Abstract
Though plans to eliminate malaria from the island of Hispaniola have recently received much attention, arbovirus surveillance continues to be largely neglected in Haiti. To support surveillance efforts and encourage vector-control strategies, a cross-sectional study of dengue virus (DENV) and West Nile virus (WNV) transmission was conducted using standard seroepidemiological methods. Blood samples (N = 673) were collected from 278 males and 395 females from three locations in the Ouest and Sud-Est Departments of Haiti. Serum was separated and tested for the presence of anti-DENV and anti-WNV immunoglobulin G (IgG) antibodies using an indirect enzyme-linked immunosorbent assay (ELISA). Anti-DENV IgG antibodies were detected in 72.1% (95% confidence interval [CI] = 68.7, 75.5) of the sample population; with no significant differences in seroprevalence by study location, participant gender, or age group (P > 0.1, in all tests). Anti-WNV IgG antibodies were detected in only 1% (95% CI = 0.3, 1.8) of the sample population, all which originated from participants located in Gressier. The high prevalence of anti-DENV IgG antibodies among all age groups, including those in the youngest age group (2–5 years of age), suggests hyperendemic transmission of DENV in the Ouest and Sud-Est Departments of Haiti. In contrast, the relative absence of anti-WNV IgG antibodies, even among older population members, further supports the notion that WNV transmission in this population is largely absent. These findings highlight the large burden of disease from DENV and the need for enhanced arbovirus surveillance and implementation of vector control strategies throughout Haiti.
Introduction
Together with Plasmodium falciparum malaria, infections from dengue virus (DENV) and West Nile virus (WNV) remain the three most commonly diagnosed mosquito-borne illnesses in the United States. In these patients, recent travel to endemic tropical countries such as Haiti is commonly identified as the source of infection.1 DENV, a positive-sense, single-stranded RNA virus of the genus Flavivirus, is transmitted to humans primarily by Aedes aegypti and Aedes albopictus mosquitoes.2,3 Previous exposure to one of four serotypes of DENV (1–4) can provide lifelong immunity, however, infection from one serotype does not provide cross-immunity for future infections by other serotypes.4 To the contrary, subsequent infections can result in more severe presentation of the disease, including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), which can be fatal.5 WNV, also a member of the genus Flavivirus, is a zoonotic arbovirus that is maintained through a mosquito-avian host cycle with Culex mosquito species responsible for the majority of transmission to humans.6 In contrast to DENV, outbreaks of WNV are often self-limiting, with humans acting as dead-end hosts for the virus, where the potential for epidemics is directly influenced by a combination of the density of mosquito vectors and the presence of avian reservoirs.7
Previous studies have identified the presence of DENV antibodies in native Haitians; however, most recent studies have focused on active infections and/or seroconversion in nongovernment organization workers or missionaries to Haiti; thus, the disease burden of DENV in the native population remains to be adequately characterized.8–10 Furthermore, even though cases of WNV have been reported in Haiti and WNV has been detected in birds and mosquitos from the Dominican Republic, no active surveillance for WNV has taken place for over a decade.11–13 Given the frequent reports of undifferentiated febrile illness, abundance of both Aedes and Culex mosquito vectors, and absence of a national arbovirus surveillance system, a cross-sectional seroepidemiological survey was conducted.14–17 By screening an indigenous sample population ranging in age from 2 to 80 years for anti-DENV and anti-WNV immunoglobulin G (IgG), this study was able to further characterize trends in the DENV transmission and for the first time, investigate the population seroprevalence of antibodies toward WNV in Haiti.
Materials and Methods
Ethical approval and sample collection.
