Abstract
A 30-year-old male, from a subtropical region of Ecuador, was hospitalized with a 5-year history of persistent cough with rusty brown sputum, chest pain, and progressive dyspnea. The patient underwent thoracic surgery 3 years ago for pleural effusion and subsequently received a 9-month regimen treatment of tuberculosis. However, there was no clinical resolution and symptoms became progressively worse. A chest radiograph and computerized tomography scan showed several small nodules in both lungs. Eggs of Paragonimus spp. were observed in sputum smears, but the smears were negative for acid-fast bacilli. Molecular characterization of eggs by the internal transcribed spacer-2 regions identified them as Paragonimus mexicanus. The patient was treated with praziquantel and tested negative parasitologically for 12 months. There was clinical resolution of the cough and expectoration, but dyspnea and chest pain persisted.
Introduction
Paragonimiasis is a food-borne trematodiasis, listed by the World Health Organization as one of the neglected tropical diseases worldwide. Infection is acquired when raw or undercooked freshwater crustaceans (crab or crayfish) infected with the metacercariae of Paragonimus spp. are ingested.1 Ecuador is considered the most endemic country in the Americas, with active transmission present in both the subtropical and tropical areas of the Pacific and Amazon regions of the country.2 Other countries in South America reporting human paragonimiasis include Peru, Colombia, Brazil, and Venezuela. Paragonimus mexicanus (synonimun Paragonimus peruvianus, Paragonimus ecuadoriensis) is considered the infective species.3
Pulmonary infection is the most common clinical form of the disease, causing a chronic productive cough with brownish sputum, accompanied by chest pain. The sputum contains rusty-brown flecks where microscopic ova of Paragonimus spp. can be observed. Fever is infrequent and generally the patient's health remains unimpaired. However, when pleural effusion occurs (pleuropulmonary paragonimiasis), chest pain is pleuritic and dyspnea is progressive, leading to hospitalization requiring thoracocentesis or surgery.4 Pleural lesions do vary according to the Paragonimus species involved; they occasionally occur in infections by Paragonimus westermani, but are more frequently observed in Chinese patients infected by Paragonimus skrjabini.4–6
The clinical presentation of pulmonary paragonimiasis mimics that of tuberculosis (TB), but does not respond to antibiotic therapy. A high percentage of patients presenting paragonimiasis with an associated pulmonary TB infection have been reported.7 In Ecuador, endemic areas of pulmonary TB and paragonimiasis overlap. As a consequence, several cases of paragonimiasis have been misdiagnosed and treated as TB.2
Imaging investigations, utilizing radiology and computerized tomography (CT), can help in diagnosing pulmonary paragonimiasis. Radiographical images could show nodular or ring shadow infiltrates, patchy infiltration, small cysts, and cavities, depending on the severity of the disease.2,8 Ten to 40% of egg-positive patients have shown to have normal chest films.4 Pleuropulmonary paragonimiasis include effusions, hydropneumothorax, and pleural thickening, which can be bilateral.4,5
Praziquantel (PZQ) is considered the drug of choice for treatment. An oral course of 75 mg/kg/day in three doses for 3 days has been shown to be nearly 100% effective,2 and is the recommended treatment by the Ministerio de Salud Pública (MSP) in Ecuador. Herein, we report a chronic case of pleuropulmonary paragonimiasis that was misdiagnosed and treated for pulmonary TB that progressed to a severe infection with dyspnea on minimal exertion, and was successfully treated with PZQ.
Case Report
The patient, a 30-year-old male, was a resident of Puerto Quito, province of Pichincha, located in the northwestern side of Ecuador, about 132 km from the capital Quito. This is a subtropical region with rapid-flowing rock-based rivers that are fed by hundreds of small tributary streams that drain the foothills of the Andes mountain range; temperatures range from 16 to 25°C. The region, once a humid rain forest area, is now fragmented by secondary plantations and urban–rural development.
