Abstract
Congenital malaria is rare in the United States, but is an important diagnosis to consider when evaluating febrile infants. Herein, we describe a case of congenital Plasmodium falciparum malaria in a 2-week-old infant born in the United States to a mother who had emigrated from Nigeria 3 months before delivery.
Introduction
Congenital malaria is the presence of asexual forms of malaria parasites in peripheral blood within the 1st week of life, or later if there is no possibility of postpartum infection by mosquito bites.1 It is the consequence of transplacental passage of parasites which infect the infant in utero or during delivery. Congenital malaria is a rare entity in the United States. In a recent review of data collected via the National Malaria Surveillance System directed by the Centers for Disease Control and Prevention (CDC), only 81 cases of congenital malaria were identified between the years 1966 and 2005.2 This is a rare diagnosis even in endemic regions, although estimating the true prevalence of congenital malaria in these areas is complicated by several factors. Herein, we describe a case of a 2-week-old infant with congenital malaria that presented to the emergency department in Washington, DC.
Case Report
A 2-week-old infant girl presented to the emergency department with fever and cough. The infant initially presented to her pediatrician for a routine newborn examination, and was found to be febrile to 102°F. She was immediately referred to Children's National Medical Center (CNMC) for further evaluation and inpatient admission.
The infant's history was notable for preterm birth in the United States at 36 weeks gestation. Her mother emigrated from Nigeria during her 6th month of pregnancy. This was the mother's first pregnancy, and there were no known prenatal complications; however, the mother had a puerperal fever, and was noted to have thrombocytopenia at the time of delivery. The infant reportedly had a normal examination at birth, and was discharged home on day 3 of life. During the 2 weeks at home, she was reportedly healthy and vigorous.
In the CNMC emergency department, a complete blood count (CBC), urinalysis, urine culture, and blood culture were obtained. Given the history of a mild cough, rapid respiratory syncytial virus (RSV) antigen testing was performed on a nasopharyngeal swab specimen, and returned positive. A significantly low platelet count of 20 K/μL prompted manual review of the infant's blood smear. Microscopic review revealed abundant malarial parasite forms with 5.4% parasitemia. A NOW® ICT Malaria Test (Binax, Inc., Portland, ME) was performed, confirming the presence of Plasmodium falciparum. The infant was admitted to the pediatric intensive care unit for further management.
In consultation with the CDC, the infant was administered loading doses of quinidine and clindamycin, and was subsequently placed on a quinidine drip (10 mg/kg/minute) and a regimen of intravenous (IV) clindamycin (5 mg/kg/dose) every 8 hours. Peripheral smears from the hospital of birth were procured from both the mother and infant for review. Malaria parasites were found on the mother's smear (Figure 1 ); however, the infant's initial blood sample at birth did not demonstrate parasites. It was later confirmed that smear of neither the infant nor the mother had been reviewed microscopically at delivery. Therefore, the diagnosis of malaria in the mother was not made until her infant presented to our institution. During initial intake of her 2-week-old daughter, the mother denied intermittent preventive therapy in pregnancy (IPTp) with sulfadoxine–pyrimethamine while in Nigeria.