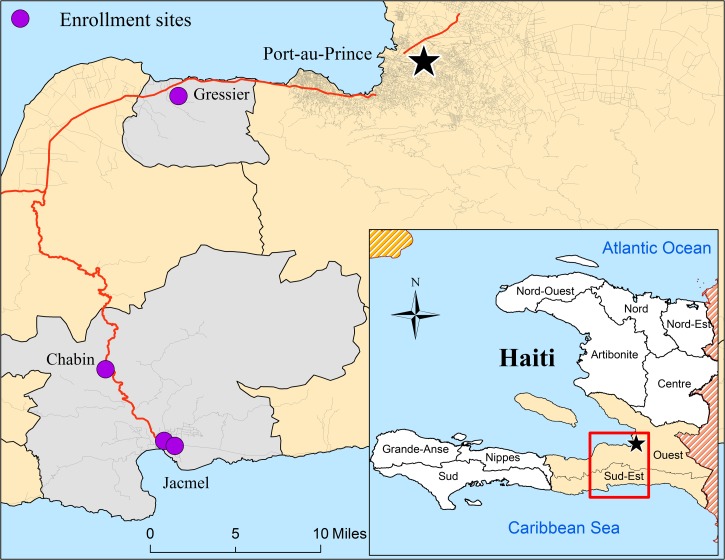
Ethical approval to conduct this research was obtained from the Haitian-based Ethical Review Committee, the University of Florida Institutional Review Board, and the Office of Research Protections, United States Army Medical Research and Materials Command. Between February and May 2013, asymptomatic (nonfebrile) participants were enrolled from two schools, a rural community, and a clinic in the Ouest and Sud-Est Departments of Haiti. A map of the enrollment locations and demographic information by enrollment site are presented in Figure 1 and Table 1. Healthy children were enrolled from the Christianville School in Gressier and from the Hossana Baptist School in Jacmel. In the rural village of Chabin, participants were enrolled from community members seeking general health services at a mobile clinic. Patients and healthy family members from the Portail Léogâne Clinic in Jacmel were enrolled on a voluntary basis. After informed consent, approximately 3 mL of blood was drawn by venipuncture, collected in 8.5-mL serum separator tubes (No. 367988, BD Vaccutainer®, Becton, Dickinson, and Co., Franklin Lakes, NJ), and immediately separated by centrifugation. Aliquots of serum were frozen at −80°C and stored in the University of Florida Field Laboratory in Gressier until shipment to the Emerging Pathogens Institute in Gainesville, FL. A total of 673 serum samples that included 278 males and 395 females aged between 2 and 80 years were available for serological analyses.
Figure 1.
Map of enrollment sites in the Ouest and Sud-Est Departments of Haiti. Serum was isolated from blood samples collected from nonfebrile study participants in the Ouest and Sud-Est Departments of Haiti (beige, inset map). Enrollment sites (purple dots) were located in the communes of Gressier and Jacmel (gray); consisting of a school in Gressier, a hospital and school in Jacmel, and a mobile clinic in the rural community of Chabin.
Table 1.
Study population characteristics by site of enrollment
| Site of enrollment | Demographic factors | ||||
|---|---|---|---|---|---|
| Sample | Age (years) | Gender | |||
| Size (n) | Average | Standard deviation | Male (%) | Female (%) | |
| Christianville school | 476 | 12.3 | 4.6 | 42.0 | 58.0 |
| Hosana Baptist school | 96 | 8.3 | 3.1 | 51.0 | 49.0 |
| Protail Leogane clinic | 63 | 27.2 | 15.3 | 30.2 | 69.8 |
| Chabin community | 38 | 29.6 | 20 | 26.3 | 73.7 |
| Total | 673 | 14.1 | 9.8 | 41.3 | 58.7 |
The sample size (n) and demographics of the study participants are listed by study enrollment site.
Enzyme-linked immunosorbent assay (ELISA) protocol.
Serum samples were screened for the presence of anti-DENV and anti-WNV antibodies using a standardized IgG ELISA protocol.18 Suckling mouse brain (smb), sucrose-acetone-extracted antigen preparations for WNV (strain NY385-99) and DENV (Dengue 1 Hawaii) viruses were obtained from the World Reference Center for Emerging Viruses and Arboviruses collection at the University of Texas Medical Branch.19 Lyophilized DENV and WNV antigens were provided along with the corresponding mouse immune ascites fluid and normal mouse brain antigen for positive and negative controls, respectively. Viral antigens were reconstituted with ultra-pure water, diluted in 50 mM bicarbonate buffer, coated on 96-well ELISA plates at a final concentration of 5 μg/mL, and allowed to incubate overnight at 4°C. After incubation, the plates were washed four times in 0.05% phosphate-buffered saline (PBS)-T and then blocked overnight at 4°C to avoid nonspecific binding. All serum samples were inactivated at 56°C for 15 minutes, diluted 1:100 in 5% nonfat skim milk-PBS before the addition of 100 μL aliquots in duplicate to each plate, and allowed to incubate for 2 hours at room temperature. After an additional wash, horseradish peroxidase conjugated rabbit antihuman IgG secondary antibody was diluted 1:500 in 5% NFSM-PBS and added to the plates. For chromatographic development, plates were washed a final time before the addition of 100 μL of 3,3′,5,5′-tetratmethylbenzidine substrate to each well, developed in the dark for 30 minutes at room temperature, and stopped with 100 μL of sulfuric acid (2 N). The absorbance values for each sample were measured at 450 nm and averaged to obtain a single absorbance value for each sample.