The patient was admitted in the Hospital “Carlos Andrade Marin” in Quito, with a 5-year history of persistent productive cough with rusty brown–colored sputum, bilateral chest pain, and progressive dyspnea. The patient was dyspneic even in a resting position. Initially, the symptoms were periodic, but 4 years earlier they became persistent and daily with progressive dyspnea. One year later, he was hospitalized in a private clinic with severe pleuritic pain and dyspnea on minimum exertion, and chest X-ray showed bilateral pleural effusion (Figure 1 ); thoracic surgery resulted in alleviated pain and dyspnea. During that admission, the pleural liquid and sputum were examined for acid-fast bacilli (AFB) and Paragonimus spp. eggs, but were reported negative; however, due to a positive Mantoux test, the patient was treated for pleuropulmonary TB. According to the guidelines of MSP, he received a 6-month course of rifampicin, isoniazid, ethambutol, and pyrazinamide. Because the clinical symptoms persisted, it was considered a drug-resistant TB strain, and a new scheme was initiated for another 3 months.
Figure 1.
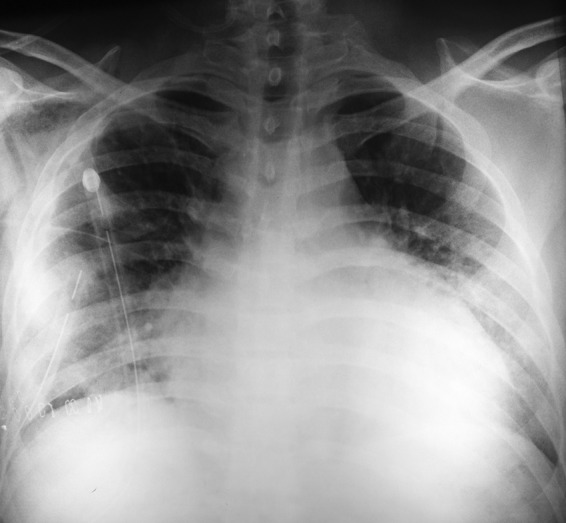
Anteroposterior chest X-ray performed in year 2011, before the patient's surgery. The image shows bilateral pleural effusions and small nodules in both lung bases. A thoracic tube was inserted in left lung to relieve the patient's symptoms.
On admission, the patient was nonfebrile, well nourished, and did not have any history of anorexia or weight loss. On questioning of his food and eating habits, he admitted eating, since his childhood, undercooked freshwater crabs and crayfish collected in his father's farm located in the Puerto Quito area. On physical examination, auscultation of the thorax revealed diffuse rales in both hemithoraces. The chest X-ray showed mixed opacities at the upper lobe of the right lung and small nodules in both lung bases with interstitial thickening. A CT scan presented several nodular images of soft tissue density bilaterally with frosted glass scattered areas (Figure 2 ). Sputum examination performed by direct microscopic examination for 3 consecutive days showed abundant operculate eggs characteristic of Paragonimus spp. (Figure 3 ), but all specimens were negative for AFB. The molecular characterization of eggs was performed by DNA isolation, amplification of the internal transcribed spacer-2 regions of the ribosomal DNA, and sequencing, as described by Sugiyama and others,9 which identified them as P. mexicanus.
