Abstract
Alcohol dependence is associated with deficits in glutamate uptake and impairment of glutamate homeostasis in different brain reward regions. Glutamate transporter subtype 1 (GLT-1), cystine-glutamate exchanger (xCT) and glutamate/aspartate transporter (GLAST) are the key players in regulating extracellular glutamate concentration in the brain. Parenteral treatment with ceftriaxone, β-lactam antibiotic, has been reported to attenuate ethanol consumption and reinstatement to cocaine-seeking behavior, in part, by restoring the expression of GLT-1 and xCT in mesocorticolimbic brain regions in rats. In this study, we focus to test Augmentin (amoxicillin/clavulanate), which can be administered orally to subjects. Therefore, we examined the effects of orally administered Augmentin on ethanol intake as well as GLT-1, xCT and GLAST expression in alcohol-preferring (P) rats. We found that orally administered Augmentin significantly attenuated ethanol consumption in P rats as compared to the vehicle-treated group. Importantly, the attenuation in ethanol consumption was associated with a significant upregulation of GLT-1 and xCT expression in nucleus accumbens (NAc) and prefrontal cortex (PFC). There was no effect of oral Augmentin on GLAST expression in either NAc or PFC. These findings present strong evidence that oral administration of Augmentin can be used as an alternative to parenteral administration.
Keywords: GLT-1, xCT, GLAST, Augmentin, oral gavage, ethanol dependence
1. Introduction
Ethanol and other drugs of abuse can induce a dysregulation of glutamatergic neurotransmission in mesocorticolimbic brain regions, including nucleus accumbens (NAc) and prefrontal cortex (PFC) (1, 2). Several studies have shown that ethanol exposure is associated with elevated extracellular glutamate concentration in central brain reward regions, such as NAc, hippocampus and ventral tegmental area (3–7). Studies from our laboratory have revealed that chronic ethanol consumption reduced the expression of the major glutamate transporters, such as glutamate transporter 1 (GLT-1, its human homolog is excitatory amino acid transporter 2, EAAT2) (8, 9). GLT-1 is responsible for the uptake of the majority of extracellular glutamate concentration (10–12). Thus, compounds that upregulate GLT-1 expression in mesocorticolimbic brain regions may serve as a potential treatment of ethanol dependence.
Cystine-glutamate exchanger (xCT) plays a major part in maintaining basal glutamate concentration (13, 14). It is important to note that xCT exchanges internal glutamate for the external cystine to maintain glutamate homeostasis (10, 15). Previous study from our laboratory demonstrated that chronic ethanol exposure downregulated xCT expression in both NAc and PFC (16). In addition, xCT was downregulated in the NAc following withdrawal of cocaine (17). Glutamate/aspartate transporter (GLAST, its human homologs is EAAT1), is another glutamate transporter that is known to control synaptic glutamate concentration and is expressed mainly in the cerebellum (18).
Alcohol-preferring (P) rats line is a selectively bred line of rats, which meets all criteria for a suitable animal model for alcoholism. P rats exhibit high alcohol-seeking behavior and consume levels of ethanol during adolescence equivalent to that observed in adulthood. The P rats line also displays excessive binge-like ethanol drinking with average ethanol intake of more than 5 g/kg/day, attaining blood ethanol concentration of 200 mg% (19, 20).
β-lactam antibiotics have been reported to reduce ethanol consumption, at least in part, through upregulation of GLT-1 and xCT expression (16, 21–25). It is noteworthy that ceftriaxone parenteral treatment attenuated ethanol consumption and cocaine-seeking behavior, in part, by restoring glutamate homeostasis in mesocorticolimbic regions in rats (16, 26). However, the unavailability of oral dosage form is considered as a major limitation for ceftriaxone treatment. To the best of our knowledge, no previous studies have investigated the effect of orally administered β-lactam antibiotic on either exposure to ethanol or other drugs of abuse. Moreover, the effects of orally administered β-lactam antibiotic on the expression of glutamate transporters have not been studied yet. Therefore, in this study, we examined the effects of orally administered Augmentin (amoxicillin/clavulanate) on ethanol intake, GLT-1, xCT and GLAST expression in alcohol-preferring (P) rats.
2. Materials and methods
2.1 Animals
Male P rats were obtained from Indiana University, School of Medicine (Indianapolis, IN, USA) at the age of 21–30 days, and housed in the Department of Laboratory Animal Resources, University of Toledo, Health Science Campus. All animals were housed in a plastic corn-cob bedding tubs and had an access to food and water ad lib throughout the experiment. The room temperature was maintained at 21°C and 50% humidity with a 12-hour light-dark cycle. At the age of 90 days, male P rats were individually housed and randomly divided into two groups; (a) control group, which received oral gavage of water 1 ml/kg/day for 5 days (n=7) (control or vehicle); (b) Treatment group, which received Augmentin (100 mg/kg/day) through oral gavage for 5 days (n=7) (Aug). It is important to note that i.p. injections of Augmentin at dose of 100 mg/kg for 5 days reduced ethanol intake and upregulated GLT-1 and xCT expression in the NAc and the PFC (25). All rats had a voluntary uninterrupted access to ethanol, water and food throughout the experiment. All animal procedures were in compliance and approved by the Institutional Animal Care and Use committee of The University of Toledo in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996).
