Abstract
The World Health Organization's (WHO) recommendation is 28-day course of meglumine antimoniate (Glucantime®, Sanofi Aventis, France) for the treatment of visceral leishmaniasis (VL). The aim of this study was to evaluate the effectiveness of a shorter duration of treatment in regions with low level of resistance to Glucantime. During 13 years, this study was conducted in three phases on 392 patients. In the pilot first phase, we performed splenic punctures in seven patients to assess the correlation between the changes in the parasite load during treatment with Glucantime and defervescence. With defervescence, parasite density was dramatically dropped (P = 0.014), propounding defervescence as a marker of parasitological response. On the basis of the results, we conducted a randomized trial on 75 patients, comparing the efficacy of continuation of Glucantime therapy for 1, 2, or 3 weeks after defervescence. The treatment course of 1 week after defervescence (mean = 11.7 days) was non-inferior to that of 3 weeks (final cure rate, 96% versus 100%; P = 0.023). The third phase was a retrospective cohort study of 302 patients treated either with the WHO's regimen or for 7 days after defervescence (intervention group). Relapse was detected in 8.3% patients of the intervention group and in 5% patients following the WHO's regimen (P = 0.006 for non-inferiority). The final duration of treatment in intervention group was significantly shorter than standard course (13.3 ± 2.6 versus 28 days; P < 0.001). In summary, treatment of VL with Glucantime for 1 week after defervescence was non-inferior to and appears to be an acceptable alternative to the standard 28-day course for patients in Iran who show a response to antimonial therapy.
Introduction
Visceral leishmaniasis (VL) is a life-threatening parasitic infection often caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi. It is endemic in the Middle East and Mediterranean regions and mainly affects children.1 In Iran, the primary foci for VL are northwestern and southern regions and L. infantum is the major causative agent.2 Leishmania tropica, which was the causative agent for leishmaniasis in American soldiers in the Persian Gulf War (1990–1991), has been confirmed as the second causative agent for VL in Iran.3,4 Leishmania major has also been reported as a causative agent for VL5 and mucocutaneous leishmaniasis in Iran.6
Among nearly 25 compounds having antileishmanial effects, antimony (sodium antimony gluconate and meglumine antimoniate) has been the therapeutic cornerstone of VL throughout the world for more than 80 years.1,7 It is still in widespread use with a cure rate of more than 90%, except in the southern Europe and Bihar state of India where low cure rates (36–69%) have been reported.8,9 The meglumine antimoniate (Glucantime®) is recommended to be administered in doses of 20 mg/kg/day (SbV) up to a maximum of 850 mg for 4 weeks by the WHO Expert Committee.10 The regimens with the lower dosage and shorter duration have been “reported to be effective” in the treatment of VL in some countries and the parasites may be more susceptible to antimony in these regions.11,12 So, there might be geographical difference in drug efficacy and there is a clear need to conduct a dose escalation study in the regions with a low level of resistance to Glucantime. Here, we have conducted a three-phase study, during 1997–2010. We aimed initially to investigate the role of defervescence as a clinical marker indicating parasitic response. Based on this, we aimed to compare prospectively three Glucantime regimens of different durations on the basis of the time of defervescence, and finally to assess treatment efficacy of two locally approved different regimens ensued from the results of the second phase, the short-duration treatment, and the regimen according to the WHO Expert Committee in a retrospective cohort.
Methods
This study is categorized into three phases. The study was conducted in three phases from 1997 to 2010 in Fars Province, the south of Iran, and 392 patients were enrolled. All the patients gave their written informed consent. The protocol was approved by the ethics committee of Shiraz University of Medical Sciences and the study conformed to the declaration of Helsinki for working with human subjects.
Initial cure was defined as defervescence with starting treatment and final cure was defined as no relapse during at least 6-month follow-up. Relapse was considered when a patient had persistent fever for more than 10 days and developed significant splenomegaly with a positive serology indirect fluorescent antibody (IFA) titer 1/128 or rK39 strip test and/or parasitological confirmation.11 Treatment failure was defined as death, lack of an initial cure, or relapse.
Phase one: Preliminary pilot study.
In the preliminary pilot study, we performed splenic punctures before starting treatment and 1–6 days after fever had subsided to assess the correlation between the parasite load before and during treatment with Glucantime and defervescence. Parasite density was graded on a log scale by pathologists who were also unaware of the treatment assignments.
Although splenic smears were routinely used in those days as one of the gold standards for diagnosis of kala-azar all around the world,1 we terminated this phase of the study with seven patients as fortunately the results showed the correlation between defervescence and a significant drop in the number of Leishman body aggregation in the spleen, and because the procedure was reported to be associated with fatal complications such as severe hemorrhage with hypotension. Consequently, we chose defervescence as a marker of parasitological response, so it was used as a guide for determining the duration of treatment of the second phase of the study.