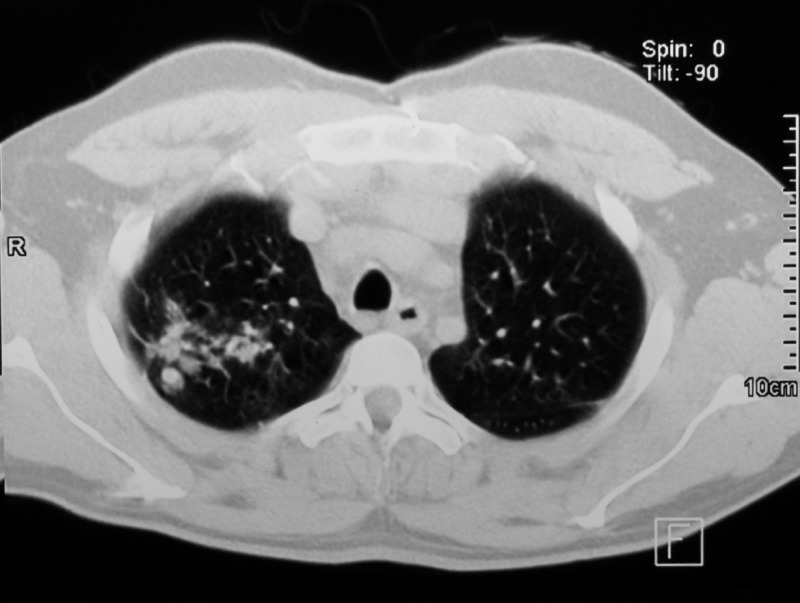
Figure 2.
Axial section thorax computerized tomography scan performed during the patient's hospitalization. Nodular images with thin and regular walls with soft tissue density can be seen in both sides surrounded by areas of scattered frosted glass. Moreover, bronchial dilatation can be appreciated next to the nodular lesions, especially in the right lung.
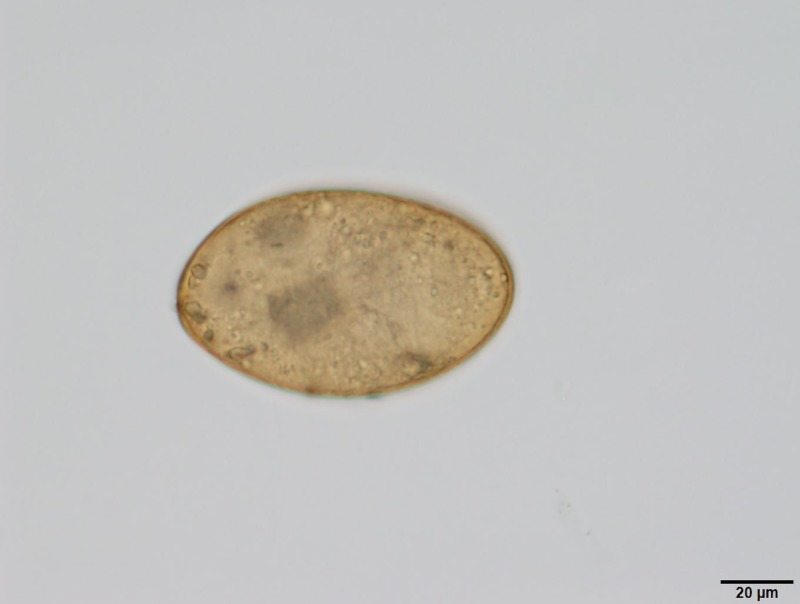
Figure 3.
An operculate egg measuring 80 × 50 μm, brownish color, observed in the sputum.
PZQ was used for treatment as recommended by the guidelines of the MSP, 75 mg/kg/day for 3 days.10 The medication was well tolerated and the patient began to progressively recover. The productive cough and rust-colored sputum resolved within 15 days of treatment. The patient was evaluated at 12 months posttreatment, and all sputum examinations were negative for Paragonimus ova and AFB. There was clinical resolution of the cough and expectoration, but dyspnea and chest pain persisted. The patient signed a consent to participate in this publication.
Discussion
Cases of severe pulmonary paragonimiasis similar to the present case are rarely encountered today. It was considered a severe infection because of the clinical symptoms; the daily and persistent productive cough with a rusty brown–colored sputum, and the progressive dyspnea, even when resting, produced by multiple lung lesions showed by the X-rays and CT scan. Reporting this unusual clinical presentation is beneficial for physicians and researchers working in areas endemic for paragonimiasis, as well as for clinicians examining migrant or returning tourist patients from subtropical regions as has occurred in the United States and Spain.11 This is the first Ecuadorian case of diagnosed pleural effusion caused by P. mexicanus. Its misdiagnosis as pulmonary TB and the consequent delay in the diagnosis of paragonimiasis led to complications requiring surgical intervention. Even though Ecuador is the most endemic country in the Americas, clinicians are unfamiliar with the disease.2 In addition, laboratory technicians are not well trained for recognizing eggs of Paragonimus. This may be due to the fact that the number of clinical cases is declining and that paragonimiasis is considered an exotic disease occurring only in indigenous people residing in remote tropical regions.