2.2 Behavioral drinking paradigms
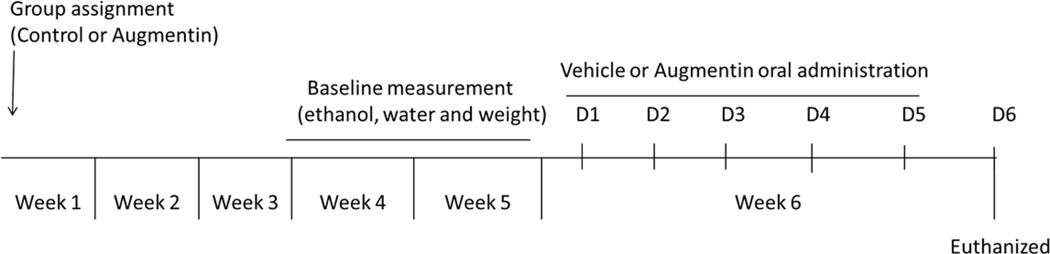
At the age of 90 days, rats were exposed to a free choice of ethanol (15% and 30% v/v), water and food for a period of five weeks. After the third week of drinking paradigm, ethanol and water intakes were measured three times a week for two weeks to obtain baseline intake. Ethanol and water intake measurements were expressed as g/kg/day. Animals with a baseline ethanol intake of less than 4 g/kg/day were not included in the study as adopted in previous studies (8, 9, 27). During Week 6, vehicle or Augmentin was administered through oral gavage for 5 consecutive days. In addition, during Week 6, ethanol and water intakes as well as animal body weight were measured every day through the last day of the experiment as illustrated in Figure 1.
Figure1.
Timeline of oral gavage of Augmentin experiment. Rats were exposed to free access of (15% and 30% v/v) ethanol, water and food for five weeks. Week 4 and 5 ethanol drinking and water measurements were used as baseline. On Week 6, rats were randomly assigned to receive either Augmentin (100 mg/kg) or vehicle through the oral gavage route for five consecutive days. Vehicle and Augmentin treated rats were then euthanized (24 hours following the last vehicle/Augmentin oral gavage) by CO2 inhalation and rapidly decapitated.
2.3. Brain tissue harvesting
Rats were euthanized using carbon dioxide inhalation 24 hours after the last administered dose of vehicle or Augmentin and rapidly decapitated. Brains were then immediately frozen at −80 °C. The NAc and PFC were micropunched using a cryostat machine according to the Rat Brain Atlas (28). Extracted brain regions were stored at −80 °C for western blot analysis.
2.4. Western blot protocol for detection of GLT-1, xCT and GLAST
Isolated NAc and PFC tissues were examined for the changes in GLT-1, xCT and GLAST expression relative to total β-tubulin using Western blotting procedure. Briefly, extracted brain regions were then lysed using regular lysis buffer as described in (27). Isolated protein samples were then mixed with 5X laemmli loading dye and loaded in polyacrylamide gels for the electrophoresis separation. Proteins were then transferred on PVDF membranes electrophoretically (Bio-Rad, Hercules, CA). Membranes were then blocked with 3% fat-free milk in TBST (50 mM Tris HCl; 150 mM NaCl, pH 7.4; 0.1% Tween 20) for 30 minutes at room temperature. Consequently, membranes were incubated overnight at 4°C with one of the following primary antibodies: guinea pig anti-GLT1 (1:5000), rabbit anti-xCT antibody (1:1,000), and rabbit anti-EAAT1 (GLAST) antibody (1:5,000). Membranes were then washed with TBST and blocked with 3% milk in TBST for 30 minutes. Membranes were further incubated with secondary antibodies for 90 minutes at room temperature. The corresponding secondary antibodies are: guinea pig anti-GLT-1 secondary antibody (1:5,000) and rabbit anti-xCT secondary antibody (1:5000). β-tubulin was used as loading control (1:5,000). Membranes were then exposed to the chemiluminescent substrate and further developed on the SRX-101A machine. The detected bands were quantified using MCID system and the results were expressed as a percentage of the ratio of tested protein/β-tubulin, relative to control group (100% control-value).
3. Statistical analyses
Statistical analysis using two-way repeated measure ANOVA was conducted to determine the main effect of day × treatment interaction on water intake, ethanol intake, ethanol preference, and body weight comparisons between control and Augmentin groups. Bonferroni multiple comparisons post-hoc test was used whenever a significant main effect or interaction was found. An unpaired t-test was used to analyze Western blot data (GLT-1/β-tubulin, xCT/β- tubulin, and GLAST/β-tubulin) between control and Augmentin groups. All statistical analyses were based on p<0.05 level of significance. Data are represented as mean ± SEM.
