Abstract
Numerous animal species display behavioral changes in response to changes in social status or territory possession. For example, in male European starlings only males that acquire nesting sites display high rates of sexual and agonistic behavior. Past studies show that mu and delta opioid receptors regulate behaviors associated with social ascension or defeat. Opioids also act at kappa receptors, with dynorphin binding with the highest affinity; however, the role of these opioids in social behavior has not been well studied. We observed flocks of male starlings during the breeding season and ran quantitative real-time polymerase chain reaction (qPCR) to measure expression of kappa opioid receptors (OPRK1) and prodynorphin (PDYN) in brain regions involved in social behavior and motivation (ventral tegmental area [VTA], medial preoptic nucleus [mPOA]) and vocal behavior (Area X). Males with nesting territories displayed more sexual/agonistic behavior than males without nesting territories. They also had lower OPRK1 expression in VTA and mPOA. OPRK1 expression in VTA correlated negatively with sexual/agonistic behaviors, consistent with past studies showing kappa receptors in VTA to inhibit sociosexual behaviors. PDYN in mPOA correlated negatively with a measure of nesting behavior that may also reflect sexual motivation. PDYN in Area X related positively to song. Distinct patterns of OPRK1 and PDYN expression in VTA, mPOA, and Area X related to gonad volume, suggesting that breeding condition may modify (or be modified by) OPRK1 and PDYN expression. Studies are now needed to further characterize the role of OPRK1 and PDYN in status-appropriate social behaviors.
Keywords: kappa receptor, dynorphin, social behavior, dominance, songbird, territoriality
1. Introduction
Many species dramatically alter vocal, sexual, and agonistic behaviors in response to changes in social status or territory acquisition (e.g., [1–6]); however, the neural mechanisms underlying these status-appropriate changes are not well characterized. Opioid neuropeptides and receptors are distributed widely in the brain and are well-studied for roles in addiction, learning and memory, feeding, analgesia, and reward (e.g., [7]). Endogenous opioid systems also regulate social behaviors, including behaviors that change in association with social ascension or defeat (e.g., sexual and agonistic behaviors), suggesting opioids as candidate modulators of status-appropriate behavior.
Most research on the role of opioids in social and sexual behavior is on mu and delta opioid receptors and their high-affinity ligands, which include beta-endorphin and enkephalin opioids. Multiple studies indicate that these receptors and ligands act region-specifically to reward and reinforce sociosexual behaviors (e.g., [8–12]). Although less studied, opioids also act at kappa receptors, with the opioid dynorphin binding with the highest affinity to these receptors [13, 14]. In contrast to mu and delta receptors and their ligands, dynorphin and the stimulation of kappa receptors induces dysphoria and place aversion (e.g., [15–18]). The kappa/dynorphin system generally inhibits sexual [19, 20], prosocial [18, 21], and agonistic behaviors [22], including vocal behaviors associated with positive social contact in rats [23]. This system intensifies submissive behavior and stress after social defeat in mice [22, 24] and is less sensitive in mice that repeatedly win social interactions [25, 26]. This suggests that the kappa/dynorphin system may be important for adjusting behavior to match changes in social status.
Male European starlings, Sturnus vulgaris, are seasonally breeding songbirds that provide an excellent study species in which to examine the neural bases of status-appropriate behavior. At the onset of the breeding season in spring, testosterone concentrations rise and males compete for nesting territories. Males that fail to acquire a nesting territory tend to avoid other males and appear to ignore females. In contrast, males that win a territory sing high rates of courtship song to females, gather green nesting material, and more often displace other males [4, 6, 27–30]. Because males are competing over a limited resource that only some are able to successfully defend, we consider males that successfully acquire and defend nest boxes to socially dominate males that fail to do so, as in prior studies of starlings (e.g., [29]).
Multiple studies implicate brain regions that are centrally involved in motivation in the regulation of status-appropriate behaviors. These areas include the ventral tegmental area (VTA) and the medial preoptic nucleus (mPOA) [31–37]. The mPOA is larger, and both the mPOA and VTA express more cFOS in male starlings with nesting territories compared to those without nesting territories [6, 38]. Mu opioid receptors and enkephalin measures are also higher in males without compared to those with nest sites [28, 39]; however the kappa/dynorphin system has not been examined previously.
In male songbirds, including starlings, dopamine, dopamine metabolites and activity in dopaminergic neurons in VTA (that project to the striatum) relate positively to production of sexually-motivated song [40–45]). In rats, kappa receptors on dopamine neurons in VTA inhibit neuronal firing [46], induce aversion [17, 47–49], and block female-directed sexual behaviors [20]. Kappa receptor mRNA levels in VTA are also reduced in male mice that repeatedly win agonistic interactions [26]. These studies lead to the prediction that the kappa/dynorphin system will be downregulated in the VTA in male starlings that win nesting sites and display high rates of sexually-motivated behavior to females. We made a similar prediction for mPOA based on past work that shows a central role for the mPOA in female-directed song in songbirds [50–52] and that kappa receptor stimulation in the mPOA inhibits male sexual behavior in rats [20]. Finally, in songbirds Area X is a striatal brain region that receives dense projections from VTA as well as the substantia nigra [53–57]. Area X regulates vocal learning and the adjustment of song structure to match specific social contexts [58–62]. Given the function of this region and the fact that it is rich in kappa opioid receptors [63], it is possible that the kappa/dynorphin system in Area X may play a role in adjusting song attributes to match social status.
If the kappa/dynorphin opioid system modifies behavior to match social status, this may be reflected in mRNA expression levels. To test this prediction, we observed flocks of male starlings in outdoor aviaries during the breeding season, collected brains and ran quantitative real-time polymerase chain reaction (qPCR) to measure expression of kappa opioid receptors (OPRK1) and prodynorphin (PDYN) in VTA, mPOA and Area X in males with and without nesting sites.
2. Material and methods
2.1 Animals and housing
Twenty male starlings were used in this study. All birds were trapped in winter 2009–2010 on a local farm in Madison, WI using baited fly-in traps. Each male was banded with stainless steel identification bands and a unique combination of plastic color bands for individual identification. Birds were housed indoors in single sex cages (91 cm x 47cm x 47 cm; 5 birds/cage) in the University of Wisconsin Department of Zoology animal facility. Birds were exposed to artificial photoperiods of 18h light (L):6h dark (D) for 6 weeks, followed by 6 weeks of 8L:16D to induce photosensitivity, a condition in which exposure to a long spring photoperiod will stimulate testosterone production and male sexual behaviors [64]. All procedures and protocols followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee.
2.2 Behavioral observations
Photosensitive males were placed into outdoor aviaries (2.13 m x 2.4 m x 1.98 m) and exposed to a natural spring photoperiod (approximately 13L:11D). Aviaries contained 4 nest boxes, perches, nesting material, baths, food and water. Initially twenty-five male starlings were randomly placed into the aviaries (5 birds / aviary) and allowed to habituate for 7-days; however, over the course of the study 5 birds were removed for various reasons (e.g., concerns about injury from agonistic interactions) leaving 20 birds. After the habituation period, male behaviors were observed in response to a female stimulus bird for 20 min on 4 consecutive days. Prior to each observation period a single observer placed a handful of green nesting material and a stimulus female into the aviary. Stimulus females consisted of 4 photosensitive females that were housed in standard cages indoors on a photoperiod mimicking the natural outdoor photoperiod. A different stimulus female was used on each test day. During each 20 min observation period, the observer noted the number of times a male 1) approached another male [landed within approx. 4cm] followed by that male’s departure (displacements), 2) waved his wings (courtship behavior), 3) gathered nest material, 4) looked into a nest box, and 5) produced a full song (song rate). The observer also used a stop watch to record the time each male spent singing in secs. Finally, the observer recorded bouts of eating and drinking, with bouts of behavior separated by at least 2 seconds. Each male was categorized as a nest box owner or non-owner, with owners identified as males that spent a majority time near a nest box opening or were observed entering and exiting the box. Males that acquired nest boxes displaced other males significantly more often than males without nest boxes (comparison of median displacement behavior in males with and without nest boxes; t1,16 = 9.83, p = 0.0064), confirming that these males were socially dominant. After each observation period, the stimulus female was returned to her home cage. The median behavior for each male across the 4 test days was used for analyses.
