Abstract
Neurocysticercosis (NCC) has been associated with hippocampal atrophy, but the prevalence and pathogenic mechanisms implicated in this relationship are unknown. Using a population-based, case–control study design, residents in a rural village (Atahualpa) aged ≥ 40 years with calcified NCC were identified as cases and paired to NCC-free individuals (control subjects) matched by age, sex, and level of education. Cases and control subjects underwent magnetic resonance imaging for hippocampal rating according to the Scheltens' scale for medial temporal atrophy and were interviewed to identify those with a clinical seizure disorder. The prevalence of hippocampal atrophy was compared between cases and control subjects by the use of the McNemar's test for correlated proportions. Seventy-five individuals with calcified NCC and their matched control subjects were included in the analysis. Hippocampal atrophy was noted in 26 (34.7%) cases and nine (12%) control subjects (odds ratio: 4.4; 95% confidence interval: 1.6–14.9, P < 0.0021). Stratification of pairs according to tertiles of age revealed an age-related trend in this association, which became significant only in those aged ≥ 68 years (P = 0.027). Only five cases and one control had recurrent seizures (P = 0.221); three of these five cases had hippocampal atrophy, and the single control subject had normal hippocampi. This study confirms an association between NCC and hippocampal atrophy, and shows that this association is stronger in older age groups. This suggests that NCC-related hippocampal atrophy takes a long time to develop.
Introduction
Hippocampal atrophy has been associated with intractable epilepsy.1 In these cases, the development of mesial temporal lobe epilepsy results from neuronal loss (mainly involving CA1 and CA3 hippocampal layers) and is often preceded by an initial precipitating injury (perinatal trauma, febrile seizures, traumatic brain injury) occurring years before.2 Hippocampal atrophy may also occur in individuals with no evidence of a seizure disorder, with one series reporting a 9.3% prevalence of fortuitously discovered hippocampal atrophy in a population younger than 50 years.3
Several clinical series and a few controlled studies suggest an association between neurocysticercosis (NCC) and hippocampal atrophy.4–7 However, it is not yet clear whether this association is related to repetitive seizure activity or to mechanisms of local or remote inflammation.8–10 We recently reported the association between calcified NCC and hippocampal atrophy in a population of 248 older adults living in rural Ecuador.11 This report expands the studied population to 663 individuals, including 318 aged 40–59 years, to explore whether this association persists at a younger age.
Methods
The study was conducted in a rural Ecuadorian village (Atahualpa) where NCC is endemic.12 The institutional review board of Hospital-Clínica Kennedy, Guayaquil (FWA 00006867), approved the study. All individuals aged ≥ 40 years enrolled in the Atahualpa Project were invited to participate and those who provided informed consent were enrolled. Women of child-bearing age underwent a pregnancy test before the study.
During the first phase of the study, all participants underwent a non-contrast head computed tomography (CT) at Hospital-Clínica Kennedy. All exams were performed with a Philips Brilliance 64 CT scanner (Philips Medical Systems, Eindhoven, The Netherlands) following a protocol described elsewhere.11 A neurologist (Oscar H. Del Brutto) and a neuroradiologist (Julio Lama), with extensive experience in NCC, independently read all CT scans. Lesions of interest included parenchymal brain calcifications as well as other intracranial lesions suggestive of NCC.13 Rounded and homogeneous nonphysiological supratentorial calcifications, measuring < 1 cm in diameter, not associated with other neuroimaging findings suggestive of alternative etiologies, and not explained by any other causes, were considered to be of cysticercotic origin.14 Cohen's kappa for inter-rater agreement was 0.91 (95% confidence interval [CI]: 0.86–0.96). Digital images corresponding to positive cases were sent to another expert (Héctor H. García) for further confirmation, and disagreements were resolved by consensus.
The second phase focused on individuals with calcified cysticerci (cases) and their control subjects, selected from participants with no evidence of NCC on CT scans. The latter were matched 1:1 by age (±2 years), sex, and level of education (up to primary or secondary/higher school education) to cases. Both cases and control subjects were invited to undergo a brain magnetic resonance imaging (MRI) with the use of a Philips Intera 1.5T machine (Philips Medical Systems, Eindhoven, The Netherlands). MRI included two-dimensional multi-slice turbo spin echo T1-weighted, fluid attenuated inversion recovery, T2-weighted, and gradient-echo sequences in the axial plane, as well as a T1-weighted sequence oriented in the sagittal plane and a T1-weighted inversion recovery sequence oriented in the coronal plane and perpendicular to the long axis of the temporal bone, as previously described.11 The latter was used for hippocampal evaluation by the use of the Scheltens' medial temporal atrophy scale (Figure 1 ).15 Each temporal lobe was rated separately, and any asymmetry ≥ 1 point was noted. Two expert neuroradiologists (Perla Salgado, Julio Lama) independently read all MRIs blinded to case–control status. Cohen's kappa for inter-rater agreement was 0.81 (95% CI: 0.71–0.91), and disagreements were resolved by consensus.