Phase two: Randomized controlled trial.
The second phase was an open-label, prospective, non-inferiority, randomized trial comparing three different regimens of Glucantime therapy with different durations in which the primary end point was efficacy. Eighty-three patients were included in the second phase and final data from 75 of them were gathered. Inclusion criteria were signs of infection (fever, splenomegaly, etc.), positive serology IFA titer 1/128 or rK39 strip test, and/or positive bone marrow microscopy for Leishman bodies.10,13 Exclusion criteria were presence of clinically obvious jaundice, disseminated intravascular coagulation (DIC), and/or shock. The patients were randomized into three groups regardless of age, sex, and duration of the disease.
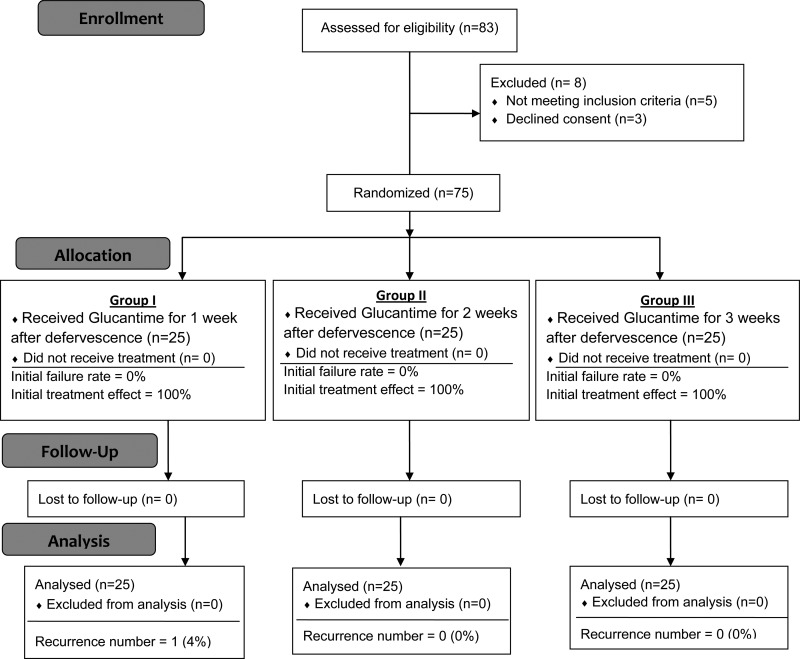
The patients admitted to Nemazee Teaching Hospital, a tertiary referral center affiliated to Shiraz University of Medical Sciences, were randomized into three groups and Glucantime was administered by deep gluteal intramuscular (IM) injection at a dose of 20 mg/kg daily for 1, 2, and 3 weeks after fever had subsided for patients in groups I, II, and III, respectively (Figure 1 ). The patients were hospitalized during the treatment and had a direct supervision from one of the study team members. All patients below the age of 1 year were treated with ampicillin and gentamicin for 10 days, as according to our experience, patients with kala-azar in this age group are at higher risk for developing occult infection.14 Other patients suspected of having septicemia were also treated accordingly.
Figure 1.
CONSORT 2010 flow diagram of phase two of the study for randomization of patients with visceral leishmaniasis to three different durations of Glucantime therapy.
The participants were followed for 6 months in this phase of the study. Because most of the participants had nomadic lifestyles and had a poor medical follow-up, the patients were followed directly by the researchers with nearly 150 trips to their residential areas and visiting them in 1, 3, and 6 months after discharging from hospital. This could help maintain the most possible follow-up data.
Phase three: a retrospective cohort study.
The third phase was a reevaluation of data from the second phase with a retrospective cohort study of patients treated with two different durations of Glucantime treatment in which the primary end point was efficacy.
On the basis of the second phase of the study, short course of Glucantime was approved as an acceptable treatment of patients with VL. In our center, physicians are free to choose short-course or standard course therapy to eliminate VL.
We gathered files of 302 patients admitted with the final diagnosis of VL in Nemazee Teaching Hospital, Shiraz, Iran (the referral center for treatment of infectious disease in the south of Iran) after completion of the second phase to compare the clinical results of patients treated with short course versus conventional course of Glucantime treatment.
Statistical analysis.
We performed a non-inferiority analysis for the primary end point. Primarily efficacy was calculated as the proportion of patients achieving a final cure and an exact confidence interval for that proportion was computed. The exact, one-sided, upper bound of the 98% confidence interval for the difference in success probabilities was compared using δ = 0.10 (the chosen margin for non-inferiority). We used Pearson's χ2 test or Fisher's exact test to compare categorical variables and the t test to compare continuous variables. Other statistical tests included were Wilcoxon singed rank test, Mann–Whitney test, and Kruskal–Wallis test. All principal analyses were performed in the intention-to-treat population, which consisted of all the patients who underwent randomization, regardless of the treatment received. The patients who were lost to follow-up and in whom no known event had occurred were not included in the denominator for calculation of binary end points. All P values except the value for non-inferiority were two tailed. The P values less than 0.05 were considered significant. Statistical package for social sciences version 17.0 (SPSS Inc., Chicago, IL) was used for statistical analysis.