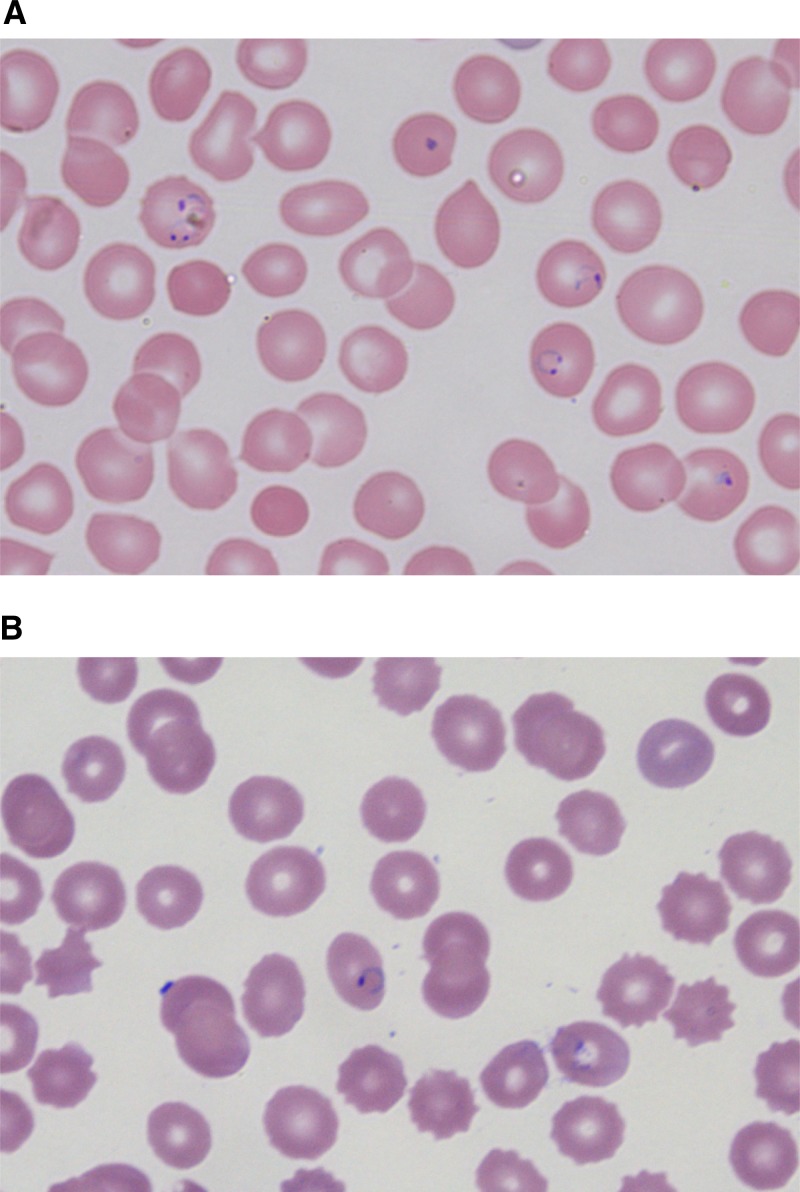
Figure 1.
Maternal and infant blood smears. (A) Infant's thin blood smear revealing Plasmodium falciparum immature trophozoites (ring forms) within erythrocytes. (B) Mother's thin blood smear, obtained on the day of delivery, documenting the presence of P. falciparum trophozoites within erythrocytes.
Repeat blood smears to evaluate parasite load, and CBCs were obtained on the infant every 4 hours. CBC demonstrated ongoing thrombocytopenia with a platelet nadir of 7 K/μL on hospital day 1, requiring platelet transfusion. After the first 4 hours on quinidine drip, parasite load had increased to 16%. The use of IV artesunate was discussed with the CDC; however, the infant did not meet all the eligibility criteria for the drug's use under emergency investigational new drug (IND) application. Aside from thrombocytopenia, the patient did not exhibit other manifestations of severe malaria (neurologic abnormalities, hypoglycemia, hemolysis, hypotension, or acute kidney failure). In the subsequent 4 hours, parasitemia steadily declined. By hospital day 2, when parasite load was confirmed as less than 1%, she was transitioned to quinine (8.3 mg base/kg [= 10 mg salt/kg]) and clindamycin (20 mg/kg/day) orally. The patient completed a total of 7 days of therapy, after which repeat smear revealed no parasites.
During treatment of severe malaria, the infant also had respiratory symptoms consistent with RSV infection. She required oxygen by nasal cannula for a total of 9 days, then weaned to room air by hospital day 11, and was discharged home.
Discussion
It is estimated that the incidence of congenital malaria in women with a history of travel to endemic areas is approximately 0.3–10%.3 Most cases of congenital malaria in nonendemic areas, such as the United States, are secondary to infection with P. vivax. It has been suggested that pregnancy is a potential cause of relapse in women harboring P. vivax hypnozoites, highlighting the importance of maternal travel history, including a remote history of travel to malaria-endemic regions.2 Congenital malaria secondary to P. falciparum should be considered in infants born to women with recent travel (shortly before or during pregnancy) to regions of the world where this parasite predominates.