4. Results
4.1. Effect of oral gavage of Augmentin on average ethanol intake
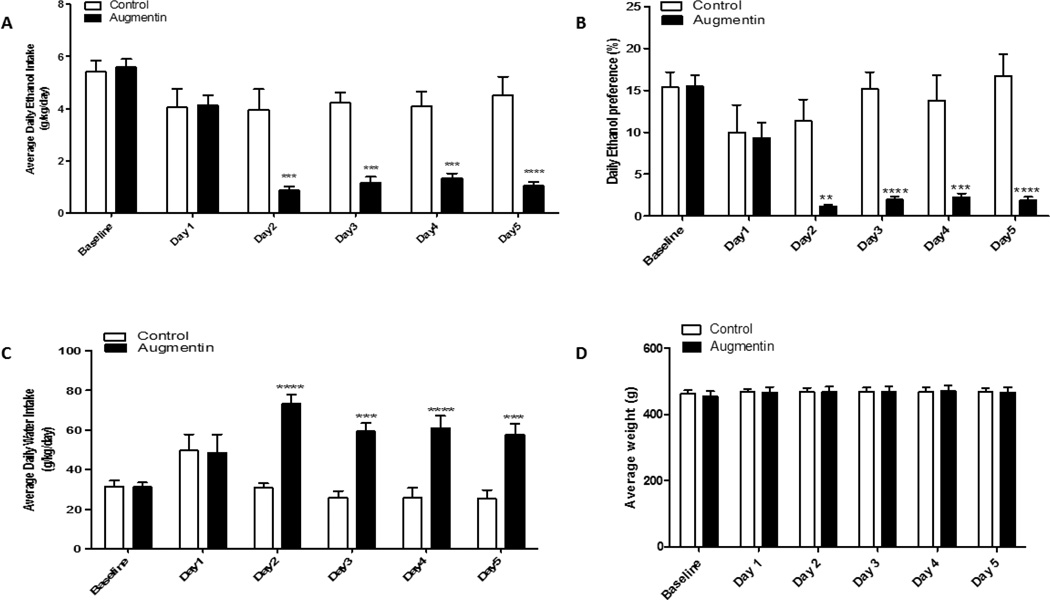
The average ethanol consumption over the last two weeks of drinking paradigm was considered as the baseline. P rats were then divided into two groups: control and oral gavage of Augmentin treatment groups. Statistical analysis using two-way repeated measure ANOVA revealed a significant main effect of Days [F (1, 5) = 3.786, p<0.01] and significant Treatment × Day interaction [F (1, 5) = 3.950, p<0.001]. Bonferroni multiple comparisons post-hoc test revealed a significant reduction in ethanol consumption in the treatment group as compared to the control group started on Day 2 (48 hours after the first oral gavage of Augmentin) through the last day of the experiment, (p<0.001), (Figure 2A).
Figure 2.
Changes in the average daily ethanol intake and water consumption following oral gavage of Augmentin in P rats exposed to free choice of ethanol and water for 5 weeks. A) Effect of Augmentin treatment on average daily ethanol intake as compared to control (g/kg/day) in P rats. Two-way repeated measure ANOVA followed by Bonferroni multiple comparisons post-hoc test revealed a significant reduction in ethanol consumption in Augmentin group as compared to control group starting 48 hours after the first day of treatment (p<0.001). B) Effect of oral gavage of Augmentin on percent of ethanol preference in male P rats. Two-way repeated measure ANOVA followed by Bonferroni multiple comparisons post-hoc test revealed a significant decrease in ethanol preference in oral gavage of Augmentin group from Day 2 through Day 5 as compared to the control group. C) Effect of Augmentin treatment on water consumption as compared to control group (g/kg/day). Two-way repeated measure ANOVA followed by Bonferroni multiple comparisons post-hoc test revealed a significant increase in water intake in all rats treated with Augmentin (p<0.001) as compared to the control group from Day 2 throughout Day 5. D) Effect of oral gavage of Augmentin on average body weight of P rats. Two-way repeated measure ANOVA followed by Bonferroni multiple comparisons post-hoc test showed no significant difference in average body weight between the Augmentin treatment and control groups. Data are represented as mean ± SEM (**p<0.01, ***p<0.001, ****p<0.0001), (n=7 for each group).
4.2. Effect of oral gavage of Augmentin on ethanol preference
The percentage of ethanol preference was calculated daily by the following equation: [total ethanol consumption / total fluid consumption × 100] using the daily ethanol and water consumption for each rat as described previously (29). Two-way repeated measure ANOVA revealed a significant main effect of Day [F (1, 5) = 7.467, p<0.0001] and significant Treatment × Day interaction [F (1, 5) = 8.111, p<0.0001]. Bonferroni multiple comparisons post-hoc test revealed a significant reduction in ethanol preference from Day 2 through the last day of the experiment in Augmentin group (p<0.01) as compared to the control group (Figure 2B).
4.3. Effect of oral gavage of Augmentin on water intake
Two-way repeated measure ANOVA demonstrated a significant effect of Days [F (1, 5) = 6.325, p<0.0001] and significant Treatment × Day interaction [F (1, 5) = 11.25, p<0.0001]. Bonferroni post-hoc test revealed a significant increase in water intake in all rats treated with Augmentin as compared to rats treated with vehicle (p<0.001). This increase in water intake in Augmentin group was detected during Week 6 (treatment administration week) as compared to the control group. However, statistical analysis revealed a significant increase in water intake from Day 2 throughout Day 5 as compared to the control group (Figure 2C).