Results
In the preliminary pilot study, it was found that after initial treatment with Glucantime and subsequent defervescence, the parasite density was dramatically dropped (Table 1). In this phase, we reached statistical significance, indicating that defervescence might be considered as a reliable sign of clearance of the body from parasites (P = 0.014).
Table 1.
Load of Leishman body aggregation in the spleen of patients with visceral leishmaniasis before treatment and after defervescence following Glucantime therapy in the first phase of the study
| Case | Leishman body in splenic puncture before treatment | Days after defervescence (after treatment) | Leishman body in splenic puncture after treatment |
|---|---|---|---|
| 1 | +2 | 6 | +1 |
| 2 | +1 | 4 | +0 |
| 3 | +4 | 2 | +1 |
| 4 | +1 | 2 | +0 |
| 5 | +1 | 2 | +0 |
| 6 | +4 | 3 | +1 |
| 7 | +1 | 1 | +0 |
On the basis of the findings of the first round, we categorized the patients into three groups for the second round of the study. After the start of treatment with Glucantime, defervescence occurred within 3 days in 42 patients (56%), and in less than 7 days in 68 patients (90.6%) (Table 2). Most of our patients had splenomegaly (Table 3), and in 32% of them, the spleen extended to more than 5 cm below the costal margin on admission. Over a 6-month follow-up period, relapse was seen in only one patient in group I. No relapse was seen in groups II and III (final cure rate, 96% versus 100%; difference, 4% points; upper bound of the 98% confidence interval, 1.9; P = 0.023 for non-inferiority and P = 0.86 for superiority). It is important to note that the treatment in group I was completed after an average of 11.7 days.
Table 2.
Time course between initiation of Glucantime therapy and defervescence
| Group | Beginning of the defervescence after treatment (day) | ||
|---|---|---|---|
| < 3 | 3–7 | > 7 | |
| I | 17 | 5 | 3 |
| II | 13 | 9 | 3 |
| III | 12 | 10 | 3 |
Table 3.
Baseline characteristics of the patients with visceral leishmaniasis, who received Glucantime for 1 week (group I), 2 weeks (group II), and 3 weeks (group III) after defervescence in the second phase of the study
| Group I (N = 25) | Group II (N = 25) | Group III (N = 25) | |
|---|---|---|---|
| Characteristic | |||
| Age (month) | 28.16 | 27.44 | 29.32 |
| Underweight (%) | 40 | 36 | 44 |
| Short stature (%) | 68 | 64 | 68 |
| Nomadic lifestyle (%) | 64 | 68 | 68 |
| Signs and symptoms | |||
| Splenomegaly (%) | 84 | 100 | 96 |
| Hepatomegaly (%) | 56 | 52 | 48 |
| Jaundice (%) | 12 | 8 | 12 |
| Ascites (%) | 12 | 8 | 12 |
| Petechia/purpura (%) | 12 | 12 | 12 |
| Fever (%) | 96 | 100 | 92 |
| Malnutrition (%) | 72 | 68 | 76 |
| Edema (%) | 16 | 12 | 8 |
| Diarrhea (%) | 12 | 12 | 16 |
| Lymphadenopathy (%) | 0 | 0 | 0 |
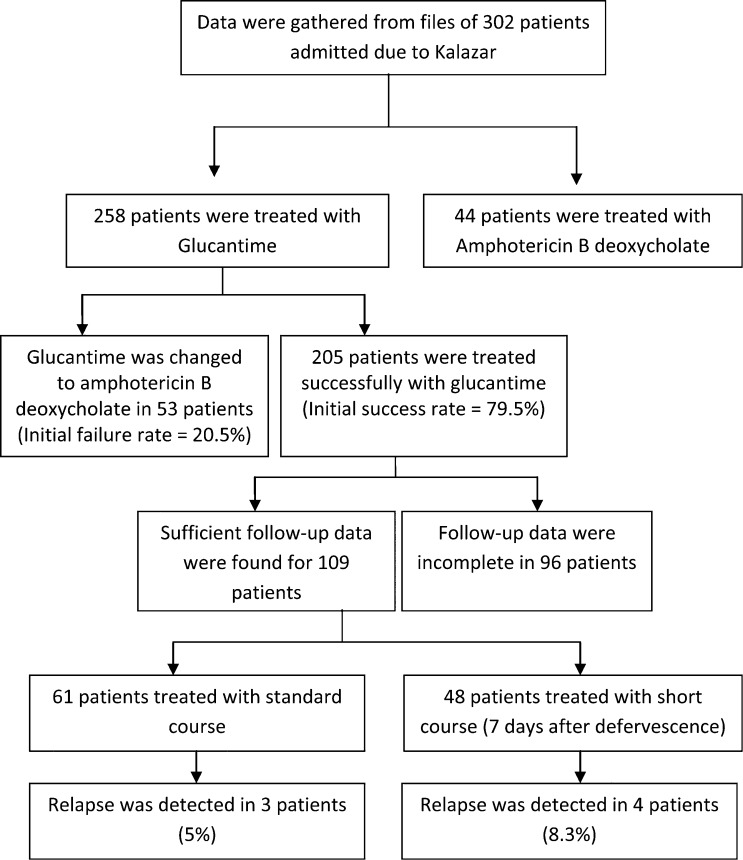
In the third phase of the study, data were gathered from 302 patients. The initial antileishmanial regimen was Glucantime in 258 (85.5%) patients and amphotericin B deoxycholate (ABD) in 44 patients (14.5%). In 53 patients (9.2%), Glucantime was changed to ABD during the treatment course due to developing conditions, such as bleeding tendency, symptomatic hepatitis, and ascites, in which Glucantime was contraindicated. Among 205 patients who had initial cure with Glucantime, accurate data regarding the uration of Glucantime treatment were available in 109 patients. The majority of patients, 61 (56%), were treated for more than 3 weeks (conventional regimen), and for 48 patients (44%), Glucantime was continued for 7 days after defervescence as the short-course regimen (Figure 2 ). Demographic, clinical, and