Abstract
Purpose
To study and compare the function and structure of the urethral sphincter in female Zucker lean and Zucker fatty (ZF) rats and to assess viability of ZF fats as a model for female obesity-associated stress urinary incontinence (OA-SUI).
Materials and Methods
Twelve16-week-old female Zucker Lean (ZUC-Leprfa 186) (ZL) rats and twelve16-week-old female Zucker Fatty (ZUC-Leprfa 185) (ZF) rats were grouped into two groups: ZL arm and ZF arm. Intraperitoneal insulin tolerance testing was carried out before functional study. Metabolic cages, conscious cystometry, and leak point pressure (LPP) were conducted. Urethral tissues were harvested for immunofluorescence staining to check intramyocellular lipid (IMCL) and sphincter muscle (smooth muscle and striated muscle) composition.
Results
The ZF rats demonstrated insulin resistance, increased voiding frequency, and decreased LPP compared to ZL rats (p<0.05) with more IMCL deposition localized in urethral striated muscle fibers of the ZF rats (p<0.05). The thickness of the striated muscle layer and the ratio of striated muscle to smooth muscle were lower in ZF rats compared to ZL rats.
Conclusion
Obesity impairs urethral sphincter function by IMCL deposition and leads to atrophy and distortion of urethral striated muscle. The ZF rats could be a consistent and reliable animal model to study obesity-associated stress urinary incontinence (OA-SUI).
Keywords: Stress Urinary Incontinence, Obesity, Intramyocellular Lipid (IMCL), Zucker Fatty (ZF) rats, Urethral Sphincter
INTRODUCTION
Obesity affects 78 million Americans and is becoming increasingly more prevalent [1].The annual cost of $147 billion for treating obesity-related illnesses is predicted to rise to $1.8 trillion by 2018. Obese women particularly risk developing stress urinary incontinence (SUI) [2]. In one study, a five point rise in body mass index (BMI) was correlated with a 20%–70% increase in incontinence [3]. The mechanism underlying this obesity-associated SUI (OA-SUI) is not well understood. Current treatments for the condition include weight loss, pelvic floor muscle training, injection of bulking agents, and sling surgeries. However, these approaches are oftentimes either ineffective or may be associated with significant post-treatment complications. Therefore, it is vital that we determine the mechanisms underlying OA-SUI so that we can develop an effective non-invasive or minimally-invasive treatment for this pervasive condition.
Although obesity has been an established risk factor for causing female SUI or contributing to the severity of the condition, little is known about the mechanism for this association. In 2010, Gasbarro et al [4] studied the obesity-associated urodynamic characteristics of Zucker Diabetic Fatty ZDF (ZDF) rats, which develop progressive insulin resistance and glucose intolerance between 3 and 8 weeks of age and become overtly diabetic between 8 and 10 weeks of age [5]. However, this is not an ideal animal model for OA-SUI due to the condition of diabetes, which itself may cause bladder dysfunction and incontinence. On the other hand, the Zucker Fatty (ZUC-FA/FA) (ZUC-Leprfa 185) (ZF) rat is considered as a model for pre-diabetes, as characterized by a genetic defect in the leptin receptor, which results in hyperphagia, insulin resistance, hyperinsulinemia, hyperlipoproteinemia, and obesity. These animals become glucose intolerant but do not develop type 2 diabetes[6]. Currently, the ZF rats are ideal for the study of genetic obesity, insulin resistance, glucose intolerance, and metabolic syndrome[7], and thus they are conceivably a good animal model for the study of OA-SUI.
Striated muscles often undergo atrophy in association with obesity [8]. The urethral rhabdosphincter is the key component involved in urinary continence during straining. However, virtually no studies have examined the relationships between obesity and urethral striated muscle dysfunction, with the exception of two review articles that suggest such a link may exist in female dogs [9, 10].
