Abstract
Mefloquine was widely prescribed to U.S. military service members until 2009 when use was limited to personnel with contraindications to doxycycline and no contraindications to mefloquine. The need to estimate the occurrence of neuropsychiatric outcomes (NPOs) in service members prescribed mefloquine warranted a comprehensive evaluation of this issue. Active component service members filling a prescription for mefloquine, doxycycline, or atovaquone/proguanil (A/P) between January 1, 2008 and June 30, 2013, were included in the analysis. The risk of developing incident NPOs and the risk of subsequent NPOs among subjects with a history of the condition were assessed. A total of 367,840 individuals were evaluated (36,538 received mefloquine, 318,421 received doxycycline, and 12,881 received A/P). Among deployed individuals prescribed mefloquine, an increased risk of incident anxiety was seen when compared with doxycycline recipients (incidence rate ratio [IRR] = 1.12 [1.01–1.24]). Among nondeployed mefloquine recipients, an increased risk of posttraumatic stress disorder (PTSD) was seen when compared with A/P recipients (IRR = 1.83 [1.07–3.14]). An increased risk of tinnitus was seen for both deployed and nondeployed mefloquine recipients compared with A/P recipients (IRR = 1.81 [1.18–2.79]), 1.51 (1.13–2.03), respectively). Six percent of the mefloquine cohort had an NPO in the year before receiving mefloquine. When comparing individuals with a prior neuropsychiatric history to those without, the ratio of relative risks for adjustment disorder, anxiety, insomnia, and PTSD were higher (not statistically significant) for mefloquine compared with doxycycline. These findings emphasize the continued need for physicians prescribing mefloquine to conduct contraindication screening.
Introduction
Mefloquine was developed by the Walter Reed Army Institute of Research as part of its malaria drug discovery program which began in the 1960s in response to the significant impact of malaria on U.S. troops during the Vietnam War.1 Mefloquine was approved for use in the United States in 1989. Since that time, numerous contraindications and enhanced warnings have been added to the drug label, including a boxed warning in 2013.2
Initially, the military used mefloquine as a first-line drug for the prevention of Plasmodium species of malaria. After the 2009 drug label warnings, the Department of Defense (DoD) issued a new policy memorandum (HA Policy 09-017) restricting use of mefloquine to personnel with contraindications to doxycycline and no contraindications to mefloquine.3 This policy made doxycycline the drug of choice to prevent malaria in deployed military in all areas other than sub-Saharan Africa. Subsequent evolution of this policy resulted in the current 2013 policy which recommends either doxycycline or atovaquone/proguanil (A/P) as first-line medications.4
Serious adverse events after mefloquine use are rare.5–7 Studies evaluating the occurrence of neuropsychiatric outcomes (NPOs) after mefloquine use report mixed results, but a large proportion of these studies found an association between mefloquine and NPOs.7–17 Due to the extensive use of mefloquine for malaria prevention among service members before 2009 and its continued, limited use, after that point, the need to evaluate adverse events in service members who took the drug is evident. Therefore, a retrospective population-based cohort study was conducted among service members. The primary objective of the study was to assess and compare the risk of NPOs after mefloquine, doxycycline, and A/P prescriptions. The secondary objective was to determine the percentage of the mefloquine and doxycycline cohorts with a neuropsychiatric diagnosis (NPD) in the year before receiving the antimalarial medication and to compare the risk of an NPO following that prescription among subjects with and without a history of an NPD in the year prior and to determine whether this difference in risk is higher among the mefloquine cohort compared with the doxycycline cohort.
Materials and Methods
Data sources.
Data from the Defense Medical Surveillance System (DMSS), the Pharmacy Data Transaction Service (PDTS), and the Theater Medical Data Store (TMDS) were used for this study. DMSS is the central repository of medical surveillance data for the U.S. Armed Forces and is maintained by the Armed Forces Health Surveillance Branch.18 DMSS contains longitudinal data on medical encounters (in both military treatment facilities [MTFs] and civilian facilities if paid for by the Military Health System), demographics, service, deployment, and immunizations for service members. TMDS contains medical encounter and pharmacy data from deployed locations. PDTS contains DoD beneficiary prescription data from MTFs, retail pharmacy networks, and mail orders, and was provided by the DoD Pharmacoeconomic Center.
Study population and design.
PDTS and TMDS were used to identify the cohort of active component service members who filled a prescription for mefloquine (250 mg), doxycycline (100 mg, tabular form, daily dose, 30-day minimum prescription), or A/P at any time between January 1, 2008 to June 30, 2013. Doxycycline and A/P prescriptions were excluded if the service member previously or concurrently received mefloquine. Doxycycline prescriptions were restricted to the dosage and regimen delineated above in an effort to identify only prescriptions for malaria prophylaxis. Because TMDS is known to have less complete capture of medical events before 2008, the study time period was restricted to begin in 2008.
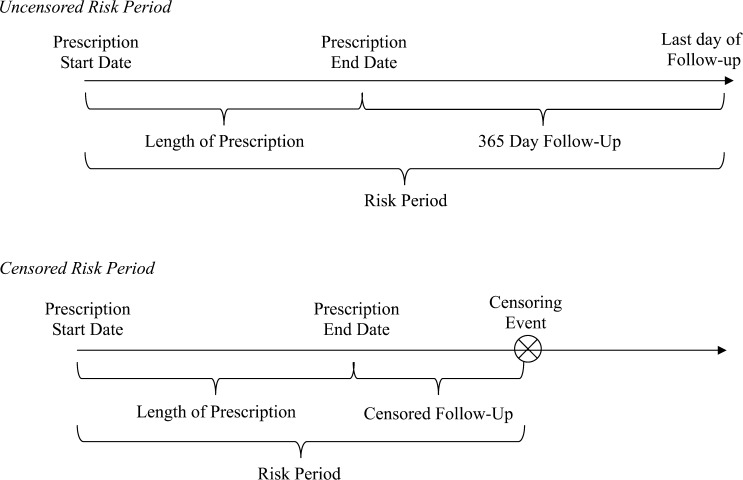
The risk period for NPOs was defined as the entire duration of the prescription plus 365 days after the end of the prescription (Figure 1 ). If an individual had overlapping or back-to-back prescriptions, the risk periods were merged. A risk period was censored when an individual had the outcome of interest, switched to a reserve component, left military service, or died. Additionally, mefloquine risk periods were censored if the service member received doxycycline or A/P during the risk period (0.06% of mefloquine prescriptions). For objective 1, service members could have multiple risk periods if they received multiple antimalarial prescriptions during the study period.
