Abstract
Moderate exposure to alcohol during development leads to subtle neurobiological and behavioral effects classified under the umbrella term fetal alcohol spectrum disorders (FASDs). Alterations in social behaviors are a frequently observed consequence of maternal drinking, as children with FASDs display inappropriate aggressive behaviors and altered responses to social cues. Rodent models of FASDs mimic the behavioral alterations seen in humans, with rats exposed to ethanol during development displaying increased aggressive behaviors, decreased social investigation, and altered play behavior. Work from our laboratory has observed increased wrestling behavior in adult male rats following prenatal alcohol exposure (PAE), and increased expression of GluN2B-containing NMDA receptors in the agranular insular cortex (AIC). This study was undertaken to determine if ifenprodil, a GluN2B preferring negative allosteric modulator, has a significant effect on social behaviors in PAE rats. Using a voluntary ethanol exposure paradigm, rat dams were allowed to drink a saccharin-sweetened solution of either 0% or 5% ethanol throughout gestation. Offspring at 6–8 months of age were implanted with cannulae into AIC. Animals were isolated for 24 hours before ifenprodil or vehicle was infused into AIC, and after 15 minutes they were recorded in a social interaction chamber. Ifenprodil treatment altered aspects of wrestling, social investigatory behaviors, and ultrasonic vocalizations in rats exposed to ethanol during development that were not observed in control animals. These data indicate that GluN2B-containing NMDA receptors in AIC play a role in social behaviors and may underlie alterations in behavior and vocalizations observed in PAE animals.
Keywords: Social behavior, agranular insular cortex, GluN2B, ifenprodil, ultrasonic vocalizations
1. Introduction
Maternal consumption of alcohol during human embryonic development results in a number of deleterious effects, including profound morphological, neurological, and behavioral deficits [1–10]. This fact is particularly relevant in the United States, where the incidence of Fetal Alcohol Spectrum Disorders (FASD), which includes Fetal Alcohol Syndrome (FAS), partial FAS (pFAS), and alcohol-related neurodevelopmental disorders (ARNDs) [11], is estimated at 2–5% [12]. While exposure to high levels of alcohol during development may cause birth defects such as facial dysmorphias, moderate exposure to alcohol can cause persistent cognitive deficits and other adverse neurobehavioral outcomes in the absence of conspicuous morphological abnormalities in humans [13–15], as well as in model organisms exposed to alcohol during prenatal development [16]. Prenatal alcohol exposure (PAE) is an issue of worldwide significance [17], with estimated rates of exposure during pregnancy ranging up to 63%. A relatively high percentage (5–30%) of children in the United States are exposed to some alcohol during gestation [18–23], and nearly 50% of women reported consuming alcohol prior to realizing they were pregnant [24, 25], which may be expected to increase the incidence of FASD in the coming years. Because most FASDs encompass the less severe forms [26] that may not be recognized early during development, it is critically important for researchers to understand the behavioral and underlying neurobiological mechanisms affected by moderate PAE.
Children with FASDs typically present with a number of cognitive and behavioral deficits, including problems in learning and memory [2, 4, 27, 28], attention [29], and difficulties in social interactions with their peers [30–33]. Utilizing rat models, many investigators have observed altered social behaviors following PAE including increased aggression [34, 35], changes in play behavior [34, 36–39], decreased social investigation and interaction [37, 40–42], changes in response to social stimuli [43–45], and deficits in socially acquired food preferences and social recognition memory [46]. These behavioral alterations have been observed across a broad range of experimental factors including the timing, dose, and duration of alcohol exposure, as well as the age at which behavioral measurements took place. While it is difficult to fully understand the neurological mechanisms that underlie social behavior deficits, it is clear that many areas of the frontal cortex are involved [47–49]. In particular, damage to orbital prefrontal cortex (oPFC) in humans [47] and primates [50], as well as corresponding agranular insular cortex (AIC) in rats [51], has been associated with an array of social behavior deficits. Therefore, it is logical to reason that alterations in the structure and function of neurons in oPFC and AIC following PAE could contribute to subsequent deficits in social behaviors. Previous work from our laboratory has documented deficits in dendritic spine formation, as well as immediate early gene (IEG) expression in AIC following a social interaction paradigm in PAE rats [40], which was associated with deficits in social behaviors [52].
Recent research from our laboratory examined alterations in excitatory neurotransmitter receptor expression and function in the frontal cortex following moderate PAE. We have observed a regionally-specific increase in GluN2B-containing NMDA receptor binding based on radiohistochemical studies in rats exposed to ethanol during gestation [53]. Further, in the same study, we demonstrated that evoked NMDA currents from layer II/III pyramidal neurons in AIC in PAE rats are more sensitive to the effects of ifenprodil, a negative allosteric modulator of GluN2B containing NMDA receptors, than saccharin controls. Given that GluN2B expression is associated with social recognition memory [54, 55], we hypothesized that PAE-induced alterations in GluN2B expression in AIC contribute to deficits in social behaviors. To evaluate this hypothesis the effects of ifenprodil locally infused bilaterally into AIC of adult PAE and control rats on social behavior were quantified. In addition, we also examined whether ifenprodil infusion affected social communication in rats by analyzing ultrasonic vocalizations (USVs). Previous work has measured elevated GluN2B expression in the AIC of PAE rats, therefore, we hypothesized that ifenprodil would have a greater effect on behaviors in PAE rats compared to saccharin exposed controls.
2. Methods
All experimental procedures included in this manuscript adhered to the Public Health Service policy on humane care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of New Mexico (IACUC protocol numbers MCC-101106 and 101166).
2.1 Subjects
Adult (6 – 8 months old) male and female Long-Evans rats born and raised at the University of New Mexico Health Sciences Center Animal Resource Facility were used in these studies. Breeding rats in-house is important because it eliminates a confound observed in rats purchased from outside sources, as these rats may display an increase in anxiety like behaviors compared to locally raised animals [56] that could potentially complicate interpretation of social behavior data following PAE and other treatments. A total of 40 rats (10 males and 10 females from each prenatal treatment condition, housed in pairs since weaning) were used for cannula implantation and subsequent behavioral studies. All rats were pair-housed with a partner of the same sex and prenatal treatment condition on a 12-hr reverse light/dark cycle with food and water available ad libitum. No more than 1–2 offspring from each litter and dam were used in these experiments to avoid potential litter effects. This study was designed so that prenatal treatment condition (two levels: exposure to either saccharin or ethanol during gestation) and sex (two levels: male and female) were between subjects factors, and drug treatment (two levels: vehicle or ifenprodil) was a within subjects factor.
2.2 Materials and Procedures
2.2.1 Breeding and Voluntary Drinking Paradigm
The voluntary drinking paradigm utilized in this study has been previously described [57, 58]. Three-to-four-month-old Long-Evans rat breeders (Harlan Industries, Indianapolis, IN) were single-housed in plastic cages at 22°C and kept on “reverse” 12-hour dark/12-hour light schedule (lights on from 2100 to 0900 hours) with Harlan Teklad rat chow and tap water ad libitum. Prior to breeding all female rats were provided 0.066% (w/v) saccharin in tap water for 4 hours each day from 1000 to 1400 hours. On Days 1 & 2, the saccharin water contained 0% ethanol and on Days 3 & 4 it contained 2.5% ethanol (v/v). On Day 5 and thereafter, the saccharin water contained 5% ethanol (v/v). Daily ethanol consumption was recorded for at least 2 weeks and mean daily ethanol consumption was calculated for each female breeder. Breeders that drank ±1 s.d. above or below the group mean were excluded from the study. The remaining females were randomly assigned to either a saccharin control group or 5% ethanol drinking group and matched such that the mean pre-pregnancy ethanol consumption by each group was comparable. Tap water was available at all times during the drinking paradigm. Females were subsequently housed with proven male breeders until pregnancy was confirmed by the presence of a vaginal plug. Female rats did not consume ethanol during breeding. Beginning on gestational Day 1 (GD1), rat dams were provided with saccharin water containing either 0% or 5 % ethanol for 4 hours per day. The volume of 0% saccharin water provided to the control group was matched to the mean volume of saccharin solution consumed by the 5% ethanol drinking group. Daily 4-hour ethanol consumption was documented for each dam. Following parturition, access to ethanol was discontinued and all litters were weighed and culled to 10 pups. Offspring were weaned at 24 days of age and pair-housed with a same-sex and prenatal treatment condition cage mate.