One explanation for OA-SUI is that obesity causes increased intra-abdominal pressure, which weakens pelvic floor innervation and musculature [11], and indeed, obesity is associated with incontinence of the urethral sphincter in experimental dogs [9, 10]. However, there are no published studies that directly address the pathological changes of the striated muscle of this sphincter. This is surprising as it is well documented that obesity produces atrophy, weakens contractility, and inhibits regeneration of skeletal muscle[12]. It is also known that obesity causes lipid deposition in striated muscle via increased uptake of circulating fatty acids. Lipids within striated muscle are comprised of two pools: extramyocellular lipids (EMCL) in adipose cells between myofibers and intramyocellular lipids (IMCL) within muscle cells [13]. Both types of lipid can weaken muscle contractility; accordingly, their accumulation in urethral striated muscle may result in sphincter dysfunction. In the other hand, IMCL could also lead to the suppression of biogenesis, inhibition of mitochondrial function, and alteration in the fiber type composition of obese rat[14].Thus, to validate the hypothesis that urethral dysfunction is a contributor to OA-SUI, the current study aims to elucidate how obesity affects the structure and function of the female urethral sphincter in a genetic obesity rat model.
MATERIALS AND METHODS
Animals and Overview
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at our institution. Twelve 16-week-old female Zucker Lean (ZUC-LEAN) (ZUC-Leprfa 186) (ZL) rats and twelve 16-week-old Zucker Fatty (ZUC-FA/FA) (ZUC-Leprfa 185) (ZF) rats were obtained from Charles River Laboratories (Wilmington, MA, USA). After a 3-day acclimation period, the rats were evaluated for insulin tolerance, micturition patterns in metabolic cages, and conscious cystometry, as well as leak point pressure (LPP).
Intraperitoneal Insulin Tolerance Test
Rats were fasted for 12 h with constant access to drinking water. The basal glucose levels were determined in each rat first. Next, 0.5 IU/kg of fast-acting insulin (Sigma, USA) was injected intraperitoneally. Blood was taken by tail puncture at 30min, 60min, 90min, and 120min after insulin injection and was measured by test strips (Accu-Check Active, Roche Diagnostics).
Metabolic Cage
All rats undergoing 24-hour voiding behavior studies were placed in a metabolic cage for 24 hours on a 12:12-hour dark/light photo cycle. Each monitoring period started at 4 p.m. Number of voids and volumes were recorded by a fluid collector connected to a pressure transducer (Utah Medical Products, Midvale, Utah). The pressure transducer was connected to a Pentium® 4 computer (Dell, Round Rock, Texas) with LaboratoryView 6.0 software (National Instruments, Austin, Texas) to monitor the cumulative weight of collected urine. All rats had free access to water and food while in the cage. Voiding frequency and volume were recorded for 24 hours.
Conscious Cystometry
Under isoflurane anesthesia, a transvesical catheter was inserted 24 hr prior to urodynamic testing. The tip of a polyethylene-90 (PE-90) tube (Clay-Adams, Parsippany, NJ) was heated to create a collar, and the catheter tip was implanted at the bladder dome. A second probe was placed in the intra-abdominal space to measure the abdominal component of the vesical pressure. A piece of PE-90 tubing was fitted with a small balloon and tied in place with a suture. The tubing was passed through the abdominal wall musculature and tunneled subcutaneously to emerge at the dorsum of the neck. For conscious cystometry, the animal was restrained in a tunnel attached to a metabolic cage grid (Braintree Scientific, Braintree, MA). The bladder was filled at a rate of 0.1 ml/min using an infusion pump (KD Scientific, Holliston, MA). Intravesical pressure changes were recorded by a computer with LabView 6.0 software (National Instruments, Austin, TX) at a rate of 10 samples/sec. The voided urine was recorded by an electronic scale connected to the LabView software. After stabilization of the micturition cycle for 10 min, the bladder was emptied by aspiration, and micturition cycles were recorded for 40 min. Multiple cystometric variables were recorded by obtaining mean values of 4 voiding cycles. Baseline pressure (BP) was defined as the lowest bladder pressure between voids. Voiding threshold pressure was the pressure where urine was first detected at the urethral meatus. The voiding function of each rat was classified as “abnormal” if bladder filling was accompanied by frequent, low volume bladder contractions with urethral leakage.
