Abstract
Cutaneous inflammation alters the function of primary afferents and gene expression in the affected dorsal root ganglia (DRGs). However specific mechanisms of injury-induced peripheral afferent sensitization and behavioral hypersensitivity during development are not fully understood. Recent studies in children suggest a potential role for growth hormone (GH) in pain modulation. GH modulates homeostasis and tissue repair after injury, but how GH effects nociception in neonates is not known. To determine if GH played a role in modulating sensory neuron function and hyper-responsiveness during skin inflammation in young mice, we examined behavioral hypersensitivity and the response properties of cutaneous afferents using an ex vivo hairy skin-saphenous nerve-dorsal root ganglion (DRG)-spinal cord preparation. Results show that inflammation of the hairy hindpaw skin initiated at either postnatal day 7 (P7) or P14 reduced GH levels specifically in the affected skin. Furthermore, pretreatment of inflamed mice with exogenous GH reversed mechanical and thermal hypersensitivity in addition to altering nociceptor function. These effects may be mediated via an upregulation of insulin-like growth factor 1 receptor (IGFr1) as GH modulated the transcriptional output of IGFr1 in DRG neurons in vitro and in vivo. Afferent-selective knockdown of IGFr1 during inflammation also prevented the observed injury-induced alterations in cutaneous afferents and behavioral hypersensitivity similar to that following GH pretreatment. These results suggest that GH can block inflammation-induced nociceptor sensitization during postnatal development leading to reduced pain-like behaviors, possibly by suppressing the upregulation of IGFr1 within DRGs.
Keywords: neonate, inflammation, electrophysiology, molecular biology, dorsal root ganglion
1. Introduction
Peripheral sensitization occurs after injury at all ages [20,50,61,73], but insults sustained during development have the potential to be much more detrimental to the long-term outcomes of neonates [14,68]. The neonatal period is a critical stage for the structural and functional reorganization of the sensory system [13,37,39–41]. For example, there is a known switch in neurotrophic factor sensitivity during the first week of life, whereby sensory neurons downregulate trkA and upregulate c-ret rendering them responsive to glial cell line-derived neurotrophic factor (GDNF) instead of nerve growth factor (NGF) [41,47]. This switch subsequently regulates how the sensory neurons relay information from the periphery to the spinal dorsal horn (DH) [28,51,54].
The appropriate interaction between primary afferents and the spinal cord during development is necessary for establishing normal responsiveness to external stimuli. Injury during early life however, has been shown to alter DH neuron development, which can induce altered behavioral responsiveness to thermal and mechanical stimuli in adulthood [36,51,68,69]. It has been hypothesized that this phenomenon is due to altered peripheral A-fiber sensitization along with improper development of glycinergic inhibition [25,27]; a notion that was recently supported by our laboratory [24]. In our previous report, we also found that developing cutaneous nociceptors were sensitized to heat and mechanical stimuli in a pattern that differed from adults [23].
As distinct mechanisms may govern injury-induced hypersensitivity in neonates, it would be important to determine if there was a unique regulator of peripheral sensitization in developing animals. It has been shown that patients with growth hormone deficiency (GHD), along with showing deficits in growth, can also display resting pain [2,7]. GH plays important roles in homeostasis and tissue repair after injury in addition to its growth promoting effects in children [18,70]. However, GH-related signaling molecules such as GH release hormone (GHRH) and ghrelin have been found to decrease mechanical and thermal hypersensitivity after inflammation [15,64] in adult rodents. In addition, recent human studies have showed that GH treatment provides analgesia in a subpopulation of adult patients with fibromyalgia [3,4,9,10,31,34,42].
GH exerts much of its effects through insulin-like growth factor (IGF)/IGF receptor (IGFr) signaling, but does not bind IGFr directly [2]. IGF-1 synthesis has been shown to increase after tissue injuries [16] and locally produced IGF-1 has been linked to the development of injury-induced hypersensitivity [46,74]. Moreover, IGFr1 antagonists block mechanical and thermal hyper-responsiveness during inflammation in adults [46]. Together, these data suggest that GH signaling may also play an important role in pain modulation during postnatal development. Nevertheless, the role of GH in the development of neonatal pain remains unclear. In the current study, we tested the hypothesis that a reduction in GH levels during neonatal cutaneous inflammation drives the sensitization of primary afferents and pain-related hypersensitivity possibly by suppressing afferent specific IGFr1 upregulation within sensory neurons.
2. Materials and Methods
2.1. Animals
Male and female Swiss Webster mice (Charles River) from postnatal day 6 through 21 (+/− ~0.5d around the specified age) were used in these studies. All animals were housed with the mother, which was provided food and water ad libitum and maintained on a 12 hour light/dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee at Cincinnati Children’s Hospital Medical Center, under AAALAC approved practices. No differences between male and female mice were detected for any tests described below and thus data was combined from both sexes throughout the manuscript. As our recent reports have documented age-related effects of injury on the excitability of primary afferent neurons, we assessed the effects of cutaneous inflammation beginning at two different postnatal ages (P7 or P14) in order to account for any potential developmental effects [24].
2.2. Carrageenan-induced inflammation
Mice were anesthetized under 3% isofluorane and 3–10 μL of 3% carrageenan (in 0.9% NaCl) was injected into the right hairy hindpaw skin by using a 30 gauge needle with syringe according to our previous procedures [24]. The carrageenan was expelled beginning at the ankle and allowed to fill under the hairy hindpaw skin towards the digits. Injections were directed towards the medial side of the hindpaw in order to target the saphenous nerve field as accurately as possible. 1μL/g body weight was used as a guide for these injections according to previous studies [65] in order to account for subtle variations in paw size that occurs as a mouse develops during this period of life. At P7, mice were approximately three to four grams in total body weight, while mice at P14 were around seven to eight grams. Using the abovementioned guide, within a given age group, only variations of approximately 1μL were made. GH treatments (below) did not alter injection volumes within a given age group. Regardless, ipsilateral and contralateral hindpaw edema was measured using calipers to ensure that a similar degree of inflammation was attained at the different ages. All behavioral, electrophysiological, anatomical or molecular analyses were performed 1d, 3d and 7d after injection of carrageenan and compared to un-anesthetized naïve mice at P7 or P14, or with each other.
2.3. Growth hormone treatments and side effects analysis
Mouse recombinant growth hormone (GH; GenScript) was diluted in H2O (50μL) in different concentrations (0.1mg/kg–0.5mg/kg) and intraperitoneally injected once per day beginning three days prior to, or once on the day of carrageenan injections at P7 or P14 as indicated above. The initial guide that was used for this injection strategy was based on the amount of GH that would be needed to begin modulating systemic GH-mediated signaling without concurrently stimulating growth [12]. The maximum serum concentration of GH detected from this injection regimen in rodents would only reach approximately 100–150ng/mL, three hours after the final injection. By 24 hours after the final dose however, which is when we begin to perform our assessments post inflammation, systemic GH levels should return to normal concentrations (~0–20ng/mL) [12]. Body weight, temperature and urine ketones were monitored to determine potential side effects of GH administration. Temperature was determined on the thorax using a contact thermocouple connected to a temperature readout device according to previous procedures [17]. Levels of ketones in the urine were determined by applying a small droplet of urine to the testing end of a Ketodiastix (Bayer) and verifying ketone concentration according to the color coded scale provided by the manufacturer.
2.4. siRNA production and in vivo nerve injections
Mice were anesthetized as described above. A small incision was made in the mid-thigh region exposing the saphenous nerve. The nerve was loosened from the adjacent connective tissue and placed onto a parafilm platform. Then 0.05–0.1μL of 20uM non-targeting control (siCON) or IGFr1 targeting (siIGFr1) siRNAs (Thermo Scientific) were pressure injected into the saphenous nerve using a quartz microelectrode connected to a pico-spritzer. The control siRNAs used in this study were a pool of four non-targeting duplexes that do not target any gene in the mouse genome (Thermo). The targeting sequences used to design each siCON duplex are as follows: 5′-UAAGGCUAUGAAGAGAUAC-3′, 5′-AUGUAUUGGCCUGUAUUAG-3′, 5′-AUGAACGUGAAUUGCUCAA-3′, 5′-UGGUUUACUGUCGACUAA-3′. The specific targeting sequence for IGFr1 used for in vivo studies was determined by selecting four different targeting sequences (Thermo Scientific; Cat#D-056843) and transfecting Neuro2A cells in vitro according to our previous reports [19] with the individual IGFr1 targeting siRNAs (1–4) and comparing them to untreated cells or those transfected with the non-targeting control siRNAs (siCON). RNA was isolated from the different culture conditions, reverse transcribed and cDNAs were used in SYBR Green realtime PCR reactions as described below. The most efficiently targeting siRNA (Sequence #1: 5′-CCAUCGAGGUUACUAAUGA-3′) was used thereafter for this report. After injections, the incision was closed with a 7-0 silk suture. For P7 mice, siRNAs were injected one day before inflammation, and for P14 mice, siRNAs were injected two days before inflammation. This strategy follows a modified version of our previous reports [21–23].