4.4. Effect of oral gavage of Augmentin on body weight
Statistical analysis revealed a significant main effect of Days [F (1, 5) = 14.42, p<0.0001]. However, there was no significant effect in Treatment × Day interaction [F (1, 5) = 2.043, p>0.05]. Additionally, Bonferroni multiple comparisons post-hoc test did not show any significant difference between the Augmentin and the control groups in the body weight during the treatment days (Figure 2D).
4.5. Effects of oral gavage of Augmentin on GLT-1, xCT and GLAST expression in NAc and PFC
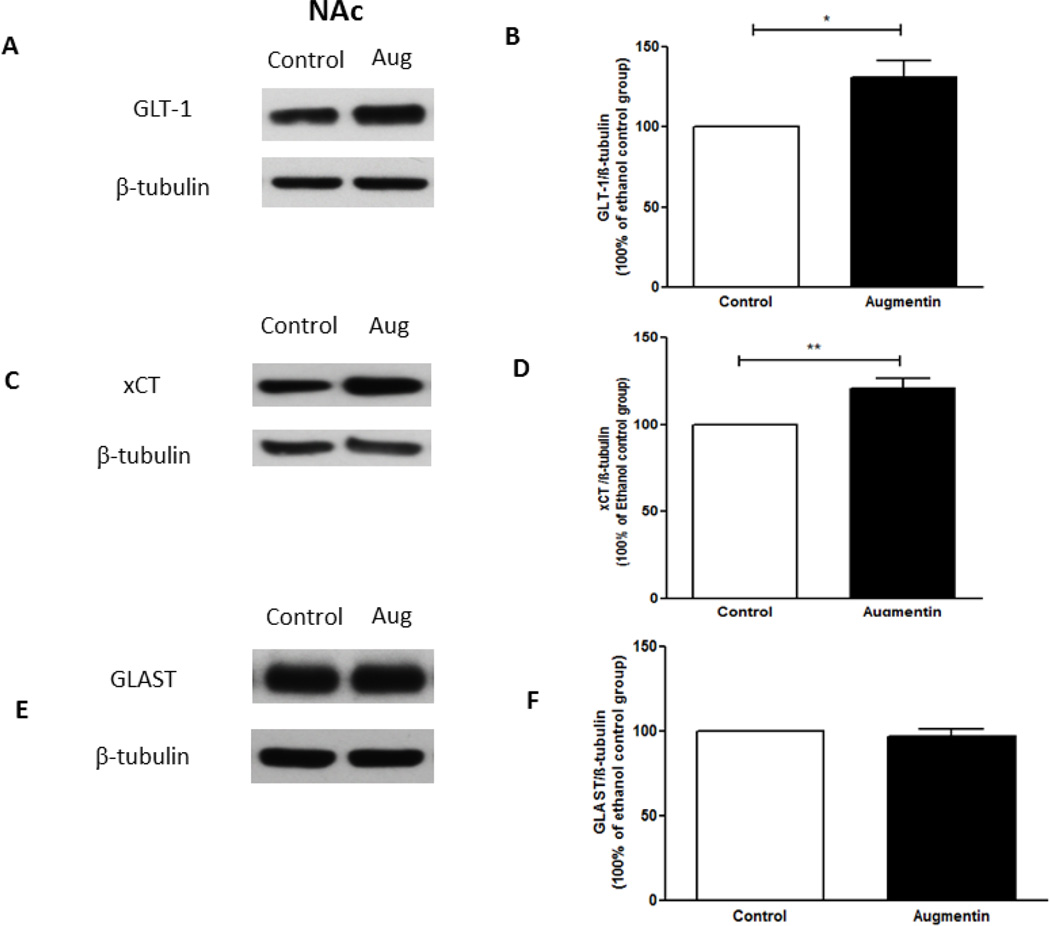
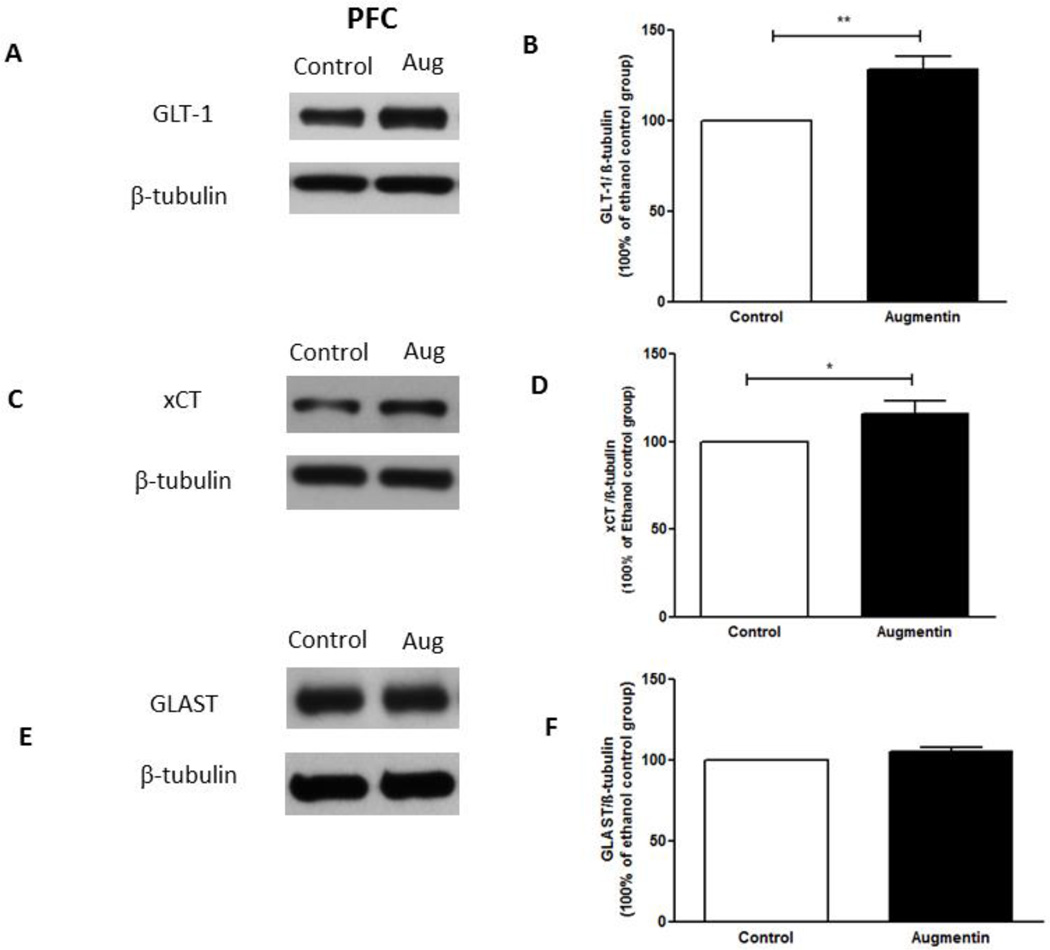
Statistical analysis using independent t-test revealed a significant upregulation of GLT-1 expression in the NAc in Augmentin-treated group as compared to the control group (Figure 3A,B), (t(12)= 3.017, p<0.05). In addition, statistical analysis using independent t-test revealed a significant upregulation of GLT-1 expression in the PFC in Augmentin-treated group as compared to control group (Figure 4A,B), (t(12)= 3.893, p<0.01).
Figure 3.
Effects of oral gavage of Augmentin on GLT-1, xCT and GLAST expression in the NAc. (A,C,E) Immunoblots for GLT-1/β-tubulin, xCT/β-tubulin, and GLAST/β-tubulin, respectively. (B) Quantitative analysis for the immunoblots revealed a significant upregulation of GLT-1 expression in the Augmentin treated group as compared to the control group in NAc. (D) Quantitative analysis for xCT immunoblots revealed a significant upregulation of xCT expression in the Augmentin treated group as compared to the control group in NAc. (F) Quantitative analysis for GLAST immunoblots revealed no significant difference in GLAST expression between Augmentin treated group and control group in NAc. Data are represented as mean ± SEM, (*p<0.05, **p<0.01); (n=7 for each group).
Figure 4.
