay, media were incubated overnight at 4°C, with or without IL-8 neutralizing antibodies (nAb; MAB208, 10 ug/ml) or with an irrelevant mouse IgG1 antibody (cAb) as a negative control. The transmigration assays with freshly isolated neutrophils were then performed. Monocyte diluent (5% FBS in RPMI 1640), with and without hrIL-8 (4 ng/ml), was used as positive and negative controls, respectively. Medium refers to the media of untreated monocytes. The means ± SEM of four independent experiments with monocytes and neutrophils from eight different blood donors are given. One hundred percent was set with the media of LPS-treated monocytes and depending on the assay, ranged from 0.34 to 0.68 × 106 neutrophils. The neutralization of IL-8 with a specific antibody significantly diminished neutrophil migration to the media of LPS- and UDP-treated monocytes but not to the media of monocytes treated with LPS in the presence of apyrase (*, P<0.05; NS, Not significant).
Fig. 3.
LPS-induced IL-8 release in human primary monocytes is abrogated by nucleotide scavenging. Indicated cells were incubated for 5 h with LPS (0.1 μg/ml), with or without apyrase (2 U/ml), a scavenger of released nucleotides. IL-8 levels of the resulted conditioned media were determined by ELISA, as described in Materials and Methods. These data represent the mean ± SD of at least three blood donors. Medium refers to the media of untreated monocytes. (A) Monocytes (open bars) and HUVEC (hatched bars; 1×106 cell per sample). (B) Neutrophils (10×106 cells per sample). LPS stimulation of neutrophils was carried out in the presence (bold hatched bars) or absence (light hatched bars) of ADA to remove adenosine, released spontaneously from these cells, which inhibits their inflammatory responses. Where indicated, neutrophils were preincubated with ADA (2 U/ml) for 30 min at 37°C prior to stimulation with LPS (*, P=0.002).
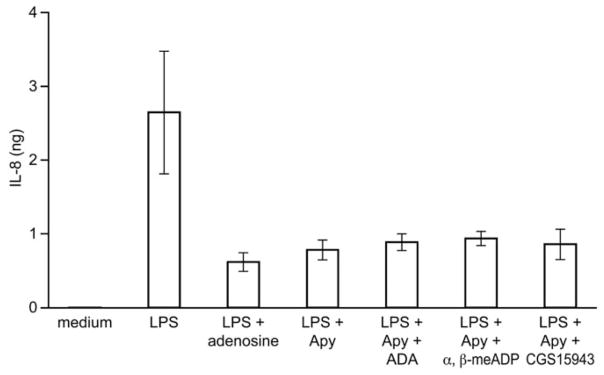
As adenosine is a potent inhibitor of IL-8 release in LPS-stimulated monocytes (Fig. 4 and ref. [21]), we tested whether it was involved in apyrase-mediated inhibition of IL-8 release. Indeed, adenosine could have been generated in our assays as a result of the hydrolysis of ATP, secreted from endotoxin-treated monocytes, by the concerted action of the exogenous apyrase and an endogenous ecto-5′-nucleotidase. To distinguish this effect, the following inhibitors of adenosine signaling were added to monocytes together with LPS and apyrase: CGS15943, a nonselective antagonist of adenosine receptors; ADA, an adenosine scavenger; and α,β-meADP, an inhibitor of ecto-5′-nucleotidase. The inhibition of IL-8 release by apyrase was not affected by any of these treatments (Fig. 4), excluding an effect of adenosine. Thus, apyrase inhibited IL-8 secretion in monocytes specifically by hydrolyzing the agonist(s) of P2 receptors.
Fig. 4.
The inhibition of LPS-activated IL-8 release by apyrase is not a result of the formation of adenosine. Freshly isolated monocytes (1×106) were incubated with LPS (0.1 μg/ml) and apyrase (2 U/ml), plus one of the following inhibitors of adenosine signaling: CGS15943 (100 μM), ADA (2 U/ml), or α,β-meADP (10 μM). The IL-8 content of the monocyte supernatants was estimated by ELISA. The media of untreated monocytes, designated as medium, were used as a control. The media of monocytes stimulated with LPS in the presence of adenosine (10 μM) were also analyzed to show inhibitory effect of adenosine on IL-8 release. The means ± SD of monocytes purified from three blood donors are shown.