2.5. Behavioral analyses
All behavioral experiments were performed in which the experimenter was blinded to the various conditions. siCON injected control mice that did not receive carrageenan injections, were performed separately by a different experimenter. Following a 15–20 minute acclimation period in the behavior chamber, the mechanical and thermal thresholds were tested as previously described [44,69]. Mechanical threshold was determined by application of an increasing series of calibrated Von Frey (VF) hairs to the medial side of the dorsal surface of the hindpaw, which is innervated by the saphenous nerve. After another 10–15 minute rest period, a water bath of varying temperatures was used to measure heat sensitivity. Mice were gently held and both hindpaws were submerged into the water. Time until a hindpaw flexion withdrawal response was detected was recorded as the latency. 20 seconds was set as a cut-off time. 40°C and 45°C were tested for P7 cohorts (1–7d post carrageenan) while 45°C and 50°C were tested for mice ≥ P14. This follows a similar strategy to that previously described by Marsh et al [44] and Walker et al [69] in which different maximal temperatures are required between these two ages. Both mechanical and thermal tests were performed 3 times at 5 minute intervals. The average of the three trials was determined per mouse, per time point/condition. The average values are reported as mean ± SEM after normalization to age-matched naives. For siRNA injected mice, DRG receptor expression (using PCR) was verified in the control or IGFr1 targeting groups to confirm validity of behavioral results obtained from these cohorts. In a few instances, individual mice did not achieve significant knockdown and were not included in the analysis.
2.6. Ex vivo preparation and intracellular recording
The ex vivo hairy hindpaw skin/saphenous nerve/dorsal root ganglion (DRG)/spinal cord (SC) somatosensory system recording preparation was performed as described previously [20,24,33,59]. Briefly, mice were anesthetized with ketamine and xylazine (90 and 10 mg/kg, respectively) and intracardially perfused with oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (aCSF; in mM: 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 D-glucose) containing 253.9 mM sucrose at a temperature of approximately 12°C. The spinal cord (caudal from ~T10) and the right hindlimb were excised and placed in a bath of this oxygenated aCSF. The hairy skin of the right hindpaw, saphenous nerve, L1-L5 DRGs and corresponding spinal cord segments were isolated in continuity and then transferred to a recording chamber containing chilled and oxygenated aCSF in which the sucrose was replaced with 127.0 mM NaCl. The skin was then pinned out on a stainless steel grid located at the bath/air interface to allow the dermal surface to be continuously perfused with the aCSF while the epidermis remained dry. The bath was finally warmed to 32°C before recording.
Intracellular single unit recording was performed in sensory neuron somata contained within the L2 or L3 DRGs using quartz microelectrodes (impedance >150MΩ) containing 5% Neurobiotin (Vector Laboratories, Burlingame, CA) in 1 M potassium acetate. Sensory neuron somata in the L2/L3 DRGs with axons in the saphenous nerve were identified by electrical simulation to the side of the nerve through a suction electrode during intracellular recording. If a cell was found to be driven by this electrical stimulus, then the cutaneous receptive fields (RF) were localized with a soft brush and/or von Frey filaments. When cells were driven by the nerve but had no mechanical RF, a thermal search was conducted by applying hot (~53°C) and/or cold (~1°C) physiological saline to the skin.
Response characteristics of individual DRG cells were determined by first applying mechanical and then thermal stimuli to the hairy skin. For mechanical stimulation, RFs were probed for 1–2s with an increasing series of calibrated VF filaments ranging from 0.07 g to 10 g. When feasible, a mechanical stimulator that delivered a digitally controlled mechanical stimulus was also employed, which consisted of a tension/length controller (Aurora Scientific) attached to a probe with a 1 mm diameter aluminum tip. Computer controlled 5s square waves of 1, 5, 10, 25, 50 and 100mN were applied to the cell’s RF in these instances. In order to compare these results to those of the VF stimulation, units in mN were converted to grams based on the 1 mm probe diameter. After mechanical stimulation, a controlled thermal stimulus was applied using a 3×5 mm contact area peltier element (Yale Univ. Machine Shop) or saline stimuli as described. The controlled thermal stimulus consisted of a variable cold ramp that started at 32°C and dropped to approximately 3–4°C, which was held for 2–3s and allowed to return slowly to the bath temperature (32°C). Bath temperature was held for a few seconds (~2–3s) and then a heat ramp was initiated, which delivered an increasing heat stimulus to the RF up to 52°C. The ramp increases in temperature from 32°C to 52°C in 12s. The 52°C stimulus was held for 5s and then the ramp returned the RF to 32°C in 12s. Adequate recovery times (approx. 20–30s) were employed between all stimulations. As discussed previously [21,24,59], the repetitive stimulation of the RFs with hot saline was not found to sensitize nociceptors during the recording experiments. No differences were found in recorded fibers from the beginning of an experiment to those obtained at the end.
When fibers were unable to be fully characterized by controlled mechanical and thermal stimulation but were partially characterized by one of the controlled stimuli and brush or saline stimuli, these cells were also included for determination of afferent subtype prevalence and for the properties in which we obtained controlled data. All responses were recorded for offline analysis (Spike2 software, Cambridge Electronic Design). Conduction velocities of the recorded afferents were then calculated from spike latency and the distance between stimulating and recording electrodes (measured directly along the nerve). Firing rates were determined by calculating the peak firing after binning the responses in 200ms bins.
A total of 738 cells were intracellularly recorded and physiologically characterized in the current study. The average number of cells recorded per condition/time point/age was 53, which were obtained from an average of 4 mice per group. The minimum number of mice per preparation was 3 and a minimum of 44 cells were obtained from each of the 14 groups analyzed. Specifically, the numbers of animals and cells recorded per condition (listed as Condition: Number of Mice: Number of Cells), were as follows: Naïve conditions: P7: 5 mice, 50 cells; P14: 4 mice, 52 cells. P7 inflammation: 1d: 4 mice, 53 cells; 3d: 4 mice, 52 cells; P14 inflammation: 1d: 5 mice 45 cells, 3d: 3 mice, 50 cells; P7 Inflammation with GH: 1d: 4 mice, 54 cells; 3d: 4 mice, 55 cells; P14 inflammation with GH: 1d: 3 mice, 52 cells, 3d: 3 mice, 44 cells; P7 siCON+inflammation: 5 mice, 84 cells; siIGFr1+inflammation: 3 mice, 50 cells; P14 siCON+inflammation: 4 mice, 44 cells; siIGFr1+inflammation: 4 mice, 55 cells.
2.7. Primary DRG cultures and single cell cDNA amplification
For primary DRG neuron cultures, we followed our originally described procedures [19,43] but used DRGs from P14 male mice. Animals were first anesthetized and intracardially perfused with Hank’s Balanced Salt Solution (HBSS). DRGs (all spinal levels) were then dissected and collected in HBSS and dissociated using cysteine/papain (0.03%, Sigma, and 20 U/mL, Worthington) followed by collagenase II (0.3%, Worthington), and then triturated with fire-polished glass pipettes in F12 complete media (F12 containing 10% fetal bovine serum and 1% penicillin/streptomycin) before plating onto poly-d-lysine/laminin (20 μg/mL each, Sigma) coated glass coverslips (Menzel, Germany) that were placed in 35mm petri dishes. Cells were allowed to incubate at 37°C/5%CO2 for 1–2 hours and then flooded with F12 complete media alone (untreated) or media containing 50 ng/mL GH (Genscript). Cells were then allowed to incubate at 37°C/5%CO2 for 24 hrs. At this time, media was removed and cells were flooded with single cell PCR buffer (In mM: 140 NaCl, 10 glucose, 5 KCl, 10 HEPES, 1 MgCl2, and 2 CaCl2). Then individual neurons (n=20) that were qualitatively determined to be in the medium to small diameter range were collected using borosilicate electrodes and Cell Tram Vario system (Eppendorf) under bright field optics using a Leica inverted microscope. Images of the various cell culture conditions were obtained using modified differential interference contrast on a Leica inverted fluorescence microscope.