Figure 1.
Schematic of uncensored and censored risk periods of follow-up. A censoring event was defined as having the outcome of interest, switching antimalarial medications, switching to a reserve component, leaving military service, or death.
Since deployment could be a confounder for many of the outcomes of interest, each antimalarial cohort was stratified into deployed and nondeployed cohorts for objective 1. Deployment was defined as being in a deployed setting according to the DoD Contingency Tracking Roster. A risk period was categorized as deployed if the prescription was filled within 30 days before the start of a deployment or if the risk period overlapped with a deployment. Since the nondeployed cohort was prescribed antimalarial medication, the assumption was made that these subjects were traveling to malaria-endemic areas for nonofficial or off-duty business or vacation/personal reasons, separate from a documented deployment.
Ascertainment of neuropsychiatric adverse events.
Ambulatory and inpatient medical encounters occurring in theater or at fixed medical facilities during a risk period were searched for International Classification of Disease–Clinical Modification, 9th Revision (ICD-9-CM) codes for an NPO. A variety of case definitions were used depending on the outcome of interest (Table 1).19–21 Completed suicide was identified using the casualty data in DMSS with suicide listed as the manner of death. For each outcome, the incident encounter was defined as the first medical encounter during military service. Objective 1 included only incident encounters in the analysis; for the psychiatric outcomes (adjustment disorder, anxiety disorder, depressive disorder, PTSD, psychosis, suicide ideation, paranoia, and confusion), subjects were excluded if they had a medical encounter for any of the psychiatric outcomes before entry into the study cohort.
Table 1.
Case definitions for neuropsychiatric diagnoses
| Diagnosis | ICD-9-CM codes | Case definition |
|---|---|---|
| Adjustment disorders | 309.XX (excluding 309.81) | One hospitalization, at least two ambulatory encounters within 180 days of each other, or at least one ambulatory encounter in a psychiatric or mental health-care specialty setting with the diagnosis of interest in the first or second diagnostic position |
| Anxiety disorders | 300.0X, 300.2X, 300.3X | |
| Depressive disorders | 296.2X, 296.3X, 296.9, 296.90, 296.99, 311.XX | |
| PTSD | 309.81 | |
| Psychoses* | 297.XX, 298.XX, 293.81, 293.82 | |
| Tinnitus | 388.3 | At least one ambulatory or hospitalization with the diagnosis of interest in any diagnostic position |
| Vertigo | 386.1, 386.2, 780.4 | |
| Suicide ideation | V62.84 | |
| Convulsions | 780.3, 780.39 | |
| Hallucinations | 780.1 | |
| Paranoia* | 297.0, 297.9, 298.3 | |
| Confusion* | 298.2 | |
| Insomnia | 307.41, 307.42, 327.00, 327.01, 372.02, 327.09, 780.52 | At least one hospitalization or at least two ambulatory encounters within 90 days of each other with the diagnosis of interest in any diagnostic position |
ICD-9-CM = International Classification of Disease–Clinical Modification, 9th Revision; PTSD = posttraumatic stress disorder.
The ICD-9-CM codes for paranoia and confusion are also contained in the ICD-9-CM code group for psychosis. However, these diagnoses were looked at individually using a separate case definition than psychosis.
Statistical analysis.
Descriptive statistics were generated for each cohort. For objective 1, incidence rates (IRs) and 95% confidence intervals (CIs) for each NPO were calculated for each cohort. The denominator was person-years (py) accrued during each risk period. Poisson regression models were used to calculate incidence rate ratio (IRR) and 95% CI comparing the mefloquine cohorts to the doxycycline and A/P cohorts. Models were adjusted for age, sex, service, grade, and year of prescription start. Deployed cohort models were additionally adjusted for deployment location (Afghanistan, Iraq, Africa, or other) and combat exposure. Combat exposure was defined as positively responding to at least one of three questions (encountering dead bodies or seeing people killed or wounded, engaged in direct combat where weapon was discharged, or felt in danger of being killed) on the Post-Deployment Health Assessment DD2796 form for the deployment of interest.22 A secondary analysis additionally stratified the cohorts by sex.
Multiple sensitivity analyses were conducted for objective 1 and included the following: 1) including only the first risk period in the analysis, 2) restricting to hospitalized NPOs, 3) using all diagnostic positions for each NPO case definition, 4) stratifying cohorts by history of traumatic brain injury, and 5) restricting the risk period to 30 days after the end of the prescription. However, none of these analyses significantly changed the results of the study and are therefore not reported.
Objective 2 was restricted to the first prescription per individual and included individuals with a prior history of an NPD. The percentage of mefloquine and doxycycline subjects with a medical encounter for an NPD during the 365 days before the start of the prescription was calculated. IR and IRR and 95% CI for each outcome comparing those with and without a prior history were calculated for the mefloquine and doxycycline cohorts separately. The A/P cohort was excluded from this objective due to insufficient sample size. Ratios of rate ratios (RRRs) were calculated comparing the mefloquine IRR to the doxycycline IRR (N. P. Klein, 17th Annual Conference on Vaccine Research, April 2014). Permutation tests were conducted to determine the statistical significance of the RRR; the nonparametric percentile bootstrap method was used to generate the 95% CI.23–28
SAS 9.4 (SAS Institute Inc., Cary, NC) was used for the analysis. The study was designated as public health practice by the U.S. Army Public Health Command Public Health Review Board and the Army Human Research Protections Office.
Results
Cohort characteristics.
A total of 36,538 individuals were included in the mefloquine cohort. The doxycycline cohort consisted of 318,421 individuals, whereas the A/P cohort consisted of 12,881 individuals (Table 2). The mefloquine and doxycycline cohorts were comparable except for service, grade, and year of prescription. Mefloquine recipients were more likely to be Air Force members(58%), senior enlisted (47%), and to have filled the prescription in 2008 or 2009 (75% combined), whereas doxycycline recipients were primarily Army members (69%), junior enlisted (48%), and filled prescriptions during 2010 or later (78% combined). The A/P cohort differed from the other two cohorts on several demographics; this cohort was older, equally distributed among Army, Navy, and Air Force members, more likely to be senior enlisted or officers, filled the prescription in 2012 or 2013, and less likely to be deployed (20%). Among deployed subjects, 29%, 43%, and 21% reported combat exposure for the mefloquine, doxycycline, and A/P cohorts, respectively.
Table 2.