The clinical symptoms of pulmonary paragonimiasis can be confusing and can lead to misdiagnosis and treatment of TB,9,12 as occurred in the present case. In Ecuador, during an epidemiological search for paragonimiasis, 12.9% of patients had or were in treatment of pulmonary TB.2 In this case, the abundant ova characteristic of Paragonimus found in sputum confirmed the diagnosis, and TB was ruled out by the negative AFB. Three years earlier, he was treated for pulmonary TB, even though all sputum specimens were negative for AFB, but had a positive Mantoux test. In Ecuador, the MSP requires that all newborns are vaccinated with Bacillus Calmette–Guerin vaccine, with a booster given 6 years later. For this reason, most Ecuadorians test positive for the Mantoux test. The number of patients treated with anti-TB drugs is increasing due to the guidelines of MSP that recommend any individual showing respiratory symptoms for a duration of 2 weeks, with suggestive X-rays and epidemiological background, even without microbiological confirmation, to be treated for TB.13 Radiological and even CT scan images, although useful, are not pathognomonic for paragonimiasis.2,8,14 Generally, individuals with pulmonary paragonimiasis exhibit good health and nutritional status in contrast to the ill health of TB patients; thus, it is important to investigate the consumption of raw or undercooked freshwater crustaceans. Because individuals with Mycobacterium tuberculosis infections can be found in endemic areas for Paragonimus, to rule out TB, it is recommended that sputum smears be stained using the Ziehl–Neelsen staining (ZNS) technique. Paragonimus eggs can readily be detected in sputum slides stained by ZNS.15 PZQ administered at recommended doses was effective for this severe case. Although PZQ is listed in the Ecuadorian National Guidelines for Basic Drugs,16 it is not available in the country.
Health education of the population in endemic regions, to discourage the consumption of raw or undercooked freshwater crabs and crayfish, would help to decrease infection rates. In addition, educating health workers on paragonimiasis in endemic areas would enhance early diagnosis and proper treatment, and decrease complications and unnecessary surgeries, as occurred in this case, in which dyspnea and chest pain persisted because of chronicity and/or pleural thickness.
ACKNOWLEDGMENTS
We thank Ronald Guderian for revising this case report and Eduardo Herrera for his advice for X-rays and CT images.
Footnotes
Financial support: This study was supported in part by the Japan Society for the Promotion of Science, JSPS (KAKENHI: Grant No. 25305011) and by grants for Research on Emerging and Re-emerging Infectious Diseases (H26-Shinko-ippan-009) from the Ministry of Health, Labor and Welfare of Japan.
Authors' addresses: Manuel Calvopina and Daniel Romero, Unidad de Parasitología Molecular y Medicina Tropical, Carrera de Medicina, Universidad De Las Américas (UDLA), Quito, Ecuador, E-mails: manuelcalvopina@gmail.com and vacdaro@hotmail.com. Ruben Macias, Hospital Carlos Andrade Marín, Instituto Ecuatoriano de Seguridad Social (IESS), Quito, Ecuador, E-mail: rubenmaciasj@hotmail.com. Hiromu Sugiyama, Department of Parasitology, National Institute of Infectious Diseases, Tokyo, Japan, E-mail: hsugi@nih.go.jp.