All collected single cells were used in realtime PCR reactions based on a modified protocol from Kurimoto et al [30] and Ross et al [55]. Single cells were first expelled from the borosilicate electrodes into individual tubes containing lysis buffer from the Message Booster cDNA Synthesis Kit (Epicentre/Illumina) and prokaryotic spike RNAs (1000 copies of LYS transcripts) were used for internal and reverse transcription controls since internal controls such as GAPDH will consistently vary from single cell to cell [58]. Single cell RNAs were reverse transcribed with Superscript III (Invitrogen) primed by an Oligo (dT) containing a T7 RNA polymerase promoter. cDNAs then underwent in vitro transcription, RNA was purified, and new cDNAs were produced using Superscript III. cDNAs were then diluted and ran in duplicate in standard SYBR Green realtime PCR reactions (20ng/reaction) on a Step-One realtime PCR machine (Applied Biosystems). The forward and reverse primer sequences for GAPDH and LYS are as follows: GAPDH: forward: 5′-ATG TGT CCG TCG TGG ATC TGA-3′; reverse: 5′-ATG CCT GCT TCA CCA CCT TCT T-3′; LYS: forward: 5′-GCC ATA TCG GCT CGC AAA TC-3′; reverse: 5′-AAC GAA TGC CGA AAC CTC CTC-3′. GAPDH was first tested in each sample to verify acquisition of single cell cDNAs. However, cycle time (Ct) values for all targets in single cell PCR reactions were normalized to the LYS spike RNA controls.
2.8. Whole DRG or skin RNA isolation, reverse transcription and realtime PCR
Animals were first anesthetized as described above. The mice were then intracardially perfused with ice cold 0.9% NaCl prior to dissection of skin or DRGs. RNA isolation from the hairy skin was performed using Trizol (Thermo) followed by RNA cleanup with the Qiagen RNeasy Mini Kits while L2 and L3 DRG RNA was isolated using Qiagen RNeasy mini kits for animal tissues using the supplied protocol (n = 3–5 per condition and time point). RNA concentrations were then determined by obtaining A260 readings on a Nanodrop spectrometer (Thermo). Purified RNA was treated with DNase I (Invitrogen) and then DNased RNA was reverse transcribed using Superscript II Reverse Transcriptase (Invitrogen). For realtime PCR, 25 ng samples of cDNA were added to a SYBR Green Master Mix (Applied Biosystems) containing the appropriate primer combinations and run in duplicate on an Applied Biosystems Step-ONE realtime PCR machine. Forward and reverse primer sequences used in realtime PCR reactions for IL1β, GDNF, NGF, IGFr1, and GAPDH were obtained from Ross et al [59], Jankowski et al [59] or Elitt et al [11]. Primer sequences for the GH receptor are as follows: Forward: 5′-GCC TCT ACA CCG ATG AGT AA-3′; Reverse: 5′-GGA AAG GAC TAC ACC ACC T-3′. Primer sequences for TNFα are: Forward: 5′-TCGGAAAGAAATGTCCCAGGTGGA-3′; Reverse: 5′-TGGAACTGGTTCTCCTTACAGCCA-3′ and sequences for IL6 are: Forward: 5′-ACTGATGCTGGTGACAAC-3′; Reverse: 5′-CCGACTTGTGAAGTGGTATAG-3′. Cycle time (Ct) values were normalized to GAPDH and changes in expression are calculated as a ΔΔCt value that is determined by subtracting the Ct values of the gene of interest from the GAPDH (or LYS) internal control for each sample and compared among samples. Fold change is described as 2ΔΔCt (Applied Biosystems) and 2-fold change equals 100% change (mean ± SEM).
2.9. Protein isolation and western blotting
Similar to RNA isolation, animals were first anesthetized as described above and intracardially perfused with ice cold 0.9% NaCl. Skin samples or L2/L3 DRGs pooled from two mice as indicated were then collected and homogenized in lysis buffer containing 1% sodium dodecyl sulfate (SDS), 10 mM Tris–HCl (pH 7.4), and protease inhibitors (1 μg/ml pepstatin,1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM sodium orthovanadate and 100 μg/ml phenylmethylsulfonyl fluoride; Sigma Biochemicals). Then 20μg samples from each condition were centrifuged and boiled 10 min in a denaturing buffer containing β-mercaptoethanol and SDS prior to gel electrophoresis. Samples were then separated on a 7.5–16% polyacrylamide SDS-PAGE gel and transferred to a PVDF (Millipore) membrane that was blocked in specialized LiCor blocking buffer. Membranes were then incubated in primary antibodies overnight at 4°C (GAPDH: 1:2000, ProSci Inc; GH: 1:2000, GenScript; IGF-1: 1:1000, Abcam; IGFr1: 1:750, Acris). Antibody binding was visualized using 680 nm or 800 nm infrared dye-conjugated donkey anti-rabbit or donkey anti-chicken secondary antibodies (1:20,000; LiCor) with detection using the LiCor Odyssey Imaging System (LiCor). Settings for detection were consistent between runs. Immunoreactive bands were analyzed by densitometry and quantified using NIH image J (RRID: nif-0000-30467) software. Band intensity was normalized to GAPDH and reported as a percent change (mean ± SEM). A negative control and a peptide block control was also performed for the GH antibody. In this latter case, an equal dilution of the manufacturer’s supplied peptide was incubated with the GH antibody (above) during blot processing. For the negative control, no primary antibody was used. Both controls followed the above procedures.
2.10 Data analysis
All data are presented as mean ± SEM. Behavioral assays were compared using one- or two-way repeated measures (RM) or standard analysis of variance (ANOVAs) with Holm Sidak or Tukey’s post hoc tests. Peak firing rates (FR), mean peak instantaneous frequencies (IF) and thresholds to mechanical or heat stimuli were compared via one-way ANOVA with Holm Sidak post hoc as appropriate or Kruskal-Wallis one-way ANOVA on ranks with Dunn’s post-hoc tests. Percent change in protein expression detected from Western blotting and gene expression changes in single cells or whole tissues were analyzed via one-way ANOVA with Tukey’s post-hoc. Critical significance level was defined at p<0.05. Rare instances of statistical outliers defined as values greater than two standard deviations away from the mean were not included in the analysis.
3. Results
3.1. Cutaneous inflammation produces a localized reduction in GH levels
In order to first determine if cutaneous inflammation altered the levels of growth hormone (GH) in the injured skin of neonates, we quantified GH protein in the hairy hindpaw skin using western blot analysis on mice with inflammation induced at P14. We found that neonatal cutaneous inflammation (n=3–4 each) significantly decreased the levels of GH protein present in this target tissue one day after carrageenan injection initiated at P14. Hairy skin GH levels returned to that of naïve mice three days after cutaneous inflammation (Fig. 1A, B).
Figure 1. Cutaneous GH protein expression with or without exogenous GH delivery after hairy skin inflammation at P14.
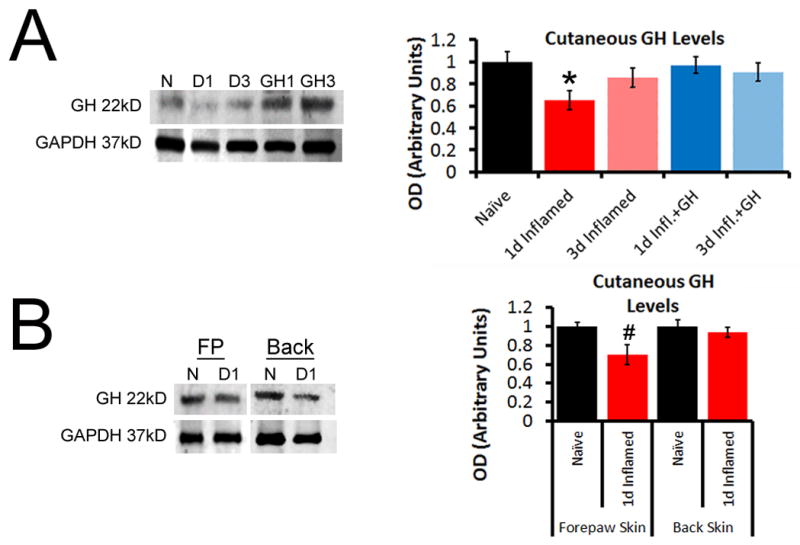
A: At P14, GH was found to be significantly reduced in the inflamed hairy hindpaw skin 1d after peripheral injury. Levels in the inflamed area were reduced 35% (±9%) relative to that observed in naïve hairy skin, but returned to naïve levels by day 3 (D3). Treatment of mice with exogenous GH for three days prior to injury was able to block the reduction in GH levels found after cutaneous inflammation. B: Analysis of naïve forepaw (FP) or back skin, or cutaneous tissue from these same regions obtained from mice 1d post carrageenan injection into the hairy hindpaw skin at P14, showed no differences in GH levels. The decrease in GH expression that was found in the FP skin after hindpaw skin inflammation, was determined to be a 29% (±10%) reduction, but this was not found to be statistically significant vs. naïve FP skin. n=3–4/ group, * p<0.05 vs. naïve, # p<0.07 vs. naive; One-way ANOVA/Tukey’s post hoc.