2.3 Quantitative real-time polymerase chain reaction preparation and analyses
After the last behavioral observation (on day 4), males were rapidly decapitated, trunk blood was collected, and brains were removed and flash frozen in isopentane (C5H12) in a container surrounded by dry ice. Brains were stored at −80°C and then sectioned coronally using a cryostat −15°C. 200μm sections were collected and placed onto microscope slides. Using a Stoelting brain punch set (#57401), 2 punches were collected from Area X and VTA (one from each hemisphere), and a single central punch was taken from mPOA (punch sizes and locations are shown in Figure 1).
Figure 1.
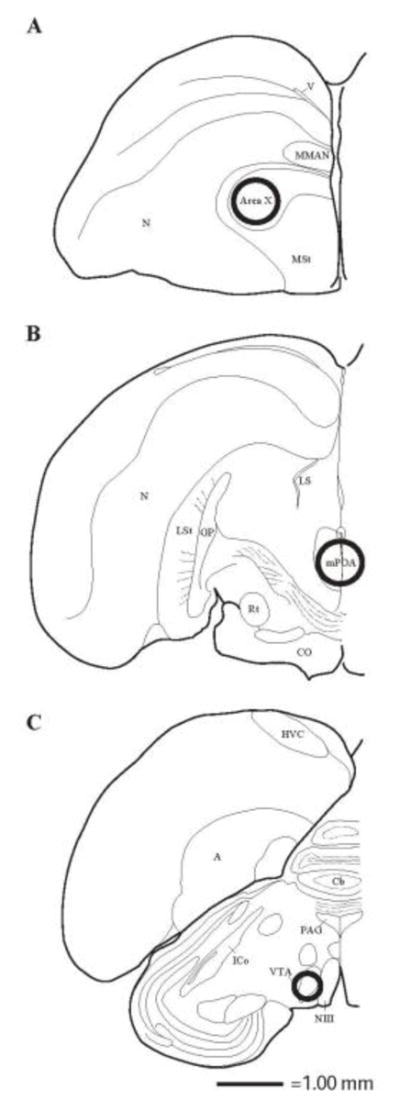
Location of tissue punches illustrated in one, coronal hemisphere of a starling brain. Punches were taken bilaterally from Area X and VTA. For mPOA a single central punch was collected. Sections progress rostrally to caudally from A–C. Approximate punch sizes and locations are represented by circles centered in Area X and mPOA (1.25 mm diameter) and VTA (0.75 mm diameter). Abbreviations listed alphabetically: A = arcopallium; Cb = cerebellum; CO = optic chiasm; CoA = anterior commissure; GP = globus pallidus; HP = hippocampus; HVC = used as a proper name; ICo = nucleus intercollicularis; LS = lateral septum; LSt = lateral striatum; MMAN = medial magnocellular nucleus of anterior nidopallium; MSt = medial striatum N = nidopallium; NIII = 3rd cranial nerve; PAG = periaqueductal gray; PVN = periventricular nucleus; Rt = nucleus rotundus; v = ventricle; VMN = ventromedial nucleus of the hypothalamus.
Tissue punches were stored in 0.5 ml microcentrifuge tubes at −80°C until RNA extraction. Punches were homogenized with an electric Dremel tool and RNA was extracted with a Bio-Rad Aurum Total RNA Fatty and Fibrous Tissue Kit (Catalog No. 732-6830; Bio-Rad, Hercules, CA). The concentration of RNA was measured with a Nanodrop system (Thermo Scientific, Wilmington, DE). RNA integrity was verified with Agilent 2100 BioAnalyzer and Agilent RNA 6000 Pico Kit (Agilent Technologies, Santa Clara, CA) on a subset of samples. The RNA (100ng) was converted into single stranded cDNA using Invitrogen SuperScript III First-Strand Synthesis System (Catalog No. 18080-051; Life Technologies, Carlsbad, CA). Following cDNA conversion, relative gene expression for kappa opioid receptor (OPRK1) and prodynorphin (PDYN) was determined for each brain region as a normalized ratio to reference genes as described below. (As part of separate studies, tissue from this set of males was also used to examine androgen receptor, endocannabinoid CB1 receptor, opioid-related, dopamine-related and neurotensin-related mRNA [65–68]).
Primers for OPRK1 and PDYN were designed (NCBI Gene Database, Primer-Blast) using the chicken (Gallus gallus) genome for qPCR analysis (Table 1). The qPCR reaction product was sequenced using Sanger sequencing with both forward and reverse primers at the University of Wisconsin Biotechnology Center (Table 1). Using NCBI BLAST this sequence matches the intended targets. Two reference genes (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]; hypoxanthine-guanine phosophoribosyltransferase [HPRT]) were also analyzed to normalize mRNA levels across samples. These reference genes were selected because expression has been found to be similar in starlings tested in different seasons and associated hormone conditions [69, 70]. The primers used for reference genes were previously shown to match the intended targets (using Sanger sequencing) in starlings [71].
Table 1.
Primer and reaction product sequences
| Gene | Origin | Accession | Forward Primer |
Reverse Primer |
Sequence | Tm |
|---|---|---|---|---|---|---|
| OPRK1 | Gallus | XM_4260 87.3 |
ACGACT TCTCTC AGGGTC TC |
TGTTTG TCCCAT CTGCTT CC |
CGCCGCATCCCAGGGTTGGTTCTTGTGGTTGTGGCAGTTTTTATCATCTGCTGGA CTCCTATTCACATTTTCGTGCTGGTTGAGGCCTTAGGGGACGTGTCCCACAGTACT GCTGCCATATCCAGCTATTACTTCTGCATTGCTTTGGGTTACACCAACAGCAGCCT CAATCCAATCCTTTATGCATTTTTGGATGAAAACTTTAAGAGATGCTTCAAGGACT TCTGCTTCCCCTTCAAGATGAGGATGGACAGGCAGAGCACCAGCAGAGTCAGAA ACACTGTGCAAGACCCAGCTTACATGAGGGAAGCAGATGGGACAAACAA |
59 |
| PDYN | Gallus | XM_0049 47136.1 |
GAGCAC AGCACA GCACAG |
CCATCC CAGCCC TTCCAC |
NNACGGGGCGCGCAGCAGGCAGCGGGGCACAATGGCACAGCGGGTGCTGGCN GCTGGTNACTCTGCCNTGTCTCTGGCTGCAACAGCCTCCGCCGACTGTGCGACCC AGTGCTCCCTCTGTG |
60 |
Samples were prepared in qPCR reaction tubes containing sample cDNA, nuclease free water, forward and reverse primers (5μM; University of Wisconsin), and SsoFast EvaGreen Supermix (Catalog No. 172-5201; Bio-Rad, Hercules, CA). Five standards were run with each plate of samples (1:10 serial dilution, starting concentration at 500ng/μl), along with a negative control (nuclease free water substituted for cDNA). Standards and samples were run in triplicate on each plate and all plates were read with the BioRad CFX96 Touch Real-Time PCR Detection System (Catalog No. 185-5195; Bio-Rad, Hercules, CA). Each qPCR run consisted of the following: an initiation step at 95°C for 30s, followed by 40 cycles of 95°C for 5s, a 30s annealing phase, a 20s elongation phase at 72°C, and a melt curve from 60°C to 88°C, 0.5 degrees for each 5s step. Plates were read following each elongation and melt curve step. Criteria for inclusion in the dataset were: run efficiencies between 90–110%, an R2 of at least 0.990, and a melt curve displaying a single peak indicative of primer specificity.