Leak Point Pressure (LPP)
The anesthetized animal was placed supine at the level of zero pressure while the bladder was filled with room temperature saline through a bladder catheter connected to a pressure transducer. LPP was tested at the half-bladder capacity. LPP was tested by manually increasing bladder pressure until a leak occured, at which time the external vesicle pressure was rapidly removed. Peak bladder pressure was recorded as LPP. Upon completion of LPP, animals were euthanized, and their urethras were harvested for histology.
Immunofluorescence and Intramyocellular Lipid Droplet Staining
Cryosections of urethral tissue samples were prepared as described previously[15]. Immunofluorescence was performed as described previously [16] using phalloidin and anti-myosin heavy chain antibody (Abcam Inc., Cambridge, MA, USA). To identify intramyocellular lipid (IMCL) within urethral striated muscle, we employed the staining procedure reported previously [13]. After washing with PBS, the slides were incubated with LipidTOX neutral lipid stain (1:1000, Invitrogen) at room temperature for 30 minutes, followed by staining with phalloidin (Invitrogen). For image analysis, five randomly selected fields per tissue per animal for each treatment group were photographed and recorded using a Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY, USA).
Image Analysis and Quantification
For image analysis 5 randomly selected fields per slide were photographed and recorded for MHC-F and phalloidin staining using a Retiga imaging camera and ACT-1 software (Nikon Instruments, Melville, New York). Image-Pro Plus (MediaCybernetics®) was used to quantify differential staining and measure the length and diameter of elastic fibers and muscular fibers.
Statistical Analysis
Data were analyzed with Prism 5 (GraphPad Software, San Diego, CA). To determine the difference between the means of two groups, t test was applied. Difference was considered significant when p<0.05. All data are shown as mean ± standard deviation (SD).
RESULTS
Zucker Fatty Rat (ZUC-FA/FA) (ZUC-Leprfa 185) - A Model of Obesity and Insulin Resistance
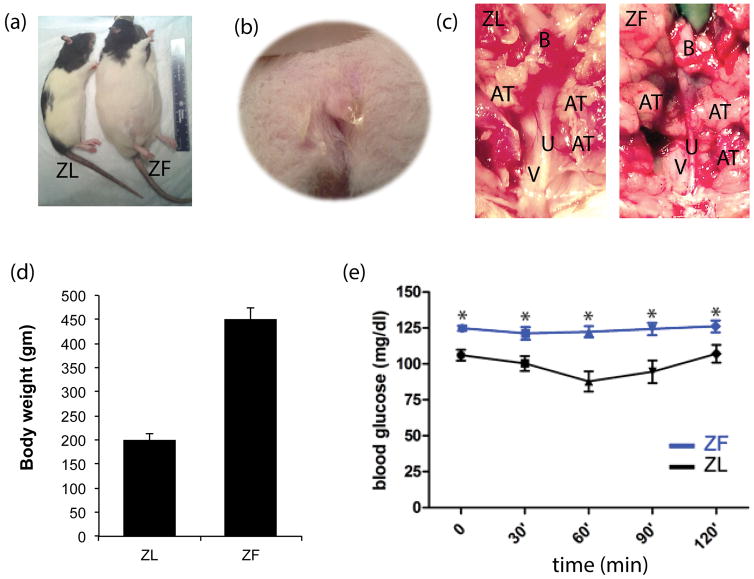
The body weight of ZF rats was higher than the body weight of ZL rats (P< 0.05) (Fig. 1). The intraperitoneal insulin tolerance test was carried out before functional tests were started. The baseline blood glucose level of ZF rats was relatively higher than that of ZL rats (P < 0.05). During the intraperitoneal insulin tolerance test, the glucose concentration declined slowly in the ZF group, and at each time point the glucose level was higher in the ZF group than in the ZL group (P < 0.05). This result demonstrated that ZF rats have insulin resistance (Fig 1). Light pressure on the suprapubic area could cause urine leakage from the external urethral orifice in ZF rats (Figure 1b). More peri-urethral adipose tissue (AT) was observed in ZF rats as compared to ZL rats (Figure 1C).