Characteristics of antimalarial medication study cohorts
| Demographic | Mefloquine | Doxycycline | Atovaquone/proguanil | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 36,538 | 100 | 318,421 | 100 | 12,881 | 100 |
| Age (years) | ||||||
| 17–19 | 831 | 2 | 16,116 | 5 | 143 | 1 |
| 20–29 | 19,814 | 54 | 192,920 | 61 | 5,139 | 40 |
| 30–39 | 11,494 | 31 | 82,433 | 26 | 4,820 | 37 |
| 40+ | 4,399 | 12 | 26,952 | 8 | 2,779 | 22 |
| Sex | ||||||
| Female | 5,589 | 15 | 36,913 | 12 | 1,975 | 15 |
| Male | 30,949 | 85 | 281,508 | 88 | 10,906 | 85 |
| Service | ||||||
| Army | 9,521 | 26 | 219,897 | 69 | 4,387 | 34 |
| Navy | 3,623 | 10 | 21,992 | 7 | 3,376 | 26 |
| Air Force | 21,185 | 58 | 58,537 | 18 | 4,168 | 33 |
| Marine Corps | 2,138 | 6 | 16,959 | 5 | 677 | 5 |
| Coast Guard | 71 | 0 | 1,036 | 1 | 273 | 2 |
| Grade | ||||||
| Junior Enlisted (E1–E4) | 11,955 | 33 | 152,110 | 48 | 2,451 | 19 |
| Senior Enlisted (E5–E9) | 17,160 | 47 | 107,692 | 34 | 4,872 | 38 |
| Junior Officers (O1–O3/W1–W3) | 4,339 | 12 | 40,693 | 13 | 2,546 | 20 |
| Senior Officers (O4–O9/W4–W5) | 3,084 | 8 | 17,926 | 5 | 3,012 | 23 |
| Year of prescription | ||||||
| 2008 | 13,610 | 37 | 22,094 | 7 | 1,331 | 10 |
| 2009 | 13,753 | 38 | 46,713 | 15 | 1,407 | 11 |
| 2010 | 6,307 | 17 | 80,471 | 25 | 1,247 | 10 |
| 2011 | 1,852 | 5 | 74,432 | 23 | 1,772 | 14 |
| 2012 | 705 | 2 | 67,071 | 21 | 3,793 | 29 |
| 2013 | 311 | 1 | 27,640 | 9 | 3,331 | 26 |
| Deployed during risk window | ||||||
| No | 10,847 | 30 | 71,635 | 23 | 10,261 | 80 |
| Yes | 25,691 | 70 | 246,786 | 77 | 2,620 | 20 |
| Combat exposed (deployed only) | ||||||
| No | 13,876 | 54 | 108,171 | 44 | 1,492 | 57 |
| Yes | 7,482 | 29 | 106,967 | 43 | 539 | 21 |
| Missing | 4,333 | 17 | 31,648 | 13 | 589 | 22 |
Risk of neuropsychiatric outcomes.
Table 3 provides the counts and crude IR for each NPO by deployed and nondeployed drug-specific cohorts. Among all deployed cohorts, adjustment disorder was the most common outcome and had the highest crude IRs (13.60–56.92 per 1,000 py) (Table 3). The next three most frequent diagnoses among the deployed cohorts included insomnia (15.78–27.53 per 1,000 py), anxiety disorder (14.51–23.53 per 1,000 py), and tinnitus (10.24–18.25 per 1,000 py). Between nondeployed cohorts, there was no consistency in the most frequent outcomes.
Table 3.
Number and IR of neuropsychiatric outcomes by antimalarial medication cohort and deployment status
| Outcome | Mefloquine | Doxycycline | Atovaquone/proguanil | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deployed | Nondeployed | Deployed | Nondeployed | Deployed | Nondeployed | |||||||
| N | IR* | N | IR* | N | IR* | N | IR* | N | IR* | N | IR* | |
| Adjustment disorder | 977 | 28.66 | 243 | 18.75 | 21,154 | 56.92 | 3,699 | 44.35 | 88 | 31.61 | 156 | 13.60 |
| Insomnia | 598 | 15.78 | 145 | 10.09 | 11,895 | 27.53 | 2,193 | 22.46 | 76 | 23.21 | 136 | 10.74 |
| Anxiety disorder | 499 | 14.51 | 121 | 9.28 | 8,948 | 23.53 | 1,569 | 18.47 | 42 | 14.97 | 100 | 8.69 |
| Tinnitus | 509 | 13.44 | 198 | 14.02 | 7,925 | 18.25 | 1,491 | 15.17 | 34 | 10.24 | 141 | 11.27 |
| Depressive disorder | 429 | 12.46 | 112 | 8.59 | 7,090 | 18.59 | 1,550 | 18.24 | 20 | 7.09 | 79 | 6.86 |
| Vertigo | 443 | 12.19 | 165 | 11.90 | 6,340 | 14.85 | 1,494 | 15.75 | 36 | 11.24 | 138 | 11.42 |
| PTSD | 382 | 11.08 | 66 | 5.05 | 5,944 | 15.55 | 775 | 9.06 | 19 | 6.74 | 44 | 3.81 |
| Suicide ideation | 71 | 2.05 | 20 | 1.53 | 1,703 | 4.43 | 363 | 4.23 | 2 | 0.71 | 17 | 1.47 |
| Convulsions | 40 | 1.03 | 25 | 1.71 | 753 | 1.67 | 220 | 2.16 | 8 | 2.31 | 9 | 0.69 |
| Psychoses | 11 | 0.32 | 6 | 0.46 | 321 | 0.83 | 60 | 0.70 | 0 | 0 | 2 | 0.17 |
| Hallucinations | 4 | 0.10 | 1 | 0.07 | 198 | 0.44 | 39 | 0.38 | 2 | 0.58 | 5 | 0.38 |
| Paranoia | 2 | 0.06 | 0 | – | 33 | 0.09 | 11 | 0.13 | 0 | – | 0 | – |
| Suicide | 1 | 0.03 | 1 | 0.08 | 11 | 0.03 | 4 | 0.05 | 0 | – | 1 | 0.09 |
| Confusion | 0 | – | 1 | 0.08 | 10 | 0.03 | 4 | 0.05 | 0 | – | 0 | – |
IR = incidence rate; PTSD = posttraumatic stress disorder.
Per 1,000 person-years.