LPS-stimulated human endothelial cells and neutrophils release IL-8 in a nucleotide-independent manner
As HUVEC and neutrophils also release IL-8 following LPS stimulation [22, 23], we examined whether this response could be affected by apyrase. In contrast to our findings in monocytes, apyrase failed to inhibit IL-8 release from LPS-stimulated HUVEC and neutrophils (Fig. 3, A and B). Following LPS stimulation, IL-8 release was as high in HUVEC as in monocytes (Fig. 3A) but was ~200-fold lower in neutrophils (Fig. 3B). As adenosine is released spontaneously by neutrophils in culture and makes these cells refractory to stimulation [24], neutrophil stimulation was also carried out in the presence of the adenosine scavenger ADA. Although LPS-activated neutrophils secreted more IL-8 when adenosine was removed from their media with ADA, nucleotides had no effect on this release, as shown with apyrase (Fig. 3B). Therefore, upon LPS stimulation, a different mechanism is responsible for IL-8 release in HUVEC and neutrophils when compared with monocytes.
P2 receptors mediate LPS- and UDP-induced IL-8 release in human primary monocytes
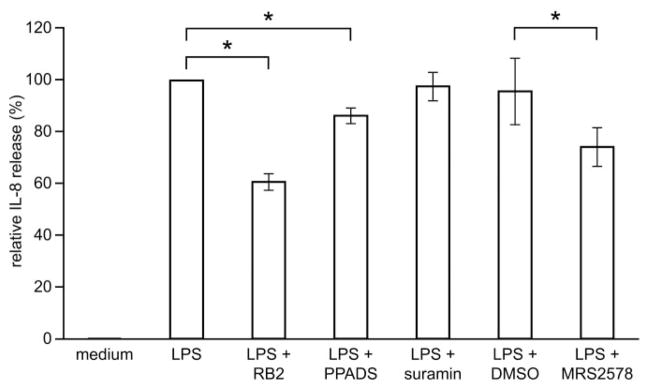
The above experiments suggest that LPS-induced IL-8 release in human monocytes is associated with the release of nucleotides and autocrine activation of P2 receptor(s). To verify this hypothesis, we measured the release of IL-8 in the presence of the following P2 receptor antagonists: RB-2, suramin, PPADS, and MRS2578 [25, 26]. RB-2 was the most efficient inhibitor of IL-8 release (40% inhibition) followed by MRS2578 (25%) and PPADS (14%; Fig. 5). This order of antagonist potencies and suramin’s inability to inhibit IL-8 release in monocytes (Fig. 5) suggest that the P2Y6 receptor is involved in LPS-induced IL-8 release by monocytes [26].
Fig. 5.
P2Y6 receptor antagonists decrease LPS-induced IL-8 release in monocytes. Samples of 1 × 106 monocytes were preincubated with 100 μM of the P2 receptor antagonists RB-2, PPADS, and suramin or with a specific P2Y6 antagonist, 10 μM MRS2578, for 30 min at 37°C and then stimulated with 0.1 μg/ml LPS for 5 h. The media of untreated monocytes, designated as medium, were also analyzed as a control. As MRS2578, as well as its vehicle (0.01% DMSO), induced some release of IL-8 when added to the cells alone, these amounts of IL-8 were estimated and subtracted where appropriate. The mean ± SD of purified monocytes from at least three blood donors is shown (*, P<0.05). One hundred percent corresponds to 2.5 ± 1.2 ng IL-8 released from 1 × 106 monocytes.