2.2.2 Maternal serum ethanol levels
A separate set of twelve rat dams for which no offspring were used in the present study were allocated to determine serum ethanol concentrations. These dams completed the same voluntary drinking paradigm as described above, except that at 45 minutes into the 4-hour ethanol consumption period on each of 3 alternate days during the third week of gestation 100 μl of whole blood was collected from the tail vein under isoflurane anesthesia and immediately mixed with 0.2 ml of 6.6% perchloric acid, frozen and stored at −20°C along with standards (0–240mg/dl) created from whole blood from untreated animals until assayed. Serum ethanol samples were assayed using a modified method of Lundquist and colleagues [59].
2.2.3 Surgery
Under isoflurane anesthesia, infusion guide cannulae (PlasticsOne, Roanoke, VA) were implanted under stereotaxic guidance. Guide cannulae (26 gauge) were implanted such that infusion cannulae were situated in AIC (+3.72 mm anterior, ±4 mm lateral, and 5.4 mm ventral relative to bregma). Guide cannulae were fixed in place using dental cement and anchored to the skull with stainless steel screws. Following surgery, animals were allowed to recover for 14 days before subsequent drug infusion and social behavior experiments.
2.2.4 Drugs
The GluN2B negative allosteric modulator ifenprodil (Sigma-Aldrich, St. Louis, MO) was dissolved at a concentration of 1μg/0.3μl in 0.9% non-pyrogenic saline (w/v) containing 5% (2-hydrocypropyl)-β-cyclodextrin adjusted to pH 7 according to the methods of Parkes and Westbrook [60]. This solution, without ifenprodil, served as the vehicle for drug infusions.
2.2.5 Social behavior and drug infusion
Social behavior observation took place in a box (95 cm × 47 cm × 43 cm) with a Plexiglass front, opaque sides, and a mirrored back wall as described in [58]. Prior to behavioral testing, pairs of animals from the same prenatal treatment condition habituated to the apparatus for 30 min on two consecutive days. Animals were tested with partners from the same prenatal treatment condition due to recent evidence indicating that a mixed prenatal treatment housing condition contributes to alterations in social behaviors in control animals [39]. At the end of the final habituation session animals were housed in isolation for 24 hours, after which cage mates were randomly assigned to be infused with either ifenprodil or vehicle to avoid potential drug order effects. Ifenprodil or vehicle (0.3 μl) was infused into AIC using 33-gauge infusion cannulae at 0.1 μl/min. Infusion cannulae were left in place for one minute following infusion. Fifteen minutes following drug infusion animal pairs were reunited and placed in the social behavior apparatus. Behavior was recorded for 12 minutes in the dark using a camera capable of recording under infrared illumination. Videos were directly digitized to a digital video file during recording. After the social interaction session was completed, pairs of animals were housed under normal conditions for 6 days. They were then again housed in isolation for 24 hours before being infused with the opposite drug treatment to achieve a within-subjects experimental design. The social behavior interaction session was repeated according to the above methods. The frequency, duration, and latency to first occurrence of the following behaviors were quantified: allogrooming, anogenital sniffing, other sniffing of the partner’s body (body sniffing), crossing over/under the partner, rearing, and wrestling (including pinning). These behaviors were selected based on existing work [61], and have been quantified in previous studies from our laboratory [35, 40]. All behaviors were analyzed by a single researcher blind to experimental conditions using software developed in-house [62].
2.2.6 Ultrasonic vocalization analysis protocol
During the social interaction sessions rat USVs were recorded to a HD-P2 audio recorder (TASCAM, Montbello, CA) using an electret ultrasound microphone (Avisoft Bioacoustics, Glienicke, Germany). USV audio files were converted to spectrograms using Audacity audio editing and recording software (Pittsburgh, PA). 50 kHz and 22 kHz USVs were recorded, however, because only one pair of animals emitted any 22 kHz USVs only 50 kHz USVs were analyzed. Specific USV waveforms were selected for analysis based on previous studies [63], and frequency of occurrence in our experiments. Total frequency, duration, average duration, and latency to first onset of the following USV waveforms were analyzed: total USVs, ascending (ascending USV pitch), atypical (not matching any other criteria), chirp (USVs under 10 ms), frequency modulated (FM) (USVs with a sinusoidal waveform), simple (USVs with a flat, unchanging pitch), and step (USVs with an abrupt, discontinuous change in pitch). Examples of USV waveforms analyzed here are provided in Supplementary Figure 1. All USVs were analyzed by a single researcher blind to the experimental conditions.
2.2.7 H&E staining protocol to analyze cannula placement
At the conclusion of the second social interaction session, animals were deeply anaesthetized with an overdose of sodium pentobarbital. Brains were extracted and flash frozen before being sectioned on a cryostat at a thickness of 20μm. Sections were then fixed in 4% PFA before being stained with Harris hematoxylin solution and Eosin Y solution according to manufacturer protocols (Sigma-Aldrich). Stained sections were then visualized under light microscopy to determine the most ventral location of infusion cannulae.
2.2.8 Statistics
All statistical tests reported here were significant at p < 0.05 unless otherwise stated. Statistical analyses were performed using SPSS version 23. Data were analyzed using repeated measures analyses of variance (ANOVAs) with prenatal treatment and sex as between-subjects factors, and drug treatment as a within-subjects factor. Planned comparisons for each behavior or USV of interest analyzed are reported in the subsections that follow. Statistical values reported include F ratio, p value, and effect size reported as partial eta squared (ηp2, which is equivalent to eta squared for one-way ANOVAs). Standard benchmarks for small (ηp2 = 0.01), medium (ηp2 = 0.06), and large (ηp2 = 0.014) effect sizes are based on the recommendations of Cohen [64] (see also refs. [65, 66]. Significant interactions between experimental factors were further explored by performing post hoc analyses of simple Drug Treatment effects within level of either Prenatal Treatment or Sex. All post hoc analyses used a Bonferroni correction (adjusted p < 0.025) to correct for the number of multiple comparisons. Due to the fact that there were a number of Sex effects on behavior observed in this study, earlier studies from our laboratory [39, 40, 53], and the work of others [38, 43, 67, 68], we also present the effects of Prenatal Treatment and Drug Treatment on behavior separately by level of sex.
3. Results
One male rat from the prenatal alcohol exposed (PAE) group died post-surgery. In addition, infusion cannula became dislodged in one male rat from the PAE group and two male rats from the saccharin control (SAC) group. These rats and their cage mates were removed from the study, leaving 6 males in the SAC group and 6 males in the PAE group.
3.1 Voluntary drinking paradigm
Maternal serum ethanol levels, as well as other effects of the voluntary drinking paradigm’s effects on rat dams and their offspring from all rats in the breeding round used in this study are presented in Table 1. There was no significant effect of prenatal treatment condition on maternal weight gain during pregnancy, litter size, or pup birth weight.
Table 1. Effects of daily four-hour consumption of 5% ethanol on rat dams and their offspring.
Data are mean (SEM) with group sample size.
| SAC | PAE (5% Ethanol) | |
|---|---|---|
|
Daily four-hour ethanol consumption (grams EtOH consumed/kg body weight/day) |
NA | 2.04(0.08) n=32 |
| Daily four-hour ethanol consumption: week 1 | NA | 1.73(0.09) n=32 |
| Daily four-hour ethanol consumption: week 2 | NA | 2.07(0.09) n=32 |
| Daily four-hour ethanol consumption: week 3 | NA | 2.04(0.08) n=32 |
|
Maternal serum EtOH concentration (mg EtOH/dL serum, 45 minutes into drinking) |
NA | 60.8(5.8) n=62 |
|
Maternal weight gain during pregnancy (grams increase in body weight) |
107(4) n=32 | 104 (5) n=32 |
| Litter size (number of live fetal pups/litter) | 12.5(0.15) n=32 | 12.2(0.29) n=32 |
| Pups birth weight (grams) | 6.17(0.13) n=41 | 6.13(0.11) n=42 |
3.2 Cannula placement
The location of infusion cannulae for all animals is shown in Figure 1. All animals had infusion cannulae situated in AIC, therefore no animals were excluded from subsequent analyses.
Figure 1. Cannula placement locations in AIC.