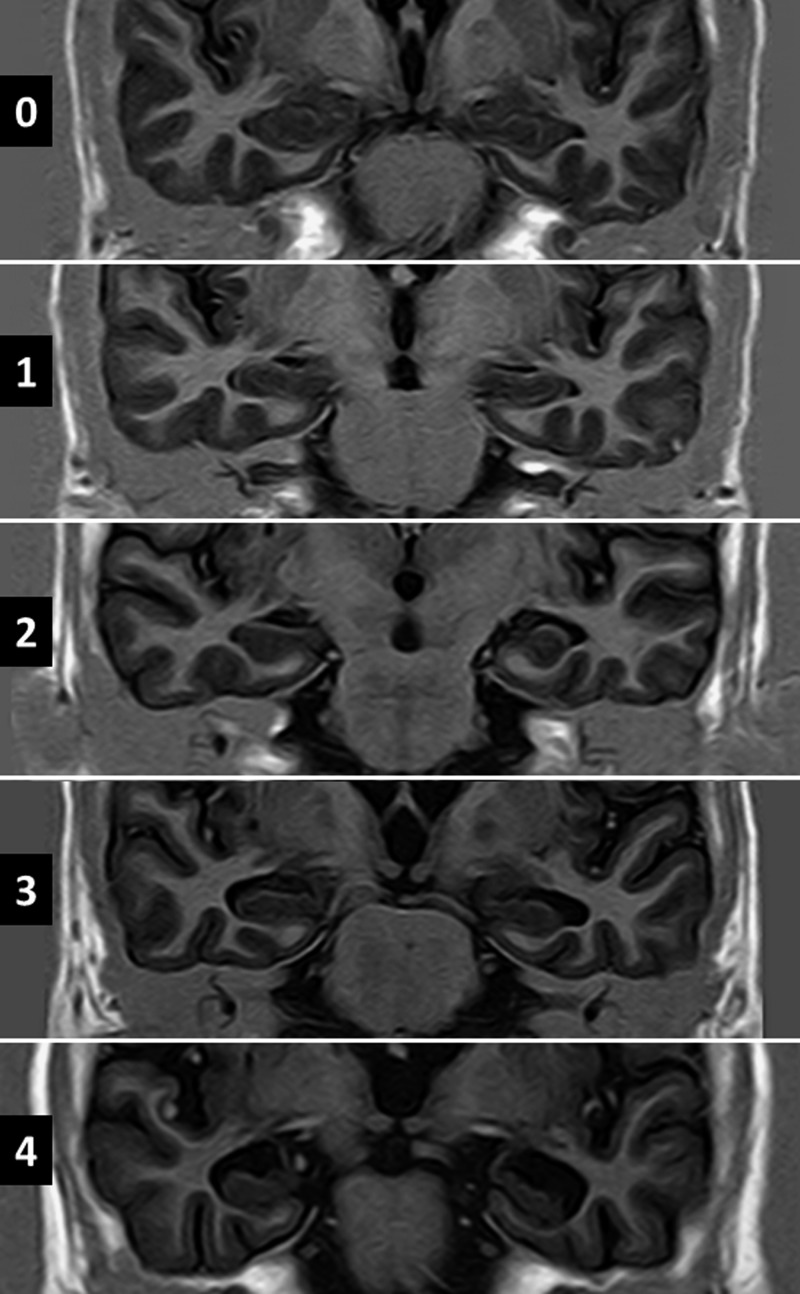
Figure 1.
T1-weighted inversion recovery magnetic resonance imagings oriented in the coronal plane showing different grades of hippocampal atrophy according to Scheltens' medial temporal atrophy scale. Width of choroid fissure, width of temporal horn, and height of hippocampus are graded on a 5-point visual rating scale ranging from 0 (no atrophy) to 4 (severe atrophy). Up to 1 point for persons < 75 years of age, and 2 points for persons ≥ 75 years are considered age-related changes.