Testosterone and Gonad Volume
Measures of plasma testosterone for these males were reported previously as part of another study, and testosterone concentrations were all in the range typical of male starlings in the breeding season [66]. We previously reported [27] that although testosterone tended to be higher in the males used in this study with nest boxes compared to those without nest boxes, this was not significant (p = 0.22 in the subset of birds from [27] reported here). We also found no relationships between testosterone and PCA factors or OPRK/PDYN expression. This is likely because in this study birds were captured and handled for varying amounts of time when blood samples were collected for the testosterone assay, and the acute stress of handling and restraint rapidly alters circulating testosterone concentrations in starlings [72]. Therefore, to gain some insight into breeding physiology, we estimated gonad volume which increases in spring and peaks when testosterone is highest at the time of breeding (e.g., [73, 74]. We used calipers to measure the length and width of the gonads for each male and calculated an estimate of gonad volume using an elliptical volume equation (Volume= 4/3π (0.5 width2 * 0.5 length)). The average of both testes for each bird was then calculated [75, 76].
2.4 Statistical analyses
The Pfaffl Method [77] was used to calculate relative levels of gene expression. This method is similar to the commonly used 2 delta-delta Ct method [78] but rather than assuming a run efficiency of 100%, it allows input of efficiencies for each individual run [77]. The mean cycle threshold (Ct; amplification threshold = 200 RFU) values of all samples were transformed using this method, and the geometric mean of the Ct values for the two reference genes for each region was used to transform the Ct values for each gene analyzed to a normalized ratio.
All data were analyzed with Statistica software (StatSoft, Tulsa, OK). For each analysis residuals were examined and outliers identified using standard residual > 2 standard deviations. We did not remove any outliers from analyses because inclusion or removal did not dramatically alter results.
Here we measured multiple variables that may be assessing similar, correlated aspects of behavior. With such data sets, principal component analysis can be used to reduce multiple variables to a smaller set of factors that explains most of the variance in the full data set. We entered the median value across the four test days for song rate, time singing, displacements, wing waving, gathering of nest material, looking in a nest box, feeding, and drinking as well as mean gonad volume into our analysis. Two of the 20 birds were dropped from analysis due to extreme behavior. One did not display any of the measured behaviors across the 4 observation days, indicating possible illness; and one became extremely aggressive on the last test day (e.g., this bird displaced others 27 times on the last day of observation compared to a mean of 2.4 times for all other birds). Inclusion of either of these birds dramatically altered patterns of behavioral and gene expression results. A MANOVA was used to compare the factors identified using principal component analysis in males with and without nesting sites.
High quality tissue punches were collected for 17 individuals for mPOA (9 without nest boxes and 8 with nest boxes), for 15 individuals from Area X (7 without nest boxes and 8 with nest boxes), and for 16 individuals from VTA (8 without nest boxes and 8 with nest boxes). Three MANOVAs (one for each brain region) were run to test for differences in OPRK1 and PDYN expression in males with and without nesting sites. We did not include all brain regions in a single MANOVA because each region was missing punches from different individuals, resulting in a drop in sample size. Sequential Bonferroni procedures were run to adjust p values to correct for multiple comparisons and this is reported in the results section. Post hoc Fisher’s LSD tests were run when ANOVA results were significant, which resulted in 4 post hoc comparisons. To assist in interpretation of results, as recommended by the American Statistical Association [79], we also report confidence intervals and effect sizes using Cohen’s d values to provide insight into the strength of differences between categorical variables with 0.20, 0.50, and 0.80 considered loose benchmarks for small, medium, and large effect sizes [80].
Standard multiple regression analyses were used to determine the statistical contributions of each factor identified using principal component analysis to mRNA expression in mPOA and VTA. Area X is a song control nucleus, and there are no data to implicate this region in any of the non-song behaviors included in the factors created using principal component analysis. Therefore, we ran a separate set of multiple regression analyses with only time spent singing and gonad volume entered as independent variables and OPRK1 and KAPPA mRNA expression in Area X entered as the outcome variables. Time singing and song rate were correlated so both could not be entered into a single multiple regression. Results were nearly identical using either song measure, and we report here only results of analysis of time singing to avoid redundancy. Finally, for this analysis we removed birds that did not sing more than once across test days. Because there are many reasons for a bird not to sing (e.g., a bird is startled by an unexpected sound, a bird is asleep or a bird simply doesn’t sing etc…) it is not possible to know in non-singers if the values are being matched to a bird’s propensity to sing. Consistent with this idea, unlike singers (results reported below), the non-singers displayed a wide range of OPRK1/PDYN values (OPRK1 mean =0.78, median = 0.91, min = 0.16, max = 1.11; PDYN mean =0.84, median = 0.88, min = 0.24, max = 1.41) that did not relate uniformly to a lack of singing behavior. Standardized Betas are reported for multiple regression analyses as indices of effect sizes (with 0.20, 0.50, and 0.80 considered loose benchmarks for small, medium, and large effect sizes) [80].
3. Results
3.1 Principal component analysis
Eigenvalues for the first 4 factors were well above 1.00 (which indicates that principal components account for more variance than that explained by one of the original variables in the original data set [81]). These 4 factors explained 83.99% of the variance in the full data set (Table 2). Eigenvector coefficients (i.e., loadings) of 0.60 or higher (which is considered an appropriate cutoff when samples sizes are <100; e.g., [82]) were found for factors 1, 3 and 4 (Table 3).The first factor had high loadings for behaviors involved in attracting females and defending nesting territories and we refer to it as Factor 1: sexual/agonistic behavior. The second factor had no loadings >0.60; however gathering nest material, looking in the nest box, song rate, and time singing had loadings >0.50, which is slightly less conservative cutoff used in similar papers [83]. For the song measures the loadings were negative and for the nesting-related behaviors the loadings were positive, meaning individuals that had high nesting scores tended to sing less. We refer to this as Factor 2: nesting behavior. The third factor had high loadings for feeding and drinking and we refer to it as Factor 3: feeding and drinking. The fourth factor had a high loading for gonad volume and we refer to it as Factor 4: gonad volume.
Table 2.
Eigenvalues and % variance explained by each factor
| Factor | Eigenvalue | % Total variance | Cumulative Eigenvalue | Cumulative % |
|---|---|---|---|---|
| 1 | 3.17 | 35.26 | 3.17 | 35.26 |
| 2 | 1.78 | 19.75 | 4.95 | 55.02 |
| 3 | 1.45 | 16.07 | 6.40 | 71.09 |
| 4 | 1.16 | 12.90 | 7.56 | 83.99 |
| 5 | 0.75 | 8.38 | 8.31 | 92.37 |
| 6 | 0.46 | 5.12 | 8.77 | 97.49 |
| 7 | 0.19 | 2.16 | 8.97 | 99.65 |
| 8 | 0.03 | 0.30 | 9.00 | 99.96 |
| 9 | 0.00 | 0.04 | 9.00 | 100.00 |
Table 3.
