Abstract
Dried blood spots (DBS) were collected from 805 children attending 42 elementary schools in the regions of Mopti, Sikasso, Koulikoro, and the regional capital of Bamako in Mali as part of an evaluation of a school health intervention. Eluted immunoglobulin (Ig) G from the DBS was assessed by a multiplex bead assay (MBA) for two filariasis antigens, Wuchereria bancrofti, Wb123, and Brugia malayi, Bm14, to determine the effectiveness of mass drug administration (MDA) programs to eliminate lymphatic filariasis (LF). The prevalence of positive IgG responses in the children to each antigen was less than 1%, indicating effectiveness of the MDA against LF. The MBA is an excellent serological platform that provides cost-effective opportunities to evaluate public health activities beyond the survey targets.
Lymphatic filariasis (LF) is a debilitating disease caused by helminths Wuchereria bancrofti, Brugia malayi, and Brugia timori with the first causing about 90% of filariasis cases.1 LF, a cause of significant chronic morbidity including elephantiasis and hydrocele, can be prevented by annual mass drug administration (MDA) with antifilarial drugs, and this strategy is the cornerstone of the Global Program to Eliminate Lymphatic Filariasis (GPELF).2 It is estimated that 120 million people in tropical and subtropical regions were infected with LF at the inception of the GPELF in 2000, but recent analyses indicate a dramatic decline as a result of MDA.3,4
In Mali, LF is considered to be endemic in all 63 health districts of the country.5,6 Despite civil strife, the national program was able to scale up and maintain regular MDA in many regions of the country. Fifteen health districts in the Sikasso and Koulikoro regions have passed the LF transmission assessment survey (TAS), and additional districts have qualified for TAS based on demonstration of reduced LF infection prevalence in sentinel and spot-check sites.7 Passing the TAS provides evidence that infection levels as assessed by antigen testing have been reduced below the targeted threshold (2%) among sampled children. Surveillance after conclusion of MDA is based on conducting repeat TAS at 2–3 year intervals to document that antigenemia has not recrudesced to levels above this threshold. We capitalized on a multiplex bead assay (MBA) serological study that evaluated immunoglobulin (Ig) G responses to antigens from a range of pathogens and vaccine-preventable diseases in Malian children attending schools with and without water, sanitation, and hygiene (WASH) improvements.8 In these children, we also assessed their LF antibody responses by including beads coupled with recombinant filarial antigens, B. malayi (Bm14) and W. bancrofti (Wb123), which serve as excellent markers of filarial infection and treatment response.9–11 The results were plotted by the school locations the children attended, which were in areas where MDA had been implemented, including health districts that had stopped MDA.
The study was reviewed and approved by the Ethics Committee of the National Institute of Public Health Research (Comité d'Ethique de l'Institute Nationale de Recherché en Santé Publique) and the Institutional Review Board of Emory University (Atlanta, GA). Laboratory staff from the Centers for Disease Control and Prevention had no contact with children nor access to personal identifiers. The trial was registered at ClinicalTrials.gov (NTC01787058).
A total of 805 children, 4–17 years of age, in 42 elementary schools, grades 1–6, in the southern regions of Mopti, Sikasso, Koulikoro, and Bamako capital in Mali were included in the study. Whole blood specimens were collected by finger prick onto circular filter paper extensions (TropBio Pty Ltd., Townsville, Australia) each designed to absorb 10 μL. After collection and drying, the dried blood spots (DBS) were stored at −20°C as previously described.12 Single DBS per child was collected between January and June 2014, the dry season.
Carboxyl groups on the surface of specifically classified-spectral magnetic polystyrene microspheres (MagPlex Beads; Luminex Corporation, Austin, TX) were converted to reactive esters using the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide method (Calbiochem, Woburn, MA). Wb123 and Bm14 were linked with glutathione-S-transferase (GST) (Wb123/GST and Bm14/GST) and were covalently linked to differently classified microspheres (12.5 million each) by amide bonds in phosphate-buffered saline (PBS), pH 7.2, using 139 μg for each antigen. Another bead classification was coupled with 15 μg GST in 50 mM 2-(N-morpholino)ethanesulfonic acid, 0.85% NaCl, pH 5.0. Coupling efficiency was determined using sera and reagent known to be highly reactive to the antigens and GST.