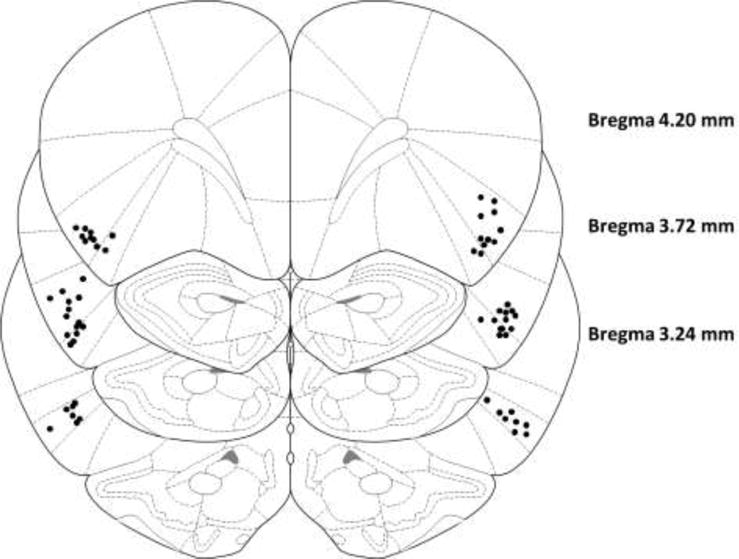
Dots represent the location of infusion cannula verified by H&E staining. Images are coronal sections (Bregma +4.20 mm, +3.72 mm, and +3.24 mm) from the stereotaxic atlas of Paxinos and Watson [106] (Reprinted with permission from Elsevier)
3.3 Social Behavior
Behavior data (frequency, total duration, and latency to onset of first occurrence) for combinations of prenatal treatment, sex, and drug treatment condition are shown in Supplementary Table 1. A summary of significant effects of drug treatment on social behaviors is presented in Table 2. For behaviors with a significant Drug Treatment × Prenatal Treatment interaction or a significant main effect of drug treatment that did not interact with prenatal treatment, planned comparisons of drug treatment effects within level of prenatal treatment are presented to explore our hypothesis that the effects of ifenprodil infusion are more prevalent in PAE animals than in SAC controls.
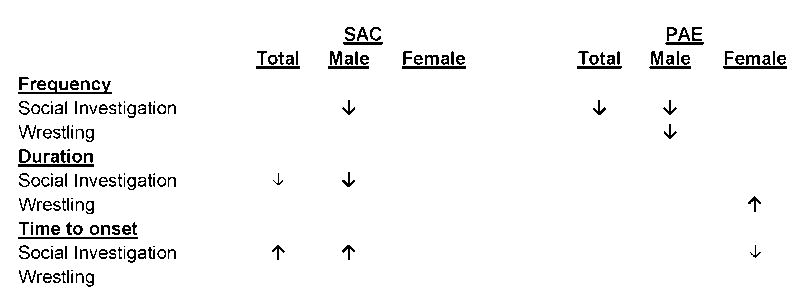
Table 2. Summary of effects of ifenprodil treatment on social behavior.
Effects of ifenprodil treatment within combinations of prenatal treatment and sex are presented for social investigation (anogenital and body sniffing, crossing over/under) and wrestling behaviors within prenatal treatment group and sex, with the arrow indicating the direction of the effect of ifenprodil treatment relative to vehicle treatment. Bold arrow (↓) indicates a significant effect of p < 0.05. Small arrow (↓) indicates a statistical trend of p < 0.06.
3.3.1. Main effects of prenatal treatment
There were two significant effects of prenatal treatment on behavior in the vehicle drug treatment group. In animals infused with vehicle, PAE decreased the total duration of crossing over/under [F(1,28) = 4.482; p = 0.043; ηp2 = 0.138], and decreased the latency to first onset of allogrooming [F(1,28) = 6.101; p = 0.020; ηp2 = 0.179]. Ifenprodil treatment did not affect these behaviors (ps > 0.36).
3.3.2. Main effects of drug treatment
There were two main effects of drug treatment on behaviors that were independent of prenatal treatment and sex. Ifenprodil treatment reduced the frequency of both anogenital sniffing [F(1,28) = 6.821; p = 0.014; ηp2 = 0.196] and body sniffing [F(1,28) = 7.265; p = 0.012; ηp2 = 0.206]. For frequency of body sniffing, a planned comparisons of drug treatment effects within level of prenatal treatment revealed that ifenprodil treatment significantly reduced the frequency of this behavior in PAE animals [F(1,14) = 7.810; p = 0.014; ηp2 = 0.358], while there was no effect in SAC controls (p > 0.79). For frequency of anogenital sniffing, the same planned comparison failed to reveal a significant effect of drug treatment in either SAC or PAE animals (ps > 0.07).
3.3.3. Main effects of sex
There were a number of sex effects on aspects of social behaviors, including: females allogrooming more frequently than males [F(1,28) = 4.867; p = 0.036; ηp2 = 0.148], females allogrooming sooner than males during the social session [F(1,28) = 8.671; p = 0.006; ηp2 = 0.236], females wrestling more frequently [F(1,28) = 6.894; p = 0.014; ηp2 = 0.198] for a longer duration [F(1,28) = 17.118, p < 0.001; ηp2 = 0.379], and earlier in the social session [F(1,28) = 9.108; p = 0.005; ηp2 = 0.245] than males.
3.3.4. Interactions of prenatal treatment and drug treatment
There were two Prenatal Treatment × Drug Treatment interactions when both sexes were analyzed together. The first Prenatal Treatment × Drug Treatment interaction was for frequency of body sniffing [F(1,28) = 6.262; p = 0.018; ηp2 = 0.183]. Analysis of this interaction indicates this effect was caused by a significant drug treatment effect in PAE animals (p = 0.014), as ifenprodil infusion reduced the frequency of body sniffing relative to vehicle infusion. This drug treatment effect was absent in SAC animals (p > 0.79). The other significant Prenatal Treatment × Drug Treatment interaction was for latency to onset of crossing over/under the partner animal [F(1,28) = 10.960; p = 0.003; ηp2 = 0.281]. Analysis of the simple effects revealed that ifenprodil infusion in SAC animals led to a significant increase in latency to crossing over/under relative to vehicle infusion (p = 0.011), an effect that was absent in PAE animals (p > 0.11).
3.3.5. Interactions of drug treatment and sex
There were a number of drug treatment effects that interacted with sex in this study. There was a Drug Treatment × Sex interaction for frequency of body sniffing [F(1,28) = 4.791; p = 0.037; ηp2 = 0.146], which post hoc analysis determined was attributable to ifenprodil treatment reducing the frequency of body sniffing in male animals (p = 0.013). There was a Drug Treatment × Sex interaction for frequency of anogenital sniffing as well [F(1,28) = 10.769; p = 0.003; ηp2 = 0.278]. Analysis of simple effects showed that this interaction was due to ifenprodil reducing the frequency of anogenital sniffing in males. In the same vein, there was also a Drug Treatment × Sex interaction for duration of anogenital sniffing [F(1,28) = 7.110, p = 0.013; ηp2 = 0.203], which again was caused by a significant simple effect in male animals (ifenprodil infusion reduced anogenital sniffing duration; p = 0.010). For latency to first onset of wrestling there was a significant Drug Treatment × Sex interaction [F(1,28) = 5.129; p = 0.031; ηp2 = 0.155]. Simple effects show that this interaction may have been due to ifenprodil reducing the latency to onset of wrestling, but this effect did not survive Bonferroni correction (p = 0.033). Another Drug Treatment × Sex interaction occurred for latency to first onset of crossing over/under [F(1,28) = 4.366; p = 0.046; ηp2 = 0.135], but post hoc testing failed to reveal simple drug treatment effects within level of sex for this behavior (ps > 0.11).
3.3.6. Interactions of prenatal treatment, drug treatment, and sex
There were two three-way interactions of Prenatal Treatment × Drug Treatment × Sex. For crossing over/under the partner animal, there was a significant three-way interaction for latency to first onset [F(1,28) = 4.309; p = 0.047; ηp2 = 0.133]. Post hoc analysis demonstrated that this interaction was due to a significant Prenatal Treatment × Drug Treatment interaction (p = 0.023) that was not present in female animals. Breaking down this effect even further suggests that this two-way interaction in males was due to ifenprodil infusion increasing the latency to onset of crossing over/under in SAC male animals, but this effect was not significant after Bonferroni correction (p = 0.026). The other significant three way interaction was a Prenatal Treatment × Drug Treatment × Sex interaction for latency to first onset of wrestling [F(1,28) = 4.533; p = 0.042; ηp2 = 0.139]. Post hoc tests suggest that this interaction may have been due to a Drug Treatment × Sex interaction in PAE animals, but this measure did not survive Bonferroni correction (p = 0.030).
3.3.7 Social behavior in females
Graphs that display significant effects of drug treatment within sex are presented in Figure 2. In female animals infused with vehicle there was a significant main effect of prenatal treatment, as PAE animals were quicker to allogroom than SAC animals [F(1,18) = 4.750; p = 0.043; ηp2 = 0.209]. There was no significant effect of ifenprodil treatment on this behavior (p > 0.53). When main effects of prenatal treatment were analyzed using drug treatment as a within-subjects factor, there was a significant prenatal treatment effect for the duration of anogenital sniffing in females [F(1,18] = 6.244; p = 0.022; ηp2 = 0.258]. PAE females engaged in anogenital sniffing behavior for a shorter duration than SAC animals.