Principal component analysis results with loadings that contribute most to each factor in bold.
| Variables Entered (Medians) | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Factor 7 | Factor 8 | Factor 9 |
|---|---|---|---|---|---|---|---|---|---|
| Song rate | 0.78 | −0.56 | 0.05 | 0.00 | 0.25 | 0.06 | −0.08 | 0.05 | −0.04 |
| Time singing | 0.78 | −0.56 | 0.01 | −0.04 | 0.22 | 0.05 | −0.11 | −0.04 | 0.04 |
| Displacements | 0.76 | 0.41 | 0.03 | 0.19 | −0.08 | 0.44 | 0.13 | −0.07 | −0.01 |
| Wing waving | 0.76 | 0.45 | −0.03 | −0.14 | −0.12 | −0.41 | −0.13 | −0.07 | −0.01 |
| Gathering nest material | 0.82 | 0.54 | 0.02 | 0.07 | −0.14 | −0.04 | 0.03 | 0.12 | 0.02 |
| Look in nest box | −0.13 | 0.51 | 0.44 | −0.18 | 0.70 | −0.05 | 0.07 | 0.00 | 0.00 |
| Feeding | −0.20 | 0.11 | 0.89 | −0.13 | −0.24 | 0.18 | −0.23 | 0.01 | 0.00 |
| Drinking | 0.18 | −0.37 | 0.65 | 0.55 | −0.12 | −0.25 | 0.20 | −0.01 | 0.00 |
| Gonad volume | −0.24 | 0.27 | −0.19 | 0.87 | 0.20 | 0.01 | −0.21 | 0.00 | 0.00 |
Nest site possession and principal components
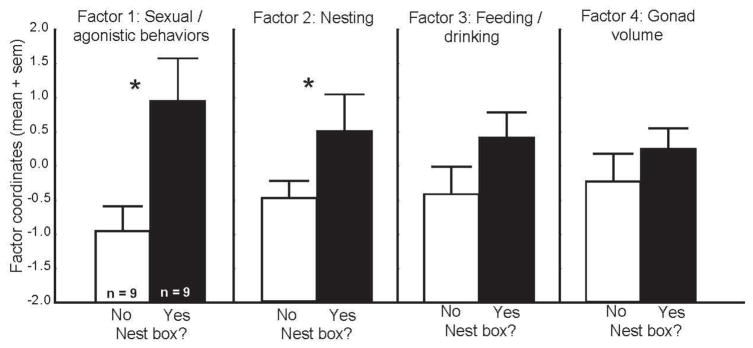
A MANOVA in which Factor 1 (sexual/agonistic behaviors), Factor 2 (nesting), Factor 3 (feeding and drinking), and Factor 4 (gonad volume) were entered as repeated measures variables and whether or not a male owned a nesting site (nest box owner versus nonowner) was entered as a between subjects variable revealed a significant main effect for nest site ownership: F1,16 = 25.62, p = 0.0001 but no significant factor effect (F3,48 = 0.00, p = 1.00) or factor x nest site ownership interaction (F3,48= 0.88, p = 0.456); Figure 2). Fisher’s LSD post hoc tests revealed that Factors 1 and 2 were significantly higher in owners compared to nonowners (Factor 1: p = 0.0003; Factor 2: p = 0.031). No differences were detected between owners and nonowners for Factor 3 (p = 0.064) or Factor 4 (p = 0.264).
Figure 2.
Territory ownership and principal components. Differences in means for the four main factors identified using principal component analysis for males with (filled bars) and those without (open bars) nest boxes. * indicates p<0.05 in posthoc comparisons. Confidence intervals and effect sizes are reported in Table 4.
3.2 Nest site possession and mRNA expression
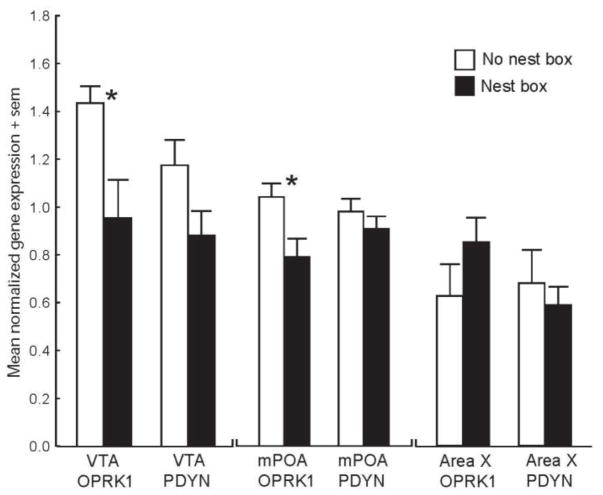
Results of an ANOVA in which measures of OPRK1 and PDYN for VTA were entered as repeated measures variables and whether or not a male owned a nesting site (nest box owner versus nonowner) was entered as a between subjects variable revealed a significant main effect for nest box ownership (F1,14= 11.09, p = 0.005; p value correction procedures for multiple comparisons are detailed below) with higher gene expression observed in males without nest boxes than males with nest boxes (Figure 3). Fisher post hoc comparison revealed that this was significant for OPRK1 (p = 0.011) but not PDYN (p = 0.096). Large effect sizes (i.e., Cohen’s d values > 0.80) were found for both OPRK1 and PDYN expression in VTA (Table 4). No significant differences were found between OPRK1 and PDYN expression (F1,14= 2.22, p = 0.159) and there was no significant expression x nest box owner interaction (F1,14= 0.70, p = 0.416; Figure 3).
Figure 3.
Mean OPRK1 and PDYN expression levels in VTA, mPOA, and Area X for males with (filled bars) or without (open bars) nest sites. * indicates p<0.05 in posthoc comparisons.
Table 4.
OPRK1 and PDYN mRNA: Confidence intervals and effect sizes for males with and without nest boxes
| VTA OPRK1 expression | CI -95 | CI +95 | Effect size (Cohen's d) |
|---|---|---|---|
| no box (n = 8) | 1.27 | 1.60 | 1.38 |
| box (n = 8) | 0.58 | 1.33 | |
| VTA PDYN expression | |||
| no box (n = 8) | 0.93 | 1.43 | 1.02 |
| box (n = 8) | 0.64 | 1.12 | |
| mPOA OPRK1 expression | |||
| no box (n = 9) | 0.90 | 1.18 | 1.27 |
| box (n = 8) | 0.64 | 0.94 | |
| mPOA PDYN expression | |||
| no box (n = 9) | 0.87 | 1.09 | 0.46 |
| box (n = 8) | 0.79 | 1.03 | |
| Area X OPRK1 expression | |||
| no box (n = 7) | 0.30 | 0.95 | 0.69 |
| box (n = 8) | 0.61 | 1.09 | |
| Area X PDYN expression | |||
| no box (n = 7) | 0.34 | 1.02 | 0.30 |
| box (n = 8) | 0.41 | 0.77 |
Results of the ANOVA for mPOA revealed a significant main effect for nest box ownership (F1,15= 5.45, p = 0.034) with higher gene expression observed in males without nest boxes than males with nest boxes (Figure 3, Table 4). Fisher post hoc comparisons revealed that this was significant for OPRK1 (p = 0.021) but not PDYN (p = 0.474). No significant differences were found between markers (F1,15= 0.34, p = 0.571) and there was no significant marker x nest box owner interaction (F1,15= 3.23, p = 0.092). A large effect size was found for OPRK1 and an effect size close to medium was found for PDYN expression in mPOA (Table 4).
Results of the ANOVA for Area X revealed no significant main effects (nest box ownership: F1,13= 0.23, p = 0.643; marker: F1,13= 1.92, p = 0.189) or interactions (F1,13= 4.43, p = 0.055) (Figure 3, Table 4).