Effects of oral gavage of Augmentin on GLT-1, xCT and GLAST expression in the PFC. (A,C,E) Immunoblots for GLT-1/β-tubulin, xCT/β-tubulin, and GLAST/β-tubulin, respectively. (B) Quantitative analysis for the immunoblots revealed a significant upregulation of GLT-1 expression in the Augmentin treated group as compared to the control group in the PFC. (D) Quantitative analysis for xCT immunoblots revealed a significant upregulation of xCT expression in the Augmentin treated group as compared to the control group in PFC. (F) Quantitative analysis for GLAST immunoblots revealed no significant difference in GLAST expression between Augmentin treated and control groups in the PFC. Data are represented as mean ± SEM; (*p<0.05, **p<0.01); (n=7 for each group).
Independent t-test revealed a significant upregulation of xCT expression in NAc in Augmentin-treated group as compared to control group (Figure 3C,D), (t(12)= 3.588, p<0.01). In addition, statistical analysis using independent t-test revealed a significant upregulation of xCT expression in the PFC in Augmentin-treated group as compared to control group (Figure 4C,D), (t(12)= 2.221, p<0.05). However, statistical analysis using independent t-test did not reveal any significant difference of GLAST expression in the NAc (Figure 3E,F; t(12)= 0.7122) and the PFC (Figure 4E,F; t(12)= 1.786) between Augmentin-treated group and control group.
5. Discussion
We report in this study that oral gavage of Augmentin (100 mg/kg) significantly reduced daily ethanol intake in male P rats. In addition, oral gavage of Augmentin results in a significant decrease in ethanol preference. However, oral gavage of Augmentin exhibited a significant increase in the water intake starting from Day 2 throughout Day 5. Oral gavage of Augmentin did not cause any change in the body weight of the treated rats as compared to the control group. Importantly, oral gavage of Augmentin upregulated the expression of GLT-1 and xCT in the NAc and the PFC. Conversely, oral gavage of Augmentin did not change the expression of GLAST in the NAc or the PFC.