One DBS from each child was placed in 0.5 mL of elution buffer consisting of PBS with 0.5% bovine serum albumin, 0.3% Tween 20, 0.1% sodium azide, 0.5% polyvinyl alcohol, 0.8% polyvinylpyrrolidone, and 0.1% casein, and allowed to elute overnight at 4°C. Afterward, the elution was further diluted 1:4 with the same elution buffer, containing sufficient amounts of crude Escherichia coli extract for a final concentration of 3 μg/mL. After overnight storage at 4°C, the eluate was exposed to antigen-coupled beads for 1.5 hours at room temperature. Bound antigen-specific IgG was detected on the coupled beads as previously described.13 Between steps, the magnetic beads were washed three times with 0.05% Tween 20 PBS, using a Bio-Plex Pro II Wash Station (Bio-Rad, Hercules, CA). Data were acquired using a Bio-Plex 100 reader with Bio-Plex Manager 6.1 software (Bio-Rad).
Cutoff for the Bm14/GST was determined using 86 serum specimens from North American adults who had not traveled outside the United States. By MBA, two outliers greater than 5 standard deviations (SD) above the mean were eliminated, and the remaining specimens were used to calculate the mean plus 3 SD as the cutoff. Using a standard serum, cutoff for the Wb123/GST was translated from a Wb123/GST standard curve that contained a previously established cutoff.
Prevalence of positive IgG responses per school was plotted for each antigen using global positioning system (GPS)–acquired latitude and longitude for each school and Google mapping software (https://mapsengine.google.com/map/). To anonymize, the exact location of each school was slightly displaced.
Serum specimens and antibodies known to be highly reactive to antigens and GST showed high median fluorescence intensity (MFI), indicating sufficient antigen coupling (MFI range, 1–32,766). Mean from duplicate wells was obtained and background (bg) from a DBS blank was subtracted (MFI-bg) and used as data. Cutoffs for Bm14/GST and Wb123/GST were 633 and 1,350 MFI-bg, respectively, and interplate percent coefficients of variation for positive controls were 16.3% and 9.3%, respectively. Beads with GST showed no appreciable reactivity: mean, 154 MFI-bg; and range, −2 to 929 MFI-bg. The DBS were in excellent condition and showed near maximum MFI-bg (IgG responses) to various nonfilarial antigens (data not shown).
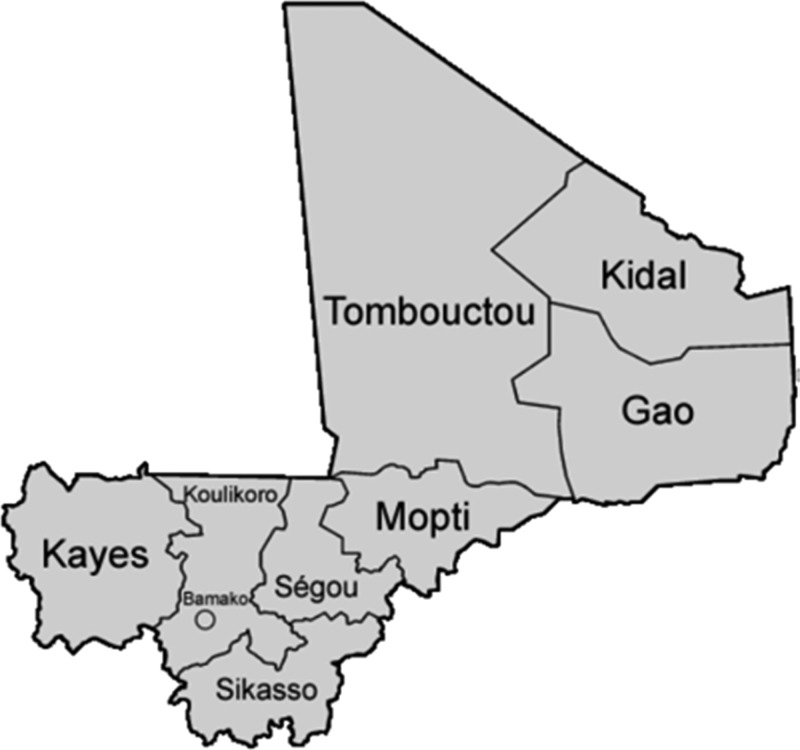
Shown in Figure 1 is a provincial map of Mali, showing Mopti, four schools; Sikasso, 16; Koulikoro, 20; and Bamako capital, 2.
Figure 1.
Map of Mali in western Africa, showing political districts. Study populations were 42 elementary schools located in Mopti, Sikasso, and Koulikoro.