Figure 1.
Body size difference between ZL and ZF rats (a). Urine leakage from the external urethral orifice after light pressure on the suprapubic area (b). More peri-urethra adipose tissue (AT) in ZF rats compared to ZL rats; V=vagina. U=Urethra; B=Bladder (c). Body weight of ZF rats is higher than ZL rats (P< 0.05) (d). Intraperitoneal insulin tolerance test: ZF rats demonstrated insulin resistance compared to ZL rats (e).
Different Micturition Patterns in ZF and ZL Rats
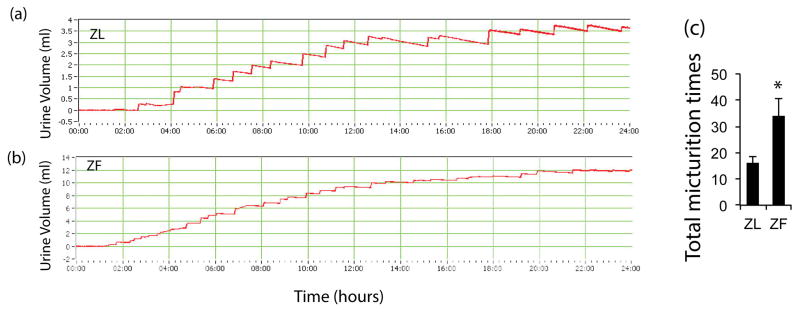
ZL rats showed a stair-like weight change in the urine chart, which was deemed a typical micturition pattern. ZF rats had more total voided volume than the lean group, which is due to higher body weight. A substantially higher micturition frequency was noted in the ZF group compared with ZL group (35 times/24 hours VS 17 times/24 hour). Some ZF rats even showed continuous urine leakage during micturition recording. (Fig 2)
Figure 2.
ZL rats showed a stair-like weight change in the urine chart; around 19 times per 24 hours (a&b). ZF rats had more micturition times than ZL rats (p<0.05), even some with continuous urine leakage (c).
ZF Rats Showed Abnormal Micturition Pattern and Lower Leak Point Pressure
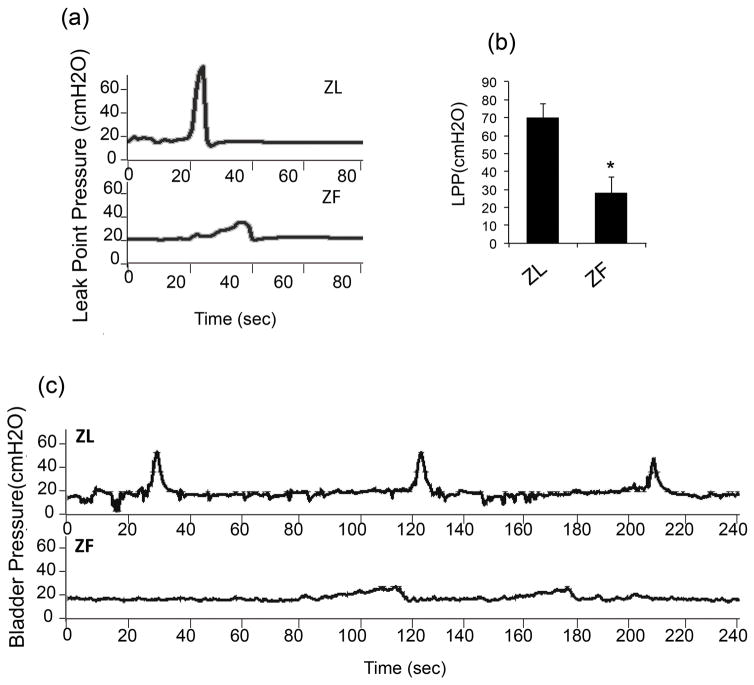
During each voiding episode, the bladder pressure of ZL rats increased to about 55 cmH2O from the baseline level of 10 cmH2O. A discrete amount of around 0.5 ml of voided volume was recorded with each bladder contraction. On the other hand, ZF rats had a lower increase of bladder pressure to 25 cmH2O from baseline and leaked continuously, without a discrete void. This abnormal voiding pattern was demonstrated in 5 of 6 rats in the ZF group and in 0 of the 6 rats of the ZL group. In addition, ZF rats had lower LPP than ZL rats (29.8 ± 8.1 vs 69.3 ± 7.7cmH2O) (Fig 3).