Central brain reward regions, particularly NAc and PFC contain a relatively high concentration of glutamate, which plays an important role in the development of dependence related changes in cognition, sensory input, emotion and subsequent motor output (30). Drug reinforcement has been suggested to involve the glutamatergic projections from the PFC, particularly to the NAc, during craving in commonly abused drugs such as nicotine, ethanol, heroin and cocaine (1, 31–36). Dysregulation of these projections from the PFC may play critical role in the development of drug dependence such as cocaine and heroin (35, 36). Moreover, PFC receives glutamatergic projections from other brain regions, including amygdala and hippocampus (37, 38). Studies from our laboratory found that chronic ethanol exposure downregulated xCT expression in PFC. Importantly, β-lactams including, ceftriaxone and Augmentin, upregulated xCT expression in PFC and this effect was associated, at least in part, with reduction of ethanol intake (16, 25). We suggest here that downregulation of xCT expression may lead to alteration in glutamate homeostasis in the PFC, and Augmentin may overcome this effect through upregulation of this xCT protein as well as GLT-1. It is well known that downregulation of GLT-1 and xCT expression was found associated with an increase in extracellular glutamate concentration in NAc in animal model of cocaine seeking (17, 39).
Several studies have demonstrated that ethanol exposure is associated with increased extracellular glutamate concentration in the NAc (5, 40, 41). In addition, downregulation of GLT-1 expression has been reported following exposure to chronic ethanol (8, 9, 16) and other drugs of abuse (39, 42). In fact, it was shown that downregulation of GLT-1 following chronic ethanol exposure was associated with an elevation in extracellular glutamate concentration in NAc (5). Interestingly, several reports have demonstrated the upregulatory effect of β-lactams parenteral administration on GLT-1 and xCT expression in the central reward brain regions (5, 24, 25, 39, 43).
Importantly, identifying an orally active compound that can be used to alleviate drug dependence is clinically relevant. Oral gavage delivery route would decrease the risk of injection site infection or tissue necrosis and this may prevent potential complications. Oral gavage of medications is considered a better alternative option to overcome the pain and discomfort associated with parenteral administrations. In addition, oral gavage of medications is cost-effective in comparison to parenteral medications, where parenteral route requires sterile and isotonic formula. Moreover, oral delivery route would increase the patient’s compliance over the parenteral route of administration (44, 45). Thus, to the best of our knowledge, this present study is the first to investigate the effect of oral gavage of β-lactam, such as Augmentin on ethanol drinking and the expression of glutamate transporters.
It is important to note that ceftriaxone 2-day treatment attenuated ethanol consumption from Day 1 (22). However, GLT-1 and xCT expression were upregulated in the PFC but not the NAc. Moreover, ceftriaxone 5-day treatment revealed reduction of ethanol consumption and upregulation of GLT-1 and xCT expression in the NAc and the PFC (22). In this study, oral gavage of Augmentin at a dose of 100 mg/kg for five consecutive days decreased the ethanol consumption from Day 2 through Day 5 compared to the control. The reduction of ethanol intake found as early as Day 2 might be due to either an increase in the activity of xCT and GLT-1 or other unknown pharmacological effects that are warranted further investigation. This attenuation in ethanol consumption was associated with significant elevation of water intake. Similarly, several studies have reported that reduction in ethanol consumption was associated with an increase of water intake (24, 27, 46). We suggest this increase in water intake is a compensatory mechanism for the reduction in ethanol intake in order to maintain the fluid intake in P rat model. This compensatory mechanism might also be compared to the increase in water intake that was observed in a previous study conducted on the high ethanol consuming rat model (47). Moreover, oral gavage of Augmentin decreased the ethanol preference.
In this study, we examined the effect of oral gavage of Augmentin on the upregulation of GLT-1 and xCT, which might restore glutamate homeostasis. We found that in association with the reduction of ethanol consumption, oral gavage of Augmentin revealed a significant upregulation of GLT-1 expression in the NAc and PFC. Moreover, several studies have reported that administration of β-Lactams upregulated GLT-1 expression in mesocorticolimbic brain regions (16, 25, 43, 48). This effect was, at least in part, associated with a reduction of ethanol consumption (16, 48) and normalization of extracellular glutamate concentration in NAc (5).
xCT plays a crucial role in regulating the extracellular glutamate homeostasis. xCT can regulate the release of glutamate indirectly (49, 50). It is noteworthy that decreased xCT expression in the NAc can lead to reduction in the glutamatergic tone on metabotropic receptor 2/3 (mGluR2/3) (51, 52). Lower glutamatergic tone on mGluR2/3 can lead to a higher release of glutamate, which plays a key role in drug dependence and reinstatement (14, 35, 51). Importantly, studies have reported significant changes in xCT activity and expression in different drug dependence and drug-relapse animal studies (17, 42, 53, 54). Moreover, xCT is proposed to play an important role in β-lactam’s neuroprotective activity (55). In addition, several studies have reported that β-lactam antibiotics induced a reduction in ethanol consumption, at least in part, by the upregulatory effect of xCT expression in the NAc and the PFC (16, 22, 24, 25). Similarly, this study showed that oral gavage of Augmentin induced an upregulation of xCT expression in the NAc and the PFC. The possibility of involvement of other mechanisms in the reduction of ethanol consumption, such as disruption of taste or smell, is a novel area of research that remains unexplored. Further investigations are warranted to examine the effect of β-lactams on taste or smell and possible interactions with ethanol consumption.