Figure 2. Graphs of behaviors with significant drug treatment effects.
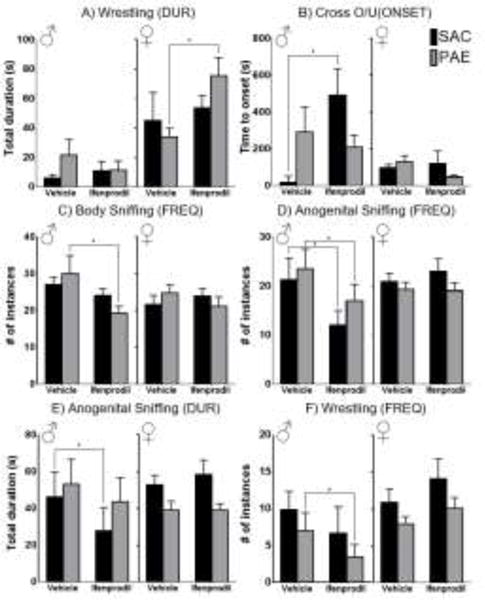
Mean (SEM) frequency (FREQ), duration (DUR), and latency to first occurrence (ONSET) of behaviors with significant effects of drug treatment are shown for males (n = 6 per prenatal treatment) and females (n = 10 per prenatal treatment). Black bars are saccharin exposed controls (SAC), and grey bars are prenatal alcohol exposed animals (PAE). A) duration of wrestling; B) latency to first occurrence of crossing over/under; C) frequency of body sniffing; D) frequency of anogenital sniffing. E) duration of anogenital sniffing; F) frequency of wrestling. Asterisk (*) denotes a significant effect of drug treatment at p < 0.05.
There were also two main effects of drug treatment in females on aspects of wrestling behavior. Ifenprodil treatment increased the total duration [F(1,18) = 5.015; p = 0.038; ηp2 = 0.218] and reduced the latency to first instance [F(1,18) = 5.354; p = 0.033; ηp2 = 0.229] of wrestling. For duration of wrestling, a planned comparison of drug treatment effects within level of prenatal treatment revealed that ifenprodil infusion increased the duration of wrestling in PAE animals [F(1,9) = 5.903; p = 0.038; ηp2 = 0.396] (Figure 2A), with no significant effects in SAC animals (p > 0.56). For latency to first instance of wrestling, planned comparisons did not detect significant effects of drug treatment in either SAC or PAE animals (ps > .08).
3.3.8 Social behavior in males
In males, there were no significant main effects of prenatal treatment on aspects of social behaviors (all ps > .067). There were, however, two Prenatal Treatment × Drug Treatment interactions in males that require further explication. For duration of wrestling there was a significant Prenatal Treatment × Drug Treatment interaction [F(1,10) = 6.612; p = 0.028; ηp2 = 0.398] (Figure 2A). Post hoc analysis of simple drug effects within prenatal treatment group suggests that this interaction was driven by ifenprodil reducing wrestling duration in PAE animals, but this effect was not significant (p = 0.061). There was also a Prenatal Treatment × Drug Treatment interaction for latency to crossing over/under the partner animal [F(1,10) = 7.216; p = 0.023; ηp2 = 0.419] (Figure 2B). A planned comparison of simple drug treatment effects within prenatal treatment demonstrated that this interaction was due to ifenprodil increasing the latency to crossing over/under in SAC males [F(1,5) = 9.735; p = 0.026; ηp2 = 0.661].
Regardless of prenatal treatment condition, there were a number of significant main effects of drug treatment in males. Ifenprodil treatment reduced the frequency of body sniffing [F(1,10) = 9.080; p = 0.013; ηp2 = 0.476] (Figure 2C). Planned comparisons demonstrate that this effect was significant to PAE animals [F(1,5) = 6.781; p = 0.048; ηp2= 0.576], and not in SAC controls (p > 0.17). Ifenprodil treatment also reduced the frequency [F(1,10) = 31.756; p < .001; ηp2 = 0.761] (Figure 2D) and duration [F(1,10) = 10.075; p = 0.010; ηp2 = 0.502] (Figure 2E) of anogenital sniffing. Planned comparisons show that ifenprodil treatment reduced the frequency of anogenital sniffing in both SAC [F(1,5) = 17.043; p = 0.009; ηp2 = 0.773] and PAE animals [F(1,5) = 15.180; p = 0.011; ηp2 = 0.752], while ifenprodil treatment only reduced the duration of anogenital sniffing in the SAC group [F(1,5) = 11.140; p = 0.021; ηp2 = 0.021] (PAE: p > 0.22). Ifenprodil reduced the frequency of wrestling [F(1,10) = 5.735; p = 0.038; ηp2 = 0.364] (Figure 2F), which planned comparison show was significant in PAE animals [F(1,5) = 15.638; p = 0.011; ηp2 = 0.758] and not in SAC controls (p > 0.89).
3.4 Ultrasonic Vocalizations
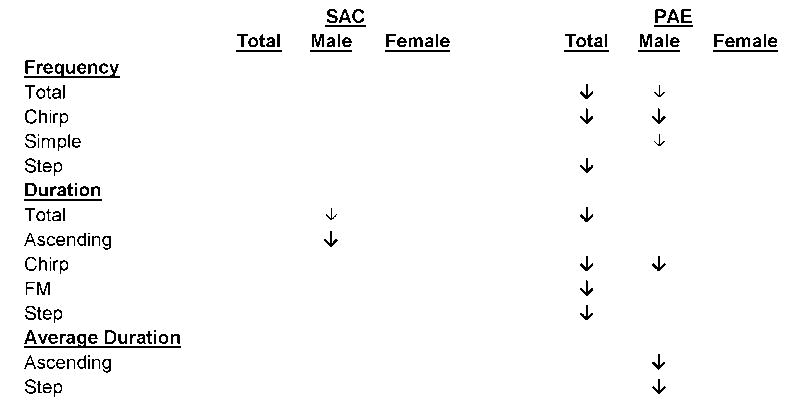
USV data (frequency, total duration) for combinations of prenatal treatment, drug treatment, and sex are detailed below. A summary of the significant effects of ifenprodil treatment on USVs is presented in Table 3. In order to simplify presentation of the effects of ifenprodil treatment on USVs, in this section only effects on total USV measures will be discussed (Table 4). For detailed information concerning the significant effects of prenatal treatment, drug treatment, and sex on the frequency, total duration, average duration, and latency to first onset of USV isotype (ascending, atypical, chirp, FM, simple, and step USVs) please see Supplementary Table 2.
Table 3. Summary of effects of ifenprodil treatment on ultrasonic vocalizations.
Effects of ifenprodil treatment are presented for characteristics of ultrasonic vocalizations within combinations of prenatal treatment and sex, with the arrow indicating the direction of the effect of ifenprodil treatment relative to vehicle treatment. Bold arrow (↓) indicates a significant effect of p < 0.05). Small arrow (↓) indicates a statistical trend of p < 0.06.
Table 4. Significant effects of ifenprodil treatment on total USV frequency and duration.
Mean (SEM) frequency (FREQ), and duration (DUR) for total ultrasonic vocalizations analyzed during the 12-minute social interaction session for saccharin (SAC) and prenatal ethanol-exposed (PAE) rats from each drug treatment condition. For each measure, means collapsed across sex (N = 8 pairs per prenatal treatment condition) are presented first in bold text followed by means for males (n = 3 pairs per prenatal treatment condition) and females (n = 5 pairs per prenatal treatment condition) presented in italicized text. Asterisk (*) indicates a significant (p < 0.05) effect of drug treatment within prenatal treatment conditions. Dagger (†) indicates an effect approaching significance (p < 0.06) of drug treatment within prenatal treatment condition. Subscripts indicate significant (p < 0.05) main effects and interactions for prenatal treatment condition (P), drug treatment condition (D), and sex (S).