References
- 1.Daumerie D, Savioli L, Crompton D, Peters P. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 2.Calvopina M, Romero D, Castañeda B, Hashiguchi Y, Sugiyama H. Current status of Paragonimus and paragonimiasis in Ecuador. Mem Inst Oswaldo Cruz. 2014;109:849–855. doi: 10.1590/0074-0276140042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acha P, Szyfres B. Zoonosis y Enfermedades Transmisibles Comunes al Hombre y a los Animales: Parasitosis. 3rd edition. Volume 3. Washington, DC: Pan American Health Organization; 2003. Paragonimiasis; pp. 158–164. [Google Scholar]
- 4.Shim YS, Cho SY, Han YC. Pulmonary paragonimiasis: a Korean perspective. Semin Respir Crit Care Med. 1991;12:35–45. [Google Scholar]
- 5.Im JG, Whang HY, Kim WS, Han MC, Shim YS, Cho SY. Pleuropulmonary paragonimiasis: radiologic findings in 71 patients. Am J Roentgenol. 1992;159:39–43. doi: 10.2214/ajr.159.1.1609718. [DOI] [PubMed] [Google Scholar]
- 6.Zhong HL, Chung HL, He LY, Ho LY, Xu ZB, Hsu CP, Cao WJ, Tsao WC. Recent progress in studies of Paragonimus and paragonimiasis control in China. Chin Med J (Engl) 1981;94:483–494. [PubMed] [Google Scholar]
- 7.Peñafiel W, Dávalos R, Coloma M. Paragonimiasis pulmonar. Revisión clínica de 92 casos. Rev Fac Cienc Med. 1981;6:253–261. [Google Scholar]
- 8.Singh TS, Mutum SS, Razaque MA. Pulmonary paragonimiasis: clinical features, diagnosis and treatment of 39 cases in Manipur. Trans R Soc Trop Med Hyg. 1986;80:967–971. doi: 10.1016/0035-9203(86)90275-0. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama H, Singh TS, Rangsiruji A. In: Molecular Detection of Human Parasitic Pathogens. Dongyou L, editor. Boca Raton, FL: CRC Press; 2013. pp. 423–436. (Paragonimus). [Google Scholar]
- 10.Díaz G, Calvopina M, Guderian R, Amunárriz M. Control de la Paragonimiasis en el Ecuador. Boletín Epidemiológico. Programa de Enfermedades Tropicales. Quito, Ecuador: Ministerio de Salud Pública; 1991. pp. 1–18. [Google Scholar]
- 11.Gómez-Seco J, Rodríguez-Guzmán MJ, Rodríguez-Nieto MJ, Gómez-Escolar PF, Presa-Abos T, Fortes-Alen J. Pulmonary paragonimiasis. Arch Bronconeumol. 2011;47:610–612. doi: 10.1016/j.arbres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Lall M, Sahni AK, Rajput AK. Pleuropulmonary paragonimiasis: mimicker of tuberculosis. Pathog Glob Health. 2013;107:40–42. doi: 10.1179/2047773212Y.0000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministerio de Salud Pública del Ecuador . Manual de Normas y Procedimientos para el Control de la Tuberculosis. Quito, Ecuador: Ministerio de Salud Pública; 2010. pp. 1–336. [Google Scholar]
- 14.Kim TS, Han J, Shim SS, Jeon K, Koh WJ, Lee I, Lee KS, Kwon OJ. Pleuropulmonary paragonimiasis: CT findings in 31 patients. Am J Roentgenol. 2005;185:616–621. doi: 10.2214/ajr.185.3.01850616. [DOI] [PubMed] [Google Scholar]
- 15.Slesak G, Inthalad S, Basy P, Keomanivong D, Phoutsavath O, Khampoui S, Grosrenaud A, Amstutz V, Barennes H, Buisson Y, Odermatt P. Ziehl-Neelsen staining technique can diagnose paragonimiasis. PLoS Negl Trop Dis. 2011;5:1–8. doi: 10.1371/journal.pntd.0001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consejo Nacional de Salud . Cuadro Nacional de Medicamentos Básicos y Registro Terapéutico. 9th edition. Quito, Ecuador: Ministerio de Salud Pública; 2014. p. 586. [Google Scholar]