Classification of seropositive and seronegative samples.
The absorbance threshold of a seropositive sample was determined using the population distributions of the ELISA results and validated using the absorbance values of the positive and negative controls. The mean (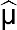 ) absorbance and sample standard deviations (
) absorbance and sample standard deviations (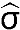 ) were estimated by fitting normal distributions to histograms of the ELISA results. A threshold value of three-sample standard deviations from the seronegative population mean (
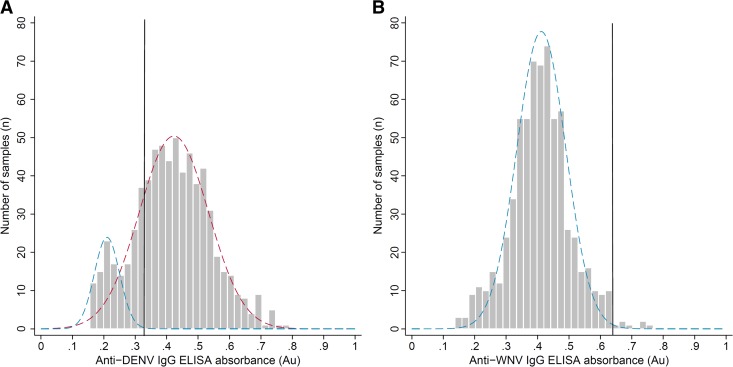
) were estimated by fitting normal distributions to histograms of the ELISA results. A threshold value of three-sample standard deviations from the seronegative population mean (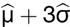 ) was selected to provide a conservative estimate of the seropositive population that excluded 99.7% of seronegative population members. The observed absorbance values, estimated seronegative and seropositive population distributions, and absorbance thresholds for seropositive samples are presented for DENV and WNV in Figure 2
. For DENV, a bimodal distribution was observed with a large peak around 0.42 absorbance units (Au) and a minor peak around 0.21 Au, which represented the seropositive and seronegative populations, respectively. The mean of the suspected seronegative population members was 0.21 Au with a standard deviation of 0.035, giving a seropositive sample threshold of 0.315 Au for the DENV ELISA. For the WNV ELISA, a uniform distribution was observed with a sample mean of 0.42 Au and a sample standard deviation of 0.07, giving a threshold absorbance value for a positive sample of 0.630 Au. The threshold absorbance values obtained from the population distributions were in agreement with the absorbance values from both the positive and negative controls.
) was selected to provide a conservative estimate of the seropositive population that excluded 99.7% of seronegative population members. The observed absorbance values, estimated seronegative and seropositive population distributions, and absorbance thresholds for seropositive samples are presented for DENV and WNV in Figure 2
. For DENV, a bimodal distribution was observed with a large peak around 0.42 absorbance units (Au) and a minor peak around 0.21 Au, which represented the seropositive and seronegative populations, respectively. The mean of the suspected seronegative population members was 0.21 Au with a standard deviation of 0.035, giving a seropositive sample threshold of 0.315 Au for the DENV ELISA. For the WNV ELISA, a uniform distribution was observed with a sample mean of 0.42 Au and a sample standard deviation of 0.07, giving a threshold absorbance value for a positive sample of 0.630 Au. The threshold absorbance values obtained from the population distributions were in agreement with the absorbance values from both the positive and negative controls.
Figure 2.
IgG enzyme-linked immunosorbent assay (ELISA) responses to dengue virus (DENV) and West Nile virus (WNV). The results from the DENV and WNV IgG ELISAs are presented in panels A and B. The average sample absorbance (OD450) from the study participants (gray bars), the distributions of the seronegative (dotted blue lines) and seropositive (dotted red line) population members, and the threshold absorbance used to classify a sample as seropositive (black line).
Statistical comparisons and seroconversion model.
