Abstract
Hookworms are enteric parasitic roundworms infecting an estimated 400 million persons worldwide. Herein, we provide the first molecular identifications of human hookworms from certain parts of rural Lower Myanmar. DNA was extracted from hookworm-positive stool samples, as determined by microscopy. DNA sequences of the partial internal transcribed spacer 1, full length 5.8S gene, and partial internal transcribed spacer 2 were determined and compared with available hookworm sequences from public databases. Of the 11 polymerase chain reaction–positive samples, eight (Bago Region, N = 4; Mon State, N = 4) yielded sequences with high similarity to those of Necator americanus. A further three sequences (Mon State, N = 2; Bago Region, N = 1) showed high similarity with those of Ancylostoma ceylanicum. The latter is primarily a parasite of dogs and represents a zoonosis. Given that different species of hookworms exhibit different epidemiological and biological characteristics, accurate identification is essential for the planning and execution of effective control programs for hookworm infections.
Human hookworm infection remains a main public health problem, particularly in developing countries in tropical and subtropical regions due to poor sanitation and hygiene practices.1 An estimated 438.9 million people are infected with hookworms globally, experiencing chronic blood loss and anemia, and often leading to retardation of physical and cognitive growth in children.2 Of the two major hookworm species that cause human infection, Necator americanus is worldwide in distribution with the highest infection rates in sub-Saharan Africa, the tropical sections of the Americas, south China, and southeast Asia. Ancylostoma duodenale is endemic in certain parts of India, China, sub-Saharan Africa, north Africa, and a few areas of the Americas.3 In recent years, zoonotic hookworm species such as Ancylostoma ceylanicum and Ancylostoma caninum have been reported as significant health problems in many areas.4,5 Ancylostoma ceylanicum is now the second most common hookworm species infecting humans in Asia and possible cause of cutaneous larvae migrans or abdominal symptoms in infected patients, with reported high egg-positivity rates in Thailand, Laos, Cambodia, and Malaysia.6–10 National surveys on the prevalence of soil-transmitted helminths (STHs) in Myanmar reported a reduction in hookworm prevalence in schoolchildren from 6.5% to 0.3% 7 years after a national deworming program.11,12 A diagnosis of hookworm infection was usually made by microscopic examination of eggs or larvae in the fecal samples. However, species identification of hookworms is difficult and cannot be done using eggs or larvae. Each genus and species of hookworm infecting humans has its own distinct biology, life cycle, pathophysiology, and epidemiology, knowledge of which therefore impacts on designing control measures.6 A molecular approach to identification is therefore imperative and copro-molecular methods for species identification of hookworms have been reported in neighboring countries (Thailand,8,13 Lao People's