We then delivered a three day pretreatment of GH (0.5mg/kg; 1x/d for 3d) and found that this was sufficient to prevent the reduction in hairy skin GH levels following carrageenan injection at P14 (Fig. 1A; p>0.05). Next, we wanted to determine if the observed GH reduction in hairy skin was specific to the inflamed target tissue or was a broad effect of peripheral inflammation. We thus performed similar western blot analyses on forepaw and back skin from mice that received carrageenan injection into the hairy hindpaw skin and found that no statistically significant differences in GH levels were detected (Fig. 1B; p>0.05). Both negative and peptide block controls verified GH antibody specificity for western blotting. GH injections into naïve neonates using this regimen were not found to increase cutaneous levels of GH (Supplementary Fig. 1). These data suggest that cutaneous inflammation produces a transient GH reduction in the affected skin and a short, low dose GH pre-treatment reverses the inflammation induced GH decrease in the skin.
3.2. Exogenous GH treatment blocks injury induced mechanical and thermal hypersensitivity
As our above results suggested that we are able to manipulate and restore the levels of GH in the inflamed hairy skin of neonatal mice, we tested the effectiveness of an exogenous GH treatment on the reported [44,54,69] mechanical and thermal hypersensitivity during cutaneous inflammation at P7 or P14. We first performed a dose response analysis based on the above information and previously reported data [12]. Mice were thus treated with one of three different doses of GH (0.1–0.5mg/kg, ip.) once a day beginning three days prior to injury up through the day of inflammation, or another cohort of mice was given a single injection of GH (at 0.5mg/kg, ip.) at the same time as cutaneous inflammation. We found that inflammation-induced mechanical and heat hypersensitivity one day after injury was blocked by varying doses of the three day pre-treatment strategy regardless of the age of initial insult. A single injection of GH at the highest dose used; however, was insufficient to block carrageenan induced hyper-responsiveness to mechanical and thermal stimuli at P14 while a single dose of GH partially blunted heat but not mechanical hypersensitivity at P7 (Supplementary Fig. 2).
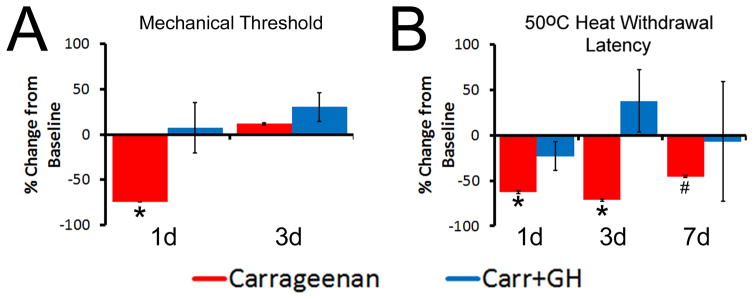
We therefore assessed the time course of GH effects on neonatal inflammatory hypersensitivity using the dosing regimen that was effective in all behavioral tests (0.5mg/kg, ip.; 1x/d for 3d prior to inflammation; see Supplementary Fig. 2). Specifically, mechanical thresholds in the ipsilateral hindpaw one day (1d) after carrageenan injection (at P14) were significantly decreased compared to baseline, but this returned to normal levels by three days (3d; Fig. 2A; n=12, p<0.05). However, GH pretreatment completely blocked this decrease at 1d and showed no differences versus baseline at 3d (Fig. 2A; n=9, p<0.05). A seven day (7d) time point was not analyzed due to the difficulty of mechanical testing on the hairy skin in older mice (≥P21). No changes were found in the contralateral limbs in any group at 1d (not shown).
Figure 2. Effects of GH pretreatment on peripheral hypersensitivity after P14 carrageenan injection.
A: Mechanical hypersensitivity during P14 inflammation (measured as a percent change in thresholds relative to baseline) was blocked by GH pretreatment at 1d. No differences were found between mice injected with carrageenan alone or those treated with carrageenan plus GH at 3d; however, both of these groups differed from mice that received carrageenan only at the 1d time point. B: Hairy skin inflammation also reduced the heat withdrawal latencies to 50°C water at 1d and 3d post carrageenan, but this was completely blocked by GH pretreatment at all time points tested. By 7d, a partial restoration of heat hypersensitivity was found in mice with carrageenan injection only; however, these mice were not different than those treated with GH at this 7d time point. GH treated mice with hairy hindpaw skin inflammation did not change relative to their baseline levels at any time point post inflammation. * p<0.05 vs. baseline and GH treated mice, # p<0.05 vs. baseline, but not time matched GH treated mice; 2-way RM ANOVA/Holm Sidak.
Heat withdrawal latencies to 50°C water were significantly decreased 1d and 3d (p<0.05) after P14 carrageenan injection compared with baseline. Heat hypersensitivity returned towards uninjured levels by 7d post inflammation, but did not fully recover to naïve levels. GH pretreatment, however, completely inhibited the carrageenan induced reduction in heat withdrawal latency at all time points (Fig. 2B). No differences were detected between any group during a 45 °C heat stimulus (p>0.05, not shown). GH pretreatment at P14 had no effect on baseline mechanical sensitivity (i.e. without injury) or heat withdrawal times compared to untreated naives (see Supplementary Fig. 3). These data suggest that an exogenous GH treatment can block mechanical and thermal hypersensitivity if given before neonatal cutaneous inflammation.
3.3. Exogenous GH pretreatment blocks cutaneous afferent sensitization during neonatal peripheral inflammation
To then determine the effects of GH pretreatment on the response properties of primary sensory neurons, we performed single unit recordings with our neonatal ex vivo hairy hindpaw skin/saphenous nerve/DRG/spinal cord recording preparation, at 1d and 3d post inflammation. Two categories of sensory afferents are detected based on conduction velocity (CV) during development due to ongoing myelination [24,28,71]. “A”-fibers were defined as those that conduct at least twice as fast as the other sensory neurons (“C”-fibers) in a given experiment. We do acknowledge however, that based on these criteria, some fibers could be classified as “A”-fibers if they conducted at the higher end of the adult C-fiber range for rodents (1.2m/s) [20,29]. However, it should be noted that only 13 cells out of 738 recorded in our study fell into this category (i.e. were classified as “A”-fibers despite conducting slower than 1.2m/s). Furthermore, this categorization is consistent with previous reports analyzing response properties of neonatal sensory neurons [24,56,71]. At P14, the average CVs for A-fibers were 5.14 m/s, and the average CVs for C-fibers were 0.56 m/s. No differences in A- or C-fiber CVs were found between any of our groups.
We detected no differences after carrageenan injection in the rapidly or slowly adapting low threshold mechanoreceptors (faster conducting cells with narrow spikes), nor were there any differences detected in the low threshold mechanically sensitive and cold sensitive C-fibers (CMC; slower conducting cells with narrow spikes). There were also no differences in the mechanically insensitive but cold (CC) or heat (CH) sensitive C-fiber neurons (slower conducting, broad spiking) among any of our experimental groups (not shown). Therefore, the remainder of the study focused on the myelinated nociceptors (faster conducting, broad spiking afferents) that were mechanically sensitive, and sometimes thermally sensitive (“A”-high threshold mechanoreceptors (A-HTMRs): AM or A-polymodal (APM)), or the unmyelinated nociceptors (slower conducting, broad spiking fibers) with these types of responses (“C”-HTMR: CM or CPM).