After classification of seropositive and seronegative participants, the ELISA results were matched to demographic information from the study participants including the geographic origin of the sample, age in years, and gender. Age groups were generated from the participants to include large enough samples for statistical comparisons and descriptive statistics. Simple logistic regression models were used to determine the association between DENV or WNV seroprevalence and geographic location, age in years, or gender using STATA v12 (StataCorp©, College Station, TX). Given the long duration of IgG antibodies, the annual seroconversion rate (SCR) for DENV was estimated using a reverse catalytic model without seroreversion as previously described.20 Age-specific seroprevalence rates were derived from population members under 5 years (mean age = 3.41 years) and by individual year for participants between the ages of 6 and 20 years. The age-specific seroprevalence was used to estimate the probability of being seropositive at age (a) by the function P(a) = [1 − exp(−λ × a)]. The sample population SCR (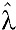 ) was generated using maximum-likelihood estimation with the “optim” function in R version 3.1.0 (www.R-project.org) and multiplied by 100 to express the SCR as a percentage of participants that seroconverted in 1 year as previously described.21 The standard error for the SCR was also generated and used to calculate 95% confidence intervals (CIs). Since the seroprevalence in the population saturated before reaching 100% seroprevalence and never exceeded 85%, the function P(a) was multiplied by 0.8 prior to maximum likelihood estimation to avoid overestimation of the seroconversion rate.
) was generated using maximum-likelihood estimation with the “optim” function in R version 3.1.0 (www.R-project.org) and multiplied by 100 to express the SCR as a percentage of participants that seroconverted in 1 year as previously described.21 The standard error for the SCR was also generated and used to calculate 95% confidence intervals (CIs). Since the seroprevalence in the population saturated before reaching 100% seroprevalence and never exceeded 85%, the function P(a) was multiplied by 0.8 prior to maximum likelihood estimation to avoid overestimation of the seroconversion rate.
Results
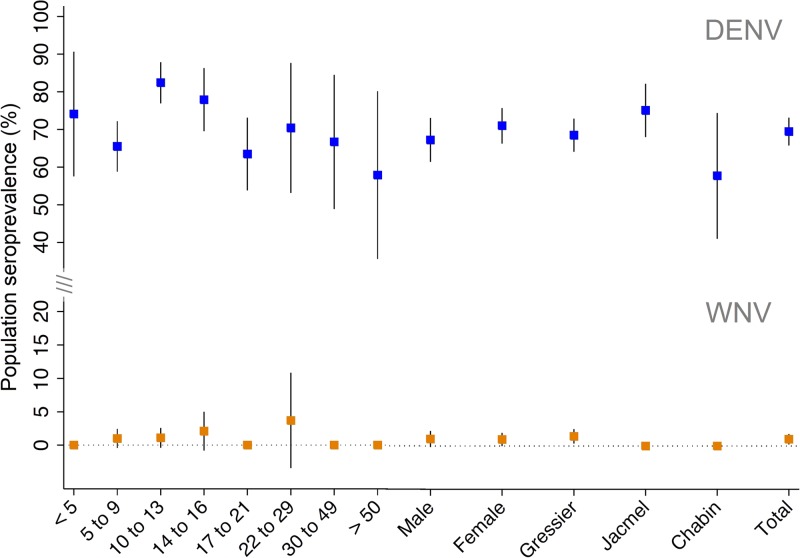
IgG antibodies toward DENV were detected in 485 out of 673 participants, giving a population seroprevalence of 72.1% (95% CI = 68.7, 75.5). IgG antibodies toward WNV were detected in only seven out of 673 participants, giving a population seroprevalence of 1.0% (95% CI = 0.3, 1.8). The sample size, number of positive samples, and seroprevalence to anti-DENV and anti-WNV IgG antibodies listed by geographic location, age group, and gender in Table 2; with point estimates and 95% CIs presented in Figure 3
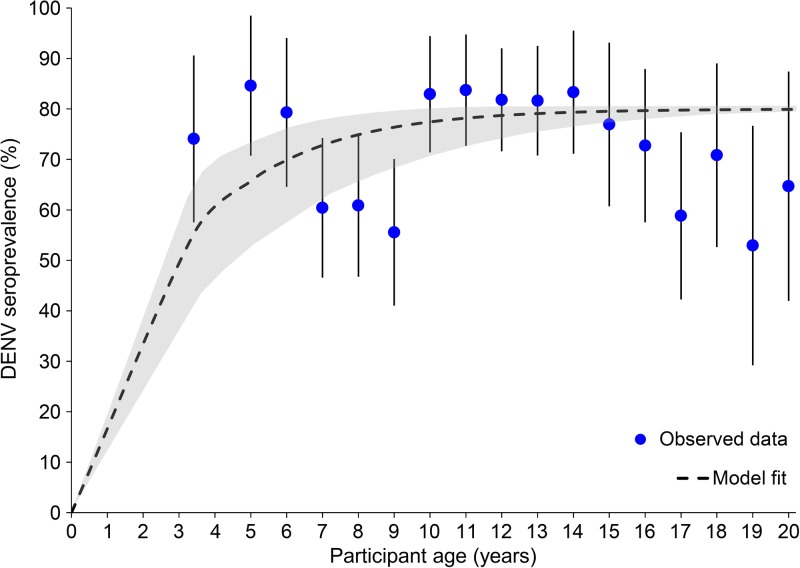