| Vehicle | Ifenprodil | Vehicle | Ifenprodil | |
|---|---|---|---|---|
| Total (FREQ)D, S, DS, SP | 474.63(180.69) | 386.63(121.10) | 787.63(294.23) | 368.25(112.62)* |
| MaleD | 747.00(399.18) | 292.00(221.27) | 1,676.00(355.51) | 583.67(137.48)† |
| Female | 311.20(160.64) | 443.40(155.65) | 254.60(122.80) | 239.00(136.76) |
| Total (DUR)D, S, DS, SP | 9.02(3.81) | 7.97(3.02) | 15.46(6.55) | 7.14(2.77)* |
| MaleD | 15.04(8.53) | 8.33(7.42)† | 33.12(11.08) | 10.35(4.93) |
| Female | 5.40(3.18) | 7.74(2.98) | 4.87(2.87) | 5.21(3.43) |
In the vehicle drug treatment group, there were no effects of prenatal treatment on total USV frequency or duration (ps > .09). There were, however, main effects of drug treatment on both the frequency [F(1,12) = 10.260; p = 0.008; ηp2 = 0.461] and duration [F(1,12) = 8.262; p = 0.014; ηp2 = 0.408] of total USVs. Ifenprodil treatment reduced both the frequency and duration of USVs compared to the vehicle infusion group. Within level of prenatal treatment, there were effects of drug treatment on the frequency [F(1,6) = 10.297; p = 0.018; ηp2 = 0.632], and duration [F(1,6) = 7.052; p = 0.038; ηp2 = 0.540] of USVs in PAE animals. These effects were not significant in SAC animals (ps > 0.30). There were also Drug Treatment × Sex interactions for both the frequency [F(1,12) = 13.877; p = 0.003; ηp2 = 0.536] and total duration [F(1,12) = 11.906; p = 0.005; ηp2 = 0.498] of total USVs. For both Drug Treatment × Sex interactions, post hoc analyses of simple drug treatment effects within sex revealed that these interactions were caused by significant drug treatment effects in males (frequency: p = 0.008; duration: p = 0.015). There were also main effects of sex for both the frequency [F(1,12) = 8.839; p = 0.012; ηp2 = 0.424] and duration [F(1,12) = 5.513; p = 0.037; ηp2 = 0.315] of total USVs. Males emitted more USVs for a longer duration than females.
4. Discussion
The aim of the present study was to determine if ifenprodil infusion in rat AIC has significant effects on the expression of social behaviors that are altered by gestational alcohol exposure [35, 40, 52]. Ifenprodil infusion in rat AIC had a significant impact on a number of social behaviors, including agonistic and social investigatory behaviors. Importantly, many of the effects of ifenprodil infusion were specific to PAE rats, including a majority of the effects involving 50 kHz USVs that occasion social interaction. These results support the hypothesis that enhanced GluN2B expression in rat ventrolateral prefrontal cortex following PAE [53] contributes to abnormal social behavior, and adds to the growing body of evidence that exposure to moderate levels of ethanol during early brain development causes persistent deficits in social behaviors and frontal cortex function.
4.1 Social Behavior
Most of the significant effects of ifenprodil infusion on behaviors were obtained for wrestling and social investigatory behaviors. For wrestling behavior, ifenprodil treatment reduced wrestling frequency in male PAE animals to levels comparable to controls, whereas there was no effect of ifenprodil on wrestling frequency in SAC exposed males. This resulted in a significant drug treatment by prenatal treatment interaction. In females, ifenprodil increased the duration of wrestling behavior in PAE animals, with no effect observed in SAC controls. There were also a number of sex effects on the frequency, duration, and time to onset of first instance of wrestling that differed by drug treatment condition. We also observed significant effects of ifenprodil infusion on social investigatory behaviors, in particular on different forms of sniffing of the partner animal. These social investigatory effects were specific to male animals. Ifenprodil infusion significantly reduced the number of instances of body sniffing in PAE males only, and significantly reduced the number of instances of anogenital sniffing in males regardless of prenatal treatment condition.
There is considerable precedent for the sexually dimorphic results obtained from the social behavior analyses conducted in the present study. Previous studies from our laboratory demonstrated that moderate PAE led to more apparent changes in social investigatory and agonistic behaviors, dendritic morphology, structural plasticity, excitatory neurotransmitter receptor physiology, and IEG expression in males [40, 53]. Other groups have also observed sexually dimorphic effects of gestational alcohol exposure on social behaviors [42, 43, 67–75]. Importantly, the majority of drug effects were observed in males, and there were no apparent differences in the variance between sexes for the measures under study. Thus, estrous cycling is not likely to represent a major determinant of drug effects on behavior in the female animals from the present study. A recent meta-analysis revealed that female rats tested without explicit staging of estrous cycles are no more variable than males with regard to a variety of biological traits [76]. Previous research from our laboratory supports this conclusion with respect to the function of GluN2B receptors in AIC. We studied excitatory neurotransmitter receptor physiology in both male and female rats, with female rats uniformly distributed throughout the various stages of estrous and observed that different characteristics of spontaneous and mini excitatory post-synaptic currents in AIC pyramidal neurons were equally variable in male and female animals [53]. Therefore, differences in aspects of neuronal physiology and social behaviors between male and female animals are likely bona fide sexually dimorphic effects, and not likely confounded by estrous.
One potentially complicating aspect affecting the interpretation of the present study concerns the fact that ifenprodil treatment resulted in a number of significant Drug Treatment × Sex interactions on aspects of social behavior. In our previous work examining receptor physiology on layer II/III pyramidal neurons in AIC, we did not observe sex differences on the expression or functioning of GluN2B containing NMDA receptors. This may confuse interpretation of the present study, as one would expect there to be no significant Drug Treatment × Sex effects if there are not differences in GluN2B expression between the sexes. These effects can be partially explained, however, by sex differences on AMPA receptor physiology that we have previously observed. In Bird et al. [53], we found that AMPAR- mediated EPSCs from male animals displayed larger amplitudes, shorter half-widths, and faster decay times than EPSCs from female animals. Because NMDA receptor activity is dependent upon initial activation of AMPA receptors to alleviate the Mg2+ block of NMDA receptors [77], it can be reasoned that differences in basic excitatory synaptic transmission between the sexes could be behind some of the sex differences observed here. Further research will be necessary to determine how AMPA receptor physiology could affect NMDA receptor activity in the AIC during social interactions.
In previous studies, we have demonstrated that gestational alcohol exposure leads to a significant increase in wrestling behavior in male rats [35, 39, 40]. Here we did not observe main effects of prenatal treatment on this aspect of social behavior. The absence of PAE treatment effects could be attributed to unintended effects caused by cannula implantation into AIC. Care was taken to prevent excess damage to AIC when performing stereotaxic surgeries, using the smallest gauge guide cannula possible and conducting experiments 1–2 weeks after implantation to prevent excess gliosis following surgery [78]. Nonetheless, unintended partial damage to AIC caused by cannula implantation may have contributed to altered social behaviors that masked some of the effects of gestational alcohol exposure. In addition, PAE rats may have altered recovery periods following surgery compared to SAC controls, which has the potential to cause functional deficits in implanted brain areas [79]. Future experiments examining social behaviors in cannulated rats will need to examine inflammatory cytokine expression in implanted brain areas to determine if PAE rats have an exaggerated immune response that could confound interpretation of experimental results. It is also possible that the presence of the implants themselves significantly altered social behavior- i.e., interacting with another rat that has a large implant on top of its head could result in atypical social interactions. There were, however, several significant prenatal treatment by drug treatment interactions for social behaviors and USVs resulting from selective alteration in PAE rats, many of which were obtained for social behaviors (wrestling, anogenital sniffing, body sniffing, and allogrooming) affected by prenatal alcohol treatment in our previous studies [35, 40]. In this study, PAE also resulted in reduced latency to the first onset of allogrooming in PAE animals. In females, there was an additional effect of PAE treatment, as it reduced the duration of social investigation via anogenital sniffing behavior.
4.2 Ultrasonic vocalizations
Rats emit USVs in one of two frequency ranges, typically associated with aversive (roughly 22 kHz) and socially appetitive (roughly 50 kHz) situations [80]. 22 kHz vocalizations occur in a variety of stressful situations, including social isolation [81], exposure to predators [82], and withdrawal from drugs [83]. Conversely, 50 kHz USVs tend to occur in more rewarding situations, such as mating, juvenile play, drug administration, and “tickling” [80, 84, 85]. We measured characteristics of USVs during social interaction to gain insight into affective states. Previous work from our laboratory has observed an increase in agonistic wrestling behavior in PAE rats as compared to controls [35]. This wrestling behavior was qualified as agonistic rather than play behavior based on the posterior target of wrestling attacks. Attacks directed at the nape of the neck are indicative of playful attacks, while attacks directed at the rump signify agonistic behavior [86, 87]. In our previous study, attacks at the nape of the neck were rare, while attacks directed at the rump were more frequent. We reasoned that these agonistic behaviors would be accompanied by 22 kHz USVs, indicating a negative affective state. Contrary to our expectations, we only observed 22 kHz USVs being emitted during a single social interaction session for one pair of animals. As this was the only instance in which 22 kHz USVs were observed we did not analyze USVs in this frequency range. On the other hand, 50 kHz USVs were emitted frequently during the social interaction tests. Systemic ifenprodil treatment (10 mg/kg) in rats has been shown to reduce 50 kHz USVs [88], however, to our knowledge the present study is the first demonstration that GluN2B receptors in AIC are implicated in modulating 50 kHz USVs. Due to the fact that most of the vocalizations were in the 50 kHz “socially appetitive” range, it may be construed that corresponding behaviors are not agonistic. This interpretation is complicated by the fact that 50 kHz calls do not selectively occur in social conditions that are appetitive. Rats frequently emit 50 kHz calls during resident-intruder tests [89], and during other aversive events [90]. Thus, a conservative conclusion is that calls within this frequency range are sensitive to social situations and the AIC contributes to this function.