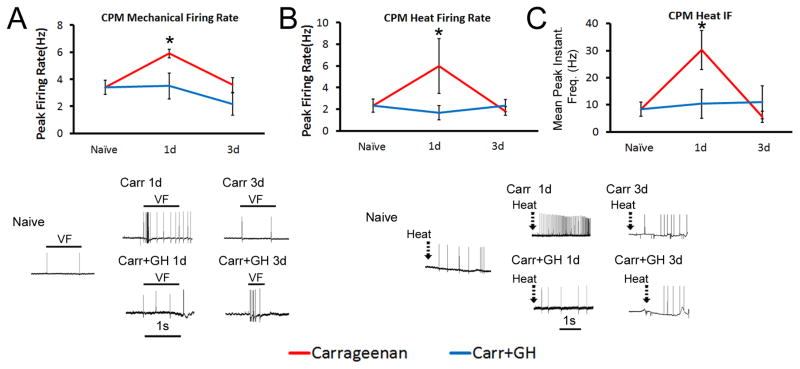
Consistent with our previous report [24] at P14, CPM mechanical firing rates (FRs), heat FRs and heat peak instantaneous frequencies (IFs) were all increased (n=7 (mechanical) and n=5 (heat)) 1d after carrageenan induced inflammation (p<0.05) compared with naïves (n=10 and n=7, respectively; p<0.05). These alterations in C-fiber response properties were all blocked by GH pretreatment (n=4 for both mechanical and heat responsive cells; p>0.05; Fig. 3A–C). No differences in mechanical or heat firing in CPMs were found between our groups at 3d post inflammation (Fig. 3; Inflammation Only: n=17 (mechanical) and n=11 (heat); Inflammation+GH: n=6 (for both mechanical and heat responsive cells)). Meanwhile, the CM mechanical FRs and thresholds had no statistical changes at any time point between any groups (n=4–9; not shown). A-fiber HTMR mechanical FRs were not found to be different post-carrageenan (n=10) compared to naïves (n=13; p>0.05; not shown). Heat FRs were also unaffected in the A-HTMR neurons at 1d (n=3–6; not shown). These data suggest that P14 cutaneous inflammation sensitizes CPM nociceptors, but not A-fiber HTMRs; however, this can be blocked by GH pretreatment.
Figure 3. Growth hormone (GH) pretreatment blocks hypersensitivity of “C”-fiber sensory neurons to mechanical and thermal stimuli during cutaneous inflammation initiated at P14.
At P14, carrageenan was found to increase the firing of slowly conducting (C-fiber) polymodal nociceptors (CPM) to both mechanical (A) and heat (B, C) stimuli. GH pretreatment in mice with hairy skin inflammation however, blocked all of the observed changes in CPM neurons at P14. Mechanical and heat hyper-responsiveness in CPM neurons resolved by 3d post inflammation while GH treated mice with inflammation showed no variations in CPM neuron responses to these stimuli at any time point relative to uninjured control cells. Examples of corresponding responses to various stimuli are presented with each panel(s). VF= von Frey filament (peak response for cell). *p<0.05 vs. naïve; # p <0.05 vs. 1d inflammation; One-way ANOVA on Ranks/Dunn’s post hoc.
3.4 A short course of low dose GH in inflamed mice does not induce classic side effects of extended GH therapy or alter cutaneous inflammatory responses to carrageenan
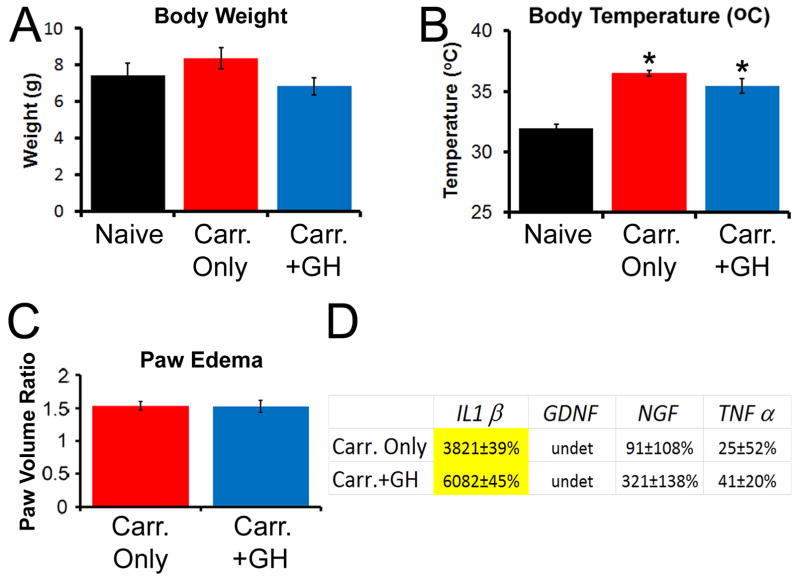
We next determined if our transient, low dose GH therapy produced any of the known side effects of prolonged GH therapy in our mouse model of cutaneous inflammation such as altered body weight, temperature or induction of hyperglycemia [6,60,63]. We found that the highest dose (0.5mg/kg ip.; 1x/d for 3d prior to inflammation) used to effectively block mechanical and thermal responsiveness in our inflamed mice was not sufficient to significantly alter body weight at P14 (Fig. 4A; n=4–14; p>0.05), nor did it produce detectable ketones in the urine (indirect measure of insulin resistance; not shown). While we did detect a significant increase in body temperature in mice that were injected with carrageenan compared to uninjured naives, this was not further affected by the injection of GH (Fig. 4B; p<0.05).
Figure 4. Analysis of body weight, temperature, paw edema and mRNA expression of select cytokines and growth factors in skin after GH pretreatment in mice after P14 inflammation.
At P14, no differences in body weight were observed in any of the three groups (A) however, carrageenan injection induced an increase in body temperature that was similarly observed in the GH treatment group (B). * p<0.05 vs. naive; #p<0.05 vs. naïve, but not Carr.+GH treated mice; n=4–11/group; One-way ANOVA/Holm Sidak post hoc. Carrageenan induced paw edema was not affected by a GH pretreatment regimen (C). Only IL1β was upregulated in the hairy hindpaw skin of P14 inflamed neonates (D). GH treated neonates with cutaneous inflammation also showed upregulation of this cytokine in the skin. Although both groups showed significant upregulation of IL1β in the skin after inflammation, there were no statistically significant differences between these groups in regards to relative gene expression. Yellow highlighted values indicate p<0.05 vs. naïve only; n=3–7 for C, D. undet= undetectable. One-way ANOVA/Tukey’s post hoc. Values are presented as a percent change from naïve.
As our results suggested robust effects of GH pretreatment on neonatal hypersensitivity to cutaneous inflammation, we wanted to determine if these effects were due to alterations in the peripheral inflammatory response. We thus measured paw edema and also performed realtime PCR on the hairy hindpaw skin for various cytokines and growth factors in our cohorts. We found that GH pretreatment did not alter carrageenan induced paw edema (Fig. 4C; p>0.8), and had no effects on the induction of cutaneous cytokines and growth factors after P14 inflammation (Fig. 4D). Of the numerous factors that were screened in this study, only the inflammatory cytokine interleukin 1β (IL1β) was found to be significantly upregulated in the skin after carrageenan injection into the hairy hindpaw skin at P14, but GH pretreatment did not prevent its upregulation. No increases in nerve growth factor (NGF) or tumor necrosis factor α (TNFα) mRNA were found in the skin after P14 inflammation (Fig. 4D). We did not detect significant levels of glial cell line-derived neurotrophic factor (GDNF) or IL6 mRNA in any group using these reaction conditions and primer sets (not shown). Thus GH does not appear to induce classic side effects of extended GH therapy, and the GH effects on peripheral hypersensitivity during neonatal inflammation are unlikely to reflect a suppression of the inflammatory response within the skin.
3.5 GH pretreatment regulates mechanical and thermal hypersensitivity after cutaneous inflammation at P7
Since our data showed a profound effect of GH on neonatal hypersensitivity to inflammation at P14, we wanted to assess whether similar results could be obtained in younger mice (P7). Overall, we found highly similar effects of GH on carrageenan induced hypersensitivity when injury was sustained at P7. We found that GH was also reduced specifically in the hairy skin after inflammation at P7 (Supplementary Fig. 4A, B; n=3–4), which could be prevented with a GH pretreatment (p>0.05). In addition, the mechanical and heat hypersensitivity (at 45°C) that was observed in these younger mice was also blocked with exogenous GH pretreatment. (Supplementary Fig. 4C, D; n=11–13, p<0.05). Similar results on heat hypersensitivity were also detected at lower temperatures analyzed. Although carrageenan injection significantly decreased withdrawal latencies in response to a 40°C heat stimulus at 1d compared to baseline (−21.7%±9.9%; p<0.05), no differences in heat withdrawal latency were detected in the GH treated group (−3.2%±10.2%; p>0.05). No differences were found among any of our groups at the 3d or 7d time points at this temperature however (not shown). Finally, no changes were found in the contralateral limbs in any group after P7 inflammation (not shown).