laboratory features of these patients are shown in Table 4. The total treatment failure in patients receiving Glucantime was 23.2% (60 of 258). Relapse was detected in four patients (8.3%) treated with short-course regimen and in three patients treated with standard course (5%) (difference, 3.3% points; upper bound of the 98% confidence interval, 2.87; P = 0.006 for non-inferiority and P = 0.49 for superiority). One death happened in conventional-regimen group and one in short-course regimen group (1.6% and 2%, respectively; difference, 0.4% points; upper bound of the 98% confidence interval, 5.9; P < 0.001 for non-inferiority and P = 0.56 for superiority). The mean days of defervescence were 5.16 ± 2.21 (3–10) days after starting treatment and 5.05 ± 2.26 (2–11) days in the standard course group. The final duration of treatment was only 13.3 ± 2.6 days in the short-course group and 23.8 ± 3.6 days in the standard course group, which was significantly shorter (P < 0.001).
Figure 2.
Flow chart and results of phase three of the study.
Table 4.
Baseline characteristics of the patients with visceral leishmaniasis who received short course and conventional course of Glucantime in the third phase of the study
| Short-course therapy group (N = 49) | Conventional course therapy group (N = 61) | P value | |
|---|---|---|---|
| Characteristic | |||
| Age (month) | 28.16 ± 26.37 (3–108) | 23.84 ± 24.89 (2–156) | 0.393 |
| Weight (kg) | 10.6 ± 4.1 (6–25) | 10.2 ± 4.4 (4.3–35) | 0.575 |
| Signs and symptoms | |||
| Palpable spleen size (cm below costal margin) | 4.9 ± 2.8 (1–12) | 4.4 ± 2.1 (0–10) | 0.849 |
| Palpable liver size (cm below costal margin) | 3.5 ± 2.1 (0–10) | 3.1 ± 1.7 (0–7) | 0.313 |
| Jaundice (%) | 4.7 | 4.5 | 0.603 |
| Ascites (%) | 0 | 1.5 | 1.000 |
| Petechia/purpura (%) | 2.3 | 0 | 0.445 |
| Fever duration before treatment (day) | 23.4 ± 12.3 (7–60) | 19.6 ± 7.8 (3–40) | 0.390 |
| Laboratory values | |||
| White blood cell count(×103/mm3) | 5,566 ± 2,250 (2,000–11,800) | 5,781 ± 3,064 (600–15,500) | 0.547 |
| Platelet count (×103/mm3) | 128 ± 83 (18–357) | 130 ± 86 (17–510) | 0.662 |
| Hemoglobin (g/dL) | 8.47 ± 2 (3.4–13.4) | 8.21 ± 1.71 (4–14.8) | 0.999 |
| Anemia (%) | 90.4 | 92.4 | 1.000 |
| Serum alanine aminotransferase (U/L) | 151 ± 176 (23–925) | 192 ± 226 (17–1,015) | 0.952 |
| Serum aspartate aminotransferase (U/L) | 74 ± 104 (5–590) | 74 ± 82 (5–462) | 0.381 |
| Total bilirubin (mg/dL) | 0.83 ± 1.5 (0.2–9.6) | 0.72 ± 1.08 (0.2–8.5) | 0.921 |
| Albumin (g/dL) | 3.58 ± 0.64 (2.1–4.9) | 3.59 ± 0.66 (2–5.2) | 0.851 |
| Prothrombin time (second) | 14.32 ± 1.26 (13–17) | 14.46 ± 2.2 (11–20) | 0.409 |
| Partial thromboplastin time (second) | 36.88 ± 13.15 (11–70) | 40.65 ± 13.9 (20–78) | 0.186 |
| Urine protein (dipstick positive plus) | 0.585 | ||
| No (%) | 61.9 | 75 | |
| 1+ (%) | 9.5 | 7.8 | |
| 2+ (%) | 4.8 | 4.7 | |
| 3+ (%) | 23.8 | 12.5 | |
| Pyuria (%) | 38.1 | 42.2 | |
| Hematuria (%) | 9.5 | 14.1 | |
| Urine culture (%) | 17.9 | 19.4 | |
| Disease indices | |||
| Indirect fluorescent antibody titer | 0.716 | ||
| Negative (%) | 10.3 | 6.7 | |
| 1/64 (%) | 17.9 | 13.3 | |
| 1/128 (%) | 33.3 | 33.3 | |
| 1/256 (%) | 12.8 | 26.7 | |
| 1/512 (%) | 17.9 | 11.7 | |
| 1/1,024 (%) | 7.7 | 8.3 | |
| Parasite found in the bone marrow (%) | 25.8 | 34.8 | |
Plus–minus values are mean ± SD. Data shown in parentheses are minimum–maximum. To convert the values for bilirubin to micromoles per liter, multiply by 17.1.
Discussion