A major goal for future research is to conduct a more detailed analysis linking USVs to behavioral processes. Due to the limited sample size of the present study, and not being able to attribute a specific USV to a particular animal, it is difficult to attribute specific USV waveforms to specific social behaviors using correlational statistics. It may be possible in the future to use a different USV recording setup and analysis approaches (e.g., multiple sensors and independent component analysis) to attribute each individual USV to a specific animal during social interaction sessions. Another alternative that would allow us to attribute USVs to a specific animal would involve devocalizing one rat in each pair [91]. Some additional limitations of the current approach are also worthy of discussion. The effects of cannula implantation could have masked some other effects of prenatal ethanol treatment on USVs in the older animals used in this study. Future studies from our lab will examine USVs in a social context from animals that have not been implanted with infusion guide cannula to determine if this is indeed the case. Another potential limitation from the analysis of USV characteristics is the limited sample size from male animals. However, even with the limited sample size, there were detectable reductions in the frequency and duration of many USV types following ifenprodil infusion in PAE males.
4.3 Future directions
The present results support the conclusion that PAE-induced alterations in GluN2B receptor expression in AIC contribute to social behaviors, and manipulations of GluN2B-containing NMDA receptor function in this brain region may normalize affected behaviors in these animals. Based on this conclusion, systemic ifenprodil treatment could be a useful tool for normalizing some of the social behavior deficits seen in rats following moderate PAE. Low dose (0.75 mg/kg) systemic ifenprodil treatment increases social activity (play, social investigation, and social contact) in naïve adolescent (P35) male rats [92], while higher doses (6–12 mg/kg) cause a decrease in social activity in adulthood (P68) [93]. The differences in the effect of ifenprodil at different doses and ages could be partially explained by the normal time course of NMDA subunit expression in rats and other mammals. Normally during early post-natal development, GluN2B subunits are preferentially expressed over GluN2A subunits, and GluN2A subunit expression becomes more dominant as the animal ages [94]. However, GluN2B expression in AIC of PAE animals remains high well into adulthood [53], suggesting that systemic low-dose administration of ifenprodil could normalize some of the social behavior deficits that PAE rats exhibit. Potential translation to functional treatments for humans is complicated by the fact that systemic ifenprodil treatment inhibits the function of GluN2B-containing NMDA receptors in other brain areas, affecting excitatory neurotransmission in these regions. For example, systemic ifenprodil treatment of 5 mg/kg impairs the acquisition of fear memory [95], and treatment of 10 mg/kg disrupts consolidation of spatial memory [96]. Both forms of memory are negatively affected by PAE [35, 57], leading to the possibility that systemic ifenprodil treatment could further compound memory deficits in PAE animals. Further, conditional loss of GluN2B receptors in the hippocampus and cortex of mice cause deficits in attentional set-shifting [97], which could also result from global GluN2B antagonism and exacerbate the effects of PAE, which has been shown to lower expression of GluN2B receptors in the hippocampus of mice [98]. Thus, systemic ifenprodil treatment must be optimized at a dose level that is sufficient to alleviate social behavior deficits, but not so high as to cause impairments in other brain functions.
Another avenue for future research motivated by the present study will be to examine the effects of a lower dose of ifenprodil infused into AIC on social behavior and communication. The dose of ifenprodil used in this study (1 μg in 0.3 μl per hemisphere) is comparable to doses of ifenprodil used by other groups to investigate how antagonizing GluN2B-containing NMDA receptors affects neuronal physiology and behaviors mediated by certain brain regions [60, 99, 100]. This dose of ifenprodil has the potential to cause off-target effects, however. In Xenopus oocytes expressing recombinant rat NMDA receptors, researchers have measured the IC50 for ifenprodil on GluN2B containing NMDA receptors to be 0.34 μM, while at GluN2A containing NMDA receptors the IC50 is 146 μM [101]. The concentration of ifenprodil used in the present study (4.16 mM) was higher than the IC50 for GluN2A containing NMDA receptors. There are mitigating factors to consider, such as the fact that the IC50s for ifenprodil were measured in Xenopus oocytes in vitro, or how far ifenprodil spread from the point of infusion thereby diluting it [102], as well as metabolic breakdown of ifenprodil in vivo after infusion took place [103] which would effectively lower the concentration of ifenprodil at neuronal synapses in AIC. While we met our goal of evaluating the differential effects of ifenprodil on behaviors and communication between control and PAE animals, the only way to be completely sure that the effects of ifenprodil were caused by inhibiting GluN2B-containing NMDA receptors will be to infuse a dose that is lower than the IC50 for GluN2A-containing NMDA receptors.
Finally, an intriguing direction for future research involves examining how PAE affects the expression and function of GABAergic interneurons in the AIC and how this could impact social behaviors and USVs. It has been demonstrated that developmental alcohol exposure increases tangential migration of GABAergic interneurons to the cortex, and that this results in increased numbers of interneurons that are observed into adulthood [104, 105]. It is possible that some of the significant results seen in this study are not the result of inhibiting GluN2B-containing NMDA receptors on layer II/III pyramidal neurons, but rather a result of inhibiting these receptors on cortical interneurons. Future work will need to determine how PAE affects GluN2B expression on interneurons and how this impacts interneuron physiology, AIC network activity, and behaviors modulated by AIC.
4.4 Conclusion
In summary, the combined data from this study demonstrate that GluN2B-containing NMDA receptors in the AIC of rats are important for regulating aspects of social behaviors and vocalization during social interaction in rats exposed to ethanol during gestation. Future work will determine if these neurotransmitter receptors will be viable targets for treatments seeking to rescue the deleterious effects of prenatal alcohol exposure on AIC-dependent social behaviors.
Supplementary Material
Highlights.
Ifenprodil infusion into agranular insular cortex alters social behaviors in rats
Many effects of ifenprodil infusion on behaviors are specific to PAE animals
Ifenprodil alters social communication, with many effects specific to PAE rats
Acknowledgments
The authors would like to thank Kara Shain for helping to section brain tissue used for H&E staining, and the team at Audacity for allowing us to use their software for USV analysis (Audacity® software is copyright © 1999–2016 Audacity Team. The name Audacity® is a registered trademark of Dominic Mazzoni.). Funding was provided by National Institutes of Health grants R01 AA019462 to DAH and R01 AA019884 to DDS.