Interestingly, when comparing raw baseline values between our groups, the mechanical withdrawal thresholds for both ipsilateral and contralateral hindpaws were decreased in the GH treated group compared to the baseline thresholds in untreated/naive neonates at P7 (see Supplementary Figure 3; p<0.05; not shown). In the absence of injury, GH pretreatment also appeared to increase the baseline withdrawal time to 45°C heat compared to baseline latencies of untreated/naive mice at P7. However, GH did not affect baseline withdrawal latencies to 40°C (see Supplementary Figure 3; p<0.05; not shown). This effect did not however alter the results found at the various time points post inflammation. In other words, these alterations in baseline at the early age cannot account for the apparent beneficial effects of GH after injury as effects on baseline responses were only found at P7 and not P14 (see Supplementary Fig. 3).
When assessing the response properties of primary afferents using ex vivo recording in these P7 cohorts, we found that A-HTMR peak mechanical FRs were increased (n=10; p<0.05) at 1d post inflammation compared to naive cells (n=7). However, GH pretreatment in the inflamed mice produced mechanical responsiveness similar to naïve levels at 1d (n=7, p>0.05). No differences in mechanical firing were found between our groups at 3d (Supplementary Fig. 5A; Inflammation Only: n=5; Inflammation+GH: n=15). Although peak heat FRs were increased in A-HTMR neurons after carrageenan injection, this did not reach statistical significance (p<0.1). Peak heat IFs however, were found to be increased in “A”-fiber nociceptors (n=3) 1d after carrageenan injection into the hairy skin (Supplementary Fig. 5B; p<0.05). GH also appeared to restore heat firing; however, we were only able to record from one heat responsive A-fiber at 1d and one A-fiber at 3d in these mice. Therefore, we combined data from both time points to try to obtain at least some basic information regarding heat hypersensitivity in A-fiber HTMR neurons from inflamed mice treated with GH. When combining both time points, GH pretreatment was found to prevent inflammation induced heat hypersensitivity in A-HTMRs during P7 inflammation (n=2; Supplementary Fig. 5B, C). The mechanical and heat FRs in the CPM and CM neurons displayed no obvious changes post-inflammation at P7 and these were also unaffected by GH pretreatment (CPM: n=4–17 (mechanical), n=4–11 (heat); CM: n=5–9; p>0.05, not shown). Similar results were also found even if we combined all C-HTMRs (CM and CPM; n=11–23; p>0.05), thus supporting the notion that alterations in primary afferents during P7 cutaneous inflammation is restricted to sensitization in the faster conducting, broad spiking sensory neurons (A-fiber nociceptors), but a GH pretreatment can potentially block these effects. The average CVs for A-fibers were 4.04 m/s and average CVs for C-fibers were 0.52 m/s at P7.
When assessing other potential effects of GH on the P7 groups, we detected a small increase in body weight in P7 mice injected with carrageenan only, but GH had no effects on body weight in inflamed mice. We did find a small increase in body temperature at P7 in inflamed mice treated with GH relative to naïve (p<0.05), however the temperatures in these mice were not different than mice that received carrageenan alone (Supplementary Fig. 6A, B; n=4–12; p>0.05). At P7, carrageenan induced a similar level of paw edema in mice with GH pretreatment to that observed in mice with carrageenan injection alone (Supplementary Fig. 6C). Finally, significant increases in cutaneous IL1β and GDNF were found in inflamed mice vs. naives at P7, but GH had no effects on the inflammation-induced upregulation of these factors in the skin. No changes in NGF or TNFα were found in the skin after P7 cutaneous inflammation and GH did not alter this result (Supplementary Fig. 6D).
3.6. GH regulates the expression of IGFr1 in sensory neurons
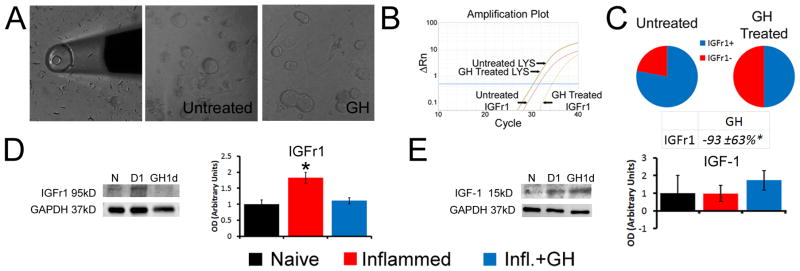
We next investigated potential downstream receptor mechanisms within sensory neurons underlying GH effects. We therefore first performed realtime PCR on the L2/L3 DRGs from mice with cutaneous inflammation. We surprisingly did not detect any differences in the expression of the GH receptor (GHr) in the DRGs of mice inflamed at P7 (−30.6±13%) or P14 (−40.7±23.3%) compared to age-matched naïve DRGs (p>0.05). Our previous data however [24] showed a significant increase in IGFr1 at both ages, which is a known downstream mediator of GH function in other systems [57] and has also been linked to mechanical and thermal hypersensitivity after inflammation [46,74]. Therefore, to test if GH regulated the expression of IGFr1 specifically in sensory neurons, we dissociated P14 DRGs and treated individual cultures with growth hormone. PCR analysis of single DRG neurons (n=20) showed that treatment of primary cultures with GH not only reduced the numbers of individual cells that expressed IGFr1, but the cells that contained IGFr1 in the GH treated cultures showed significantly reduced expression of this receptor (p<0.05) compared to untreated neurons (Fig. 5A–C). Using WB, we found that carrageenan induced an increase in IGFr1 protein in the DRGs of mice at P14. This increase however, was blocked by pretreatment with GH (Fig. 5D). Interestingly, we found no changes in IGF-1 in the skin from any group (Fig. 5E). These data suggest that IGFr1 may be one target by which GH could regulate peripheral hypersensitivity within sensory neurons during neonatal cutaneous inflammation.
Figure 5. Growth hormone (GH) regulates the expression of insulin like growth factor 1 receptor in vitro and after inflammation in vivo at P14.
Examples of the single cell collection method used for analysis and examples of primary dorsal root ganglion (DRG) cultures treated with or without GH (A). Single cell PCR results from the various culture conditions show that treatment of primary P14 DRG neurons (n=20) with GH significantly reduces the expression of IGFr1 in single cells (B, C). Example of an amplification plot obtained from a cell treated with GH compared to an untreated DRG neuron shows a rightward shift in the Ct value for IGFr1 in the GH treated neuron while the LYS normalization control gene remained constant in each cell (B). In addition to significantly reduced relative expression, the number of cells that express IGFr1 (IGFR1+) at detectable levels in GH treated cultures was also lower than the number of cells containing IGFr1 in untreated DRG neuron cultures (C). One day (D1) after carrageenan induced inflammation of the hairy hindpaw skin, a significant increase in IGFr1 protein is detected in the DRGs; however this is completely prevented in mice treated with GH at P14 (D; n=3–4 for each age). No changes in the ligand IGF-1 however, were detected in the skin among any of the experimental groups tested (E; n=3–4). Examples of IGFr1 and IGF-1 western blots along with their GAPDH are provided in panels D and E. * p < 0.05 vs. naïve (N); One-way ANOVA/Tukey’s post hoc test. Value in C is presented as a percent change from naïve.