IRRs comparing mefloquine to doxycycline for both deployed and nondeployed cohorts revealed statistically significant reduced risks for a large number of outcomes (Table 4). However, after adjustment, these outcomes were no longer statistically significant for the deployed cohort. Among the deployed cohorts, anxiety disorder had an elevated adjusted IRR of 1.12 (95% CI = 1.01–1.24) comparing mefloquine to doxycycline. Among the nondeployed cohorts, the adjusted IRRs for adjustment disorder, insomnia, anxiety disorder, depressive disorder, vertigo, and PTSD all remained statistically significantly protective for mefloquine compared with doxycycline. Among the nondeployed cohorts, the mefloquine cohort did not demonstrate a significantly elevated risk for any outcome. When comparing the mefloquine cohorts to the A/P cohorts for both deployers and nondeployers, tinnitus had a statistically significant elevated adjusted IRR (Table 5). The adjusted IRR was 1.81 (95% CI = 1.18–2.79) and 1.51 (95% CI = 1.13–2.03) for the deployed and nondeployed cohorts, respectively. Additionally, the IRR for PTSD was statistically significantly elevated among the nondeployed cohort (IRR = 1.83 [95% CI = 1.07–3.14]).
Table 4.
IRR of each neuropsychiatric outcome comparing the mefloquine cohort to the doxycycline cohort by deployment status
| Outcome | Deployed | Nondeployed | ||
|---|---|---|---|---|
| Unadjusted IRR (95% CI) | Adjusted IRR* (95% CI) | Unadjusted IRR (95% CI) | Adjusted IRR* (95% CI) | |
| Adjustment disorder | 0.50 (0.47–0.54) | 0.95 (0.88–1.02) | 0.42 (0.37–0.48) | 0.69 (0.60–0.80) |
| Insomnia | 0.57 (0.53–0.62) | 1.04 (0.95–1.14) | 0.45 (0.38–0.53) | 0.67 (0.56–0.81) |
| Anxiety disorder | 0.62 (0.56–0.67) | 1.12 (1.01–1.24) | 0.50 (0.42–0.60) | 0.70 (0.57–0.86) |
| Tinnitus | 0.74 (0.67–0.81) | 1.02 (0.92–1.13) | 0.92 (0.80–1.07) | 0.94 (0.80–1.11) |
| Depressive disorder | 0.67 (0.61–0.74) | 1.02 (0.92–1.15) | 0.47 (0.39–0.57) | 0.68 (0.55–0.84) |
| Vertigo | 1.19 (0.93–1.52) | 1.05 (0.79–1.40) | 0.57 (0.35–0.93) | 0.52 (0.31–0.88) |
| Posttraumatic stress disorder | 0.71 (0.64–0.79) | 1.08 (0.96–1.22) | 0.56 (0.43–0.72) | 0.69 (0.52–0.91) |
| Suicide ideation | 0.46 (0.37–0.59) | 1.03 (0.79–1.34) | 0.36 (0.23–0.57) | 0.90 (0.55–1.47) |
| Convulsions | 0.62 (0.45–0.85) | 0.83 (0.58–1.19) | 0.79 (0.52–1.19) | 1.10 (0.69–1.76) |
| Psychosis | 0.38 (0.21–0.69) | 0.64 (0.33–1.24) | 0.66 (0.28–1.52) | 1.13 (0.43–2.97) |
| Hallucinations | 0.23 (0.09–0.63) | 0.48 (0.17–1.39) | 0.18 (0.02–1.30) | 0.24 (0.03–1.99) |
| Paranoia | 0.67 (0.16–2.81) | 1.27 (0.24–6.57) | – | – |
| Suicide | 1.01 (0.13–7.83) | 0.87 (0.08–9.19) | 1.64 (0.18–14.67) | 2.94 (0.27–31.67) |
| Confusion | – | – | 1.64 (0.18–14.67) | 3.28 (0.21–50.02) |
CI = confidence interval; IRR = incidence rate ratio.
Models adjusted for age, sex, service, grade, year of prescription start; deployed model also adjusted for location and combat exposure.
Table 5.
IRR of each neuropsychiatric outcome comparing the mefloquine cohort to the atovaquone/proguanil cohort by deployment status
| Outcome | Deployed | Nondeployed | ||
|---|---|---|---|---|
| Unadjusted IRR (95% CI) | Adjusted IRR* (95% CI) | Unadjusted IRR (95% CI) | Adjusted IRR* (95% CI) | |
| Adjustment disorder | 0.91 (0.73–1.13) | 1.01 (0.74–1.37) | 1.38 (1.13–1.69) | 1.31 (0.99–1.74) |
| Insomnia | 0.68 (0.54–0.86) | 0.80 (0.58–1.10) | 0.94 (0.74–1.19) | 1.07 (0.78–1.48) |
| Anxiety disorder | 0.97 (0.71–1.33) | 0.97 (0.64–1.48) | 1.07 (0.82–1.39) | 0.98 (0.67–1.43) |
| Tinnitus | 1.31 (0.93–1.86) | 1.81 (1.18–2.79) | 1.24 (1.00–1.54) | 1.51 (1.13–2.03) |
| Depressive disorder | 1.76 (1.12–2.75) | 1.56 (0.89–2.74) | 1.25 (0.94–1.67) | 1.36 (0.90–2.04) |
| Vertigo | 1.27 (0.51–3.14) | 1.24 (0.34–4.54) | 0.83 (0.43–1.61) | 1.04 (0.43–2.49) |
| Posttraumatic stress disorder | 1.65 (1.04–2.61) | 1.31 (0.75–2.29) | 1.32 (0.90–1.94) | 1.83 (1.07–3.14) |
| Suicide ideation | 2.90 (0.71–11.82) | 3.56 (0.45–28.40) | 1.04 (0.54–1.98) | 1.00 (0.36–2.79) |
| Convulsions | 0.44 (0.21–0.95) | 0.39 (0.14–1.12) | 2.46 (1.15–5.27) | 1.72 (0.60–4.92) |
| Psychosis | – | – | 2.64 (0.53–13.10) | 7.18 (1.00–51.65) |
| Hallucinations | 0.18 (0.03–0.97) | 0.09 (0.01–1.20) | 0.18 (0.02–1.52) | 0.43 (0.02–8.40) |
| Paranoia | – | – | – | – |
| Suicide | – | – | 0.88 (0.06–14.09) | 2.59 (0.03–225.47) |
| Confusion | – | – | – | – |
CI = confidence interval; IRR = incidence rate ratio.
Models adjusted for age, sex, service, grade, year of prescription start; deployed model also adjusted for location and combat exposure.