Meglumine antimoniate (Glucantime) remains the first line of treatment of VL in the majority of regions throughout the world for more than 80 years.1,7 Administration of 20 mg/kg/day Glucantime for 20–30 days leads to a cure rate of about 90% in these regions.1,8 As for therapeutic regimens, the dosage and duration of treatment vary from country to country, due to the differences in the sensitivity of parasites to antileishmanial compounds.11,15–17 Considering the regional and species variability in the treatment, it is suggested that the choice of treatment strategy should be based on geographical location and the infecting species.5,18 In India, whereas a 10-day treatment course has been shown to be efficient by one study group, a 30-day course has also been advocated by others.10,19 To the best of our knowledge, this is the first study of the rate of response to Glucantime in patients with VL from Iran. This study shows that treatment of VL with a dose of 20 mg/kg/day Glucantime for 1 week after defervescence appears to be an effective treatment for nonsevere VL in Iran. The duration of treatment with short-course protocol (daily for mean 11.7 days in the second phase of the study and 13.3 days in the third phase) is shorter than other regimens, such as conventional course of Glucantime (daily for 28 days), paromomycin (intramuscularly daily for 21 days), and amphotericin B deoxycholate (intravenously every other day for 30 days). It is also shorter than miltefosine (orally daily for 28 days), although the visit burden may be higher.12,20 Another therapeutic regimen with even shorter course is liposomal formulation of amphotericin B (LAmB) as a single dose, which has been shown to have promising results in some regions.21–24 Some clinical trials aiming to shorten the duration of antileishmanial treatment had not shown promising results. The definitive cure rate in eastern Africa was 85% in patients treated with multiple doses of liposomal formulation and only 40% in single doses.25 It was also not successful to shorten the duration of IM paromomycin from 21 to 14 days in India and it was accompanied by significantly less cure rate (92% and 82%, respectively).20
In the preliminary phase, defervescence after treatment of VL was strongly correlated with a decrease in the parasite load of the spleen. It has been documented that the few remaining organisms that survive after treatment will be trapped by the immune system.26–28 To assure complete parasite control after defervescence, in this study we continued Glucantime for another week. A rapid decrease in L. infantum kinetoplast DNA loads in the blood, evaluated using real-time polymerase chain reaction (PCR) assay, was also observed with defervescence during treatment with Glucantime.26 A good correlation between peripheral blood parasite load detected by quantitative PCR and splenic parasite score has also been recently shown.29
In the third phase of the study, the failure rates including both failure of initial response and relapse were as high as 24% among patients receiving Glucantime, despite no failure rate in phase two. Excluding patients with severe VL in phase two can explain this difference. Worldwide, various failure rates have been reported (1–60%), which mainly depend on geographic area and Glucantime regimen (dose and duration).30,31 Among those who responded, the overall relapse rate was 8.3% with the use of short-course regimen, and the difference in relapse rates between patients treated with short-course protocol or the conventional course was consistently less than 3.3% points. So, the overall relapse rate among patients treated with short-course protocol was acceptable for a program to eliminate VL to succeed prompt treatment.