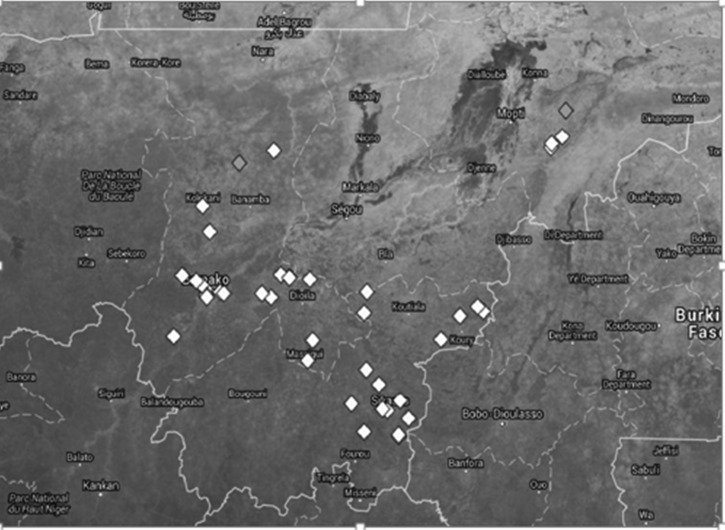
The proportion of children IgG positive for Wb123/GST by school is shown in a satellite image in Figure 2 . Each school, located by GPS, is represented by a gray-scale diamond symbol: medium gray, 5% prevalence; and white, 0%. There are two medium-gray and 40 white symbols. Some schools overlap. From 805 children, only two were positive for Wb123/GST, and they showed low MFI-bg: mean, 2,066; range, 1,401–2,718 (maximum MFI-bg is 32,766–bg).
Figure 2.
Prevalence of positive IgG responses to Wb123 per school. Satellite image of southern Mali (https://mapsengine.google.com/map/) country border (solid white lines), showing political districts (broken white lines) of Mopti, Sikasso, and Koulikoro, and the location of 42 elementary schools studied (coordinates by global positioning system [location intentionally displaced to prevent identification]). Each school, average of 19 children (range, 14–20), is represented by a gray-scale diamond symbol: medium gray, 5% prevalence; and white, 0%. There are two medium-gray and 40 white symbols. Some schools overlap.
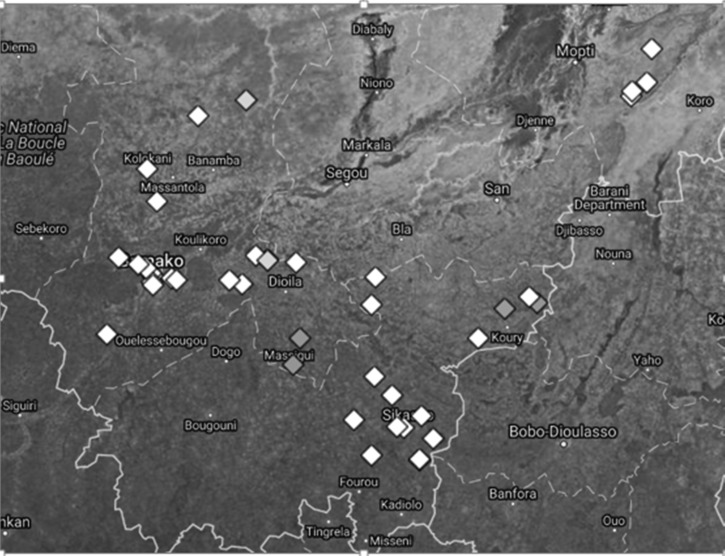
Shown in Figure 3 is a satellite image displaying the prevalence of children positive for IgG to Bm14/GST by school. Each school, located by GPS, is represented by a gray-scale diamond symbol: light gray, 10% prevalence; medium gray, 5%, and white, 0%. There are two light-gray, four medium-gray, and 36 white symbols. From 805 children, only eight were positive for Bm14/GST, and they showed low MFI-bg: mean, 1,502; range, 684–3,178. None of the children who were Bm14/GST positive were Wb123/GST positive.
Figure 3.
Prevalence of positive IgG responses to Bm14 per school. Satellite image of southern Mali (https://mapsengine.google.com/map/) country borders (solid white lines), showing political districts (broken white lines) of Mopti, Sikasso, and Koulikoro, and the location of 42 elementary schools studied (coordinates by global positioning system [location intentionally displaced to prevent identification]). Each school, average of 19 children (range, 14–20), is represented by a gray-scale diamond symbol: light gray, 10% prevalence; medium gray, 5%, and white, 0%. There are two light-gray, four medium-gray, and 36 white symbols. Some schools overlap.