Oral gavage of Augmentin did not change the expression of GLAST in the NAc and the PFC. This finding might be explained by the fact that GLAST is predominant glutamate transporter in the cerebellum as compared to the forebrain regions (18). Moreover, studies have demonstrated the important role of GLAST in other peripheral organs such as the retina (56) and the inner ear (57). Furthermore, previous studies demonstrated also that other β-lactam antibiotics treatment did not reveal any difference in GLAST expression as compared to the control group (24, 58, 59).
Augmentin contains amoxicillin and clavulanic acid in a 4:1 (amoxicillin: clavulanate) ratio. Both compounds have a central β-lactam core which is proposed to be the reason for the upregulatory effect on GLT-1 (21). Importantly, clavulanic acid also has been reported to upregulate GLT-1 expression, which was associated with reduction in reinforcing effects of cocaine in mice (43). Studies are warranted to investigate the role of clavulanic acid in ethanol dependence. Our findings provide the first evidence that orally administered β-lactam compounds can reduce ethanol consumption presumably by restoring glutamate homeostasis due to the upregulatory effects on GLT-1 and xCT expression in mesocorticolimbic brain regions.
Acknowledgments
This work was supported by the National Institutes on Alcohol Abuse and Alcoholism (Award Number R01AA019458 to Y.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, et al. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33(11):1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 2005;29(3):326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- 4.Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology. 2007;190(4):415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- 5.Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding ZM, Engleman EA, Rodd ZA, McBride WJ. Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcoholism: Clinical and Experimental Research. 2012;36(4):633–640. doi: 10.1111/j.1530-0277.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neuroscience letters. 1994;178(1):99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 8.Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–335. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sari Y, Sreemantula SN, Lee MR, Choi D-S. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. Journal of Molecular Neuroscience. 2013;51(3):779–787. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 12.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 13.Baker D, Shen H, Kalivas P. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino acids. 2002;23(1–3):161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- 14.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. The Journal of neuroscience. 2005;25(27):6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volterra A, Bezzi P, Rizzini BL, Trotti D, Ullensvang K, Danbolt NC, et al. The competitive transport inhibitor L-trans-pyrrolidine-2-4-dicarboxylate triggers excitotoxicity in rat cortical neuron-astrocyte co-cultures via glutamate release rather than uptake inhibition. European Journal of Neuroscience. 1996;8(9):2019–2028. doi: 10.1111/j.1460-9568.1996.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 16.Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014;231(20):4049–4057. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 18.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18(21):8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. REVIEW: The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction biology. 2006;11(3–4):270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 20.McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li T-K. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats-animal models of alcoholism. Alcohol. 2014;48(3):209–215. doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 22.Rao PS, Saternos H, Goodwani S, Sari Y. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao PS, Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Curr Med Chem. 2012;19(30):5148–5156. doi: 10.2174/092986712803530511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alasmari F, Rao P, Sari Y. Effects of cefazolin and cefoperazone on glutamate transporter 1 isoforms and cystine/glutamate exchanger as well as alcohol drinking behavior in male alcohol-preferring rats. Brain Research. 2016 doi: 10.1016/j.brainres.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakami AY, Hammad AM, Sari Y. Effects of amoxicillin and Augmentin on cystine-glutamate exchanger and glutamate transporter 1 isoforms as well as ethanol intake in alcohol-preferring rats. Frontiers in neuroscience. 2016;10 doi: 10.3389/fnins.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29(29):9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol and alcoholism. 2011;46(3):239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Compact. Vol. 1. San Diego: Academic Press Figure; 1997. pp. 25–30. [Google Scholar]