Figure 3.
Compared to ZL rats, with infusion of saline into the bladder, ZF rats had a lower increase of bladder pressure from baseline, many without a discrete void but instead with continuous leakage. Leak point pressures in ZF rats were much lower than that in ZL rats (n=12, p<0.05).
Obesity Structurally Impairs Urethral Striated Muscle
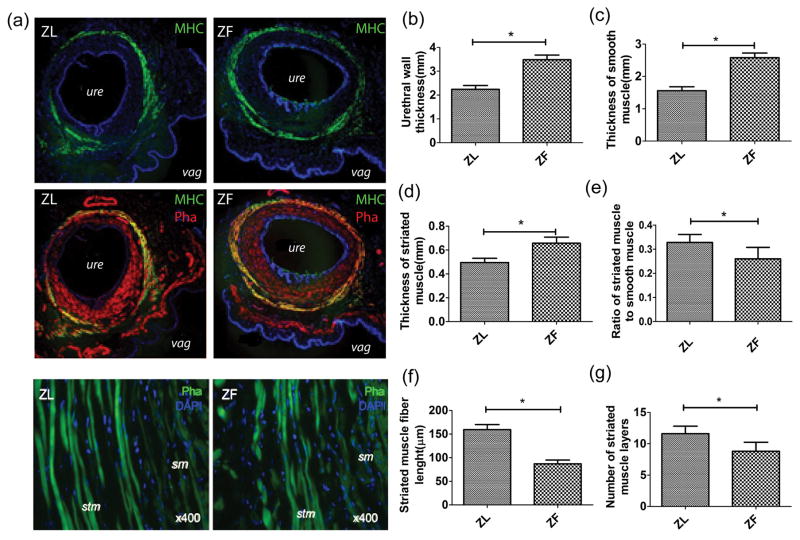
To elicit the mechanism of the decreased leak point pressures in the ZF rats, urethral tissues were stained with striated muscle marker MHC (myosin heavy chain) and F-actin (phalloidin). The results revealed a much thinner urethral wall and thinner urethral striated muscle layer in ZL rats than in ZF rats. In addition, the length of individual muscular fibers was shorter in ZF rats than in ZL rats (Fig 4).
Figure 4.
Cross sections of middle urethral segments and part of the anterior vaginal wall (a). Although ZF rats have a thicker urethral wall (b), smooth muscle layers (c), and striated muscle layers (d) compared to ZL rats, the ZL rats had a relatively higher ratio of striated muscle to smooth muscle (e), longer striated muscle fiber length (f), and more striated muscle layers (g) (n=12, P< 0.05).