All individuals undergoing MRI were then interviewed by a neurology resident (Victor J. Del Brutto) blinded to neuroimaging findings. History taking focused on any evidence of a seizure disorder. In addition, cognitive performance was assessed by the use of the Spanish version of the Montreal Cognitive Assessment (MoCA) test (www.mocatest.org;© Z. Nasreddine MD, version 07, November 2004).
Descriptive statistics are presented as means with standard deviations (SDs) for continuous variables and as percentages with 95% CI for categorical variables. In univariate analyses, continuous variables were compared by linear models and categorical variables by χ2 or Fisher's exact test as appropriate. Using the McNemar's test for correlated proportions (matched-pair analysis), we evaluated differences between the prevalence of hippocampal atrophy and a seizure disorder across cases and their matched control subjects.
Results
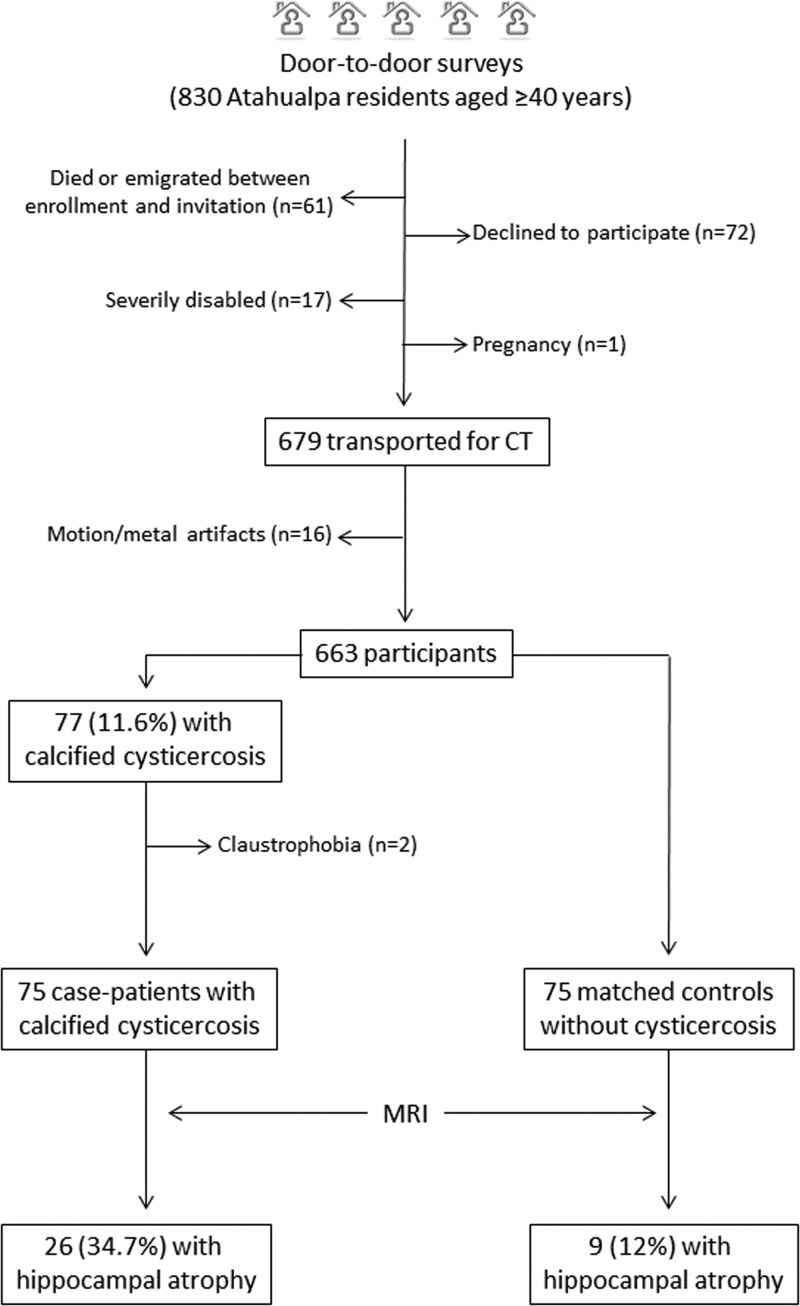
Figure 2 shows enrollment and reasons for not including potentially eligible individuals. Of 830 individuals aged ≥ 40 years enrolled in the Atahualpa Project from June 2012 to December 2015, 679 (82%) underwent a head CT. Reasons for not performing CTs included refusal to participate (N = 72), death or emigration between enrollment and the invitation (N = 61), severe disability (N = 17), and pregnancy (N = 1). Motion and metal artifacts (mostly dental implants) precluded accurate CT interpretation in 16 of the scanned persons, and these subjects were not included.