Similar results were seen when the cohorts were stratified by sex. There were no differences in the IRR for any of the outcomes between males and females (data not shown).
History of neuropsychiatric diagnoses and subsequent risk.
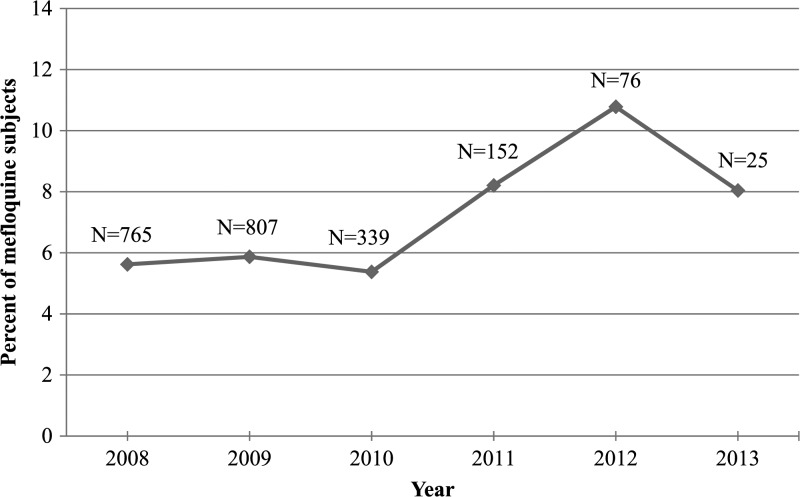
Overall, 5.9% of the mefloquine cohort had at least one NPD in the year before prescription, compared with 9.2% in the doxycycline cohort (Table 6). The most frequent prior diagnosis for both cohorts was adjustment disorder (mefloquine: 2.1%; doxycycline: 4.6%). The proportion of mefloquine subjects with an NPD in the year prior was stable from 2008 to 2010 (5.7%), but increased to 10.8% in 2012 and 8.0% for the partial 2013 year (Figure 2 ).
Table 6.
Number and percentage of subjects in the mefloquine and doxycycline cohorts with a neuropsychiatric outcome diagnoses in the 1 year before the first antimalarial prescription
| Outcome* | Mefloquine | Doxycycline | ||
|---|---|---|---|---|
| N | % | N | % | |
| Adjustment disorder | 764 | 2.1 | 14,614 | 4.6 |
| Anxiety | 341 | 0.9 | 5,652 | 1.8 |
| Insomnia | 253 | 0.7 | 3,371 | 1.1 |
| Depressive disorder | 359 | 1.0 | 6,390 | 2.0 |
| PTSD | 131 | 0.4 | 2,671 | 0.8 |
| Tinnitus | 332 | 0.9 | 3,239 | 1.0 |
| Vertigo | 460 | 1.3 | 3,997 | 1.3 |
| Suicide ideation | 17 | 0.1 | 844 | 0.3 |
| Convulsions | 32 | 0.1 | 375 | 0.1 |
| Psychosis | 7 | 0.0 | 125 | 0.0 |
| Hallucinations | 1 | 0.0 | 41 | 0.0 |
| Any† | 2,164 | 5.9 | 29,405 | 9.2 |
PTSD = posttraumatic stress disorder.
Outcomes not listed were not diagnosed in the 365 days before prescription.
Individuals with a prior history of multiple outcomes are only counted once in the “Any” category.
Figure 2.
Percentage of the mefloquine cohort subjects with a neuropsychiatric outcome diagnosed in the 1 year before first mefloquine prescription by year of prescription start. *Data for 2013 is only through June 30, 2013.
For both the mefloquine and doxycycline cohorts, individuals with an NPD in the year prior had a statistically significant elevated risk for a subsequent diagnosis of the same condition compared with individuals without that diagnosis in the year prior (Table 7). This was true for all outcomes investigated in the analysis. The adjusted IRR for the mefloquine cohort ranged from 4.32 (95% CI = 3.15–5.93; vertigo) to 122.7 (95% CI = 51.42–292.78; convulsions). The adjusted IRR for the doxycycline cohort ranged from 4.86 (95% CI = 4.73–5.00; adjustment disorder) to 134.80 (95% CI = 110.13–164.99; convulsions). The RRR point estimates were equal to or greater than 1.00 for all psychiatric outcomes investigated when comparing mefloquine to doxycycline (Table 7). However, none of the outcomes reached statistical significance.
Table 7.
IRR of each neuropsychiatric outcome comparing individuals with a 1-year prior history to those without: stratified and comparing mefloquine and doxycycline
| Outcome* | Diagnosis vs. no diagnosis of the condition in the 1 year before antimalarial medication | Mefloquine IRR compared with doxycycline IRR | ||
|---|---|---|---|---|
| Mefloquine cohort | Doxycycline cohort | Bootstrap RRR (95% CI) | Permutation test P value | |
| Adjusted IRR (95% CI)† | Adjusted IRR (95% CI)† | |||
| Adjustment disorder | 5.47 (4.69–6.36) | 4.86 (4.73–5.00) | 1.13 (0.94–1.34) | 0.81 |
| Anxiety | 16.95 (13.86–20.73) | 13.50 (12.92–14.11) | 1.26 (0.98–1.59) | 0.17 |
| Insomnia | 7.62 (5.57–10.44) | 6.66 (6.20–7.15) | 1.14 (0.80–1.59) | 0.61 |
| Depressive disorder | 12.54 (10.04–15.67) | 12.58 (12.04–13.14) | 1.00 (0.78–1.29) | 0.38 |
| PTSD | 27.98 (20.71–37.82) | 24.60 (23.17–26.12) | 1.14 (0.78–1.65) | 0.88 |
| Tinnitus | 7.52 (5.70–9.91) | 8.05 (7.43–8.71) | 0.93 (0.69–1.23) | 0.60 |
| Vertigo | 4.32 (3.15–5.93) | 5.90 (5.39–6.46) | 0.73 (0.50–1.00) | 0.06 |
| Convulsions | 122.7 (51.42–292.78) | 134.80 (110.13–164.99) | 0.91 (0.25–2.34) | 0.73 |
CI = confidence interval; IRR = incidence rate ratio; PTSD = posttraumatic stress disorder; RRR = ratio of rate ratios.
Outcomes not listed were not diagnosed in the 365 days before prescription.
Models adjusted for age, sex, service, grade, year of prescription start, deployment status.