. Compared with female participants, the seroprevalence of IgG antibodies in males was not significantly different for either DENV (odds ratio [OR] = 0.83; P = 0.27) or WNV (OR = 1.1; P = 0.93). For DENV, the seroprevalence of IgG antibodies was not significantly different by geographic location (P = 0.96); however, all seven samples with anti-WNV IgG antibodies were collected from Gressier. The seroprevalence of anti-DENV IgG antibodies in different age groups was highest in those between 10 and 14 years (82.4%; 95% CI = 77.0, 88.0) and lowest in those over 50 years (57.9%; 95% CI = 33.4, 82.3), however, was not significantly different by age group (P = 0.3). Likewise, the seroprevalence of anti-WNV IgG antibodies was not significantly different by age group (P = 0.9), with half of the age groups having no seropositive samples. All of the 95% CIs for the seroprevalence of WNV by age group included zero (Figure 3). Given the high seroprevalence of anti-DENV IgG antibodies in participants under 20 years of age, a reverse catalytic model was used to estimate to annual SCR in this population. The age-specific seroprevalence of anti-DENV IgG antibodies for participants less than 5 years of age (mean age 3.41 years) and the ages of 6–20, and the model fit are presented in Figure 4
. From the model, the estimated annual SCR for DENV was 34.4% (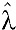 = 0.344; 95% CI = 0.212, 0.477). Since only seven individuals were classified as seropositive for WNV and the seroprevalence of anti-WENV IgG antibodies in different age groups was not significantly different from zero, a seroconversion model was not considered for WNV.
= 0.344; 95% CI = 0.212, 0.477). Since only seven individuals were classified as seropositive for WNV and the seroprevalence of anti-WENV IgG antibodies in different age groups was not significantly different from zero, a seroconversion model was not considered for WNV.
Table 2.
Seroprevalence of antibodies to dengue and WNV
| Demographics factors | Sample size (n) | DEN | WNV | |||
|---|---|---|---|---|---|---|
| No. pos. | Prevalence (%) | No. pos. | Prevalence (%) | |||
| Gender | Male | 278 | 194 | 69.8 | 3 | 1.1 |
| Female | 395 | 291 | 73.7 | 4 | 1.0 | |
| City | Gressier | 476 | 339 | 71.2 | 7 | 1.5 |
| Jacmel | 159 | 123 | 77.4 | 0 | 0.0 | |
| Chabin | 38 | 23 | 60.5 | 0 | 0.0 | |
| Age (years) | 2–5 | 27 | 20 | 74.1 | 0 | 0.0 |
| 6–10 | 194 | 127 | 65.5 | 2 | 1.0 | |
| 10–14 | 188 | 155 | 82.4 | 2 | 1.1 | |
| 14–17 | 95 | 74 | 77.9 | 2 | 2.1 | |
| 17–22 | 96 | 61 | 63.5 | 0 | 0.0 | |
| 22–30 | 27 | 19 | 70.4 | 1 | 3.7 | |
| 30–50 | 27 | 18 | 66.7 | 0 | 0.0 | |
| Over 50 | 19 | 11 | 57.9 | 0 | 0.0 | |
| Total | 673 | 485 | 72.1 | 7 | 1.0 | |
DENV = dengue virus; WNV = West Nile virus. The sample size (n), number of positive samples, and the seroprevalence (%) of anti-DENV and anti-WNV virus antibodies are presented by demographic factors including gender, geographic location, and age group.
Figure 3.
Seroprevalence of dengue virus (DENV) and West Nile virus (WNV) by demographic factors. The proportions of the study population with IgG antibodies toward DENV (blue) and WNV (orange) are presented by age group, gender, and commune of enrollment with 95% confidence intervals.
Figure 4.
Dengue virus (DENV) seroconversion model for participants under 20 years of age. The seroprevalence of anti-DENV antibodies is presented with 95% confidence intervals (CIs) (blue circles) for participants between the ages of 2 and 5 years (average 3.4), individually between the ages of 5 and 19 years, and for those 20 years and above. The fit of the seroconversion model (black dotted line) appears with 95% CIs (gray shaded region) for the predicted population seroprevalence at each age.