3.7 Primary afferent knockdown of IGFr1 blocks peripheral hypersensitivity and alterations in sensory neuron response properties during neonatal cutaneous inflammation
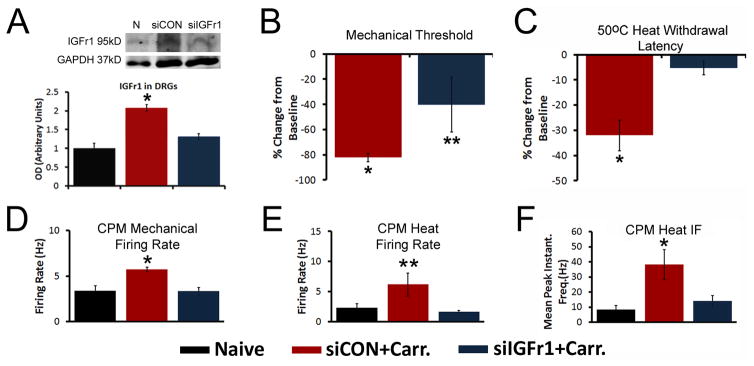
Based on the above information, we then utilized a nerve-specific siRNA mediated knockdown strategy to specifically inhibit the inflammation induced upregulation of IGFr1 in injured saphenous afferents. IGFr1 expression in the DRGs (n=6–14) of mice injected with non-targeting siRNAs (siCON) plus inflammation confirms our previous report of a significant increase in IGFr1 mRNA 1d after inflammation at P14 (95.3%±14.8%; p<0.05 vs naïve). Injection of IGFr1 targeting siRNAs (siIGFr1) in inflamed mice prevented this increase (49.7%±14.9%; p>0.05). WB results (n=3–4) also shows that siIGFr1 injection successfully blocked the inflammation-induced increase in IGFr1 protein in the DRGs (p<0.05, Fig. 6A).
Figure 6. Selective insulin like growth factor 1 receptor (IGFr1) knockdown inhibits mechanical and heat hypersensitivity during inflammation at P14.
A: Western blot results show that saphenous nerve injection of siRNAs targeting IGFr1 (siIGFr1) successfully blocks the increase in IGFr1 expression found in the DRGs of mice with saphenous nerve injection of control, non-targeting siRNAs (siCON) in addition to hairy skin carrageenan injection at P14 (n=3–4/group). Example western blots of IGFr1 and GAPDH are provided from each condition. B: At P14, siIGFr1 partially reversed the carrageenan induced reduction in ipsilateral mechanical withdrawal thresholds 1d after inflammation. C: The inflammation induced decrease in heat withdrawal latency at 50°C was completely blocked by siIGFr1 injection. Using ex vivo recording, the inflammation induced increase in polymodal “C”-fiber (CPM) neuron firing rates to both mechanical (D) and heat (E) stimuli and the mean peak instantaneous frequencies (IFs) to heat (F) were all blocked by afferent targeted IGFr1 siRNAs during inflammation. Results are consistent to that described for GH treatment above. A–C: * p<0.05 vs. naïve or baseline; **p<0.05 vs. baseline and 1d siCON+Carr.; One-way ANOVA with Holm Sidak or Tukey’s post hoc tests. D–F: *p<0.05 vs. Naïve and siCON+Carr.; **p<0.05 vs. siIGFr1+Carr. and p<0.06 vs. naïve; One-way ANOVA on Ranks/Dunn’s post hoc.
We then assessed behavioral hypersensitivity during cutaneous inflammation, in addition to afferent sensitization at P14. Ipsilateral hindpaw mechanical thresholds at 1d post inflammation were again decreased from baseline in the siCON+Carrageenan group (n=11; p<0.05), while in the siIGFr1+carrageenan group (n=10), the thresholds only partially decreased from their baseline (p<0.05, Fig. 6B). Again no changes were found in the contralateral limbs in any group at 1d (not shown). At 50°C, the heat withdrawal latencies were significantly decreased from baseline in the siCON group (p<0.05), but no significant changes were found in the siIGFr1 injected mice with inflammation (p>0.05, Fig. 6C, D). The heat withdrawal latencies at 45°C were not different between baseline and 1d inflammation for either siRNA injected group at P14 (not shown). No effects of siCON injection were detected in regards to mechanical sensitivity in mice at P14. The siRNA injection strategy also did not alter behavioral responses to heat stimulation (Supplementary Fig. 7).
Single unit recording using our ex vivo preparation showed no differences in mice with siCON injection plus carrageenan compared to mice that only received carrageenan injection (Table 1), therefore data from these two groups were combined to enhance statistical power. At P14, CPM mechanical FRs (n=14), heat FRs and heat IFs (n=12) all increased in the siCON group (p<0.05) after carrageenan compared to naïves (n=10 (mechanical) and n=7 (heat)). siIGFr1 injection prevented the inflammation induced alterations in the CPM neurons at this age (n=15 (mechanical) and n=11 (heat); p>0.05, Fig. 6D–F). Similar effects of IGFr1 knockdown were found on the CM neurons at this age in regards to the mechanical FRs (Naïve: 1.8±0.3Hz, n=8; siCON+carrageenan: 4.9±0.7Hz, n=12, p<0.05 vs. naive; siIGFr1+carrageenan: 3.5± 0.6Hz, n=6, p>0.05 vs. naive). A-HTMR mechanical FRs, however, were not different between any group (n=6–15; not shown); Finally, we were only able to record from one A-fiber that responded to heat at P14 from each of the siCON and siIGFr1 groups, thus we were unable to assess the effects of IGFr1 knockdown on A-fiber heat responses at this age.
Table 1.
Comparison of the response properties of A-HTMRs, CPM or CM fibers in mice at P7 or P14 with hairy skin inflammation alone or those with siCON injection plus inflammation as assessed with ex vivo recording. No differences detected.
| Mechanical Thresholds (g) | Mechanical Firing Rates (Hz) | Heat Thresholds (°C) | Heat Firing Rates (Hz) | |||||
|---|---|---|---|---|---|---|---|---|
| P7 | 1d Inflammation | siCON+Infl. | 1d Inflammation | siCON+Infl. | 1d Inflammation | siCON+Infl. | 1d Inflammation | siCON+Infl. |
| A-HTMR | 47.6±14.6 | 34.6±7.2 | 11.1±3.0 | 6.3±1.0 | nt | 39.9±6.7 | 12.7±6.2 | 8.3±2.6 |
| CPM | 24.6±12.6 | 28.5±7.6 | 6.5±1.3 | 4.2±0.9 | 45.9±0.9 | 45.8±1.4 | 1.5±0.3 | 2.2±0.3 |
| CM | 57.5±12.6 | 31.6±7.1 | 4.2±1.6 | 3.6±0.5 | nt | nt | nt | nt |
| P14 | 1d Inflammation | siCON+Infl. | 1d Inflammation | siCON+Infl. | 1d Inflammation | siCON+Infl. | 1d Inflammation | siCON+Infl. |
| A-HTMR | 19.5±7.5 | 37.8±21.5 | 9.0±1.3 | 6.9±1.5 | 47.5 | 45.6 | nt | nt |
| CPM | 15.4±7.8 | 29.8±9.2 | 5.9±0.3 | 5.7±0.3 | 40.3±4.0 | 42.1±1.9 | 6.0±2.5 | 6.2±1.9 |
| CM | 41.3±7.2 | 42.0±13.9 | 4.3±0.8 | 5.3±1.0 | nt | nt | nt | nt |
In order to again assess whether similar effects of GH could be found on IGFr1 expression or whether nerve specific IGFr1 inhibition could also blunt peripheral hypersensitivity found in younger neonates with inflammation, we performed similar experiments described above in these younger cohorts. As was found with P14 inflammation, P7 carrageenan injection upregulated IGFr1 in the DRGs, which was prevented by GH pretreatment, but inflammation did not alter cutaneous IGF-1 levels (Supplementary Fig. 8; n=3–4). In addition, siRNA mediated knockdown of IGFr1 in sensory neurons prevented the development of pain-related hypersensitivity (n=9–10), and the sensitization of A-HTMRs, normally seen after P7 inflammation (Supplementary Fig. 8). siCON injection alone slightly increased mechanical thresholds at P7, but did not alter heat (45°C) withdrawal latencies (see Supplementary Fig. 7). These data suggest that afferent selective knockdown of IGFr1 blocks peripheral hypersensitivity and alterations in sensory neuron response properties during neonatal cutaneous inflammation similar to GH pretreatment.
4. Discussion
4.1. Growth hormone and nociceptor sensitization
The initial postnatal period of life is a critical stage for the development of the peripheral nervous system [1,8,30,31,62], and early life injury produces long-term alterations in nociceptive processing [14,68]. Pain also occurs after injury in pediatric patients at all ages and we observe related effects in developing mice after injury [19] (Fig. 2, Supplementary Fig. 4). Furthermore, insults sustained during development induce patterns of sensory neuron sensitization [8,19,21,24] that differ from those seen in adults [48]. Thus the specific mechanisms by which pain may develop in neonates could be unique. Here we found that cutaneous inflammation in neonatal mice produced a transient reduction in GH levels selectively in the affected skin, which corresponded with the development and resolution of mechanical and thermal hypersensitivity during inflammation. Pretreatment of inflamed neonatal mice with exogenous GH prevented this hypersensitivity and blocked all of the injury-induced alterations in cutaneous afferents (Figs. 1–3, Supplementary Figs. 4, 5), possibly by preventing the upregulation of IGFr1 within sensory neurons (Figs. 5, 6, Supplementary Fig. 8).