Of the four regions included in the survey, all have either passed the TAS and entered the posttreatment phase or qualified for TAS based on sentinel and spot-checks. A passing result with the TAS indicates that antigen prevalence in the 6- to 7- year-old cohort included in the survey is less than 2%. In this study of 805 Malian children in 42 elementary schools in the regions of Mopti, Sikasso, Koulikoro, and the Bamako capital, we used antibody testing for LF to assess MDA program impact. LF antibody assays provide a more sensitive indicator of LF exposure and/or infection than antigen testing.10 Our results document that overall antibody prevalence was less than 1% (Figures 2 and 3). Although our survey was not as large as a typical TAS, our data, generated opportunistically as part of a WASH project, provide confirmatory evidence of LF program success, using a more conservative indicator of LF transmission. There are currently no antibody prevalence thresholds that have been established as markers of program success; however, Weil and others proposed that a prevalence of 2% in children might be appropriate for Bm14.14 Inclusion of antibody testing in the TAS may provide an independent measure of program impact and a more sensitive tool for monitoring potential recrudescence. It is noteworthy that these results were generated independent of the LF program and without any resource demands on program staff, illustrating the utility of surveys using the MBA to generate integrated surveillance data.
Wb123 was originally selected because of its specificity for W. bancrofti.15 Bm14, in contrast, is less specific and therefore considered to be less useful for monitoring LF in Africa where LF might overlap with onchocerciasis or other filarial species.10 LF and onchocerciasis do overlap in some of the areas included in our surveys; however, Wb123 and Bm14 prevalences were very similar, arguing that lower Bm14 specificity was not an issue in this post-MDA setting.
The MBA is an excellent serological technique that collects data on multiple antigens simultaneously, thus conserving specimens, as well as cost, and labor when compared with enzyme-linked immunosorbent assay (ELISA).11,13 The MBA is at least as sensitive as ELISA.16–18 Funding for neglected tropical disease surveillance is limited. In the present study, we included 38 antigens from 22 different pathogens. Here, the MBA is notable in that it not only used DBS from a school-surveillance study for evaluating effectiveness of WASH improvements in the schools, but also provided valuable serological information on the effectiveness of MDA against LF.
ACKNOWLEDGMENTS
We would like to thank the parents, students, and teachers who allowed us to conduct this work, as well as the Government of Mali. We would also like to thank the research team, including Abdoulaye Sow, Seydou Samaké, Salif Ismaïla Traoré, Fatoumata Habib Traoré, Karim Diamoutene, Yacouba Sogore, Alpha Oumar Haidara, as well as Niélé Hawa Diarra and Samba Diop from the University of Bamako. Thanks as well to the UNICEF, WaterAid, CARE, Oxfam, and Save the Children teams for their support, specifically Jérémie Toubkiss, Yagouba Diallo, Seydou Niafo, Touréba Keïta, Assitan Sogoré, Salimata Togola, Fatoumata Haïdara, Mamadou Diallo, Zoumana Cissé, Ousmane Haïdara, and Thierno Bocoum. We thank Tom Nutman for providing the Wb123 antigen.
Disclaimer: Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Service. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The funder had no involvement in its design, in collection, analysis, or interpretation of the data or in the preparation of this manuscript.
Footnotes
Financial support: Funding for this study was provided by the Dubai Cares Foundation.
Authors' addresses: Delynn M. Moss, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: dmm3@cdc.gov. Anna N. Chard, Victoria Trinies, and Matthew C. Freeman, Department of Environmental Health, Rollins School of Public Health, Emory University, Atlanta, GA, E-mails: achard@emory.edu, vtrinies@gmail.com, and mcfreem@emory.edu. Seydou Doumbia, Faculty of Medicine and Odontostomatology, Malaria Research and Training Center, University of Sciences, Techniques and Technologies of Bamako (USTTB), Bamako, Mali, E-mail: sdoumbi@icermali.org. Patrick J. Lammie, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: pjl1@cdc.gov.
References
- 1.World Health Organization Lymphatic Filariasis Epidemiology. 2015. http://www.who.int/lymphatic_filariasis/epidemiology/en/ Available at. Accessed December 30, 2015.
- 2.World Health Organization Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation. 2012. http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf Available at. Accessed February, 2016.