To determine if a P2 receptor can trigger the release of IL-8 in monocytes, we next incubated these cells with the P2 agonists: 100 μM ATP, ADP, UTP, or UDP. As a result, monocytes released important amounts of IL-8 when incubated with UDP (Fig. 6A) but not with the other nucleotides (data not shown). These data were in favor of the P2Y6 receptor, which is activated selectively by UDP. It is interesting that HUVEC and human primary neutrophils also express P2Y6 mRNA [4]. However, these cells did not release any IL-8 in response to UDP (Fig. 6A). In further support for the P2Y6 receptor in IL-8 release in monocytes, MRS2578, a selective antagonist of this receptor, decreased UDP-induced IL-8 release by 90% (Fig. 6B).
Fig. 6.
UDP induces IL-8 release in human primary monocytes via the activation of P2Y6. (A) Human neutrophils (10×106 cells per sample), monocytes, and HUVEC (1×106 cells per sample) were stimulated with 100 μM UDP for 5 h as described in Materials and Methods. The stimulation of neutrophils was carried out in the presence of ADA. The secretion of IL-8 in the media of these cells was evaluated by ELISA. The media of untreated cells, designated as medium, were also analyzed as a control. UDP activated the release of IL-8 in monocytes (open bars), but not in HUVEC (light hatched bars) and neutrophils (bold hatched bars). (B) Freshly isolated monocytes (1×106) were preincubated with 10 μM of the P2Y6 antagonist MRS2578 for 30 min (37°C) and then stimulated with 100 μM UDP for 5 h. The media of untreated monocytes, designated as medium, were used as a control. Other controls with 10 μM MRS2578 and 0.01% DMSO were also performed, and their IL-8 contents were subtracted where appropriate. The mean ± SD of three cell (blood) donors or three different HUVEC donors is shown. One hundred percent corresponds to 0.5 ± 0.32 ng IL-8 released.
As UDP-stimulated monocytes released important amounts of IL-8, the media of these cells were tested for neutrophil migration. As anticipated, they were chemotactic for neutrophils (Fig. 1), which was in large part a result of IL-8, as shown with IL-8 neutralizing antibodies (Fig. 2). However, even in the presence of neutralizing antibodies, these media still retained ~50% of their chemotactic activity. This remaining activity could not be attributed to UDP, as this nucleotide was not a chemoattractant for neutrophils in our transmigration model (see Results, Extracellular nucleotides regulate neutrophil transmigration in vitro). In addition, the treatment of the conditioned media from UDP-stimulated monocytes with apyrase, to deplete remaining UDP molecules prior to the transmigration experiment, had no effect on neutrophil transmigration (data not shown).
Additional and confirmatory experiments were performed using monocytes purified by MACS®. As expected, this highly purified monocyte preparation (>95%) released IL-8 in response to UDP, and LPS-induced IL-8 release was inhibited by apyrase and RB-2 to a similar degree observed for the monocytes purified by adherence (data not shown). Thus, extracellular nucleotides regulate LPS responses in adherent and circulating monocytes.
Extracellular nucleotides mediate LPS-induced neutrophil migration in vivo
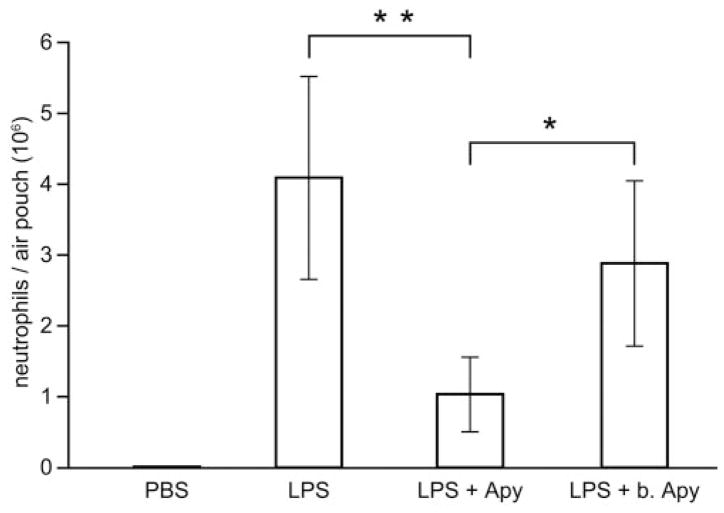
As extracellular nucleotides mediate LPS-induced neutrophil migration in vitro, we next investigated if these molecules play a similar role in vivo using the mouse air-pouch model. This model allows precise quantification of extravasated neutrophils in the pouch. LPS injection into an air pouch induced robust neutrophil migration (4×106 cells, n=6; Fig. 7). Coadministration of LPS and apyrase resulted in a fourfold decrease in neutrophil migration (1×106 cells, n=6, P=0.0003). Heat-inactivated apyrase had no effect on LPS-induced neutrophil migration. These results suggest that extracellular nucleotides are instrumental in mediating neutrophil recruitment induced by LPS into a mouse air pouch.