Discussion
In this large, retrospective cohort study of U.S. military service members, rates of NPOs among mefloquine recipients were similar or less than the rates among two other antimalarial prescribed cohorts for the majority of outcomes investigated. This is the largest study that the authors are aware of evaluating potential NPOs after mefloquine exposure among U.S. military service members prescribed antimalarial medications.
Mefloquine recipients had similar rates to doxycycline and A/P recipients for the majority of outcomes. However, mefloquine recipients were at increased risk of three outcomes and at decreased risk for six outcomes. The IRR for anxiety disorder, tinnitus, and PTSD were all statistically significantly elevated among mefloquine users in certain cohorts; the elevated risk for anxiety disorder was only found in comparison to doxycycline among the deployed cohort, tinnitus was only found in comparison to A/P, and the risk of PTSD was only found among the nondeployed cohort in comparison to A/P. All three outcomes are known adverse events of mefloquine.29 In comparison to doxycycline recipients in the nondeployed cohort, mefloquine recipients were at a statistically significant lower risk for adjustment disorder, insomnia, anxiety disorder, depressive disorder, vertigo, and PTSD. The reason for this finding is not completely understood. Due to the nonexperimental nature of this study and the use of administrative data to assess exposure and outcomes, it is possible that residual and/or unmeasured confounding could be affecting the results. Although cases of other NPOs occurred in the mefloquine cohort, on a population level, no increased risk was identified as compared with the other cohorts. This null finding is similar to reports from other observational studies.5,8,15,17,30 Specifically among U.S. military deployers, Wells and others reported no association between mefloquine prescriptions and hospitalizations for a wide range of outcomes.5 Additional studies among deployed military populations reported mefloquine to be well tolerated, with the exception of individuals previously diagnosed with an NPO.15,17,30 Contrary to our findings, other studies have reported an association between NPOs and mefloquine.6,10,11,13,31–33 The study by Schlagenhauf and others was a randomized, double-blinded study among travelers and found mefloquine to have the highest proportion of moderate to severe NPOs compared with chloroquine/proguanil, doxycycline, and A/P.6 However, this study had small numbers and the outcomes were defined by a subjective questionnaire as opposed to medical diagnoses of the outcomes.
The finding of similar IRRs for males and females is contrary to other studies which found females to have higher risks of NPOs than males.6,8,10,31,34 However, this difference may be explained by factors such as entrance screening for mental health issues, physical fitness requirements, and combat exposures that distinguish military females from the general female population.
Among individuals with a prior history of an NPD, the study did not identify a statistically significant increased risk for subsequent diagnoses of the same condition among mefloquine subjects compared with doxycycline subjects. However, nonsignificant elevated risks of four outcomes and nonsignificant decreased risks for three outcomes were seen. It is likely that, with a larger sample size, anxiety (higher risk) and vertigo (lower risk) might reach statistical significance. Van Riemsdijk and others reported more definitive findings in a Dutch study which evaluated the risk of serious psychiatric events while taking mefloquine, reporting more than double the risk of a psychiatric event in those with versus without a history of psychiatric disease.10 Findings like these and those of other studies are the basis for the mefloquine package insert statement that a history of psychiatric illness is a contraindication for use of the drug.29
This issue is especially relevant for the military since the proportion of mefloquine prescriptions to individuals with NPOs in the year prior has nearly doubled since 2010. However, it should be noted that not all of the NPOs investigated in this study are classified as contraindications to mefloquine. Before 2011, the percent of mefloquine recipients with a prior NPO diagnosis was similar to the percentage of mefloquine recipients with a contraindication reported among U.S. military deployers to Afghanistan in 2007 (4.8%), but lower than the percentage of contraindications reported among U.S and United Kingdom civilians (7.5–9%).35–37 In 2012 and 2013, prior NPO diagnoses among mefloquine recipients were higher than any of these previous reports. This rising proportion of mefloquine prescriptions for individuals with a history of NPOs suggests the potential need for improved scrutiny of each service member's medical history before prescribing mefloquine. It may be beneficial to implement patient alert cards for contraindication before being able to prescribe mefloquine.
The findings of this study should be interpreted in light of its limitations. The use of electronic medical data archived in DMSS allowed for near complete capture of diagnoses recorded during medical encounters; however, these data are dependent upon the accuracy of ICD-9 coding. These data may be inaccurate due to miscoding, may reflect “rule out” diagnoses or may be subject to other error. In an effort to minimize some of these potential issues, standardized case definitions were used and typically required more than one encounter for the same medical condition. Additionally, service members may have experienced outcomes for which they never sought medical care, or received care from sources not documented in DMSS. Such outcomes would not be captured in the analysis and would result in under-ascertainment of the NPOs. It is not expected that such misclassification of the outcome would differ by drug type, making the misclassification nondifferential and biasing the results toward the null. Some medical care administered to deployed personnel at level 1 or role 1 facilities (immediate first aid delivered at the scene/Battalion Aid Stations), may also not be captured in the electronic medical data. This may result in under-ascertainment of NPOs treated in a level 1 or role 1 facility. Given the 365-day postprescription follow-up period, it is expected that persistent and more severe NPOs will get captured from medical care administered after return from deployment. Additionally, anecdotal reports indicate that antimalarial medications were provided to entire deploying units for force health protection measures and were not documented as an individual medical prescription. If this occurred, there is no way to capture those prescriptions and this study is missing those individuals in the analysis. However, the risk of developing NPOs among the group with undocumented prescriptions is not expected to be different than the risk among service members given an actual prescription. Temporal trends in deployment locations, combat exposure, and type of antimalarial medication prescribed had the potential to confound these results. However, we attempted to account for these factors by adjusting our models by year of prescription start and deployment status, location, and combat exposure.
Potentially one of the most significant limitations of this study is the lack of data on prescription compliance. Service members were assumed to have taken the prescription in its entirety; however, it is unlikely that complete chemoprophylaxis adherence was achieved. Self-reported compliance with antimalarial medications among military personnel was found to be 60% among a group of Afghanistan deployers, and in one report, self-reported compliance for mefloquine (48.5%) was much lower than for doxycycline (78.4%) and other antimalarials.38,39 However, other studies have found that compliance with mefloquine is higher than with doxycycline (American Soldiers: 80% versus 60%; Turkish troops: 61% versus 56%; Australian travelers: 78% versus 68%).40–42 However, how compliance is defined is important in interpreting these findings. In the cited studies, estimated compliance rates correspond to taking the medication as prescribed without missing a dose. This is different than completely stopping the medication, which was only reported between 3% and 5% for either medication among service members.41,42 Complete cessation of the medication would impact this study more profoundly than missing doses, but this is also expected to occur among a small percentage of subjects. If subjects stopped taking prescribed prophylaxis due to adverse events and if the adverse events resulted in a medical encounter, then it would have been captured and the risk period would have been censored appropriately. However, if the adverse events were not reported and the individual did not switch antimalarial medications, then the risk period would have been overestimated.