Figure 2.
Flow diagram showing the process of enrollment and the number of participants in this study.
Mean ± SD age of the 663 participants was 60.4 ± 12.8 years, 374 (56%) were women, and 387 (58%) had primary school education only. CT showed calcified cysticerci in the brain parenchyma in 77 individuals (11.6%, 95% CI: 9.4–14.3%). There was a single cerebral calcification in 58 cases, between two to four calcifications in 16, and the remaining three had five or more calcifications. Degenerating cysticerci (granular cysts) were noticed in only one individual with a single calcification and in another with multiple calcifications. There were no living parenchymal brain cysts or extraparenchymal NCC in any case. The prevalence of individuals with calcifications was evenly distributed across groups of scanned individuals stratified by decades: 14/157 (8.9%) in individuals aged 40–49 years, 20/161 (12.4%) in those aged 50–59 years, 22/191 (11.5%) in those aged 60–69 years, and 21/154 (13.6%) in those aged ≥ 70 years.
MRI was performed in 75 of the 77 individuals with calcifications (claustrophobia precluded the practice of MRI in two of them). MRI identified the calcifications in 45 cases (60%), particularly in the T2-weighted and gradient-echo sequences. The two degenerating cysticerci seen on CT were also identified on MRI. No additional cysticerci-related lesions were noticed on MRI. Seventy-five control subjects were selected from the 586 individuals with no evidence of cysticercosis on CT. MRIs confirmed a cysticercosis-free status in all of them. As expected, cases and their control subjects were similar in regard to the matched variables, including age (61.4 ± 12.1 versus 60.8 ± 11.4 years), sex (67% women in each group), and education (57% primary school in each group). Twenty-eight of these 75 matched pairs (cases and control subjects) had been included in our preliminary report of hippocampal atrophy and NCC conducted in older adults.11
Five cases and only one control subject had history of recurrent seizures (P = 0.221; McNemar's test). Age of onset ranged from 12 to 62 years. All cases with epilepsy had a single parenchymal brain calcification, and the control subject with epilepsy had a cavernous angioma as the most likely cause of the seizure disorder. Four of these individuals had > 20 lifetime seizures and three of them had active epilepsy. Seizures were tonic–clonic generalized in five cases (one with complex partial seizures) and simple partial in one; no individual had a history of status epilepticus. Mean scores on the MoCA were similar between cases and control subjects (21.5 ± 4.8 versus 21.9 ± 4.6, respectively; P = 0.603).
Twenty-six of 75 cases (34.7%, 95% CI: 24.9–45.9%) and 9/75 control subjects (12%, 95% CI: 6.4–21.3%) had hippocampal atrophy. According to the McNemar's test, there were 48 concordant pairs (four pairs in which both the case patient and the control subject had hippocampal atrophy, and 44 pairs in which neither did), as well as 27 discordant pairs; in 22 (81.5%) pairs, the case patient had hippocampal atrophy but the control did not (odds ratio [OR]: 4.4, 95% CI: 1.6–14.9, P = 0.0021).
Mean ± SD points in the Scheltens' scale for all rated hippocampi (N = 150 in each group) were 3.1 ± 1.3 for cases and 2.4 ± 0.9 for control subjects (P < 0.001). Of the 35 individuals with hippocampal atrophy, 14 cases and seven control subjects had symmetrical atrophy, and 12 cases and two control subjects had asymmetrical or strictly unilateral atrophy. All cases with asymmetrical atrophy had at least one calcification ipsilateral to the more affected hippocampus (Figure 3 ). However, calcification burden in cases with hippocampal atrophy was similar to the burden in cases with normal hippocampi (1.5 ± 1.2 versus 1.5 ± 1.0 calcifications per person, respectively).
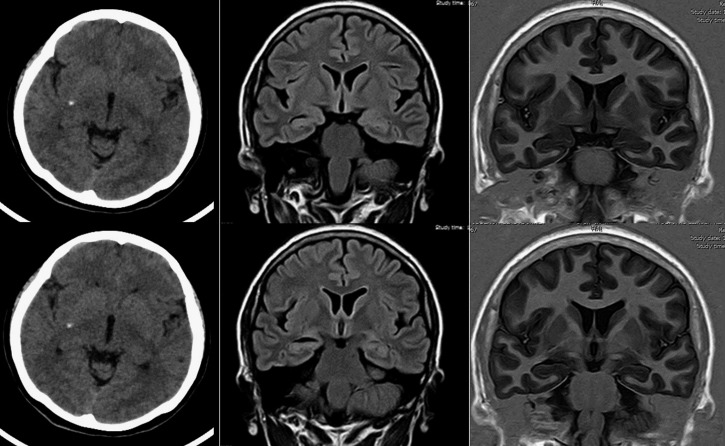
Figure 3.