Fig. 7.
Extracellular nucleotides mediate LPS-induced neutrophil migration to mouse air pouches, which were raised on the backs of female CD-1 mice. Six days later, mice were split in three groups and were injected in the air pouches with 1 ml PBS (control) and LPS (0.2 μg), with and without apyrase (2 U) in PBS, respectively. The cells (largely neutrophils) accumulated in the pouches were collected 4 h later and analyzed as described in Materials and Methods. These data represent the mean ± SD of at least five mice per group (**, P=0.0003; *, P=0.01).
DISCUSSION
This study shows that LPS-elicited neutrophil migration is mediated by extracellular nucleotides in vitro and in vivo. Indeed, the stimulation of monocytes with LPS in the presence of apyrase significantly decreased the release of chemotactic activity by these cells. In vitro experiments using human neutrophils revealed that this chemotactic activity was mainly a result of the chemokine IL-8. We also showed that the media of UDP-treated monocytes exhibited significant chemotactic activity, mainly as a result of the presence of IL-8. As for the media of LPS-treated monocytes, IL-8 neutralizing antibodies did not completely prevent neutrophil migration to these media. This suggests that LPS- and UDP-treated monocytes released more than one neutrophil chemoattractant. It is noteworthy that UDP per se did not activate any neutrophil migration in our system, although it was shown by others to trigger the migration of immature dendritic cells [27].
It is interesting that extracellular nucleotides were instrumental in LPS-elicited neutrophil migration into the mouse air pouch. LPS is a potent inducer of neutrophil infiltration in this model [28]. The striking and novel observation made here is that neutrophil migration into the pouches can be almost totally abrogated by apyrase. This suggests that LPS induces the release of nucleotide(s), which in turn trigger neutrophil infiltration by activating some P2 receptors. It is conceivable that nucleotides could be released from the air-pouch resident cells, most of which are fibroblasts and macrophages [29]. In agreement with this hypothesis, mouse peritoneal cells, which contain a majority of macrophages, released ATP following incubation with LPS [30]. It has not been reported whether LPS can induce the release of nucleotides other than ATP.
Further experiments performed on human primary monocytes pointed to P2Y6 as the nucleotide receptor mediating IL-8 release. First, this could be suspected from the susceptibility of this response to P2 receptor antagonists. RB-2 and MRS2578, both being potent P2Y6 antagonists [26], were the most potent inhibitors of LPS-induced IL-8 release, and PPADS and suramin had a weak or no effect, respectively (Fig. 5). Second, IL-8 secretion in monocytes was induced selectively by UDP (Fig. 6 and ref. [30]), a specific agonist of P2Y6 [1]. Neutrophils and HUVEC also express P2Y6 mRNA [4], but unlike monocytes, these cells did not respond to UDP by secreting IL-8. This suggests that these cells lack functional P2Y6 or that their receptor is not coupled to signaling pathways leading to IL-8 release. The ability of apyrase and P2Y6 antagonists to significantly decrease IL-8 release from LPS-treated monocytes supports the hypothesis of autocrine/paracrine P2Y6 activation. RB-2 and MRS2578 are the best commercially available P2Y6 blockers, but they inhibited LPS-induced IL-8 release less efficiently than apyrase. This could have been predicted from their limited potencies [26]. It is noteworthy that the properties of RB-2 vary from batch to batch [31], and MRS2578 is not stable in aqueous solutions [26]. MRS2578 blocked IL-8 release more efficiently in UDP-stimulated monocytes than in LPS-treated cells. We speculate that the intracellular signaling pathways induced by LPS alone may amplify the signal of a weak P2Y6 stimulation.