Democratic Republic [PDR],14 and Cambodia6,15). Molecular methods were used to identify as A. ceylanicum a case of hookworm infection in a French individual who had visited Myanmar. This is the only previous report on molecular characterization of hookworm species in Myanmar.16 In the present study, molecular identification of hookworms that infect humans in rural parts of Lower Myanmar is reported for the first time. This genetic data is of value in continuing explorations into epidemiology of hookworms in Myanmar populations.
This study protocol was approved by the Khon Kaen University Ethics Committee for Human Research (HE581396) and the Research and Ethical Committee of the University of Medicine 1, Yangon, Myanmar (5695/Research and Ethics 2015). Each participant was informed of study methods, risks, and benefits of the process. Before enrollment, written consent was obtained from all adult participants and from parents or legal guardians of minors. All infected persons were treated according to the World Health Organization guideline for STH control programs in Myanmar.17
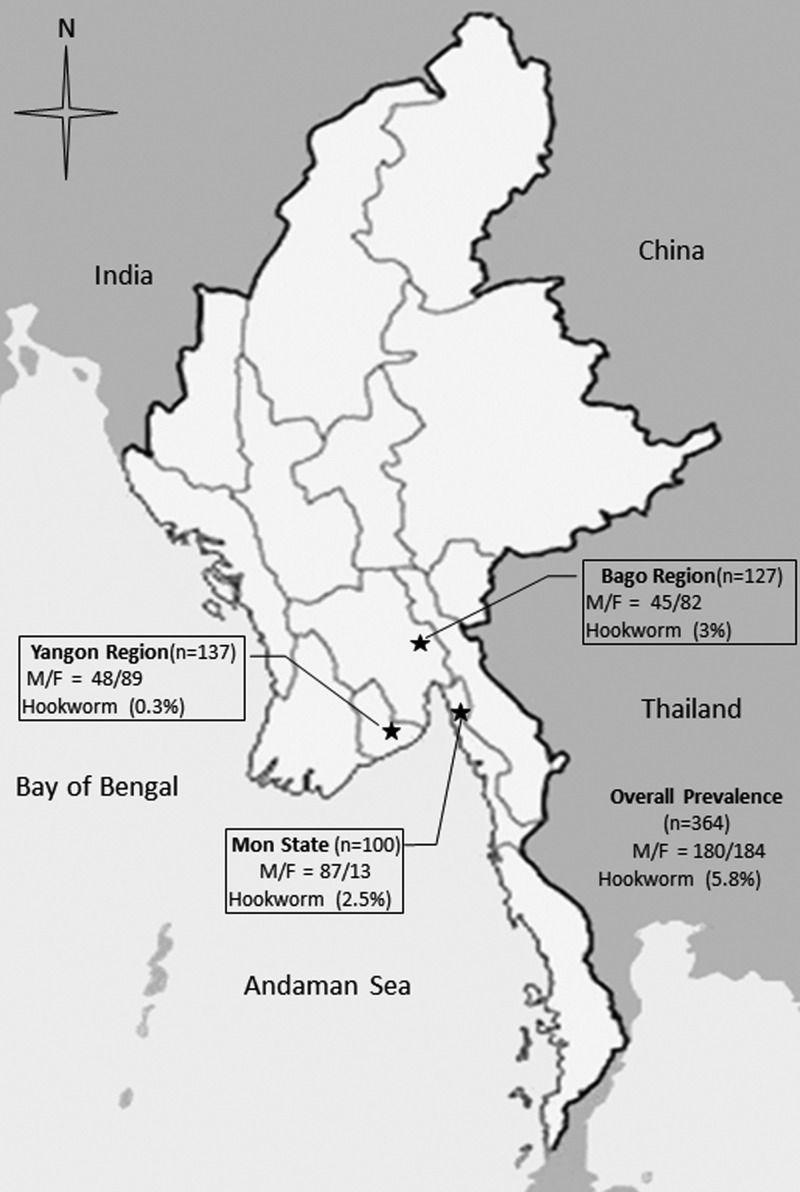
A total of 364 fecal samples was collected from individuals between 5 and 60 years of age living in three different rural study areas of Lower Myanmar (Kyaik Hto Township, Mon State, N = 100; Shwe Kyin Township, Bago Region, N = 127; Thone Gwa Township, Yangon Region, N = 137) (Figure 1 ) during June and December 2015. Twenty-one samples (5.8%) were positive for hookworm eggs after stool examination by formalin ethyl acetate concentration technique. Of these, 11 (3.0%) were from the Bago Region, nine (2.5%) from Mon State, and one (0.3%) from the Yangon Region. DNA was extracted from all positive fecal samples (preserved in 95% ethanol and stored at −20°C) using a QIAamp® DNA stool mini kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. The polymerase chain reaction (PCR) was carried out using a GeneAmp® PCR System 9700 (Applied Biosystems, Singapore City, Singapore). Primers used were specific for Ancylostoma as well as Necator species and amplified most of the ribosomal internal spacer region (partial internal transcribed spacer 1, 5.8S rRNA gene, and partial internal transcribed spacer 2). Primer sequences are RTHW1F (forward): 5′-GAT GAG CAT TGC WTG AAT GCC G-3′ and RTHW1R (reverse): 5′-GCA AGT RCC GTT CGA CAA ACA G- 3′.8 The PCR reaction and conditions were as reported previously.13 PCR products were run on a 1% agarose gel to demonstrate amplicons of approximately 485 base pairs (bp) (typical for N. americanus) or 380 bp (typical for Ancylostoma spp.). Eleven samples yielded positive PCR results. DNA sequencing of these was performed using an Applied Biosystems 3730 + I DNA Analyzer and ABI big dye Version 3.1 (Applied Biosystems, Foster City, CA). Sequencing was done in both directions, using the PCR primers as sequencing primers. The nucleotide sequences obtained were compared with those available in the GenBank database by BLAST-N search via NCBI. Sequences were aligned using ClustalW (Belfield, Dublin, Ireland).
Figure 1.
Study areas in Lower Myanmar.