Limitations of the third phase of the study include high variation at the patients' files since these data were not documented by the researchers but by other physicians, making the data collection not standardized for this specific outcome. Moreover, although the patients were followed directly by the researchers by traveling to their residential areas and visiting them in the second phase of the study, the patient follow-up in the third phase of the study was done in a passive manner in which the patients refer to physicians, and this may lead to loss of some of the follow-up data. But follow-up conditions were similar for both groups in phase three, so we can accept the results of this phase.
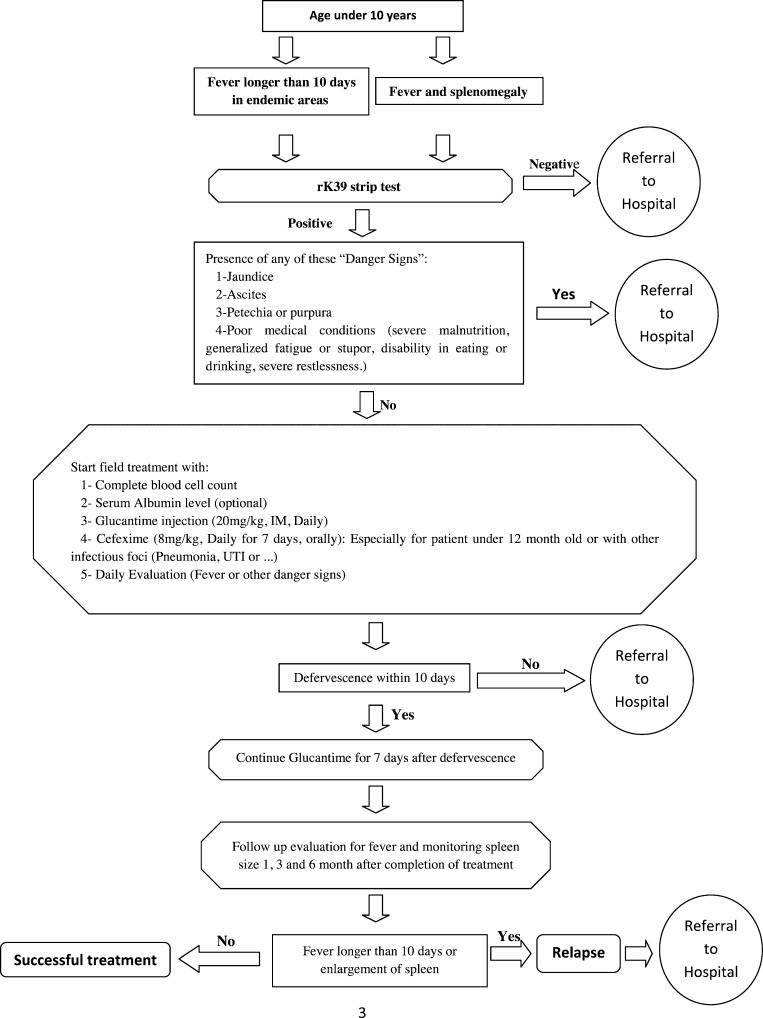
It is shown that using rK39 strip test can lead to rapid accurate field diagnosis of VL in adults and pediatrics.12,13 Furthermore, Glucantime can be administered intramuscularly according to body weight (20 mg/kg) to patients with VL. Considering the results of this study and the fact that the costs of long duration of treatment in standard course causes poor compliance for admission and a need for referral to local centers for IM injection, we adopted an algorithmic approach to diagnose the patients in the fields rapidly and choose the appropriate patients for local treatment with daily Glucantime IM injections (Figure 3 ), omitting the costs of hospitalization and risks of traveling with a better cost–benefit yield.32 This approach will need further clinical evaluations to confirm its validity.
Figure 3.
Proposed flow chart of the approach for rapid field diagnosis and treatment of visceral leishmaniasis in endemic areas.
It can be concluded that among patients who responded to treatment of VL with 20 mg/kg/day Glucantime, extending the treatment for 1 week after defervescence was non-inferior to the standard course and is thoroughly acceptable in regions with low antimonial resistance, especially in patients not presenting with a severe leishmaniasis. Since most of the patients become afebrile during the first week of the treatment, this regimen shortens the duration of therapy and hospitalization, translating into a better cost–benefit yield and less drug complication. Other antileishmanial drugs, such as liposomal amphotericin B, should be further investigated as first-line therapeutic regimen for VL in Iran, especially in patients with severe and complicated VL.
ACKNOWLEDGMENTS
We would like to thank Nasrin Shokrpour at Center for Development of Clinical Research of Nemazee Teaching Hospital for editorial assistance and Hassan Khajehei for copy-editing the manuscript.
Footnotes
Financial support: This investigation is the result of two theses supported by vice-chancelleries of research in Shiraz University of Medical Sciences and Fasa University of Medical Sciences.