Neuroimaging of a study participant with unilateral hippocampal atrophy and calcified neurocysticercosis. Plain computed tomography (left column) shows single calcified cysticercus deep in the right side of the brain. Fluid attenuated inversion recovery (center) and T1-weighted inversion recovery magnetic resonance imaging sequence (right) show ipsilateral hippocampal atrophy, characterized by increased width of the choroid fissure and decreased height of hippocampal formation.
Cases with hippocampal atrophy were older (69 ± 9.7 versus 57.4 ± 11.2 years, P < 0.001) and less educated (77% versus 49% subjects with primary school education only, P = 0.019) than those with normal hippocampi, but the percentage of women (65.4% versus 67.3%, P = 0.864) was similar across both groups. Control subjects with hippocampal atrophy were also older (68.3 ± 10.1 versus 59.8 ± 11.2 years, P = 0.034) and had a nonsignificant trend for being less educated (77.8% versus 50% subjects with primary school education only, P = 0.052) than those without atrophy, but there were no sex-related differences across both groups (66.7% women in each group). Among cases, epilepsy was present in three with bilateral hippocampal atrophy and in two with normal hippocampi. The single individual with epilepsy from the control group had normal hippocampi.
Exploratory analysis demonstrated an influence of age on the relationship between case–control status and the presence of hippocampal atrophy. When stratified above and below the median age (60.5 years), the frequency of hippocampal atrophy was not statistically different between younger cases and control subjects (10.5% versus 5.4%; OR: 2.1, 95% CI: 0.35–12, P = 0.674). In older individuals, however, hippocampal atrophy was significantly more prevalent among cases than control subjects (59.4% versus 18.4%; OR: 6.5, 95% CI: 2.3–18.6, P < 0.001). Stratifying pairs of participants into tertiles of age showed that the odds for hippocampal atrophy among cases compared with control subjects increased with age (Table 1).
Table 1.
Percentages (with 95% CI) of participants with hippocampal atrophy according to case–control status
| Tertiles of age | Hippocampal atrophy% (95% CI) | Significance* | |
|---|---|---|---|
| Cases | Controls | ||
| 40–54 years (26 pairs) | 7.7 (2.1–24.1) | 3.9 (0.7–18.9) | OR = 2; 95% CI = 0.1–118; P = 1 |
| 55–67 years (25 pairs) | 40 (23.4–59.3) | 12 (4.2–29.9) | OR = 4.5; 95% CI = 0.93–42.8; P = 0.07 |
| 68–86 years (24 pairs) | 58.3 (38.8–75.5) | 20.8 (9.2–40.5) | OR = 5.5; 95% CI = 1.2–51; P = 0.027 |
CI = confidence interval; OR = odds ratio. Stratification of pairs according to tertiles of age reveals an age-related trend in the association between neurocysticercosis and hippocampal atrophy.
Analyses performed by the use of the McNemar's test for correlated proportions (matched-pair analyses).
Discussion
This population-based study shows a robust association between NCC and hippocampal atrophy and confirms the findings of our initial study.11 The addition of a younger study population in this report allowed us to assess the effect of age on NCC-associated hippocampal atrophy. To begin with, the prevalence of NCC was similar across age groups, which together with the fact that all NCC cases identified in the study population had calcifications and no viable cysts suggests that infection occurred during the first decades of life, an expected finding due to transmission dynamics of helminths observed in highly endemic villages.16 Second, despite a similar prevalence of NCC across age groups, hippocampal atrophy was only more prevalent in cases than in control subjects among older individuals. This age dependence suggest that NCC-associated hippocampal atrophy takes years to develop.