Main effects for nest box ownership remained significant for VTA when a sequential Bonferroni correction was used to adjust the p value for running 3 separate ANOVAs for each brain region (p for nest box main effects shifted to 0.017 for lowest p value [which was 0.005 for VTA]); however for mPOA the p value (0.034) was not under the adjusted p value of 0.025. The post hoc result for VTA OPRK1 also remained significant when a sequential Bonferroni correction was used to adjust the p value for the 4 post hoc comparisons run for the 2 significant overall ANOVAs (the VTA and mPOA ANOVAs). This shifted p to 0.0125 for the lowest p value [which was 0.011 for VTA] and to 0.017 for the second lowest value [which was 0.021 for mPOA]). Significance was thus lost for the second highest p value for mPOA OPRK1; however the effect size in this case was large (Table 4).
3.3 Nest site possession, principal components, and mRNA expression
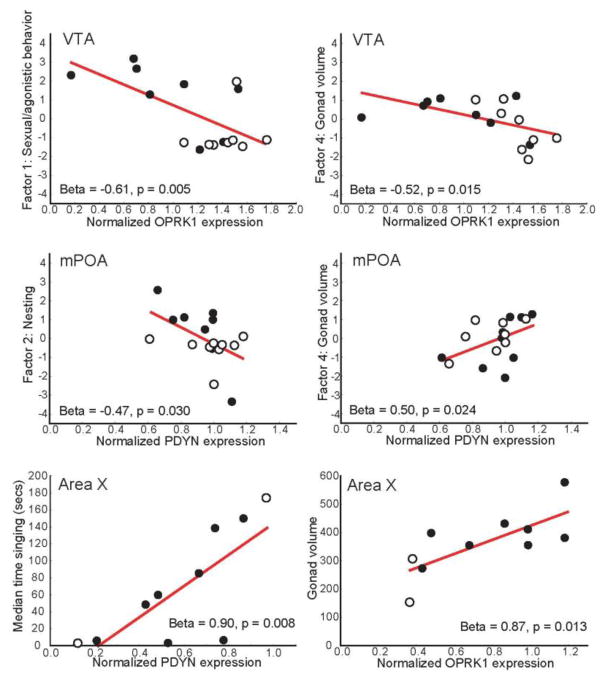
A standard multiple regression analysis in which OPRK1 expression in VTA was entered as the outcome variable and Factor 1, Factor 2, Factor 3, and Factor 4 were entered as independent variables was significant (Table 5) with Factor 1 (sexual/agonistic behaviors) and Factor 4 (gonad volume) relating negatively to OPRK1 expression in VTA (Figure 4). The same analysis run with PDYN in VTA as the outcome variable was not significant (Table 5).
Table 5.
Multiple regression results VTA and POM: OPRK1, PDYN, factors and nest box possession
| VTA OPRK1 (dependent variable) | Independent Variables | Beta | std error of Beta | t (df) | p |
|---|---|---|---|---|---|
| adj R2 = 0.54, p = 0.012 | F1: sexual/agonistic behaviors | − 0.61 | 0.18 | 3.45 (11) | 0.005 |
| n = 16 | F2: nesting | − 0.02 | 0.18 | 0.10 (11) | 0.920 |
| F3: feeding and drinking | 0.03 | 0.18 | 0.18 (11) | 0.862 | |
| F4: gonad volume | − 0.52 | 0.18 | 2.89 (11) | 0.015 | |
| VTA PDYN (dependent variable) | |||||
| adj R2 = −0.03, p = 0.505 | F1: sexual/agonistic behaviors | − 0.12 | 0.26 | 0.45 (11) | 0.658 |
| n = 16 | F2: nesting | − 0.20 | 0.26 | 0.75 (11) | 0.471 |
| F3: feeding and drinking | − 0.40 | 0.27 | 1.49 (11) | 0.165 | |
| F4: gonad volume | − 0.13 | 0.27 | 0.47 (11) | 0.647 | |
| mPOA OPRK1 (dependent variable) | |||||
| adj R2 =0.25, p = 0.113 | F1: sexual/agonistic behaviors | − 0.44 | 0.22 | 2.05 (12) | 0.063 |
| n = 17 | F2: nesting | 0.03 | 0.22 | 0.16 (12) | 0.879 |
| F3: feeding and drinking | − 0.52 | 0.22 | 2.40 (12) | 0.034 | |
| F4: gonad volume | 0.08 | 0.22 | 0.35 (12) | 0.731 | |
| mPOA PDYN (dependent variable) | |||||
| adj R2 = 0.42, p = 0.031 | F1: sexual/agonistic behaviors | −0.25 | 0.19 | 1.32 (12) | 0.210 |
| n = 17 | F2: nesting | −0.47 | 0.19 | 2.43 (12) | 0.032 |
| F3: feeding and drinking | −0.18 | 0.19 | 0.96 (12) | 0.358 | |
| F4: gonad volume | 0.50 | 0.19 | 2.57 (12) | 0.024 |
Figure 4.
Scatterplots showing relationships between OPRK1 and PDYN expression in VTA and mPOA and principal components; and between expression in Area X and median time singing and gonad volume. Each point represents data for an individual male. Filled points indicate males with nest sites; open points indicate males without nest sites. Regression line indicates p < 0.05.
For the same analyses run with OPRK1 in mPOA as the outcome variable the overall multiple regression was not significant (Table 5); however, one variable (Factor 3: feeding and drinking) related negatively to mPOA OPRK1 expression. With PDYN in mPOA as the outcome variable, the same analysis was significant (Table 5) with both Factor 2 (nesting) relating negatively and Factor 4 (gonad volume) relating positively to mPOA PDYN expression.
A standard multiple regression analysis in which OPRK1 expression in Area X was entered as the outcome variable and median time spent singing and mean gonad volume entered as independent variables was significant (Table 6) with gonad volume contributing positively (Figure 4). Results of the same analysis with PDYN in Area X entered as the outcome variable were also significant (Table 6) with median time singing contributing positively (Figure 4).
Table 6.
Multiple regression results Area X: OPRK1, PDYN, singing and nest box possession
| Area X OPRK1 (dependent variable) | Independent Variables | Beta | std error of Beta | t (df) | p |
|---|---|---|---|---|---|
| adj R2 = 0.50, p = 0.036 | Median time singing | 0.30 | 0.26 | 1.14 (7) | 0.291 |
| n = 10 | Gonad volume | 0.87 | 0.26 | 3.31 (7) | 0.013 |
| Area X PDYN (dependent variable) | |||||
| adj R2 = 0.56, p = 0.024 | Median time singing | 0.90 | 0.25 | 3.62 (7) | 0.008 |
| n = 10 | Gonad volume | 0.32 | 0.25 | 1.30 (7) | 0.235 |
4. Discussion
The results presented here show strong associations between status-appropriate behaviors and kappa/dynorphin-related gene expression in brain regions involved in social motivation (VTA and mPOA) and vocal behavior (Area X). Based on past studies demonstrating a causal role for the kappa/dynorphin system in sociosexual behaviors [18–23], we interpret our results to support a causal role for this system in the behaviors examined here. However, these findings are correlational and may reflect the non-mutually exclusive possibility that agonistic and sexual behaviors modify kappa/dynorphin-related gene expression (or that an unidentified third variable is involved).