A strength of this analysis is the large sample size which allowed for investigation of NPOs which are infrequently diagnosed (i.e., “rare” outcomes). However, some outcomes were so infrequent (e.g., suicide), that estimates derived for these outcomes may be unreliable. The use of robust statistical methods allowed for complex comparisons between subcohorts of the mefloquine and doxycycline cohorts. Use of the electronic medical encounter and pharmacy data allowed for near complete capture of medical encounters and removed potential reporting bias.
In summary, on a population level, this study did not find an association between mefloquine and NPOs among U.S. military service members, with the exception of anxiety, tinnitus, and PTSD for some subcohorts. Among service members with a history of an NPD during the year before beginning chemoprophylaxis, mefloquine was associated with an increased risk of subsequent diagnosis of the same outcome. These findings emphasize the need for appropriate screening for contraindications when physicians prescribe mefloquine.
Disclaimer: The views expressed herein are the views of the authors and do not reflect the official policy of the Department of Defense or the U.S. Government.
Footnotes
Authors' addresses: Angelia A. Eick-Cost, Zheng Hu, Patricia Rohrbeck, and Leslie L. Clark, Armed Forces Health Surveillance Branch, Defense Health Agency, Silver Spring, MD, E-mails: angelia.a.cost.ctr@mail.mil, zheng.hu.ctr@mail.mil, patricia.rohrbeck.mil@mail.mil, and leslie.l.clark6.ctr@mail.mil.
References
- 1.Kitchen LW, Vaughn DW, Skillman DR. Role of US military research programs in the development of US Food and Drug Administration–approved antimalarial drugs. Clin Infect Dis. 2006;43:67–71. doi: 10.1086/504873. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration FDA Approves Label Changes for Antimalarial Drug Mefloquine Hydrochloride due to Risk of Serious Psychiatric and Nerve Side Effects. 2013. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM362232.pdf Available at. Accessed April 7, 2016.
- 3.Office of the Assistant Secretary of Defense (Health Affairs) Memorandum: Policy Memorandum on the Use of Mefloquine (Lariam) in Malaria Prophylaxis. 2009. http://www.health.mil/∼/media/MHS/Policy%20Files/Import/09-017.ashx Available at. Accessed April 7, 2016.
- 4.Office of the Assistant Secretary of Defense (Health Affairs) Memorandum: Guidance on Medications for Prophylaxis of Malaria. 2013 http://www.health.mil/∼/media/MHS/Policy%20Files/Import/13-002.ashx HA Policy 13-002. Available at. Accessed April 7, 2016. [Google Scholar]
- 5.Wells TS, Smith TC, Smith B, Wang LZ, Hansen CJ, Reed RJ, Goldfinger WE, Corbell TE, Spooner CN, Ryan MA. Mefloquine use and hospitalizations among US service members, 2002–2004. Am J Trop Med Hyg. 2006;74:744–749. [PubMed] [Google Scholar]
- 6.Schlagenhauf P, Tschopp A, Johnson R, Nothdurft HD, Beck B, Schwartz E, Herold M, Krebs B, Veit O, Allwinn R, Steffen R. Tolerability of malaria chemoprophylaxis in non-immune travelers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ. 2003;327:1078. doi: 10.1136/bmj.327.7423.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlagenhauf P, Adamcova M, Regep L, Schaerer MT, Rhein HG. The position of mefloquine as a 21st century malaria chemoprophylaxis. Malar J. 2010;9:357–371. doi: 10.1186/1475-2875-9-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider C, Adamcova M, Jick SS, Schlagenhauf P, Miller MK, Rhein HG, Meier CR. Antimalarial chemoprophylaxis and the risk of neuropsychiatric disorders. Travel Med Infect Dis. 2013;11:71–80. doi: 10.1016/j.tmaid.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Overbosch D, Schilthuis H, Bienzle U, Behrens RH, Kain KC, Clarke PD, Toovey S, Knobloch J, Nothdurft HD, Shaw D, Roskell NS, Chulay JD, Malarone International Study Team Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin Infect Dis. 2001;33:1015–1021. doi: 10.1086/322694. [DOI] [PubMed] [Google Scholar]
- 10.van Riemsdijk MM, Sturkenboom MC, Pepplinkhuizen L, Stricker BH. Mefloquine increases the risk of serious psychiatric events during travel abroad: a nationwide case-control study in The Netherlands. J Clin Psychiatry. 2005;66:199–204. doi: 10.4088/jcp.v66n0207. [DOI] [PubMed] [Google Scholar]
- 11.van Riemsdijk MM, Sturkenboom MC, Ditters JM, Ligthelm RJ, Overbosch D, Stricker BH. Atovaquone plus chloroguanide versus mefloquine for malaria prophylaxis: a focus on neuropsychiatric adverse events. Clin Pharmacol Ther. 2002;72:294–301. doi: 10.1067/mcp.2002.127113. [DOI] [PubMed] [Google Scholar]
- 12.Jacquerioz FA, Croft AM. Drugs for preventing malaria in travellers. Cochrane Database Syst Rev. 2009;4:CD006491. doi: 10.1002/14651858.CD006491.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Barrett PJ, Emmins PD, Clarke PD, Bradley DJ. Comparison of adverse events associated with use of mefloquine and combination of chloroquine and proguanil as antimalarial prophylaxis: postal and telephone survey of travellers. BMJ. 1996;313:525–528. doi: 10.1136/bmj.313.7056.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korhonen C, Peterson K, Bruder C, Jung P. Self-reported adverse events associated with antimalarial chemoprophylaxis in peace corps volunteers. Am J Prev Med. 2007;33:194–199. doi: 10.1016/j.amepre.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Fujii T, Kaku K, Jelinek T, Kimura M. Malaria and mefloquine prophylaxis use among Japan Ground Self-Defense Force personnel deployed in east Timor. J Travel Med. 2007;14:226–232. doi: 10.1111/j.1708-8305.2007.00122.x. [DOI] [PubMed] [Google Scholar]
- 16.Naing C, Aung K, Ahmed SI, Mak JW. Signal detection to identify serious adverse events (neuropsychiatric events) in travelers taking mefloquine for chemoprophylaxis of malaria. Drug Healthc Patient Saf. 2012;4:87–92. doi: 10.2147/DHPS.S34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaspers CA, Hopperus Buma AP, van Thiel PP, van Hulst RA, Kager PA. Tolerance of mefloquine chemoprophylaxis in Dutch military personnel. Am J Trop Med Hyg. 1996;55:230–234. doi: 10.4269/ajtmh.1996.55.230. [DOI] [PubMed] [Google Scholar]
- 18.Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900–1904. doi: 10.2105/ajph.92.12.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armed Forces Health Surveillance Center (AFHSC) Mental disorders and mental health problems, active component, U.S. Armed Forces, 2000–2011. MSMR. 2012;19:11–17. [Google Scholar]
- 20.Armed Forces Health Surveillance Center Insomnia, active component, U.S. Armed Forces, January 2000–December 2009. MSMR. 2010;17:12–15. [Google Scholar]
- 21.Armed Forces Health Surveillance Center Case Definitions for Data Analysis and Health Reports: Section 12, Mental Health. 2012. https://www.afhsc.mil/documents/pubs/documents/CaseDefs/Web_12_MENTAL%20HEALTH_DEC15.pdf Available at. Accessed April 7, 2016.