Consistent with the data presented here, UDP has previously been reported to stimulate the secretion of IL-8 in human primary monocytes [30], and P2Y6 has been implicated in the release of IL-8 in mature dendritic and epithelial cells [27, 30, 32]. However, the role of this receptor in IL-8 release in THP-1 cells is a more controversial issue. Warny et al. showed that UDP was a potent agonist of IL-8 release in this cell line [30], but this result could not be reproduced by Cox and co-workers [33]. It is likely that the differences between the cells used in these two works were responsible for these discrepancies, even if both originated from the same parental cell line.
In summary, the data presented in this work suggest that extracellular nucleotides mediate neutrophil migration in LPS-induced inflammation in vitro and in vivo. In extension, this finding reveals an important function of extracellular nucleotides in innate immune defenses against pathogenic, Gram-negative bacteria. It is interesting that another nucleotide receptor, P2Y2, expressed on murine neutrophils, has recently been involved in neutrophil migration caused by the Gram-positive bacteria Staphylococcus aureus [5]. Altogether, these data suggest a general implication of nucleotides in neutrophil migration toward bacterial infections.
Acknowledgments
This work was supported by grants to J. S. from the Canadian Institutes of Health Research (CIHR; MOP-68957) and from The Arthritis Society (TAS; 01/0078). F. K. was a recipient of a fellowship from the CIHR/Wyeth Pharmaceuticals, F. B. Y. of a scholarship from “Fonds de la Recherche sur l’Arthrite et les Maladies Rhumatismales” (FRAMR), J. L. of a scholarship from the CIHR, P. A. T. of a scholarship from the “Fonds de la Recherche en Santé du Québec,” and J. S. of a New Investigator award from the CIHR. The authors are grateful to Dr. M. Dufour for flow cytometry analysis.
References
- 1.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 2.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 3.Dubyak GR. Purinergic signaling at immunological synapses. J Auton Nerv Syst. 2000;81:64–68. doi: 10.1016/s0165-1838(00)00155-7. [DOI] [PubMed] [Google Scholar]
- 4.Jin J, Dasari VR, Sistare FD, Kunapuli SP. Distribution of P2Y receptor subtypes on haematopoietic cells. Br J Pharmacol. 1998;123:789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 6.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Shukla A, Namiki S, Insel PA, Junger WG. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukoc Biol. 2004;76:245–253. doi: 10.1189/jlb.0204066. [DOI] [PubMed] [Google Scholar]
- 8.Meshki J, Tuluc F, Bredetean O, Ding Z, Kunapuli SP. Molecular mechanism of nucleotide-induced primary granule release in human neutrophils: role for the P2Y2 receptor. Am J Physiol Cell Physiol. 2004;286:C264–C271. doi: 10.1152/ajpcell.00287.2003. [DOI] [PubMed] [Google Scholar]
- 9.Kukulski F, Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, Kirley TL, Sévigny J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure, function, relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in RAW 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1-β release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai M, Goepfert C, Kaczmarek E, Robson SC. CD39 modulates IL-1 release from activated endothelial cells. Biochem Biophys Res Commun. 2000;270:272–278. doi: 10.1006/bbrc.2000.2410. [DOI] [PubMed] [Google Scholar]
- 16.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1β and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 17.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 18.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 19.Issekutz AC, Chuluyan HE, Lopes N. CD11/CD18-independent transendothelial migration of human polymorphonuclear leukocytes and monocytes: involvement of distinct and unique mechanisms. J Leukoc Biol. 1995;57:553–561. doi: 10.1002/jlb.57.4.553. [DOI] [PubMed] [Google Scholar]