Authors' addresses: Abdolvahab Alborzi, Gholamreza Pouladfar, Zahra Jafarpour, and Mohammad Rahim Kadivar, Department of Paediatrics, Professor Aborzi Clinical Microbiology Research Center, Nemazee Teaching Hospital, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, E-mails: alborziiraj2004@yahoo.com, pooladfar@sums.ac.ir, kadivarmr@sums.ac.ir, and zjafarpoor54@yahoo.com. Armin Attar, Cardiovascular Research Center, TAHA Clinical Trial Group, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, E-mail: attarar@sums.ac.ir. Fatemeh Falahi, Student Research Committee, Shiraz and Fasa University of Medical Sciences, Fasa, Iran, E-mail: f.fallahi58@yahoo.com. Abdollah Karimi, Pediatric Infectious Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran, E-mail: dr_aKarimi@yahoo.com.
References
- 1.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 2.Alborzi A, Pouladfar GR, Aelami MH. Visceral leishmaniasis; literature review and Iranian experience. Iran J Clinic Infect Dis. 2007;2:99–108. [Google Scholar]
- 3.Alborzi A, Rasouli M, Shamsizadeh A. Leishmania tropica-isolated patient with visceral leishmaniasis in southern Iran. Am J Trop Med Hyg. 2006;74:306–307. [PubMed] [Google Scholar]
- 4.Magill AJ, Grogl M, Gasser RA, Sun W, Oster CN. Visceral infection caused by Leishmania tropica in veterans of operation desert storm. N Engl J Med. 1993;328:1383–1387. doi: 10.1056/NEJM199305133281904. [DOI] [PubMed] [Google Scholar]
- 5.Karamian M, Motazedian MH, Mehrabani D, Gholami K. Leishmania major infection in a patient with visceral leishmaniasis: treatment with Amphotericin B. Parasitol Res. 2007;101:1431–1434. doi: 10.1007/s00436-007-0649-x. [DOI] [PubMed] [Google Scholar]
- 6.Alborzi A, Pouladfar GR, Moghadam AG, Attar A, Drakhshan N, Khosravi Maharlooei M, Kalantari M. First molecular-based detection of mucocutaneous leishmaniasis caused by Leishmania major in Iran. J Infect Dev Ctries. 2013;7:413–416. doi: 10.3855/jidc.2754. [DOI] [PubMed] [Google Scholar]
- 7.Mandal G, Mandal S, Sharma M, Charret KS, Papadopoulou B, Bhattacharjee H, Mukhopadhyay R. Species-specific antimonial sensitivity in Leishmania is driven by post-transcriptional regulation of AQP1. PLoS Negl Trop Dis. 2015;9:e0003500. doi: 10.1371/journal.pntd.0003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman J. Amphotericin B formulations and other drugs for visceral leishmaniasis. Am J Trop Med Hyg. 2015;92:471–473. doi: 10.4269/ajtmh.14-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, Kumar PC, Murray HW. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–1106. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- 10.WHO Report of the Informal Meeting on the Chemotherapy of Visceral Leishmaniasis. 1982. TDR/CHEMLEISH/VL/82.3.
- 11.Chulav JD, Bhatt SM. A comparison of 3 dosage regimens of sodium stibogluconate in the treatment of visceral leishmaniasis in Kenya. J Infect Dis. 1983;148:148–155. doi: 10.1093/infdis/148.1.148. [DOI] [PubMed] [Google Scholar]
- 12.Sundar S, Reed SG, Singh VP, Kumar CKP, Murray HW. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet. 1998;351:563–565. doi: 10.1016/S0140-6736(97)04350-X. [DOI] [PubMed] [Google Scholar]
- 13.Alborzi A, Rasouli M, Nademi Z, Kadivar MR, Pourabbas B. Evaluation of rK39 strip test for the diagnosis of visceral leishmaniasis in infants. East Mediterr Health J. 2006;12:294–299. [PubMed] [Google Scholar]
- 14.Kadivar MR, Kajbaf TZ, Karimi A, Alborzi A. Childhood visceral leishmaniasis complicated by bacterial infections. East Mediterr Health J. 2000;6:879–883. [PubMed] [Google Scholar]
- 15.Kirk R, Sati MH. Observation on the use of sodium antimony gluconte (sodium stibogluconate) in treatment of kala-azar. Ann Trop Med Parasitol. 1947;41:14–21. doi: 10.1080/00034983.1947.11685306. [DOI] [PubMed] [Google Scholar]
- 16.Leng YJ. A review of kala-azar in China from 1949–1959. Trans R Trop Med Hyg. 1982;76:531–537. doi: 10.1016/0035-9203(82)90157-2. [DOI] [PubMed] [Google Scholar]