The pathophysiology of congenital malaria is poorly understood. Possible mechanisms include maternal transfusion into the fetal circulation during pregnancy or at the time of delivery, direct penetration through the chorionic villi, or through premature separation of the placenta.4 A number of studies support each of these theories, though no study has completely elucidated the key factors that contribute to congenital malaria. Because congenital malaria is a rare event, even in endemic regions, it is widely believed that the placenta acts as an effective barrier preventing the transfer of parasites from mother to child. This suggests that transmission during labor may be the most likely mechanism. It has been hypothesized that acute fever in a pregnant woman may result in increased friability of the placenta, leading to an increased risk of transmission.1
Placental malaria, where parasitic forms directly infect the placenta, may be another predisposing factor. Malaria infection of the placenta can be accompanied by intervillous infiltrates of mononuclear cells. This intervillous mononuclear inflammation induces an alteration in the cytokine balance of the placenta which can damage the syncytiotrophoblastic membrane, compromise the integrity of the placenta, and increase the risk of transmission.1 This mechanism, however, is incompletely understood and to a large extent unverified as studies looking at the correlation between placental maternal cord and neonatal parasitemia are scarce and contradictory. In studies in which both cord and the infant's peripheral blood have been collected, the frequency of parasitemia has always been lower in the baby's blood than in cord blood, if the infant becomes infected at all.1 Nonetheless, placental malaria carries significant perinatal morbidity and mortality; while it may not always lead to overt malaria infection in the newborn, it has been consistently associated with low birth weight, intrauterine growth restriction, preterm labor, and intrauterine fetal death.5 In fact, it has been estimated that 11% of neonatal deaths in malaria-endemic areas of Africa are due to low birth weight secondary to P. falciparum infection in pregnancy.6 This highlights the importance of preventive measures such as administration of IPTp with sulfadoxine–pyrimethamine to pregnant women. In a recent placebo-controlled trial in Mozambique, IPTp was found to reduce neonatal mortality by as much as 61%.7
Another reason why congenital malaria is a rare occurrence may be due to the protective effect of maternal immunity. Maternal immunoglobulin G antibodies (IgG) against malaria are transmitted via the placenta to the fetus in utero,8 although there is little evidence suggesting a protective role of these passively acquired antibodies.9 Other studies, however, have suggested that maternal IgG and fetal hemoglobin (HbF) act cooperatively to impair the cytoadherence of parasitized red blood cells, which helps prevent high parasite densities and the symptoms of malaria.10 As IgG and HbF disappear from circulation, infant susceptibility to P. falciparum malaria is thought to increase. These factors may also explain in part, why most infants with congenital malaria do not exhibit symptoms or demonstrable malarial forms in the peripheral blood immediately after birth, but days to weeks later, as passive immunity wanes.
Treatment of young infants with malaria is complicated by developmental features unique to children less than 6 months of age. For instance, intestinal motor activity matures by week 20 of life, affecting absorption of most drugs.11 Our patient required parenteral treatment due to high-density parasitemia (> 5%); however, one might consider treatment of very young infants with IV quinidine gluconate in combination with clindamycin 10 mg base/kg loading dose and then 5 mg base/kg IV every 8 hours irrespective of parasitemia given their intestinal immaturity. The use of IV artesunate under emergency IND through the CDC is also a treatment option for young infants who require parenteral therapy and either cannot tolerate quinidine or when there is quinidine shortage. Treatment with artesunate was discussed with the CDC due to our patient's severe thrombocytopenia and high-level parasitemia after 4 hours of IV quinidine. However, the parasite load sharply dropped after this, and while quinidine has been associated with thrombocytopenic purpura (a contraindication to its use), severe thrombocytopenia due to malaria infection without overt bleeding, such as in our patient, is not considered a contraindication to quinidine.