- 20.Galligan CL, Coomber BL. Effects of human IL-8 isoforms on bovine neutrophil function in vitro. Vet Immunol Immunopathol. 2000;74:71–85. doi: 10.1016/s0165-2427(00)00162-8. [DOI] [PubMed] [Google Scholar]
- 21.Bouma MG, Stad RK, Van den Wildenberg FA, Buurman WA. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol. 1994;153:4159–4168. [PubMed] [Google Scholar]
- 22.Beck GC, Rafat N, Brinkkoetter P, Hanusch C, Schulte J, Haak M, van Ackern K, van der Woude FJ, Yard BA. Heterogeneity in lipopolysaccharide responsiveness of endothelial cells identified by gene expression profiling: role of transcription factors. Clin Exp Immunol. 2006;143:523–533. doi: 10.1111/j.1365-2249.2006.03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattar K, Fink L, Fietzner K, Himmel B, Grimminger F, Seeger W, Sibelius U. Cell density regulates neutrophil IL-8 synthesis: role of IL-1 receptor antagonist and soluble TNF receptors. J Immunol. 2001;166:6287–6293. doi: 10.4049/jimmunol.166.10.6287. [DOI] [PubMed] [Google Scholar]
- 24.Flamand N, Lefebvre J, Lapointe G, Picard S, Lemieux L, Bourgoin SG, Borgeat P. Inhibition of platelet-activating factor biosynthesis by adenosine and histamine in human neutrophils: involvement of cPLA2α and reversal by lyso-PAF. J Leukoc Biol. 2006;79:1043–1051. doi: 10.1189/jlb.1005614. [DOI] [PubMed] [Google Scholar]
- 25.Mamedova LK, Joshi BV, Gao ZG, von Kugelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1770. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunschweiger A, Muller CE. P2 receptors activated by uracil nucleotides—an update. Curr Med Chem. 2006;13:289–312. doi: 10.2174/092986706775476052. [DOI] [PubMed] [Google Scholar]
- 27.Idzko M, Panther E, Sorichter S, Herouy Y, Berod L, Geissler M, Mockenhaupt M, Elsner P, Girolomoni G, Norgauer J. Characterization of the biological activities of uridine diphosphate in human dendritic cells: influence on chemotaxis and CXCL8 release. J Cell Physiol. 2004;201:286–293. doi: 10.1002/jcp.20070. [DOI] [PubMed] [Google Scholar]
- 28.Vandal K, Rouleau P, Boivin A, Ryckman C, Talbot M, Tessier PA. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171:2602–2609. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- 29.Willoughby DA, Sedgwick AD, Giroud JP, Al-Duaij AY, de Brito F. The use of the air pouch to study experimental synovitis and cartilage breakdown. Biomed Pharmacother. 1986;40:45–49. [PubMed] [Google Scholar]
- 30.Warny M, Aboudola S, Robson SC, Sévigny J, Communi D, Soltoff SP, Kelly CP. P2Y6 nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J Biol Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- 31.Glanzel M, Bultmann R, Starke K, Frahm AW. Constitutional isomers of reactive blue 2—selective P2Y-receptor antagonists? Eur J Med Chem. 2003;38:303–312. doi: 10.1016/s0223-5234(02)01449-6. [DOI] [PubMed] [Google Scholar]
- 32.Khine AA, Del Sorbo L, Vaschetto R, Voglis S, Tullis E, Slutsky AS, Downey GP, Zhang H. Human neutrophil peptides induce interleukin-8 production through the P2Y6 signaling pathway. Blood. 2006;107:2936–2942. doi: 10.1182/blood-2005-06-2314. [DOI] [PubMed] [Google Scholar]
- 33.Cox MA, Gomes B, Palmer K, Du K, Wiekowski M, Wilburn B, Petro M, Chou CC, Desquitado C, Schwarz M, Lunn C, Lundell D, Narula SK, Zavodny PJ, Jenh CH. The pyrimidinergic P2Y6 receptor mediates a novel release of proinflammatory cytokines and chemokines in monocytic cells stimulated with UDP. Biochem Biophys Res Commun. 2005;330:467–473. doi: 10.1016/j.bbrc.2005.03.004. [DOI] [PubMed] [Google Scholar]