- 17.Copeland NK, Aronson NE. Leishmaniasis: treatment updates and clinical practice guidelines review. Curr Opin Infect Dis. 2015;28:426–437. doi: 10.1097/QCO.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 18.Croft SL, Coombs GH. Leishmaniasis current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19:502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Wigers DJB. A ten year study of kala-azar in Tharke (Meru District Kenya) relapses. East Afr Med J. 1971;48:551–558. [PubMed] [Google Scholar]
- 20.Jamil KM, Haque R, Rahman R, Faiz MA, Bhuiyan AT, Kumar A, Hassan SM, Kelly H, Dhalaria P, Kochhar S, Desjeux P, Bhuiyan MA, Khan MM, Ghosh RS. Effectiveness study of paromomycin IM injection (PMIM) for the treatment of visceral leishmaniasis (VL) in Bangladesh. PLoS Negl Trop Dis. 2015;9:e0004118. doi: 10.1371/journal.pntd.0004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundar S, Pandey K, Thakur CP, Jha TK, Das VN, Verma N, Lal CS, Verma D, Alam S, Das P. Efficacy and safety of amphotericin B emulsion versus liposomal formulation in Indian patients with visceral leishmaniasis: a randomized, open-label study. PLoS Negl Trop Dis. 2014;8:e3169. doi: 10.1371/journal.pntd.0003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondal D, Alvar J, Hasnain MG, Hossain MS, Ghosh D, Huda MM, Nabi SG, Sundar S, Matlashewski G, Arana B. Efficacy and safety of single-dose liposomal amphotericin B for visceral leishmaniasis in a rural public hospital in Bangladesh: a feasibility study. Lancet Glob Health. 2014;2:e51–e57. doi: 10.1016/S2214-109X(13)70118-9. [DOI] [PubMed] [Google Scholar]
- 23.Maintz EM, Hassan M, Huda MM, Ghosh D, Hossain MS, Alim A, Kroeger A, Arana B, Mondal D. Introducing single dose liposomal amphotericin B for the treatment of visceral leishmaniasis in rural bangladesh: feasibility and acceptance to patients and health staff. J Trop Med. 2014;2014:676817. doi: 10.1155/2014/676817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucero E, Collin SM, Gomes S, Akter F, Asad A, Kumar Das A, Ritmeijer K. Effectiveness and safety of short course liposomal amphotericin B (AmBisome) as first line treatment for visceral leishmaniasis in Bangladesh. PLoS Negl Trop Dis. 2015;9:e0003699. doi: 10.1371/journal.pntd.0003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalil EA, Weldegebreal T, Younis BM, Omollo R, Musa AM, Hailu W, Abuzaid AA, Dorlo TP, Hurissa Z, Yifru S, Haleke W, Smith PG, Ellis S, Balasegaram M, EL-Hassan AM, Schoone GJ, Wasunna M, Kimutai R, Edwards T, Hailu A. Safety and efficacy of single dose versus multiple doses of AmBisome for treatment of visceral leishmaniasis in eastern Africa: a randomised trial. PLoS Negl Trop Dis. 2014;8:e2613. doi: 10.1371/journal.pntd.0002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pourabbas B, Ghadimi Moghadam A, Pouladfar G, Rezaee Z, Alborzi A. Quantification of Leishmania infantum kinetoplast DNA for monitoring the response to meglumine antimoniate therapy in visceral leishmaniasis. Am J Trop Med Hyg. 2013;88:868–871. doi: 10.4269/ajtmh.12-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyakundi PM, Wasunna KM, Rashid Jr, Gachihi GS, Mbugua J, Kirigi G, Muttunga J. Is one year follow up justified in kala-azar post-treatment? East Afr Med J. 1994;71:453–459. [PubMed] [Google Scholar]
- 28.Saha S, Mondal S, Banerjee A, Ghose J, Bhowmick S, Ali N. Immune responses in kala-azar. Indian J Med Res. 2006;123:245–266. [PubMed] [Google Scholar]
- 29.Sudarshan M, Singh T, Chakravarty J, Sundar S. A correlative study of splenic parasite score and peripheral blood parasite load estimation by qPCR in visceral leishmaniasis. J Clin Microbiol. 2015;53:3905–3907. doi: 10.1128/JCM.01465-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aikat BK, Sahaya S, Pathania AG, Bhattacharya PK, Desai N, Prasad LS, Mishra S, Jain S. Clinical profile of cases of kala-azar in Bihar. Indian J Med Res. 1979;70:563–570. [PubMed] [Google Scholar]
- 31.Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, Kumar PC, Murray HW. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–1107. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- 32.Coura-Vital W, Araújo VE, Reis IA, Amancio FF, Reis AB, Carneiro M. Prognostic factors and scoring system for death from visceral leishmaniasis: an historical cohort study in Brazil. PLoS Negl Trop Dis. 2014;8:e3374. doi: 10.1371/journal.pntd.0003374. [DOI] [PMC free article] [PubMed] [Google Scholar]