- 29.Lee MR, Ruby CL, Hinton DJ, Choi S, Adams CA, Kang NY, et al. Striatal adenosine signaling regulates EAAT2 and astrocytic AQP4 expression and alcohol drinking in mice. Neuropsychopharmacology. 2013;38(3):437–445. doi: 10.1038/npp.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. The Journal of Neuroscience. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999 doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dom G, Sabbe B, Hulstijn W, Van Den Brink W. Substance use disorders and the orbitofrontal cortex. The British Journal of Psychiatry. 2005;187(3):209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- 33.Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, et al. Functional magnetic resonance imaging of cocaine craving. American Journal of Psychiatry. 2001 doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Z, Lee T, Zhang JX, Wu Q, Wu R, Weng X, et al. Thirsty heroin addicts show different fMRI activations when exposed to water-related and drug-related cues. Drug and alcohol dependence. 2006;83(2):157–162. doi: 10.1016/j.drugalcdep.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 35.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. The journal of neuroscience. 2008;28(12):3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience. 2003;23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald AJ. Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: colocalization of excitatory amino acids and projections to the limbic circuit. Journal of Comparative Neurology. 1996;365(3):367–379. doi: 10.1002/(SICI)1096-9861(19960212)365:3<367::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Gigg J, Tan AM, Finch DM. Glutamatergic hippocampal formation projections to prefrontal cortex in the rat are regulated by GABAergic inhibition and show convergence with glutamatergic projections from the limbic thalamus. Hippocampus. 1994;4(2):189–198. doi: 10.1002/hipo.450040209. [DOI] [PubMed] [Google Scholar]
- 39.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67(1):81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffin WC, 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39(3):707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapasova Z, Szumlinski KK. Strain Differences in Alcohol-Induced Neurochemical Plasticity: A Role for Accumbens Glutamate in Alcohol Intake. Alcoholism: Clinical and Experimental Research. 2008;32(4):617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- 42.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J, John J, Langford D, Walker E, Ward S, Rawls SM. Clavulanic acid enhances glutamate transporter subtype I (GLT-1) expression and decreases reinforcing efficacy of cocaine in mice. Amino acids. 2015:1–8. doi: 10.1007/s00726-015-2117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen KM, Paladino JA. Cost-effectiveness of abbreviating the duration of intravenous antibacterial therapy with oral fluoroquinolones. Pharmacoeconomics. 1997;11(1):64–74. doi: 10.2165/00019053-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 45.Kuper KM. Intravenous to oral therapy conversion. Competence assessment tools for health-system pharmacies 4th ed American Society of Health-System Pharmacists. 2008:349–351. [Google Scholar]
- 46.Alasmari F, Abuhamdah S, Sari Y. Effects of ampicillin on cystine/glutamate antiporter and glutamate transporter 1 isoforms as well as ethanol drinking in male P rats. Neurosci Lett. 2015;600:148–152. doi: 10.1016/j.neulet.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell RL, Rodd ZA, Schultz JA, Peper CL, Lumeng L, Murphy JM, et al. Effects of short deprivation and re-exposure intervals on the ethanol drinking behavior of selectively bred high alcohol-consuming rats. Alcohol. 2008;42(5):407–416. doi: 10.1016/j.alcohol.2008.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sari Y, Franklin KM, Alazizi A, Rao PS, Bell RL. Effects of ceftriaxone on the acquisition and maintenance of ethanol drinking in peri-adolescent and adult female alcohol-preferring (P) rats. Neuroscience. 2013;241:229–238. doi: 10.1016/j.neuroscience.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bridges R, Lutgen V, Lobner D, Baker DA. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (system xc−) to normal and pathological glutamatergic signaling. Pharmacological reviews. 2012;64(3):780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22(20):9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dietrich D, Kral T, Clusmann H, Friedl M, Schramm J. Presynaptic group II metabotropic glutamate receptors reduce stimulated and spontaneous transmitter release in human dentate gyrus. Neuropharmacology. 2002;42(3):297–305. doi: 10.1016/s0028-3908(01)00193-9. [DOI] [PubMed] [Google Scholar]
- 52.Manzoni O, Michel JM, Bockaert J. Metabotropic glutamate receptors in the rat nucleus accumbens. European Journal of Neuroscience. 1997;9(7):1514–1523. doi: 10.1111/j.1460-9568.1997.tb01506.x. [DOI] [PubMed] [Google Scholar]
- 53.Baker DA, McFarland K, Lake RW, Shen HUI, Toda S, Kalivas PW. N-Acetyl Cysteine-Induced Blockade of Cocaine-Induced Reinstatement. Annals of the New York Academy of Sciences. 2003;1003(1):349–351. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 54.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. The Journal of Neuroscience. 2007;27(51):13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewerenz J, Albrecht P, Tien MLT, Henke N, Karumbayaram S, Kornblum HI, et al. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. Journal of neurochemistry. 2009;111(2):332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- 56.Lehre KP, Davanger S, Danbolt NC. Localization of the glutamate transporter protein GLAST in rat retina. Brain Res. 1997;744(1):129–137. doi: 10.1016/s0006-8993(96)01022-0. [DOI] [PubMed] [Google Scholar]
- 57.Takumi Y, Matsubara A, Danbolt NC, Laake JH, Storm-Mathisen J, Usami S, et al. Discrete cellular and subcellular localization of glutamine synthetase and the glutamate transporter GLAST in the rat vestibular end organ. Neuroscience. 1997;79(4):1137–1144. doi: 10.1016/s0306-4522(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 58.Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, et al. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci. 2014;8:366. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen T, Zhang D, Dragomir A, Kobayashi K, Akay Y, Akay M. Investigating the influence of PFC transection and nicotine on dynamics of AMPA and NMDA receptors of VTA dopaminergic neurons. Journal of neuroengineering and rehabilitation. 2011;8(1):1. doi: 10.1186/1743-0003-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]