The design of the present study reduces the possibility that other causes of hippocampal atrophy could otherwise obscure the importance of NCC-associated atrophy. Patients with NCC were matched to NCC-free individuals for age, sex, and levels of education. In addition, all the subjects lived in a similar rural setting where ethnicity, lifestyle, and socioeconomic factors were nearly homogeneous.17 More than 95% of the participants were born in Atahualpa and had always lived in the village. Cognitive performance was also similar among the case and control groups, making it unlikely that fewer individuals with degenerative diseases of the central nervous system (associated with hippocampal atrophy) were inadvertently allocated to the control group. The MoCA is highly reliable for the detection of individuals with mild cognitive impairment,18 and has proven to be of value for the recognition of this condition and its correlation with structural brain damage among Atahualpa residents.19,20 In addition, a previous study showed that cognitive performance is not compromised in individuals with hippocampal atrophy associated with NCC.21 Given the similarity in the demographics of the case and control groups, there is no apparent reason—other than NCC—to explain the higher prevalence of hippocampal atrophy in the case-patient group.
While this is a cross-sectional study and a cause-and-effect relationship cannot be definitively established, the abovementioned evidence—together with biological plausibility—suggest that cysticercosis infection of the brain parenchyma occurs first and then over time induces the development of hippocampal atrophy. Indeed, some authors have suggested that NCC itself can act as an initial precipitating injury for the subsequent development of hippocampal atrophy.8 These results are in line with recent studies showing that other infective agents, particularly viruses, may be related to the late development of hippocampal atrophy.22 However, the mechanism by which infection induces hippocampal atrophy is unclear. In patients with viral infections, the most likely mechanism is the occurrence of prolonged febrile seizures, which, together with chronic inflammation, can lead to hippocampal damage.23
Data from a tertiary care epilepsy surgical service in Brazil suggest that hippocampal atrophy in patients with NCC is related to repetitive seizure activity.4–6 Although this is a plausible pathogenic trigger for hippocampal atrophy, it is difficult to draw broad conclusions from a population limited to cases of medically intractable epilepsy. A plausible alternative involves inflammation-mediated damage as the causative mechanism for hippocampal atrophy. Periodic release of antigens trapped within calcified cysticerci might lead to inflammation-mediated hippocampal damage, not requiring recurrent seizures as a causative factor.8 In a murine model, recurrent exposure to endotoxins and increased levels of pro-inflammatory cytokines correlate with hippocampal damage independent of seizures.24 In another murine model, intraperitoneal inoculation of Taenia crassiceps metacestode factor (a parasite akin to Taenia solium) in a mice model induces apoptosis of hippocampal cells due to complex inflammatory mechanisms.25 The low prevalence of individuals with recurrent seizures in our series would favor the above-described seizure-independent inflammatory hypothesis, although we cannot rule out the presence of subclinical paroxysmal activity.
Strengths of the present study include the unbiased selection of participants, the homogeneous characteristics of Atahualpa residents regarding race/ethnicity and living habits, the case–control design, and the high rates of inter-rater agreement for both CT and MRI readings. On the other hand, our study has some limitations. As stated, subclinical seizure activity in individuals with brain calcifications cannot be ruled out. The use of a visual scale for rating hippocampal atrophy, instead of volumetric assessment, could be seen as another potential limitation. However, the reliability of the Scheltens' visual scale for grading hippocampal atrophy is comparable to that of volumetric assessment.26 Although visual assessment would not be appropriate for categorizing subfield hippocampal atrophy (which requires high-resolution MRI including voxel-based morphometric analyses of hippocampi), it is sufficient for estimating the severity of atrophy. Because of the population-based design of our study, we did not perform contrast-enhanced MRIs. However, the use of gadolinium might have shown additional evidence of blood–brain barrier disruption or inflammation.
This study adds to a growing body of evidence pointing to an association between NCC and hippocampal atrophy. Longitudinal studies are needed to determine whether a causal relationship exists, to characterize the pathogenetic mechanisms involved and to identify potential biomarkers of neuronal damage in patients with NCC-related hippocampal atrophy.
Footnotes
Financial support: This study was partially supported by Universidad Espíritu Santo – Ecuador, Guayaquil – Ecuador. Hector H. Garcia is supported by a Wellcome Trust International Senior Fellowship in Public Health and Tropical Medicine.