Discussion
The observation that over 70% of the sample population had evidence of previous exposure to DENV, even in children less than 5 years of age, indicated high rates of DENV transmission in the recent past. These findings complement other surveys conducted in nearby Leogane, where 100% of middle-aged adults (mean age 33) had serological markers of DENV exposure, as well as in Port-au-Prince, where, by 3 years of age, 65% of the study participants were seropositive, with all four DENV serotypes identified.8,22 In 2001, Halstead and others found that 85% of the population had antibodies to two or more DENV serotypes estimated an annual SCR of 30%; which is nearly identical to the seroprevalence and SCR estimated by the current study.10 Though the SCR suggests hyperendemic transmission of DENV, it was notable that the seroprevalence saturated around 80–85% and never approached 100%. Whether this observation is due to differences in susceptibility of a proportion of the population, differences in the transmission of DENV in over the past several decades, the potential for seroreversion, or modification of the concentration of circulating DENV antibodies from infections by multiple serotypes was unable to be determined.
To our knowledge, this study represents the only serosurvey for WNV, which has been conducted in Haiti, or on the island of Hispaniola for that matter. Using sensitive serological methods in a population ranging in age from 20 to 80 years, the presence of anti-WNV IgG antibodies was identified in only 1% of samples (7/673). The low seroprevalence of WNV found by this study could be the result of multiple environmental factors. Since WNV needs an intermediate host, with the large majority of hosts suspected to be various species of birds, the rampant deforestation present in Haiti could contribute to a lower density of migrating passerine birds, thus limiting the potential introductions of WNV from avian hosts.23–25 This does not completely rule out the potential for viral reservoirs in Haiti, especially considering that WNV antibodies have been identified in migratory birds from the neighboring country of the Dominican Republic, and outside of the typical passerine hosts, there are 29 species of birds endemic to Haiti.12,26 However, given that there have only been two cases of WNV ever reported in Haiti, we conclude that the transmission of WNV in Haiti is rather unlikely.
Besides the contrast in the transmission of DENV and WENV in Haiti, another unique aspect of the current study is that this population was also previously screened for IgG antibodies to P. falciparum, allowing for a comparison between these three mosquito-borne illnesses in the Ouest and Sud-Est Departments of Haiti. Compared with the 20–30% seroprevalence of IgG antibodies toward P. falciparum antigens previously identified in this study population, over 70% of the population was seropositive for anti-DENV IgG antibodies and only 1% for anti-WENV IgG antibodies.27 Similarly, the SCR for P. falciparum was between 2% and 5%, which is far less than the estimated 20–40% SCR for DENV identified in the current study. This not only suggests that the transmission of DENV is much higher than that of P. falciparum malaria, but could explain many of the cases of undiagnosed febrile illness reported from clinics in Gressier and elsewhere in Haiti.15,28
Limitations
One of the primary limitations of this study was that the serum samples originated from a convenience sample that consisted of school-, clinic-, and community-based enrollment sites, which limited our ability to infer how this sample population represents Haiti as a whole. Though the ELISA protocol was validated with the appropriate controls obtained from a standardized reference collection, only a single strain of DENV (serotype 1) was used and plaque reduction and neutralization assays were not conducted to determine the serotype-specific seroprevalence to DENV (1–4). Finally, since a population absorbance threshold approach was used to define participants as seropositive or seronegative for WNV, the seven WNV positives could have resulted from cross-reactivity with DENV antibodies in those participants. These limitations aside, we believe this study provides valuable insights into the transmission of arboviruses in the Ouest Department of Haiti.
Conclusion
This study adds to the body of literature that suggests ongoing, hyperendemic levels of DENV in Haiti, while also documenting the relative absence of WNV transmission in the same sample population. Although the transmission rate of DENV among the indigenous population is high, visitors to Haiti are unlikely to have acquired immunity and are at the greatest risk for contracting dengue, DSS, and DHF. Since an effective DENV vaccine is still in development, wide-scale vector control strategies may be the most appropriate tool to mitigate burden of illness caused by dengue fever, while also reducing the transmission of other mosquito-borne illnesses currently circulating in Haiti.
ACKNOWLEDGMENTS
We would like to extend a special thanks to the dedicated staff at Community Coalition for Haiti and the Christianville Foundation for without their support this study would not be possible. The authors would like to acknowledge the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) and Robert Tesh at the University of Texas Medical Branch (UTMB) in Galveston, TX, for the serological reagents.