4.1 Nest site possession, OPRK1/PDYN expression, and behavior
Principal component analysis revealed four robust factors that we describe as Factor 1: sexual/agonistic behavior, Factor 2: nesting behavior, Factor 3: feeding and drinking, and Factor 4: gonad volume based on the primary variables loading onto each factor. As expected based on multiple past studies [4, 6, 27–30], sexual/agonistic and nesting behaviors were significantly higher in males that acquired nest boxes than males without nest boxes. Males with nest boxes had lower relative expression of OPRK1 in both VTA and mPOA than males without nest boxes (with a similar trend observed for VTA PDYN). No differences were found for PDYN in mPOA or either measure in Area X. (Note, although the p value for the mPOA analysis was not significant after sequential Bonferroni correction, the effect size was large, which when paired with a borderline p value suggests a biologically relevant effect [84].) These findings suggest that acquisition of a nest box may reduce kappa/dynorphin activity to promote sexual/agonistic and nesting behaviors. However, it is also possible that individuals that already had low kappa/dynorphin activity were more likely to obtain nesting sites.
Although, the causal relationships between mRNA expression and behavior remain to be determined, prior studies in male mice demonstrate that winning agonistic interactions causes a reduction in kappa mRNA expression in VTA [26]. Furthermore, kappa receptors on dopaminergic neurons in VTA inhibit neuronal firing and sexually-motivated behaviors in rats [20, 46]. If in male starlings the acquisition of a nesting site (which involves winning agonistic interactions against male rivals) reduces kappa expression in VTA, this may lead to a reduction of kappa-inhibition of dopamine release, which would lead to increased production of sexually-motivated behavior. This prediction is supported in our study by the strong negative correlation that we found between the sexual/agonistic behavior factor and OPRK1 expression in VTA. Studies are now needed to test the prediction that blocking kappa receptor inhibition of dopaminergic neurons in VTA will facilitate dopamine release and female-directed song.
The finding that OPRK1 mRNA was also lower in mPOA in males with nest sites (i.e., males that show sexually-motivated behavior) compared to those without nest sites, is also consistent with prior studies showing that injection of a kappa receptor-selective agonist into mPOA suppresses sexually-motivated behavior in male rats [20]. We also found a negative correlation between the factor that we refer to as “nesting” and PDYN expression in mPOA. If nesting is considered a reflection of sexual motivation, then this is consistent with an inhibitory role for dynorphin in mPOA in male sexually-motivated behavior [20]. However, for this factor loadings for singing behavior (another reflection of sexual motivation) contrasted with nesting behaviors. This means that although PDYN expression in mPOA was lower in birds that showed high rates of nesting behavior, it was higher in birds singing at high rates. This may reflect a trade-off as a bird cannot sing and carry nesting material at the same time; however, interpretation is not straightforward. Furthermore, past studies show that in contrast to OPRK1 receptor specific manipulation, dynorphin in the mPOA [85] (as well as VTA [86]) stimulates sexual behavior in male rats. This may be because dynorphin (as opposed to kappa-specific agonists) also binds to delta and mu receptors [20]. Given that male starlings with nest sites demonstrate high levels of sexually-motivated behavior, it may be that acquiring a nesting site reduces kappa expression as well as PYDN in mPOA to reduce kappa-mediated inhibition of sexual behavior; however, this must be tested using experimental manipulations in future studies.
In Area X there were no significant differences in OPRK1 or PDYN expression in males with or without nest sites; however we did see a positive correlation between PDYN expression in the striatal region, Area X, and the amount of time spent singing (in singing males). In mammals, dynorphin-containing neurons in the striatum that project directly to the substantia nigra are thought to act as part of a negative feedback system to inhibit excessive activation of substantia nigra neurons by dopamine [87]. In songbirds Area X does not appear to project directly to substantia nigra (or to VTA). Instead, it projects to each of these regions indirectly via the ventral pallidum, which sends projections that terminate in the substantia nigra and VTA that then carry information back to Area X [88]. This suggests that, similar to what is observed in rats, dynorphin is positioned to influence activity in an anatomical loop in which the striatum (Area X) can control its own dopaminergic input, which may then modulate context-dependent song variability [62, 89]. In addition to VTA (reviewed above), catecholamine systems in substantia nigra relate to female-directed singing behavior in songbirds [43, 90]. It is thus possible that the positive relationship that we observe between PDYN in Area X and singing behavior relates to indirect PDYN projections from Area X to substantia nigra and VTA acting to fine tune dopamine release to optimize singing behavior to match social status.
4.2 OPRK1 expression and gonad volume
Our results revealed positive correlations between measures of gonad volume and OPRK1 expression in both mPOA and Area X. We additionally report a negative relationship between gonad volume and OPRK1 expression in VTA. These findings are similar to those of a study in dark-eyed juncos (seasonally breeding songbirds) in which densities of kappa receptors measured using autoradiography tended to be higher in mPOA and lower in VTA in summer when gonads were large compared to fall when they were fully regressed [91]; however, in Area X they did not change in another junco study [63]. These findings may relate to testosterone influencing OPRK1 expression, given past studies in rodents and rams that show that testosterone and its metabolite estradiol can region-specifically alter mRNA expression for kappa opioid receptors or prodynorphin [92, 93]. However, given that testosterone and corresponding large gonads generally relate positively to sexually-motivated behavior, it is difficult to understand why gonad volume would relate positively to kappa receptors in mPOA (given that they act in this region to inhibit sexual behavior [20]). It is also possible that singing behavior contributed to differences in OPRK1 and PDYN expression. Future studies are now needed to understand these results.
4.3 Interpretational Considerations
We interpret our data to suggest that high OPRK1 and PDYN mRNA expression reflects high kappa receptor and dynorphin synthesis and subsequent translation into protein; however, there are several important caveats. First, qPCR alone does not provide information about translational and post-translational regulation of kappa receptors or dynorphin, including availability of mRNA for translation or post-translational receptor migration and turnover. Second, with the existing study design, we do not know whether preexisting OPRK1 and PDYN differences in the brain drive behavioral differences or whether behavior or other variables influence OPRK1 and PDYN expression. Future studies using measures and targeted manipulations of kappa receptors and dynorphin in VTA, mPOA, and Area X are now needed.
4.4 Conclusions
Past data already strongly demonstrate a causal role for mu opioid receptor stimulation in the male starling mPOA in singing behavior and differences have been found in mu and enkephalin protein measures in mPOA and VTA in males with and without nesting sites [28, 39]. The present results for the first time also suggest potential causal relationships between the kappa/dynorphin system in mPOA, VTA, and Area X and these behaviors. Mu and kappa receptors can have opposing actions [21, 94] and bind different opioids with different affinities. Distinct opioid receptor subtypes can also heterodimerize [95]. This suggests that a complex interplay among the specific opioids released (e.g., dynorphin, enkephalin, beta-endorphin), the levels of opioid release (e.g., at low levels dynorphin primarily binds to kappa receptors, at higher levels it binds to other receptors), and the concentrations of different opioid receptors may function to fine tune status-appropriate behavior.
Highlights.
Dominant male starlings had lower OPRK1 mRNA in VTA and mPOA than subordinates
OPRK1 in VTA related negatively to sexual/agonistic behaviors and gonad volume
PDYN in mPOA related negatively to nesting behaviors and positively to gonad volume
Area X PDYN related positively to singing; OPRK1 related positively to gonad volume
Kappa receptors/dynorphin relate to individual differences in sociosexual behaviors
Acknowledgments
This work was supported by the National Institutes of Health [NIMH grant R01 MH080225 to L. V. Riters]. The authors gratefully acknowledge Chris Elliott for animal care and Caroline Angyal for primer design and validation.