Of the 11 samples from Lower Myanmar, eight (Bago Region, N = 4; Mon State, N = 4) showed high sequence similarity (99-100%) to those of N. americanus from GenBank (accession nos. LC036565, from Lao PDR; LC036563, from Japan; KM891738 of unknown geographical origin; AB793527, from Central African Republic; AF217891, from Guatemala), and three (Mon State, N = 2; Bago Region, N = 1) showed 99–100% similarity with sequences of A. ceylanicum from GenBank accession nos. LC036567, Papua New Guinea; AB501355, Lao PDR; KF279134, China; KM066110 of unknown geographical origin). All sequences obtained in this study were deposited in the GenBank database under the accession numbers for N. americanus (KX577783 and KX577786) and for A. ceylanicum (KX577784 and KX577785).
The present study provides the first molecular confirmation of the identities of human hookworm species that are prevalent in Myanmar. Necator americanus, a species that commonly infects humans and A. ceylanicum, a common parasite of animals, were found in the study area of Lower Myanmar. Ancylostoma duodenale, reported from humans in many parts of the world, was not found in this study. Several molecular epidemiologic surveys have also reported that N. americanus and A. ceylanicum are the two most common hookworm species infecting humans and dogs in Asia.6–10 Prevalences of hookworms in Cambodia are reportedly very high,18 and are predominantly due to N. americanus and A. ceylanicum (51.6% equal prevalence): only 3.2% of people were found infected with A. duodenale.6 In Thailand, the principal hookworm species infecting humans was confirmed as N. americanus. However, A. duodenale, A. ceylanicum, and A. caninum were also detected in different parts of the country.8,13 The predominance of N. americanus in Lower Myanmar is similar to a report from Peninsular Malaysia where N. americanus was more common than A. ceylanicum, but A. duodenale infection was not discovered.10 In contrast, a report from Lao PDR people found that infections due to A. duodenale and the animal hookworms, A. caninum and A. ceylanicum, were slightly more prevalent than those due to N. americanus.14 Hookworm infections constitute a significant neglected tropical disease in the human populations of southeast Asia.2 Our study has been the first to use molecular techniques to identify the species of hookworms, including the zoonotic A. ceylanicum, infecting rural people in Lower Myanmar. Although animal hookworm infections of people have been regarded as rare and overlooked, A. ceylanicum has been reported from a French traveler who had visited Myanmar and presented with bloody diarrhea.16 The present study also identified A. ceylanicum from asymptomatic rural people from the study area in Myanmar. Zoonotic awareness should be a concern because domestic animals such as dogs and cats act as natural hosts.19 The geographical differences in species of hookworm causing infections in humans are probably related to climate, temperature, environmental factors, parasite behavior, and ethnicity.5 The extent of variation within the cox1 sequence of human hookworm still needs further investigation as reported previously16 to explore potential haplotype-linked differences in zoonotic, epidemiological, and pathological characteristics of human hookworm in different localities of Myanmar.
ACKNOWLEDGMENTS
We would like to thank Kyi Kyi Thinn, University of Medicine 1, Yangon, Ministry of Health and Sport, Myanmar, and staffs from Department of Parasitology, National Health Laboratory, Myanmar, for assisting field trip and laboratory. We also would like to thank David Blair for valuable suggestions and assistance with the presentation of this paper through Khon Kaen University Publication Clinic.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Footnotes
Financial support: This study was supported by a TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5880001; the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Thailand, through the Health Cluster (SHeP-GMS); the Faculty of Medicine, Khon Kaen University (TR57201) through Wanchai Maleewong and Pewpan M. Intapan. Win Pa Pa Aung was partially supported by the Faculty of Medicine, Khon Kaen University (IN59211). Oranuch Sanpool was supported by Scholarship under the Post-Doctoral Training Program from Research Affairs and Graduate School, Khon Kaen University (58101).
Authors' addresses: Win Pa Pa Aung, Department of Parasitology and Research and Diagnostic Center for Emerging Infectious Disease, Faculty of Medicine, Khon Kaen University, Khon Kaen Thailand and Department of Microbiology, University of Medicine 2, Ministry of Health and Sport, Yangon, Myanmar, E-mail: eipamicro@gmail.com. Thi Thi Htoon and Htay Htay Tin, Department of Parasitology, National Health Laboratory, Myanmar, E-mails: thithihtoon@gmail.com and drhtayhtaytin@gmail.com. Oranuch Sanpool, Jurairat Jongthawin, Lakkhana Sadaow, Issarapong Phosuk, Rutchanee Ropai, Pewpan M. Intapan, and Wanchai Maleewong, Department of Parasitology and Research and Diagnostic Center for Emerging Infectious Disease, Faculty of Medicine, Khon Kaen University, Thailand, E-mails: sanpoolor@yahoo.com, jurairat_kku@hotmail.com, sadaow1986@gmail.com, issarapong2oum@gmail.com, rutchanee5020@gmail.com, pewpan@kku.ac.th, and wanch_ma@kku.ac.th.