In summary, congenital malaria is a rare diagnosis owing in part perhaps to the placental barrier and developmental factors such as HbF and maternal antibodies to malaria parasites. However, it is an important diagnosis to consider in any febrile infant born to a mother with recent or remote travel to malaria-endemic countries.
Footnotes
Authors' addresses: Melissa Del Castillo, Division of Pediatric Infectious Diseases, Children's National Medical Center, Washington, DC, and Office of Vaccines Research and Review, Food and Drug Administration, Silver Spring, MD, E-mail: mdelcast@cnmc.org. Ann Marie Szymanski and Ariella Slovin, Division of General Pediatrics, Children's National Medical Center, Washington, DC, E-mails: aszymans@cnmc.org and aslovin@cnmc.org. Edward C. C. Wong, Division of Laboratory Medicine, Children's National Medical Center, Washington, DC, E-mail: ewong@cnmc.org. Roberta L. DeBiasi, Department of Microbiology, Immunology, and Tropical Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC, and Division of Pediatric Infectious Diseases, Children's National Medical Center, Washington, DC, E-mail: rdebiasi@cnmc.org.
References
- 1.Menendez C, Mayor A. Congenital malaria: the least known consequence of malaria in pregnancy. Semin Fetal Neonatal Med. 2007;12:207–213. doi: 10.1016/j.siny.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Lesko CR, Arguin PM, Newman RD. Congenital malaria in the United States: a review of cases from 1966 to 2005. Arch Pediatr Adolesc Med. 2007;161:1062–1067. doi: 10.1001/archpedi.161.11.1062. [DOI] [PubMed] [Google Scholar]
- 3.Vottier G, Arsac M, Farnoux C, Mariani-Kurkdjian P, Baud O, Aujard Y. Congenital malaria in neonates: two case reports and review of the literature. Acta Paediatr. 2008;97:500–512. doi: 10.1111/j.1651-2227.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 4.De Silva DH, Mendis KN, Premaratne UN, Jayatilleke SM, Soyza PE. Congenital malaria due to Plasmodium vivax: a case report from Sri Lanka. Trans R Soc Trop Med Hyg. 1982;76:33–35. doi: 10.1016/0035-9203(82)90011-6. [DOI] [PubMed] [Google Scholar]
- 5.Osungbade KO, Oladunjoye OO. Prevention of congenital transmission of malaria in sub-Saharan African countries: challenges and implications for health system strengthening. J Trop Med. 2012;2012:648456. doi: 10.1155/2012/648456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisele TP, Larsen DA, Anglewicz PA, Keating J, Yukich J, Bennett A, Hutchinson P, Steketee RW. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis. 2012;12:942–949. doi: 10.1016/S1473-3099(12)70222-0. [DOI] [PubMed] [Google Scholar]
- 7.Menendez C, Bardaji A, Sigauque B, Sanz S, Aponte JJ, Mabunda S, Alonso PL. Malaria prevention with IPTp during pregnancy reduces neonatal mortality. PLoS One. 2010;5:e9438. doi: 10.1371/journal.pone.0009438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley EM, Wagner GE, Akanmori BD, Koram KA. Do maternally acquired antibodies protect infants from malaria infection? Parasite Immunol. 2001;23:51–59. doi: 10.1046/j.1365-3024.2001.00364.x. [DOI] [PubMed] [Google Scholar]
- 9.Riley EM, Wagner GE, Ofori MF, Wheeler JG, Akanmori BD, Tetteh K, McGuinness D, Bennett S, Nkrumah FK, Anders RF, Koram KA. Lack of association between maternal antibody and protection of African infants from malaria infection. Infect Immun. 2000;68:5856–5863. doi: 10.1128/iai.68.10.5856-5863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaratunga C, Lopera-Mesa TM, Brittain NJ, Cholera R, Arie T, Fujioka H, Keefer JR, Fairhurts RM. A role for fetal hemoglobin and maternal immune IgG in infant resistance to Plasmodium falciparum malaria. PLoS One. 2011;6:e14798. doi: 10.1371/journal.pone.0014798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]