Abbreviations
- mPOA
medial preoptic nucleus
- OPRK1
kappa opioid receptor
- PDYN
prodynorphin
- qPCR
quantitative real-time polymerase chain reaction
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oyegbile TO, Marler CA. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm Behav. 2005;48(3):259–67. doi: 10.1016/j.yhbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Maruska KP, Fernald RD. Behavioral and physiological plasticity: rapid changes during social ascent in an African cichlid fish. Horm Behav. 2010;58(2):230–40. doi: 10.1016/j.yhbeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3(11):e363. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gwinner H, Gwinner E, Dittami J. Effects of nestboxes on LH, testosterone, testicular size, and the reproductive behavior of male European starlings in spring. Behaviour. 1987;103(1–3):68–82. [Google Scholar]
- 5.Korzan WJ, Summers TR, Summers CH. Manipulation of visual sympathetic sign stimulus modifies social status and plasma catecholamines. Gen Comp Endocrinol. 2002;128(2):153–61. doi: 10.1016/s0016-6480(02)00077-1. [DOI] [PubMed] [Google Scholar]
- 6.Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38(4):250–61. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar RJ. Endogenous opiates and behavior: 2014. Peptides. 2016;75:18–70. doi: 10.1016/j.peptides.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21(3):309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 9.Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ. Nucleus accumbens mu-opioid receptors mediate social reward. J Neurosci. 2011;31(17):6362–70. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22(3):437–52. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 11.Paredes RG. Opioids and sexual reward. Pharmacol Biochem Behav. 2014;121:124–31. doi: 10.1016/j.pbb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Van Ree JM, Niesink RJ, Van Wolfswinkel L, Ramsey NF, Kornet MM, Van Furth WR, Vanderschuren LJ, Gerrits MA, Van den Berg CL. Endogenous opioids and reward. Eur J Pharmacol. 2000;405(1–3):89–101. doi: 10.1016/s0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- 13.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–5. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 14.Chavkin C. Dynorphin--still an extraordinarily potent opioid peptide. Mol Pharmacol. 2013;83(4):729–36. doi: 10.1124/mol.112.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27(43):11614–23. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chefer VI, Backman CM, Gigante ED, Shippenberg TS. Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharmacology. 2013;38(13):2623–31. doi: 10.1038/npp.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robles CF, McMackin MZ, Campi KL, Doig IE, Takahashi EY, Pride MC, Trainor BC. Effects of kappa opioid receptors on conditioned place aversion and social interaction in males and females. Behav Brain Res. 2014;262:84–93. doi: 10.1016/j.bbr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deviche P, Moore FL. Opioid kappa-receptor agonists suppress sexual behaviors in male rough-skinned newts (Taricha granulosa) Horm Behav. 1987;21(3):371–83. doi: 10.1016/0018-506x(87)90021-3. [DOI] [PubMed] [Google Scholar]
- 20.Leyton M, Stewart J. The stimulation of central kappa opioid receptors decreases male sexual behavior and locomotor activity. Brain Res. 1992;594(1):56–74. doi: 10.1016/0006-8993(92)91029-e. [DOI] [PubMed] [Google Scholar]
- 21.Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur J Pharmacol. 1995;276(3):257–66. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- 22.Benton D. Mu and kappa opiate receptor involvement in agonistic behaviour in mice. Pharmacol Biochem Behav. 1985;23(5):871–6. doi: 10.1016/0091-3057(85)90085-1. [DOI] [PubMed] [Google Scholar]
- 23.Hamed A, Szyndler J, Taracha E, Turzynska D, Sobolewska A, Lehner M, Krzascik P, Daszczuk P. kappa-opioid receptor as a key mediator in the regulation of appetitive 50-kHz ultrasonic vocalizations. Psychopharmacology (Berl) 2015;232(11):1941–55. doi: 10.1007/s00213-014-3824-7. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31(6):1241–8. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudryavtseva NN, Gerrits MA, Avgustinovich DF, Tenditnik MV, Van Ree JM. Modulation of anxiety-related behaviors by mu- and kappa-opioid receptor agonists depends on the social status of mice. Peptides. 2004;25(8):1355–63. doi: 10.1016/j.peptides.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Goloshchapov AV, Filipenko ML, Bondar NP, Kudryavtseva NN, Van Ree JM. Decrease of kappa-opioid receptor mRNA level in ventral tegmental area of male mice after repeated experience of aggression. Brain Res Mol Brain Res. 2005;135(1–2):290–2. doi: 10.1016/j.molbrainres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Cordes MA, Stevenson SA, Riters LV. Status-appropriate singing behavior, testosterone and androgen receptor immunolabeling in male European starlings (Sturnus vulgaris) Horm Behav. 2014;65(4):329–39. doi: 10.1016/j.yhbeh.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelm CA, Forbes-Lorman RM, Auger CJ, Riters LV. Mu-opioid receptor densities are depleted in regions implicated in agonistic and sexual behavior in male European starlings (Sturnus vulgaris) defending nest sites and courting females. Behav Brain Res. 2011;219(1):15–22. doi: 10.1016/j.bbr.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119(1):233–44. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- 30.Eens M, Pinxten R, Verheyen RF. Function of the song and song repertoire in the European starling (Sturnus vulgaris): An aviary experiment. Behaviour. 1993;125:51–66. [Google Scholar]
- 31.Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Front Neuroendocrinol. 2012;33(2):194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Will RG, Hull EM, Dominguez JM. Influences of dopamine and glutamate in the medial preoptic area on male sexual behavior. Pharmacol Biochem Behav. 2014;121:115–23. doi: 10.1016/j.pbb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28(4):161–78. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519(18):3599–639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- 35.Goodson JL, Eibach R, Sakata J, Adkins-Regan E. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla) Behav Brain Res. 1999;98(1):167–80. [PubMed] [Google Scholar]
- 36.Blanchard DC, Blanchard RJ, Takahashi LK, Takahashi T. Septal lesions and aggressive behavior. Behav Biol. 1977;21(1):157–61. doi: 10.1016/s0091-6773(77)92407-5. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez JM, Salas C, Portavella M. Offense and defense after lateral septal lesions in Columba livia. Int J Neurosci. 1988;41(3–4):241–50. doi: 10.3109/00207458808990730. [DOI] [PubMed] [Google Scholar]
- 38.Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65(3):207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- 39.Kelm-Nelson CA, Stevenson SA, Cordes MA, Riters LV. Modulation of male song by naloxone in the medial preoptic nucleus. Behav Neurosci. 2013;127(3):451–7. doi: 10.1037/a0032329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heimovics SA, Salvante KG, Sockman KW, Riters LV. Individual differences in the motivation to communicate relate to levels of midbrain and striatal catecholamine markers in male European starlings. Horm Behav. 2011;60(5):529–539. doi: 10.1016/j.yhbeh.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci U S A. 2009;106(21):8737–42. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95(1–2):258–66. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25(11):3406–16. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kubikova L, Kostal L. Dopaminergic system in birdsong learning and maintenance. J Chem Neuroanat. 2010;39(2):112–23. doi: 10.1016/j.jchemneu.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang YC, Hessler NA. Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS ONE. 2008;3(10):e3281. doi: 10.1371/journal.pone.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23(31):9981–6. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA., Jr Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111(16):E1648–55. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264(1):489–95. [PubMed] [Google Scholar]