References
- 1.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Diemert DJ. Hookworm. In: Satoskar AR, Simon GL, Hotez PJ, Tsuji M, editors. Medical Parasitology. Austin, TX: Landes Bioscience; 2009. pp. 21–30. [Google Scholar]
- 5.Mahdy MA, Lim YA, Ngui R, Siti Fatimah MR, Choy SH, Yap NJ, Al-Mekhlafi HM, Ibrahim J, Surin J. Prevalence and zoonotic potential of canine hookworms in Malaysia. Parasit Vectors. 2012;5:88. doi: 10.1186/1756-3305-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, Sok D, Marti H, Muth S, Odermatt P, Traub RJ. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg Infect Dis. 2014;2:976–982. doi: 10.3201/eid2006.131770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, Traub RJ, Piyaraj P, Naaglor T, Taamasri P, Leelayoova S. Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg. 2011;84:594–598. doi: 10.4269/ajtmh.2011.10-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traub RJ, Inpankaew T, Sutthikornchai C, Sukthana Y, Thompson RC. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet Parasitol. 2008;155:67–73. doi: 10.1016/j.vetpar.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Conlan JV, Khamlome B, Vongxay K, Elliot A, Pallant L, Sripa B, Blacksell SD, Fenwick S, Thompson RC. Soil-transmitted helminthiasis in Laos: a community-wide cross-sectional study of humans and dogs in a mass drug administration environment. Am J Trop Med Hyg. 2012;86:624–634. doi: 10.4269/ajtmh.2012.11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngui R, Lim YA, Traub R, Mahmud R, Mistam MS. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl Trop Dis. 2012;6:e1522. doi: 10.1371/journal.pntd.0001522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montresor A, Zin TT, Padmasiri E, Allen H, Savioli L. Soil-transmitted helminthiasis in Myanmar and approximate costs for countrywide control. Trop Med Int Health. 2004;9:1012–1015. doi: 10.1111/j.1365-3156.2004.01297.x. [DOI] [PubMed] [Google Scholar]
- 12.Tun A, Myat SM, Gabriell AF, Montresor A. Control of soil-transmitted helminthiasis in Myanmar: results of 7 years of deworming. Trop Med Int Health. 2013;18:1017–1020. doi: 10.1111/tmi.12130. [DOI] [PubMed] [Google Scholar]
- 13.Phosuk I, Intapan PM, Thanchomnang T, Sanpool O, Janwan P, Laummaunwai P, Aamnart W, Morakote N, Maleewong W. Molecular detection of Ancylostoma duodenale, Ancylostoma ceylanicum, and Necator americanus in humans in northeastern and southern Thailand. Korean J Parasitol. 2013;51:747–749. doi: 10.3347/kjp.2013.51.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato M, Sanguankiat S, Yoonuan T, Pongvongsa T, Keomoungkhoun M, Phimmayoi I, Boupa B, Moji K, Waikagul J. Copro-molecular identification of infections with hookworm eggs in rural Lao PDR. Trans R Soc Trop Med Hyg. 2010;104:617–622. doi: 10.1016/j.trstmh.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Schär F, Odermatt P, Khieu V, Panning M, Duong S, Muth S, Marti H, Kramme S. Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop. 2013;126:89–92. doi: 10.1016/j.actatropica.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Brunet J, Lemoine JP, Lefebvre N, Denis J, Pfaff AW, Abou-Bacar A, Traub RJ, Pesson B, Candolfi E. Bloody diarrhea associated with hookworm infection in traveler returning to France from Myanmar. Emerg Infect Dis. 2015;21:1878–1879. doi: 10.3201/eid2110.150695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . Deworming for Health and Development. Reports on the Third Global Meeting of the Partners for Parasite Control. Geneva, Switzerland: World Health organization; 2005. https://extranet.who.int/iris/restricted/bitstream/10665/69005/1/WHO_CDS_CPE_PVC_2005.14.pdf Available at. Accessed September 17, 2016. [Google Scholar]
- 18.Yong TS, Chai JY, Sohn WM, Eom KS, Jeoung HG, Hoang EH, Yoon CH, Jung BK, Lee SH, Sinuon M, Socheat D. Korean J Parasitol. 2014;52:661–666. doi: 10.3347/kjp.2014.52.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traub RJ. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol. 2013;43:1009–1015. doi: 10.1016/j.ijpara.2013.07.006. [DOI] [PubMed] [Google Scholar]