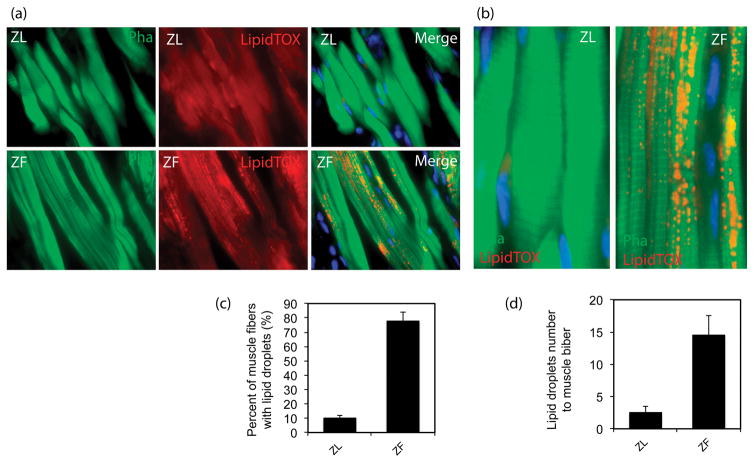
Intramyocellular Lipid Droplets in Urethral Striated Muscle
The results revealed abundant lipid droplet accumulation within the urethral striated muscle myofibers in ZF rats, while few lipid droplets were observed within the urethral striated muscle myofibers of ZL rats. Compared to ZL rats, ZF rats had a higher percentage of muscle fibers containing lipid droplets and a higher ratio of lipid droplets to muscle fibers (P<0.05). (Fig 5).
Figure 5.
Abundant IMCL accumulated within the urethral striated muscle myofibers in ZF rats while fewer IMCL accumulated in ZL rats. ZF rats had a higher percentage of urethral muscle fibers containing IMCL and a higher ratio of IMCL droplets to muscle fibers as compared to ZL rats (n=12, p<0.05).
DISCUSSION
It is well-known that the obese women are at high risk of developing SUI [2]. The association of SUI with obesity has long been recognized, but due to the lack of suitable animal models, the pathogenesis of OA-SUI remains poorly understood. As OA-SUI is such a prevalent condition, it is essential to have a good animal model to employ in studying this important and prevalent medical condition. However, no such well-established animal model for OA-SUI exists. Several animal models of SUI have been reported, and the vaginal distension (VD) methodology has been widely employed to create a model of SUI in rats [17]. We hypothesized that the ZF rat, a widely studied model of obesity and insulin resistance, might also be characterized by SUI. Our results showed decreased LPP and loss of the normal stairway-like micturition pattern in ZF rats.
Anatomically, the urinary continence control system can be divided into two parts: the urethral supportive system and the urethral sphincteric closure system[18]. Pelvic floor muscle, the major component of the supportive system, consists of three main parts: pubococcygeus, puborectalis, and iliococcygeus muscles. The pubococcygeus and the puborectalis muscles form a U-shaped structure as they originate from the pubic bone on either side of the midline and pass behind the rectum to form a sling. It is the constant tone of this sling that normally keeps the urogenital hiatus closed. The sphincteric closure of the urethra is normally provided by the urethral striated muscles, the urethral smooth muscles, and the vascular elements within the submucosa. Striated muscles are found throughout the entire urethra, being most prominent around the middle urethra. Thus, this layer of external striated muscles that runs the entire length of the urethra, except for the neck region, might play a sphincteric function for the bulk of the entire urethra. In addition, previous studies also suggest that the middle urethra might be more important in modulating micturition [19].
Historically, it was believed that obesity primarily impairs the urethral supportive system by increasing the intra-abdominal pressure on the pelvic floor musculature and innervation [1];[11]; [20]. However, few studies have examined voiding function and the pathological changes in the urethral sphincteric closure mechanism, especially the urethral striated muscle, which is closely related to continence. Gasbarro et al reported that the LPP in female Zucker diabetic fatty (ZDF) rats was decreased compared with control rats, but this difference was not statistically significant [4]. However, our results showed that ZF rats had lower LPP than that of ZL rats with statistical significance. This disparity might be explained by the different animal model utilized in each experiment in terms of genotype and phenotype. However, despite the difference in animal model, LPP is similar in ZDF and ZF rats. It was around ~30 cmH2O in ZDF, 29.8±8.1 cmH2O in ZF, and 69.3 ±7.7cmH2O in ZL. ZDF rats developed progressive insulin resistance and glucose intolerance between 3 and 8 weeks of age and become overtly diabetic between 8 and 10 weeks of age[5]. The ZF rat is considered as a model for pre-diabetes, as characterized by a genetic defect in the leptin receptor, which results in hyperphagia, insulin resistance, hyperinsulinemia, hyperlipoproteinemia, and obesity. ZF rats become glucose intolerant but do not develop type 2 diabetes[6].