Footnotes
Financial support: This study was funded by the Armed Forces Health Surveillance Center, Global Emerging Infections Surveillance and Response Division to B.A.O. and by University of Florida, College of Public Health and Health Profession funds to M.E.V.
Authors' addresses: Thomas A. Weppelmann, Herbert Wertheim College of Medicine, Florida International University, Miami, FL, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, E-mail: alexwepp@yahoo.com. Alexandra Burne, Infectious Diseases and Pathology, College of Veterinary Medicine, University of Florida, Gainesville, FL, and Emerging Pathogens Institute, University of Florida, Gainesville, FL. E-mail: burnea@ufl.edu. Michael E. von Fricken, Department of Global and Community Health, College of Health and Human Services, George Mason University, Fairfax, VA, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, E-mail: mvonfric@gmu.edu. Maha A. Elbadry and Bernard A. Okech, Department of Environmental and Global Health, College of Public Health and Health Professions, University of Florida, Gainesville, FL, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, E-mails: eldadrym@ufl.edu and bokech@ufl.edu. Madsen Beau De Rochars, Department of Health Services Research Management and Policy, College of Public Health and Health Professions, University of Florida, Gainesville, FL, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, E-mail: madsenbeau@phhp.ufl.edu. Jacques Boncy, Laboratoire National de Santé Publique (LNSP), Ministère de la Santé et de la Population (MSPP), Route de Tabarre, Port au Prince, Haiti, E-mail: jboncy2001@yahoo.fr.
References
- 1.Caraballo H, King K. Emergency department management of mosquito-borne illness: malaria, dengue, and West Nile virus. Emerg Med Pract. 2014;16:1–23. [PubMed] [Google Scholar]
- 2.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 3.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikin-Beers R, Ciupe SM. The role of antibody in enhancing dengue virus infection. Math Biosci. 2015;263:83–92. doi: 10.1016/j.mbs.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Quijano FA, Waldman EA. Factors associated with dengue mortality in Latin America and the Caribbean, 1995–2009: an ecological study. Am J Trop Med Hyg. 2012;86:328–334. doi: 10.4269/ajtmh.2012.11-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray TJ, Webb CE. A review of the epidemiological and clinical aspects of West Nile virus. Int J Gen Med. 2014;7:193–203. doi: 10.2147/IJGM.S59902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daep CA, Munoz-Jordan JL, Eugenin EA. Flaviviruses, an expanding threat in public health: focus on dengue, West Nile, and Japanese encephalitis virus. J Neurovirol. 2014;20:539–560. doi: 10.1007/s13365-014-0285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salyer SJ, Ellis EM, Salomon C, Bron C, Juin S, Hemme RR, Hunsperger E, Jentes ES, Magloire R, Tomashek KM, Desormeaux AM, Munoz-Jordan JL, Etienne L, Beltran M, Sharp TM, Moffet D, Tappero J, Margolis HS, Katz MA. Dengue virus infections among Haitian and expatriate non-governmental organization workers in Leogane and Port-au-Prince, Haiti. PLoS Negl Trop Dis. 2012;8:e3269. doi: 10.1371/journal.pntd.0003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp TM, Pillai P, Hunsperger E, Santiago GA, Anderson T, Vap T, Collinson J, Buss BF, Safranek TJ, Sotir MJ, Jentes ES, Munoz-Jordan JL, Arquello DF. A cluster of dengue cases in American missionaries returning from Haiti. Am J Trop Med Hyg. 2012;86:16–22. doi: 10.4269/ajtmh.2012.11-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halstead SB, Streit TG, Lafontant JG, Putvatana R, Russell K, Sun W, Kanesa-Thasan N, Hayes CG, Watts DM. Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission. Am J Trop Med Hyg. 2001;65:180–183. doi: 10.4269/ajtmh.2001.65.180. [DOI] [PubMed] [Google Scholar]