- 49.Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, Zweifel LS, Kieffer BL, Phillips PE, Chavkin C. Kappa Opioid Receptor-Induced Aversion Requires p38 MAPK Activation in VTA Dopamine Neurons. J Neurosci. 2015;35(37):12917–31. doi: 10.1523/JNEUROSCI.2444-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36(3):276–86. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- 51.Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behav Neurosci. 2006;120(6):1326–36. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alger SJ, Maasch SN, Riters LV. Lesions to the medial preoptic nucleus affect immediate early gene immunolabeling in brain regions involved in song control and social behavior in male European starlings. European Journal of Neuroscience. 2009;29(5):970–982. doi: 10.1111/j.1460-9568.2009.06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol. 1981;196(2):347–54. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- 54.Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279(2):312–26. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- 55.Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24(1):51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- 56.Soha JA, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol. 1996;29(4):473–89. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 57.Person AL, Gale SD, Farries MA, Perkel DJ. Organization of the songbird basal ganglia, including area X. J Comp Neurol. 2008;508(5):840–66. doi: 10.1002/cne.21699. [DOI] [PubMed] [Google Scholar]
- 58.Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224(4651):901–3. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 59.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165(4):457–86. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 60.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11(9):2896–913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biology. 1990;53(1):51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 62.Leblois A, Wendel BJ, Perkel DJ. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J Neurosci. 2010;30(16):5730–43. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gulledge CC, DeViche P. Autoradiographic localization of opioid receptors in vocal control regions of a male passerine bird (Junco hyemalis) J Comp Neurol. 1995;356(3):408–17. doi: 10.1002/cne.903560308. [DOI] [PubMed] [Google Scholar]
- 64.Dawson A. Effect of daylength on the rate of recovery of photosensitivity in male starlings (Sturnus vulgaris) J Reprod Fertil. 1991;93(2):521–4. doi: 10.1530/jrf.0.0930521. [DOI] [PubMed] [Google Scholar]
- 65.Cordes MA, Stevenson SA, Driessen TM, Eisinger BE, Riters LV. Sexually-motivated song is predicted by androgen-and opioid-related gene expression in the medial preoptic nucleus of male European starlings (Sturnus vulgaris) Behav Brain Res. 2015;278:12–20. doi: 10.1016/j.bbr.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeVries MS, Cordes MA, Stevenson SA, Riters LV. Differential relationships between D1 and D2 dopamine receptor expression in the medial preoptic nucleus and sexually-motivated song in male European starlings (Sturnus vulgaris) Neuroscience. 2015;301:289–97. doi: 10.1016/j.neuroscience.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merullo DP, Cordes MA, Susan DeVries M, Stevenson SA, Riters LV. Neurotensin neural mRNA expression correlates with vocal communication and other highly-motivated social behaviors in male European starlings. Physiol Behav. 2015;151:155–161. doi: 10.1016/j.physbeh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeVries MS, Cordes MA, Rodriguez JD, Stevenson SA, Riters LV. Neural endocannabinoid CB1 receptor expression, social status, and behavior in male European starlings. Brain Res. doi: 10.1016/j.brainres.2016.05.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bentley GE, Perfito N, Calisi RM. Season- and context-dependent sex differences in melatonin receptor activity in a forebrain song control nucleus. Horm Behav. 2013;63(5):829–35. doi: 10.1016/j.yhbeh.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 70.Bentley GE, Tucker S, Chou H, Hau M, Perfito N. Testicular growth and regression are not correlated with Dio2 expression in a wild male songbird, sturnus vulgaris, exposed to natural changes in photoperiod. Endocrinology. 2013;154(5):1813–9. doi: 10.1210/en.2013-1093. [DOI] [PubMed] [Google Scholar]
- 71.Riters LV, Stevenson SA, DeVries MS, Cordes MA. Reward associated with singing behavior correlates with opioid-related gene expression in the medial preoptic nucleus in male European starlings. PLoS One. 2014;9(12):e115285. doi: 10.1371/journal.pone.0115285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Hout AJ, Eens M, Darras VM, Pinxten R. Acute stress induces a rapid increase of testosterone in a songbird: implications for plasma testosterone sampling. Gen Comp Endocrinol. 2010;168(3):505–10. doi: 10.1016/j.ygcen.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen Comp Endocrinol. 1983;49(2):286–94. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- 74.Dawson A, Goldsmith AR. Prolactin and gonadotrophin secretion in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to nesting, incubation, and rearing young. Gen Comp Endocrinol. 1982;48(2):213–21. doi: 10.1016/0016-6480(82)90019-3. [DOI] [PubMed] [Google Scholar]
- 75.McGuire NL, Koh A, Bentley GE. The direct response of the gonads to cues of stress in a temperate songbird species is season-dependent. PeerJ. 2013;1:e139. doi: 10.7717/peerj.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dawson A. The effect of temperature on photoperiodically regulated gonadal maturation, regression and moult in starlings - potential consequences of climate change. Funct Ecol. 2005;19(6):995–1000. [Google Scholar]
- 77.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 79.Wasserstein RL, Lazar NA. The ASA's statement on p-values: context, process, and purpose. The American Statistician. 2016 [Google Scholar]
- 80.Cohen J. Statistical power analysis for the behavioral sciences. 2. L Erlbaum Associates; Hillsdale, N.J: 1988. [Google Scholar]
- 81.Kaiser HF. The Application of Electronic-Computers to Factor-Analysis. Educ Psychol Meas. 1960;20(1):141–151. [Google Scholar]
- 82.Budaev SV. Using Principal Components and Factor Analysis in Animal Behaviour Research: Caveats and Guidelines. Ethology. 2010;116(5):472–480. [Google Scholar]
- 83.Kelly AM, Goodson JL. Functional interactions of dopamine cell groups reflect personality, sex, and social context in highly social finches. Behav Brain Res. 2015;280:101–12. doi: 10.1016/j.bbr.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Garamszegi LZ. Comparing effect sizes across variables: generalization without the need for Bonferroni correction. Behav Ecol. 2006;17(4):682–687. [Google Scholar]
- 85.Band LC, Hull EM. Morphine and dynorphin(1–13) microinjected into the medial preoptic area and nucleus accumbens: effects on sexual behavior in male rats. Brain Res. 1990;524(1):77–84. doi: 10.1016/0006-8993(90)90494-v. [DOI] [PubMed] [Google Scholar]
- 86.Mitchell JB, Stewart J. Facilitation of sexual behaviors in the male rat associated with intra-VTA injections of opiates. Pharmacol Biochem Behav. 1990;35(3):643–50. doi: 10.1016/0091-3057(90)90302-x. [DOI] [PubMed] [Google Scholar]
- 87.Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123(1–2):60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- 88.Gale SD, Person AL, Perkel DJ. A novel basal ganglia pathway forms a loop linking a vocal learning circuit with its dopaminergic input. J Comp Neurol. 2008;508(5):824–39. doi: 10.1002/cne.21700. [DOI] [PubMed] [Google Scholar]
- 89.Leblois A, Perkel DJ. Striatal dopamine modulates song spectral but not temporal features through D1 receptors. Eur J Neurosci. 2012;35(11):1771–81. doi: 10.1111/j.1460-9568.2012.08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matheson LE, Sakata JT. Catecholaminergic contributions to vocal communication signals. Eur J Neurosci. 2015;41(9):1180–94. doi: 10.1111/ejn.12885. [DOI] [PubMed] [Google Scholar]
- 91.Woods JK, Deviche P, Corbitt C. Opioid receptor densities analyzed across seasons in the POM and VTA of the dark-eyed junco, Junco hyemalis. J Chem Neuroanat. 2010;40(2):123–9. doi: 10.1016/j.jchemneu.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 92.Yukhananov RY, Handa RJ. Alterations in kappa opioid receptor mRNA levels in the paraventricular nucleus of the hypothalamus by stress and sex steroids. Neuroreport. 1996;7(10):1690–4. doi: 10.1097/00001756-199607080-00033. [DOI] [PubMed] [Google Scholar]
- 93.Scott CJ, Clarke IJ, Tilbrook AJ. The effect of testosterone and season on prodynorphin messenger RNA expression in the preoptic area-hypothalamus of the ram. Domest Anim Endocrinol. 2008;34(4):440–50. doi: 10.1016/j.domaniend.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 94.Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244(3):1067–80. [PubMed] [Google Scholar]
- 95.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399(6737):697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]