Abbreviations
- PAE
Prenatal alcohol exposure
- AIC
Agranular insular cortex
- USV
Ultrasonic vocalization
- FASDs
Fetal alcohol spectrum disorders
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Autti-Rämö I, Granström ML. The psychomotor development during the first year of life of infants exposed to intrauterine alcohol of various duration. Fetal alcohol exposure and development. Neuropediatrics. 1991;22:59–64. doi: 10.1055/s-2008-1071418. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 3.Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Mattson SN, Riley EP. Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. J Int Neuropsychol Soc. 1999;5:462–71. doi: 10.1017/s1355617799555082. [DOI] [PubMed] [Google Scholar]
- 5.Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, et al. Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2013;37:517–28. doi: 10.1111/j.1530-0277.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen TT, Ashrafi A, Thomas JD, Riley EP, Simmons RW. Children with heavy prenatal alcohol exposure have different frequency domain signal characteristics when producing isometric force. Neurotoxicol Teratol. 2013;35:14–20. doi: 10.1016/j.ntt.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roebuck-Spencer TM, Mattson SN, Marion SD, Brown WS, Riley EP. Bimanual coordination in alcohol-exposed children: role of the corpus callosum. J Int Neuropsychol Soc. 2004;10:536–48. doi: 10.1017/S1355617704104116. [DOI] [PubMed] [Google Scholar]
- 9.Simmons RW, Thomas JD, Levy SS, Riley EP. Motor response programming and movement time in children with heavy prenatal alcohol exposure. Alcohol. 2010;44:371–8. doi: 10.1016/j.alcohol.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams L, Jackson CP, Choe N, Pelland L, Scott SH, Reynolds JN. Sensory-motor deficits in children with fetal alcohol spectrum disorder assessed using a robotic virtual reality platform. Alcohol Clin Exp Res. 2014;38:116–25. doi: 10.1111/acer.12225. [DOI] [PubMed] [Google Scholar]
- 11.Chasnoff IJ, Wells AM, Telford E, Schmidt C, Messer G. Neurodevelopmental functioning in children with FAS, pFAS, and ARND. J Dev Behav Pediatr. 2010;31:192–201. doi: 10.1097/DBP.0b013e3181d5a4e2. [DOI] [PubMed] [Google Scholar]
- 12.May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–66. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcohol Clin Exp Res. 1990;14:650–5. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 14.Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA. 1991;265:1961–7. [PubMed] [Google Scholar]
- 15.Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14:662–9. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 16.Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends Neurosci. 2012;35:284–92. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miguez HA, Magri R, Suarez M. Consumo de tabaco y bebidas alcohólicas durante el embarazo. Acta Psiquiátrica y Psicológica de América Latina. 2009;55:76–83. [Google Scholar]
- 18.(CDC) CfDCaP. Alcohol use among women of childbearing age–United States, 1991–1999. MMWR Morb Mortal Wkly Rep. 2002;51:273–6. [PubMed] [Google Scholar]
- 19.Day NL, Cottreau CM, Richardson GA. The epidemiology of alcohol, marijuana, and cocaine use among women of childbearing age and pregnant women. Clin Obstet Gynecol. 1993;36:232–45. doi: 10.1097/00003081-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Peadon E, Payne J, Henley N, D’Antoine H, Bartu A, O’Leary C, et al. Attitudes and behaviour predict women’s intention to drink alcohol during pregnancy: the challenge for health professionals. BMC Public Health. 2011;11:584. doi: 10.1186/1471-2458-11-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker MJ, Al-Sahab B, Islam F, Tamim H. The epidemiology of alcohol utilization during pregnancy: an analysis of the Canadian Maternity Experiences Survey (MES) BMC Pregnancy Childbirth. 2011;11:52. doi: 10.1186/1471-2393-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(CDC) CfDCaP. Alcohol use and binge drinking among women of childbearing age–United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2012;61:534–8. [PubMed] [Google Scholar]
- 23.(CDC) CfDCaP. Alcohol use among pregnant and nonpregnant women of childbearing age - United States, 1991–2005. MMWR Morb Mortal Wkly Rep. 2009;58:529–32. [PubMed] [Google Scholar]
- 24.Floyd RL, Decouflé P, Hungerford DW. Alcohol use prior to pregnancy recognition. Am J Prev Med. 1999;17:101–7. doi: 10.1016/s0749-3797(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 25.Grant TM, Huggins JE, Sampson PD, Ernst CC, Barr HM, Streissguth AP. Alcohol use before and during pregnancy in western Washington, 1989–2004: implications for the prevention of fetal alcohol spectrum disorders. Am J Obstet Gynecol. 2009;200:278.e1–8. doi: 10.1016/j.ajog.2008.09.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.May PA, Fiorentino D, Coriale G, Kalberg WO, Hoyme HE, Aragón AS, et al. Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: new estimated rates are higher than previous estimates. Int J Environ Res Public Health. 2011;8:2331–51. doi: 10.3390/ijerph8062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uecker A, Nadel L. Spatial locations gone awry: object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia. 1996;34:209–23. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- 28.Uecker A, Nadel L. Spatial but not object memory impairments in children with fetal alcohol syndrome. Am J Ment Retard. 1998;103:12–8. doi: 10.1352/0895-8017(1998)103<0012:SBNOMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol. 1999;21:109–18. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 30.Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–9. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcoholism NIoAAa. 10th special report to the US congress on alcohol and health. Washington, DC: National Institutes of Health; 2000. [Google Scholar]
- 32.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 33.Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. 1998;22:528–33. [PubMed] [Google Scholar]
- 34.Royalty J. Effects of prenatal ethanol exposure on juvenile play-fighting and postpubertal aggression in rats. Psychol Rep. 1990;66:551–60. doi: 10.2466/pr0.1990.66.2.551. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, et al. Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav Brain Res. 2014;269:44–54. doi: 10.1016/j.bbr.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer LS, Riley EP. Social play in juvenile rats prenatally exposed to alcohol. Teratology. 1986;34:1–7. doi: 10.1002/tera.1420340102. [DOI] [PubMed] [Google Scholar]
- 37.Middleton FA, Varlinskaya EI, Mooney SM. Molecular substrates of social avoidance seen following prenatal ethanol exposure and its reversal by social enrichment. Dev Neurosci. 2012;34:115–28. doi: 10.1159/000337858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz MR, Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol on gestational day 12 elicits opposing deficits in social behaviors and anxiety-like behaviors in Sprague Dawley rats. Behav Brain Res. 2016;310:11–9. doi: 10.1016/j.bbr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez CI, Magcalas CM, Barto D, Fink BC, Rice JP, Bird CW, et al. Effects of sex and housing on social, spatial, and motor behavior in adult rats exposed to moderate levels of alcohol during prenatal development. Behavioural Brain Research. 2016 doi: 10.1016/j.bbr.2016.07.018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, et al. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tunc-Ozcan E, Ullmann TM, Shukla PK, Redei EE. Low-dose thyroxine attenuates autism-associated adverse effects of fetal alcohol in male offspring’s social behavior and hippocampal gene expression. Alcohol Clin Exp Res. 2013;37:1986–95. doi: 10.1111/acer.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varlinskaya EI, Mooney SM. Acute exposure to ethanol on gestational day 15 affects social motivation of female offspring. Behav Brain Res. 2014;261:106–9. doi: 10.1016/j.bbr.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly SJ, Dillingham RR. Sexually dimorphic effects of perinatal alcohol exposure on social interactions and amygdala DNA and DOPAC concentrations. Neurotoxicol Teratol. 1994;16:377–84. doi: 10.1016/0892-0362(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 44.Lugo JN, Marino MD, Cronise K, Kelly SJ. Effects of alcohol exposure during development on social behavior in rats. Physiol Behav. 2003;78:185–94. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- 45.Lugo JN, Marino MD, Gass JT, Wilson MA, Kelly SJ. Ethanol exposure during development reduces resident aggression and testosterone in rats. Physiol Behav. 2006;87:330–7. doi: 10.1016/j.physbeh.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly SJ, Tran TD. Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicol Teratol. 1997;19:383–9. doi: 10.1016/s0892-0362(97)00064-0. [DOI] [PubMed] [Google Scholar]
- 47.Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 48.Berthoz S, Armony JL, Blair RJ, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- 49.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 50.Butter CM, Snyder DR. Alterations in aversive and aggressive behaviors following orbital frontal lesions in rhesus monkeys. Acta Neurobiol Exp (Wars) 1972;32:525–65. [PubMed] [Google Scholar]
- 51.Kolb B. Social behavior of rats with chronic prefrontal lesions. J Comp Physiol Psychol. 1974;87:466–74. doi: 10.1037/h0036969. [DOI] [PubMed] [Google Scholar]
- 52.Hamilton DA, Candelaria-Cook FT, Akers KG, Rice JP, Maes LI, Rosenberg M, et al. Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behav Brain Res. 2010;214:66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bird CW, Candelaria-Cook FT, Magcalas CM, Davies S, Valenzuela CF, Savage DD, et al. Moderate prenatal alcohol exposure enhances GluN2B containing NMDA receptor binding and ifenprodil sensitivity in rat agranular insular cortex. PLoS One. 2015;10:e0118721. doi: 10.1371/journal.pone.0118721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobs SA, Tsien JZ. genetic overexpression of NR2B subunit enhances social recognition memory for different strains and species. PLoS One. 2012;7:e36387. doi: 10.1371/journal.pone.0036387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobs SA, Tsien JZ. Overexpression of the NR2A subunit in the forebrain impairs long-term social recognition and non-social olfactory memory. Genes Brain Behav. 2014;13:376–84. doi: 10.1111/gbb.12123. [DOI] [PubMed] [Google Scholar]