- 11.Beatty ME, Hunsperger E, Long E, Schürch J, Jain S, Colindres R, Lerebours G, Bernard Y-M, Dobbins JG, Brown M, Clark GG. Mosquitoborne infections after Hurricane Jeanne, Haiti, 2004. Emerg Infect Dis. 2007;13:308–310. doi: 10.3201/eid1302.061132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komar O, Robbins MB, Klenk K, Blitvich BJ, Marlenee NL, Burkhalter KL, Gubler DJ, Gonzálvez G, Peña CJ, Peterson AT, Komar N. West Nile virus transmission in resident birds, Dominican Republic. Emerg Infect Dis. 2003;9:1299–1302. doi: 10.3201/eid0910.030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komar O, Robbins MB, Contreras GG, Benz BW, Klenk K, Blitvich BJ, Marlenee NL, Burkhalter KL, Beckett S, Gonzalvez G, Pena CJ, Peterson AT, Komar N. West Nile virus survey of birds and mosquitoes in the Dominican Republic. Vector Borne Zoonotic Dis. 2005;5:120–126. doi: 10.1089/vbz.2005.5.120. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Launching a National Surveillance System after an earthquake: Haiti. Morb Mortal Wkly Rep. 2010;59:933–938. [PubMed] [Google Scholar]
- 15.Beau De Rochars VE, Alam MT, Telisma T, Masse R, Chavannes S, Anilis MG, Guillaume HJ, Gelin G, Kirkpatrick EL, Okech BA, Weppelmann TA, Rashid M, Karst S, Johnson JA, Ali A, Morris JG. Spectrum of outpatient illness in a school-based cohort in Haiti, with a focus on diarrheal pathogens. Am J Trop Med Hyg. 2015;92:752–757. doi: 10.4269/ajtmh.14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuberger A, Tenenboim S, Golos M, Pex R, Krakowsky Y, Urman M, Vernet S, Schwartz E. Infectious diseases seen in a primary care clinic in Leogane, Haiti. Am J Trop Med Hyg. 2012;86:11–15. doi: 10.4269/ajtmh.2012.11-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samson DM, Archer RS, Alimi TO, Arheart KK, Impoinvil DE, Oscar R, Fuller DO, Qualls WA. New baseline environmental assessment of mosquito ecology in northern Haiti during increased urbanization. J Vector Ecol. 2015;40:46–58. doi: 10.1111/jvec.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miagostovich MP, Nogueira RM, dos Santos FB, Schatzmayr HG, Araujo ES, Vorndam V. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J Clin Virol. 1999;14:183–189. doi: 10.1016/s1386-6532(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 19.Beasley DWC, Barrett ADT. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol. 2002;76:13097–13100. doi: 10.1128/JVI.76.24.13097-13100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam CC, Tissera H, de Silva AM, De Silva AD, Margolis HS, Amarasinge A. Estimates of dengue force of infection in children in Colombo, Sri Lanka. PLoS Negl Trop Dis. 2013;7:e2259. doi: 10.1371/journal.pntd.0002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold BF, Priest JW, Hamlin KL, Moss DM, Colford JM, Jr, Lammie PJ. Serological measures of malaria transmission in Haiti: comparison of longitudinal and cross-sectional methods. PLoS One. 2014;9:e93684. doi: 10.1371/journal.pone.0093684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rioth M, Beauharnais CA, Noel F, Ikizler MR, Mehta S, Zhu Y, Long CA, Pape JW, Wright PF. Serologic imprint of dengue virus in urban Haiti: characterization of humoral immunity to dengue in infants and young children. Am J Trop Med Hyg. 2011;84:630–636. doi: 10.4269/ajtmh.2011.10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brault AC. Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility. Vet Res. 2009;40:43. doi: 10.1051/vetres/2009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubenstein DR, Chamberlain CP, Holmes RT, Ayres MP, Waldbauer JR, Graves GR, Tuross NC. Linking breeding and wintering ranges of a migratory songbird using stable isotopes. Science. 2002;295:1062–1065. doi: 10.1126/science.1067124. [DOI] [PubMed] [Google Scholar]
- 25.Churches CE, Wampler PJ, Sun W, Smith AJ. Evaluation of forest cover estimates for Haiti using supervised classification of Landsat data. Int J Appl Earth Obs Geoinf. 2014;30:203–216. [Google Scholar]
- 26.Lepage D. Checklist of Birds of Haiti From Bird Checklists of the World. 2003. http://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=ht&list=clements Available at. Accessed May 5, 2016.
- 27.von Fricken ME, Weppelmann TA, Eaton WT, Masse R, Beau de Rochars MV, Okech BA. Performance of the CareStart glucose-6-phosphate dehydrogenase (G6PD) rapid diagnostic test in Gressier, Haiti. Am J Trop Med Hyg. 2014;91:77–80. doi: 10.4269/ajtmh.14-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landman KZ, Jean SE, Existe A, Akom EE, Chang MA, Lemoine JF, Mace KE. Evaluation of case management of uncomplicated malaria in Haiti: a national health facility survey, 2012. Malar J. 2012;14:394. doi: 10.1186/s12936-015-0901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]