Authors' addresses: Oscar H. Del Brutto, School of Medicine, Universidad Espíritu Santo–Ecuador, Guayaquil, Ecuador and Department of Neurological Sciences, Hospital-Clínica Kennedy, E-mail: oscardelbrutto@hotmail.com. Naoum P. Issa and Victor J. Del Brutto, Department of Neurology, University of Chicago, Chicago, IL, E-mails: naoum.issa@uchospitals.edu and vjdelbrutto@gmail.com. Perla Salgado, Neuroimaging Unit, National Institute of Neurology and Neurosurgery, Mexico City, Mexico, E-mail: pmtsalgado@yahoo.com.mx. Mauricio Zambrano, The Atahualpa Project, Community Center, Atahualpa, Ecuador, E-mail: zamaleon@hotmail.com. Julio Lama, Imaging Department, Hospital-Clínica Kennedy, Guayaquil, Ecuador, E-mail: julama54@hotmail.com. Héctor H. García, Department of Microbiologia, School of Sciences, Universidad Peruana Cayetano Heredia, Lima, Peru, Department of Microbiology, School of Sciences, Universidad Peruana Cayetano Heredia, Lima, Perú, and Cysticercosis Unit, Instituto Nacional de Ciencias Neurológicas, Lima, Perú, E-mail: hgarcia1@jhu.edu.
References
- 1.Engel J, Jr, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy with hippocampal sclerosis. In: Engel J Jr., Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd edition. Philadelphia: Lippincott-Raven; 2008. pp. 2479–2486. [Google Scholar]
- 2.Dutras JR, Cortés EP, Vonsattel JPG. Update on hippocampal sclerosis. Curr Neurol Neurosci Rep. 2015;15:67. doi: 10.1007/s11910-015-0592-7. [DOI] [PubMed] [Google Scholar]
- 3.Benbadis SR, Wallace J, Murtagh FR, Martinez C, Tatum WO, Vale FL. MRI evidence of mesial temporal sclerosis in subjects without seizures. Seizure. 2002;11:340–343. doi: 10.1053/seiz.2001.0613. [DOI] [PubMed] [Google Scholar]
- 4.Velasco TR, Zanello PA, Dalmagro CL, Araújo D, Jr, Santos AC, Bianchin MM, Alexande V, Jr, Walz R, Assirati JA, Carlotti GG, Jr, Takayanagui OM, Sakamoto AC, Leite JP. Calcified cysticercosis lesions and intractable epilepsy: a cross sectional study of 512 patients. J Neurol Neurosurg Psychiatry. 2006;77:485–488. doi: 10.1136/jnnp.2005.078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchin MM, Velasco TR, Coimbra ER, Gargaro AC, Escorsi-Rosset SR, Wichert-Ana L, Terra VC, Alexandre V, Jr, Araujo D, Jr, dos Santos AC, Fernandes RM, Assirati JA, Jr, Carlotti GG, Jr, Leite JP, Takayanagui OM, Markowitsch HJ, Sakamoto AC. Cognitive and surgical outcome in mesial temporal lobe epilepsy associated with hippocampal sclerosis plus neurocysticercosis: a cohort study. PLoS One. 2013;8:e60949. doi: 10.1371/journal.pone.0060949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchin MM, Velasco TR, Wichert-Ana L, Alexandre V, Jr, Araujo D, Jr, dos Santos AC, Carlotti GG, Jr, Takayanagui OM, Sakamoto AC. Characteristics of mesial temporal lobe epilepsy associated with hippocampal sclerosis plus neurocysticercosis. Epilepsy Res. 2014;108:1889–1895. doi: 10.1016/j.eplepsyres.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Rathore C, Thomas B, Kesavadas C, Radhakrishnan K. Calcified neurocysticercosis lesions and hippocampal sclerosis: potential dual pathology? Epilepsia. 2012;53:e60–e62. doi: 10.1111/j.1528-1167.2011.03386.x. [DOI] [PubMed] [Google Scholar]
- 8.Bianchin MM, Velasco TR, Santos AC, Sakamoto AC. On the relationship between neurocysticercosis and mesial temporal lobe epilepsy associated with hippocampal sclerosis: coincidence or a pathogenic relationship. Pathog Glob Health. 2012;106:280–285. doi: 10.1179/2047773212Y.0000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Brutto OH, Engel J, Jr, Eliashiv DS, Salamon N, Garcia HH. Hippocampal sclerosis: the missing link of cysticercosis epileptogenesis? Epilepsia. 2014;55:2077–2078. doi: 10.1111/epi.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Brutto OH, Engel J, Jr, Eliashiv DS, Garcia HH. Update on cysticercosis epileptogenesis: the role of the hippocampus. Curr Neurol Neurosci Rep. 2016;16:1. doi: 10.1007/s11910-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Brutto OH, Salgado P, Lama J, Del Brutto VJ, Campos X, Zambrano M, Garcia HH. Calcified neurocysticercosis associates with hippocampal atrophy: a population-based Study. Am J Trop Med Hyg. 2015;91:64–68. doi: 10.4269/ajtmh.14-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Brutto OH, Santibáñez R, Idrovo L, Rodríguez S, Díaz-Calderón E, Navas C, Gilman RH, Cuesta F, Mosquera A, Gonzalez AE, Tsang VC, García HH. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46:583–587. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 13.Kimura-Hayama ET, Higuera JA, Corona-Cedillo R, Chávez-Macias L, Perochena A, Quiroz-Rojas LY, Rodriguez-Carbajal J, Criales JL. Neurocysticercosis: radiologic-pathologic correlation. Radiographics. 2010;30:1705–1719. doi: 10.1148/rg.306105522. [DOI] [PubMed] [Google Scholar]
- 14.Del Brutto OH, Rajshekhar V, White AC, Jr, Tsang VC, Nash TE, Takayanagui OM, Schantz PM, Evans CA, Flisser A, Correa D, Botero D, Allan JC, Sarti E, Gonzalez AE, Gilman RH, Garcia HH. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–183. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheltens PH, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Walk J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Brutto OH, Peñaherrera E, Ochoa E, Santamaría M, Zambrano M, Del Brutto VJ. Door-to-door survey of cardiovascular health, stroke, and ischemic heart disease in rural coastal Ecuador—The Atahualpa Project: methodology and operational definitions. Int J Stroke. 2014;9:367–371. doi: 10.1111/ijs.12030. [DOI] [PubMed] [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 19.Del Brutto OH, Mera RM, Zambrano M, Soriano F, Lama J. Global cortical atrophy (GCA) associates with worse performance in the Montreal Cognitive Assessment (MoCA). A population-based study in community-dwelling elders living in rural Ecuador. Arch Gerontol Geriatr. 2015;60:206–209. doi: 10.1016/j.archger.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Del Brutto OH, Mera RM, Del Brutto VJ, Sedler MJ. The bicaudate index inversely associates with performance in the Montreal Cognitive Assessment (MoCA) in older adults living in rural Ecuador. The Atahualpa Project. Int J Geriatr Psychiatry. 2016;31:944–950. doi: 10.1002/gps.4419. [DOI] [PubMed] [Google Scholar]
- 21.Terra-Bustamante VC, Coimbra ER, Rezek KO, Escorsi-Rosset SR, Guarnieri R, Dalmagro CL, Inuzuka LM, Bianchin MM, Wichert-Ana L, Alexandre V, Takayanagui OM, Araújo D, dos Santos AC, Carlotti CG, Walz R, Markowitsch HJ, Sakamoto AC. Cognitive performance of patients with mesial temporal lobe epilepsy and incidental calcified neurocysticercosis. J Neurol Neurosurg Psychiatry. 2005;76:1080–1083. doi: 10.1136/jnnp.2004.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura Y, Nakayama A, Kato T, Miura H, Ishihara N, Ihira M, Takahashi Y, Matsuda K, Yoshikawa T. Pathogenic role of human herpesvirus 6B infection in mesial temporal lobe epilepsy. Clin Infect Dis. 2015;212:1014–1021. doi: 10.1093/infdis/jiv160. [DOI] [PubMed] [Google Scholar]
- 23.Leibovitch EC, Jacobson S. Human herpesvirus 6 as a trigger in mesial temporal lobe epilepsy. Clin Infect Dis. 2015;212:1011–1013. doi: 10.1093/infdis/jiv162. [DOI] [PubMed] [Google Scholar]
- 24.Kahn MS, Kranjac D, Alonzo CA, Haase JH, Cedillos RO, McLinden KA, Boehm GW, Chumley MJ. Prolonged elevation in hippocampal Aβ and cognitive deficit following repeated endotoxin exposure in the mouse. Behav Brain Res. 2012;229:176–184. doi: 10.1016/j.bbr.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Zepeda N, Solano S, Copitin N, Chávez JL, Fernández AM, García F, Tato P, Molinari JL. J Helminthol. 2016. Apoptosis of mouse hippocampal cells induced by Taenia crassiceps metacestode factor. doi: https://doi.org/10.1017/S0022149X16000146. [DOI] [PubMed] [Google Scholar]
- 26.Boutet C, Chupin M, Colliot O, Sarazin M, Mutlu G, Drier A, Pellot A, Dormont D, Lehércy S. Alzheimer's Disease Neuroimaging Initiative Is radiological evaluation as good as computer-based volumetry to assess hippocampal atrophy in Alzheimer's disease? Neuroradiology. 2012;54:1321–1330. doi: 10.1007/s00234-012-1058-0. [DOI] [PubMed] [Google Scholar]