- 56.Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E394–403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, Staples MC, et al. Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcohol Clin Exp Res. 2010;34:1793–802. doi: 10.1111/j.1530-0277.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamilton DA, Magcalas CM, Barto D, Bird CW, Rodriguez CI, Fink BC, et al. Moderate prenatal alcohol exposure and quantification of social behavior in adult rats. J Vis Exp. 2014 doi: 10.3791/52407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lundquist F, Fugmann U, Klaning E, Rasmussen H. The metabolism of acetaldehyde in mammalian tissues: reactions in rat-liver suspensions under anaerobic conditions. Biochem J. 1959;72:409–19. doi: 10.1042/bj0720409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parkes SL, Westbrook RF. The basolateral amygdala is critical for the acquisition and extinction of associations between a neutral stimulus and a learned danger signal but not between two neutral stimuli. J Neurosci. 2010;30:12608–18. doi: 10.1523/JNEUROSCI.2949-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnett SA. A study in behaviour: principles of ethology and behavioural physiology displayed mainly in the rat. London, UK: Camelot Press; 1963. [Google Scholar]
- 62.Barto D, Bird CW, Hamilton DA, Fink BC. The Simple Video Coder: A free tool for efficiently coding social video data. Behav Res Methods. 2016 doi: 10.3758/s13428-016-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright JM, Gourdon JC, Clarke PB. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology (Berl) 2010;211:1–13. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- 64.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic; 1988. [Google Scholar]
- 65.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maxwell SE, Delaney HD. Designing experiments and analyzing data: a model comparison perspective. 2nd. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. [Google Scholar]
- 67.Weinberg J. Prenatal ethanol effects: sex differences in offspring stress responsiveness. Alcohol. 1992;9:219–23. doi: 10.1016/0741-8329(92)90057-h. [DOI] [PubMed] [Google Scholar]
- 68.Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: effects of age, sex, and timing of exposure. Behav Brain Res. 2011;216:358–64. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64:265–75. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- 70.Hannigan JH, Pilati ML. The effects of chronic postweaning amphetamine on rats exposed to alcohol in utero: weight gain and behavior. Neurotoxicol Teratol. 1991;13:649–56. doi: 10.1016/0892-0362(91)90049-3. [DOI] [PubMed] [Google Scholar]
- 71.McGivern RF, Ervin MG, McGeary J, Somes C, Handa RJ. Prenatal ethanol exposure induces a sexually dimorphic effect on daily water consumption in prepubertal and adult rats. Alcohol Clin Exp Res. 1998;22:868–75. [PubMed] [Google Scholar]
- 72.Osborn JA, Kim CK, Steiger J, Weinberg J. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcohol Clin Exp Res. 1998;22:685–96. [PubMed] [Google Scholar]
- 73.Zimmerberg B, Mickus LA. Sex differences in corpus callosum: influence of prenatal alcohol exposure and maternal undernutrition. Brain Res. 1990;537:115–22. doi: 10.1016/0006-8993(90)90347-e. [DOI] [PubMed] [Google Scholar]
- 74.Patten AR, Sickmann HM, Dyer RA, Innis SM, Christie BR. Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure. Neurosci Lett. 2013;551:7–11. doi: 10.1016/j.neulet.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 75.Sickmann HM, Patten AR, Morch K, Sawchuk S, Zhang C, Parton R, et al. Prenatal ethanol exposure has sex-specific effects on hippocampal long-term potentiation. Hippocampus. 2014;24:54–64. doi: 10.1002/hipo.22203. [DOI] [PubMed] [Google Scholar]
- 76.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Nikkhah G, Olsson M, Eberhard J, Bentlage C, Cunningham MG, Björklund A. A microtransplantation approach for cell suspension grafting in the rat Parkinson model: a detailed account of the methodology. Neuroscience. 1994;63:57–72. doi: 10.1016/0306-4522(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 79.Terasaki LS, Schwarz JM. Effects of Moderate Prenatal Alcohol Exposure during Early Gestation in Rats on Inflammation across the Maternal-Fetal-Immune Interface and Later-Life Immune Function in the Offspring. J Neuroimmune Pharmacol. 2016 doi: 10.1007/s11481-016-9691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122:357–67. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- 81.Francis RL. 22-kHz calls by isolated rats. Nature. 1977;265:236–8. doi: 10.1038/265236a0. [DOI] [PubMed] [Google Scholar]
- 82.Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50:967–72. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- 83.Barros HM, Miczek KA. Withdrawal from oral cocaine in rate: ultrasonic vocalizations and tactile startle. Psychopharmacology (Berl) 1996;125:379–84. doi: 10.1007/BF02246021. [DOI] [PubMed] [Google Scholar]
- 84.Mällo T, Matrov D, Herm L, Kõiv K, Eller M, Rinken A, et al. Tickling-induced 50-kHz ultrasonic vocalization is individually stable and predicts behaviour in tests of anxiety and depression in rats. Behav Brain Res. 2007;184:57–71. doi: 10.1016/j.bbr.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 85.Waddell J, Yang T, Ho E, Wellmann KA, Mooney SM. Prenatal Ethanol Exposure and Whisker Clipping Disrupt Ultrasonic Vocalizations and Play Behavior in Adolescent Rats. Brain Sci. 2016;6 doi: 10.3390/brainsci6040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blanchard RJ, Blanchard DC, Takahashi T, Kelley MJ. Attack and defensive behaviour in the albino rat. Anim Behav. 1977;25:622–34. doi: 10.1016/0003-3472(77)90113-0. [DOI] [PubMed] [Google Scholar]
- 87.SM P, VC P. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggressive Behavior. 1987;13:227–42. [Google Scholar]
- 88.Burgdorf J, Kroes RA, Weiss C, Oh MM, Disterhoft JF, Brudzynski SM, et al. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience. 2011;192:515–23. doi: 10.1016/j.neuroscience.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vivian JA, Miczek KA. Morphine attenuates ultrasonic vocalization during agonistic encounters in adult male rats. Psychopharmacology (Berl) 1993;111:367–75. doi: 10.1007/BF02244954. [DOI] [PubMed] [Google Scholar]
- 90.Vivian JA, Miczek KA. Ultrasounds during morphine withdrawal in rats. Psychopharmacology (Berl) 1991;104:187–93. doi: 10.1007/BF02244177. [DOI] [PubMed] [Google Scholar]
- 91.Kisko TM, Himmler BT, Himmler SM, Euston DR, Pellis SM. Are 50-kHz calls used as play signals in the playful interactions of rats? II. Evidence from the effects of devocalization. Behav Processes. 2015;111:25–33. doi: 10.1016/j.beproc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 92.Morales M, Varlinskaya EI, Spear LP. Low doses of the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, induces social facilitation in adolescent male rats. Behav Brain Res. 2013;250:18–22. doi: 10.1016/j.bbr.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morales M, Spear LP. The effects of an acute challenge with the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, on social inhibition in adolescent and adult male rats. Psychopharmacology (Berl) 2014;231:1797–807. doi: 10.1007/s00213-013-3278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shipton OA, Paulsen O. GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130163. doi: 10.1098/rstb.2013.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–40. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- 96.Ma YY, Yu P, Guo CY, Cui CL. Effects of ifenprodil on morphine-induced conditioned place preference and spatial learning and memory in rats. Neurochem Res. 2011;36:383–91. doi: 10.1007/s11064-010-0342-9. [DOI] [PubMed] [Google Scholar]
- 97.Thompson SM, Josey M, Holmes A, Brigman JL. Conditional loss of GluN2B in cortex and hippocampus impairs attentional set formation. Behav Neurosci. 2015;129:105–12. doi: 10.1037/bne0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brady ML, Diaz MR, Iuso A, Everett JC, Valenzuela CF, Caldwell KK. Moderate prenatal alcohol exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrus. J Neurosci. 2013;33:1062–7. doi: 10.1523/JNEUROSCI.1217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sachser RM, Santana F, Crestani AP, Lunardi P, Pedraza LK, Quillfeldt JA, et al. Forgetting of long-term memory requires activation of NMDA receptors, L-type voltage-dependent Ca(2+) channels, and calcineurin. Sci Rep. 2016;6:22771. doi: 10.1038/srep22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang CM, Yang YJ, Zhang JT, Liu J, Guan XL, Li MX, et al. Regulation of emotional memory by hydrogen sulfide: role of GluN2B-containing NMDA receptor in the amygdala. J Neurochem. 2015;132:124–34. doi: 10.1111/jnc.12961. [DOI] [PubMed] [Google Scholar]
- 101.Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–9. [PubMed] [Google Scholar]
- 102.Routtenberg A. Intracranial chemical injection and behavior: a critical review. Behav Biol. 1972;7:601–41. doi: 10.1016/s0091-6773(72)80073-7. [DOI] [PubMed] [Google Scholar]
- 103.Falck E, Begrow F, Verspohl E, Wünsch B. Metabolism studies of ifenprodil, a potent GluN2B receptor antagonist. J Pharm Biomed Anal. 2014;88:96–105. doi: 10.1016/j.jpba.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 104.Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH. Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci. 2008;28:1854–64. doi: 10.1523/JNEUROSCI.5110-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skorput AG, Gupta VP, Yeh PW, Yeh HH. Persistent Interneuronopathy in the Prefrontal Cortex of Young Adult Offspring Exposed to Ethanol In Utero. J Neurosci. 2015;35:10977–88. doi: 10.1523/JNEUROSCI.1462-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. 5th. Elsevier Academic Press; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.