In middle-aged mice, obesity from chronic high-fat feeding was reported to decrease myofiber area, satellite cells, and myonuclei of the gastrocnemius muscle [21]. Similarly, tenuous-appearing muscle fibers with obvious thinning and atrophy of the striated muscle were observed in the urethra of ZF rats compared with the ZL rats in our study. Thus, the relatively thinner striated muscles in mid-urethra probably contribute to incontinence in ZF rats.
Skeletal muscle can take up plasma free fatty acids (FFAs) and then allocate them to either mitochondrial oxidation and triglyceride (TG) synthesis or lipid droplet storage[22]. The vast majority of FAs taken up by muscle are either oxidized by mitochondria to synthesize ATP or stored as intramyocellular lipid (up to 90% in the soleus muscle) [23]. In obesity, the oversupply of fatty acid during low energetic demand results in lipid accumulation, incomplete β-oxidation, and reactive oxygen species (ROS) production, which may then interfere with muscular contraction. ZF rats showed insulin resistance during intraperitoneal insulin tolerance testing. This is in line with the concept that IMCL can act as a surrogate for ceramide and diacylglycerol (DAG), which are implicated in insulin resistance by perturbing insulin signaling pathways. Our results also showed that abundant IMCL accumulated within the urethral striated muscle myofibers in ZF rats, while little IMCL accumulated in the urethral striated muscle of ZL rats. Consequently, IMCL accumulation might explain the incontinence in ZF rats by causing the dysfunction of striated muscle along the urethra.
CONCLUSION
Obesity impairs urethral sphincter function by intramyocellular lipid deposition and leads to atrophy and distortion of urethral striated muscle. ZF rats can serve as a novel animal model to study obesity-associated stress urinary incontinence.
Acknowledgments
Research reported in this publication was supported by NIDDK of the National Institutes of Health under award number R56DK105097. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: Tom F. Lue is a consultant for Pfizer, Eli Lilly, and Boston Scientific and chief medical officer and stock holder for AWCT, Inc.
References
- 1.Osborn DJ, Strain M, Gomelsky A, Rothschild J, Dmochowski R. Obesity and female stress urinary incontinence. Urology. 2013;82:759–63. doi: 10.1016/j.urology.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Hannestad YS, Rortveit G, Daltveit AK, Hunskaar S. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG : an international journal of obstetrics and gynaecology. 2003;110:247–54. [PubMed] [Google Scholar]
- 3.Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. The Journal of urology. 2009;182:S2–7. doi: 10.1016/j.juro.2009.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasbarro G, Lin DL, Vurbic D, Quisno A, Kinley B, Daneshgari F, et al. Voiding function in obese and type 2 diabetic female rats. American journal of physiology Renal physiology. 2010;298:F72–7. doi: 10.1152/ajprenal.00309.2009. [DOI] [PubMed] [Google Scholar]
- 5.Katsuda Y, Ohta T, Miyajima K, Kemmochi Y, Sasase T, Tong B, et al. Diabetic complications in obese type 2 diabetic rat models. Experimental animals /Japanese Association for Laboratory Animal Science. 2014;63:121–32. doi: 10.1538/expanim.63.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augstein P, Salzsieder E. Morphology of pancreatic islets: a time course of pre-diabetes in Zucker fatty rats. Methods in molecular biology (Clifton, NJ) 2009;560:159–89. doi: 10.1007/978-1-59745-448-3_12. [DOI] [PubMed] [Google Scholar]
- 7.Kava R, Greenwood M, Johnson P. Zucker (fa/fa) rat. ILAR Journal. 1990;32:4–8. [Google Scholar]