- 22.Department of Defense Deployment Health Clinical Center Enhanced Post-Deployment Health Assessment (PDHA) Process (DD Form 2796) 2006. http://www.dtic.mil/whs/directives/forms/eforms/dd2796.pdf Available at. Accessed April 7, 2016.
- 23.Zelen M. Berkeley, CA: University of California Press; 1972. Exact Significance Tests for Contingency Tables Embedded in a 2nd Classification. The Sixth Berkeley Symposium on Mathematical Statistics and Probability; pp. 737–757. [Google Scholar]
- 24.Good P. Permutation, Parametric, and Bootstrap Tests of Hypotheses. New York, NY: Springer-Verlag; 2005. [Google Scholar]
- 25.Ernst MD. Permutation methods: a basis for exact inference. Stat Sci. 2004;19:676–685. [Google Scholar]
- 26.DiCiccio TJEB. Bootstrap confidence intervals. Stat Sci. 1996;11:189–228. [Google Scholar]
- 27.Rizzo RL. Statistical Computing with R. Boca Raton, FL: Chapman and Hall/CRC; 2007. [Google Scholar]
- 28.Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 29.Roche USA Lariam Brand of Mefloquine Hydrochloride Tablets (Package insert) 2004. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019591s026s028lbl.pdf Available at. Accessed April 7, 2016.
- 30.Kitchener SJ, Nasveld PE, Gregory RM, Edstein MD. Mefloquine and doxycycline malaria prophylaxis in Australian soldiers in east Timor. Med J Aust. 2005;182:168–171. doi: 10.5694/j.1326-5377.2005.tb06647.x. [DOI] [PubMed] [Google Scholar]
- 31.van Riemsdijk MM, Ditters JM, Sturkenboom MC, Tulen JH, Ligthelm RJ, Overbosch D, Strickler BH. Neuropsychiatric events during prophylactic use of mefloquine before travelling. Eur J Clin Pharmacol. 2002;58:441–445. doi: 10.1007/s00228-002-0492-z. [DOI] [PubMed] [Google Scholar]
- 32.Ringqvist A, Bech P, Glenthoj B, Petersen E. Acute and long-term psychiatric side effects of mefloquine: a follow-up on Danish adverse event reports. Travel Med Infect Dis. 2014;13:80–88. doi: 10.1016/j.tmaid.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Peterson AL, Seegmiller RA, Schindler LS. Severe neuropsychiatric reaction in a deployed military member after prophylactic mefloquine. Case Rep Psychiatry. 2011;2011:350–417. doi: 10.1155/2011/350417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Riemsdijk MM, Sturkenboom MC, Ditters JM, Tulen JH, Ligthelm RJ, Overbosch D, Strickler BH. Low body mass index is associated with an increased risk of neuropsychiatric adverse events and concentration impairment in women on mefloquine. Br J Clin Pharmacol. 2004;57:506–512. doi: 10.1046/j.1365-2125.2003.02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nevin RL, Pietrusiak PP, Caci JB. Prevalence of contraindications to mefloquine use among USA military personnel deployed to Afghanistan. Malar J. 2008;7:30–34. doi: 10.1186/1475-2875-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill DR. Pre-travel health, immunization status, and demographics of travel to the developing world for individuals visiting a travel medicine service. Am J Trop Med Hyg. 1991;45:263–270. doi: 10.4269/ajtmh.1991.45.263. [DOI] [PubMed] [Google Scholar]
- 37.Bloechliger M, Schlagenhauf P, Toovey S, Schnetzler G, Tatt I, Tomianovic D, Jick SS, Meier CR. Malaria chemoprophylaxis regimens: a descriptive drug utilization study. Travel Med Infect Dis. 2014;12:718–725. doi: 10.1016/j.tmaid.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Brisson M, Brisson P. Compliance with antimalaria chemoprophylaxis in a combat zone. Am J Trop Med Hyg. 2012;86:587–590. doi: 10.4269/ajtmh.2012.11-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armed Forces Health Surveillance Center Surveillance snapshot: self-reported malaria prophylaxis compliance among U.S. service members with diagnosed malaria, 2008–2013. MSMR. 2014;21:15. [PubMed] [Google Scholar]
- 40.Phillips MA, Kass RB. User acceptability patterns for mefloquine and doxycycline malaria chemoprophylaxis. J Travel Med. 1996;3:40–45. doi: 10.1111/j.1708-8305.1996.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 41.Saunders DL, Garges E, Manning JE, Bennett K, Schaffer S, Kosmowski AJ, Magill AJ. Safety, tolerability, and compliance with long-term antimalarial chemoprophylaxis in American soldiers in Afghanistan. Am J Trop Med Hyg. 2015;93:584–590. doi: 10.4269/ajtmh.15-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonmez A, Harlak A, Kilic S, Polat Z, Hayat L, Keskin O, Dogru T, Yilmaz MI, Acikel CH, Kocar IH. The efficacy and tolerability of doxycycline and mefloquine in malaria prophylaxis of the ISAF troops in Afghanistan. J Infect. 2005;51:253–258. doi: 10.1016/j.jinf.2005.01.014. [DOI] [PubMed] [Google Scholar]