- 3.Hooper PJ, Chu BK, Mikhailov A, Ottesen EA, Bradley M. Assessing progress in reducing the at-risk population after 13 years of the global programme to eliminate lymphatic filariasis. PLoS Negl Trop Dis. 2014;8:e3333. doi: 10.1371/journal.pntd.0003333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramaiah KD, Ottesen EA. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Negl Trop Dis. 2014;8:e3319. doi: 10.1371/journal.pntd.0003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulibaly YI, Dembele B, Diallo AA, Konate S, Dolo H, Coulibaly SY, Doumbia SS, Soumaoro L, Coulibaly ME, Bockarie MJ, Molyneux D, Nutman TB, Klion AD, Toure YT, Traore SF. The impact of six annual rounds of mass drug administration on Wuchereria bancrofti infections in humans and in mosquitoes in Mali. Am J Trop Med Hyg. 2015;93:356–360. doi: 10.4269/ajtmh.14-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulibaly YI, Dembele B, Diallo AA, Kristensen S, Konate S, Dolo H, Dicko I, Sangare MB, Keita F, Boatin BA, Traore AK, Nutman TB, Klion AD, Toure YT, Traore SF. Wuchereria bancrofti transmission pattern in southern Mali prior to and following the institution of mass drug administration. Parasit Vectors. 2013;6:247. doi: 10.1186/1756-3305-6-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Envision Mali Work Plan, FY 2016, Project Year 5. October 2015–September 2016. 2016. http://ntdenvision.org/sites/default/files/docs/envision_fy_16_mali_country.pdf Available at. Accessed February 2016.
- 8.Trinies V, Garn JV, Chang HH, Freeman MC. The impact of a school-based water, sanitation, and hygiene program on absenteeism, diarrhea, and respiratory infection: a matched-control trial in Mali. Am J Trop Med Hyg. 2016;94:1418–1425. doi: 10.4269/ajtmh.15-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamlin KL, Moss DM, Priest JW, Roberts J, Kubofcik J, Gass K, Streit TG, Nutman TB, Eberhard ML, Lammie PJ. Longitudinal monitoring of the development of antifilarial antibodies and acquisition of Wuchereria bancrofti in a highly endemic area of Haiti. PLoS Negl Trop Dis. 2012;6:e1941. doi: 10.1371/journal.pntd.0001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lammie PJ, Weil G, Noordin R, Kaliraj P, Steel C, Goodman D, Lakshmikanthan VB, Ottesen E. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis: a multicenter trial. Filaria J. 2004;3:9. doi: 10.1186/1475-2883-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss DM, Priest JW, Boyd A, Weinkopff T, Kucerova Z, Beach MJ, Lammie PJ. Multiplex bead assay for serum samples from children in Haiti enrolled in a drug study for the treatment of lymphatic filariasis. Am J Trop Med Hyg. 2011;85:229–237. doi: 10.4269/ajtmh.2011.11-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodhew EB, Priest JW, Moss DM, Zhong G, Munoz B, Mkocha H, Martin DL, West SK, Gaydos C, Lammie PJ. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis. 2012;6:e1873. doi: 10.1371/journal.pntd.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss DM, Priest JW, Hamlin K, Derado G, Herbein J, Petri WA, Jr, Lammie PJ. Longitudinal evaluation of enteric protozoa in Haitian children by stool exam and multiplex serologic assay. Am J Trop Med Hyg. 2014;90:653–660. doi: 10.4269/ajtmh.13-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weil GJ, Ramzy RM. Diagnostic tools for filariasis elimination programs. Trends Parasitol. 2007;23:78–82. doi: 10.1016/j.pt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Kubofcik J, Fink DL, Nutman TB. Identification of Wb123 as an early and specific marker of Wuchereria bancrofti infection. PLoS Negl Trop Dis. 2012;6:e1930. doi: 10.1371/journal.pntd.0001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane BE. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 2001;45:27–36. doi: 10.1002/1097-0320(20010901)45:1<27::aid-cyto1141>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Martins TB, Jaskowski TD, Tebo A, Hill HR. Development of a multiplexed fluorescent immunoassay for the quantitation of antibody responses to four Neisseria meningitidis serogroups. J Immunol Methods. 2009;342:98–105. doi: 10.1016/j.jim.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Pickering JW, Martins TB, Schroder MC, Hill HR. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae Type b. Clin Diagn Lab Immunol. 2002;9:872–876. doi: 10.1128/CDLI.9.4.872-876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]