- 8.Lee SR, Khamoui AV, Jo E, Park BS, Zourdos MC, Panton LB, et al. Effects of chronic high-fat feeding on skeletal muscle mass and function in middle-aged mice. Aging clinical and experimental research. 2015;27:403–11. doi: 10.1007/s40520-015-0316-5. [DOI] [PubMed] [Google Scholar]
- 9.Gregory SP. Developments in the understanding of the pathophysiology of urethral sphincter mechanism in competence in the bitch. The British veterinary journal. 1994;150:135–50. doi: 10.1016/S0007-1935(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 10.Janssens LA, Peeters S. Comparisons between stress incontinence in women and sphincter mechanism incompetence in the female dog. The Veterinary record. 1997;141:620–5. [PubMed] [Google Scholar]
- 11.Hunskaar S. A systematic review of overweight and obesity as risk factors and targets for clinical intervention for urinary incontinence in women. Neurourology and urodynamics. 2008;27:749–57. doi: 10.1002/nau.20635. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen MH, Cheng M, Koh TJ. Impaired muscle regeneration in ob/ob and db/db mice. TheScientificWorldJournal. 2011;11:1525–35. doi: 10.1100/tsw.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitnick MT, Basantani MK, Cai L, Schoiswohl G, Yazbeck CF, Distefano G, et al. Skeletal muscle triacylglycerol hydrolysis does not influence metabolic complications of obesity. Diabetes. 2013;62:3350–61. doi: 10.2337/db13-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneko S, Iida RH, Suga T, Fukui T, Morito M, Yamane A. Changes in triacylglycerol-accumulated fiber type, fiber type composition, and biogenesis in the mitochondria of the soleus muscle in obese rats. Anatomical record (Hoboken, NJ : 2007) 2011;294:1904–12. doi: 10.1002/ar.21472. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi N, Bella AJ, Wang G, Lin G, Deng DY, Nunes L, et al. Effect of extended-term estrogen on voiding in a postpartum ovariectomized rat model. Can Urol Assoc J. 2007;1:256–63. doi: 10.5489/cuaj.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, et al. Defining stem and progenitor cells within adipose tissue. Stem cells and development. 2008;17:1053–63. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang HH, Damaser MS. Animal Models of Stress Urinary Incontinence. Handbook of experimental pharmacology. 2011:45–67. doi: 10.1007/978-3-642-16499-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashton-Miller JA, Howard D, DeLancey JO. The functional anatomy of the female pelvic floor and stress continence control system. Scandinavian journal of urology and nephrology Supplementum. 2001:1–7. doi: 10.1080/003655901750174773. discussion 106–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim SH, Wang TJ, Tseng GF, Lee YF, Huang YS, Chen JR, et al. The distribution of muscles fibers and their types in the female rat urethra: cytoarchitecture and three-dimensional reconstruction. Anatomical record. 2013;296:1640–9. doi: 10.1002/ar.22740. [DOI] [PubMed] [Google Scholar]
- 20.Ramalingam K, Monga A. Obesity and pelvic floor dysfunction. Best practice & research Clinical obstetrics & gynaecology. 2015;29:541–7. doi: 10.1016/j.bpobgyn.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Lee SR, Khamoui AV, Jo E, Park BS, Zourdos MC, Panton LB, et al. Effects of chronic high-fat feeding on skeletal muscle mass and function in middle-aged mice. Aging clinical and experimental research. 2015 doi: 10.1007/s40520-015-0316-5. [DOI] [PubMed] [Google Scholar]
- 22.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 23.Dyck DJ, Peters SJ, Glatz J, Gorski J, Keizer H, Kiens B, et al. Functional differences in lipid metabolism in resting skeletal muscle of various fiber types. Am J Physiol. 1997;272:E340–51. doi: 10.1152/ajpendo.1997.272.3.E340. [DOI] [PubMed] [Google Scholar]