Children with GHD [2,7,18] display a resting pain [2], which suggests that GH levels may have robust effects on sensory function during early life when the peripheral nervous system is undergoing normal functional and neurochemical changes [24,26,41,47]. During development, sensory neurons change their phenotype [45,71] as myelinated afferents lose heat sensitivity during the first week of life [71] while “C”-fibers gain heat sensitivity during the second week [24]. NGF and GDNF likely play a role in this normal phenotypic switch [24,41,47,55] in addition to afferent sensitization after injury [e.g. 20,22,24,56]. However, our data also suggests that GH may be one additional factor involved in shaping sensory responsiveness specifically in regards to how the afferents respond to neonatal injuries and generate a pain state (Figs. 1–3; Supplementary Figs. 3, 5). Since GH can influence the functional responsiveness of primary afferents during developmental inflammation, it will be important in the future to determine if GH also has the ability to affect normal development of the peripheral sensory system and whether GH has similar effects after injury at other ages (i.e. adults).
Interestingly, we observed an effect of GH on normal behavioral responses at P7 specifically. GH pretreatment reduced mechanical thresholds and increased heat withdrawal latencies in uninjured mice only at P7 (Suppl. Fig. 4). This change in mechanical and thermal sensitivity in the P7 mice essentially produced thresholds/latencies that were similar to naive P14 mice. As GH levels are known to increase over time during early life to enhance normal growth and development, artificial delivery of GH to a very young mouse may hasten the development of the peripheral sensory system even under our conditions in which we are using very low doses [57,62]. However, the fact that we are performing these tests on the hairy skin and not the glabrous skin may be another factor, as this technique becomes increasingly more difficult to perform as the animals get older. This is also the reason we did not perform these mechanical tests at 7d post P14 inflammation. Regardless, mice treated with GH still displayed a complete block of mechanical and heat hypersensitivity provoked by cutaneous inflammation (Fig. 2, Supplementary. Fig. 4).
GH has well-documented effects on growth and metabolism [18,70] and extended GH treatment in patients is known to produce side effects such as increased weight gain, transient fever or hyperglycemia. We found that a short course of low dose (0.5mg/kg, ip.; 1x/d for 3d prior to inflammation) GH was able to reverse pain-like behaviors (Fig. 2, Supplementary. Fig. 4) induced by cutaneous inflammation without inducing these classic side effects or growth promoting effects [12] of extended GH delivery in mice. Furthermore, the GH pretreatment regimen did not alter paw edema during inflammation, nor was it able to block select cytokine/growth factor production (IL1β, GDNF) in the skin (Figs. 4, Supplementary Fig. 6; not shown). Although it unclear how GH is reduced in the periphery during inflammation, our data suggests that subtle changes in peripheral GH levels may have profound effects on sensory function after injury possibly via direct effects on the afferents (Figs. 5, 6, Supplementary Fig. 8). Future studies will be needed however to fully confirm this notion.
4.2. GH regulates afferent sensitization possibly by suppressing IGFr1 upregulation
The GHRH-GH-IGF-IGFr system is important for the development of body growth and repair after tissue injury [32,57]. Similar to GH releasing molecules such as ghrelin or GHRH [10, 54], IGF-1 and its receptor IGFr1, have also been demonstrated to be involved in nociceptive processing [16,46] in adult rodents possibly by modulating neuronal excitability [74]. Interestingly, in mice overexpressing IGF-2, studies have found reduced sensory innervation of the skin, which may play a role in peripheral responsiveness [52]. Although we did not assess IGF-2, we did not detect any significant alterations in IGF-1 in the skin during neonatal cutaneous inflammation, nor was our transient, low dose GH therapy able to alter IGF-1 in the skin (Fig. 5, Supplementary Fig. 8). However, we did observe an upregulation of IGFr1 in the DRGs [24] (Fig. 5, Supplementary Fig. 8). IGFr1 is expressed in medium and small diameter DRG neurons [8], which are likely the cells that are experiencing sensitization during inflammation. Since GH can directly modulate the expression of IGFr1 in single primary afferent neurons (Fig. 5), we sought to determine if IGFr1 upregulation was one possible mediator of the observed hyper-responsiveness within sensory neurons that was downstream of reduced GH levels in the skin. Similar to GH pretreatment, we found that afferent selective knockdown of IGFr1 during neonatal inflammation also prevented the injury-induced alterations in cutaneous afferents in addition to mechanical and thermal hypersensitivity (Fig. 6, Supplementary Fig. 8).
Mechanistically, this may due to the fact that GH can modulate IGFr1 transcription through one of the many of the transcription factors that are known to be activated by GH signaling such as serum response factor or ELK1 [67]. Each of these factors have binding sites in the upstream promotor region of IGFr1 (MatInspector software) and have been shown to act as transcriptional repressors under certain contexts [35], which could be at play in our sensory neurons. Thus loss of GH could reduce the activation of the transcriptional repressors, subsequently permitting IGFr1 upregulation in the DRGs. IGFr1 would then have the ability to increase the responsiveness of DRG neurons to mechanical and thermal stimuli [46,74] by modulating excitability [74] or by regulating many of the transcription factors activated from IGFr1 signaling such as ELK-1, CREB, or NFAT [2,56,65; MatInspector] which can regulate the expression of various other sensory transduction receptors/channels (e.g. TRPM3, ASIC3, P2X3, TRPV1, Piezo2, etc) that are modulated during inflammation [24]. However, this will need to be confirmed in future experimentation. While a loss of cutaneous GH may seem counterintuitive during injury since it is a known tissue repair molecule [35], the transient nature of this loss may serve a role in dynamic IGFr1 upregulation in the DRGs to subsequently modulate mechanical and thermal responsiveness (i.e. pain), which is itself a protective measure for tissue repair. Although our results do not completely confirm whether GH is acting directly or indirectly on the sensory neurons, one plausible mechanism by which GH acts within sensory neurons to mediate hypersensitivity to peripheral inflammation in neonates is via its effects on IGFr1 upregulation.
4.3. Clinical Significance
GH has been reported to be an effective pain therapy for GHD children and patients with erythromelalgia [7,49]. Other reports have also shown that GH treatment is an effective pain therapy for patients with fibromyalgia [3,4,9,10,31,34,42]. However the effect in fibromyalgia patients was mainly found in those with corresponding low IGF-1 levels. Our data shows no effects of neonatal inflammation or GH pretreatment on IGF-1 in the skin (Fig. 5, Supplementary Fig. 8). Although future studies would be needed to confirm, this may suggest that the link to IGF-1 levels and the effectiveness of a GH therapy may be specific to widespread muscle pain disorders in adults and not neonatal inflammatory pain states. Nevertheless, our results show GH has profound effects on injury responses during postnatal development, suggesting that a short course of low dose GH replacement therapy may eventually be used as an effective pharmacological treatment strategy for neonatal pain that does not cause unwanted side effects typically seen with extended GH delivery in humans [2,53]. As neonatal injury is known to alter primary afferent function [24] which can reorganize the developing DH if initiated at early ages [68], this strategy may also mitigate the long-term consequences of early life insult on adult nociceptive processing [14,68]. Studies on the effects of localized GH delivery in acute pediatric pain or the effects of early life GH therapies on long-term hyperalgesic priming will be important in the future.
Supplementary Material
Supplementary Figure 1: Antibody controls for use of growth hormone (GH) antibody in western blotting and effects of GH treatment on cutaneous GH levels in naïve neonates.
A: In normal skin at P7 or P14, GH protein is detected at the predicted weight around 22kD using a GH antibody. Negative control experiments show that without primary antibody incubation, no bands are detected for GH at 22kD while GAPDH protein is readily detected in these samples. Processing blots with both the GH antibody and a GH blocking peptide also eliminates band detection at 22kD while GAPDH is still detected. B: Treating naïve neonates with GH for three days prior to analysis at 24hrs after the final dose, does not significantly alter the levels of cutaneous GH detected in either P7 or P14 mice. One-way ANOVA with Tukey’s post hoc test.
Supplementary Figure 2: Dose response analysis of the effectiveness of growth hormone (GH) treatment on mechanical and thermal sensitivity 1d post inflammation at P7 or P14.
Three different doses of GH (0.1, 0.25 and 0.5mg/kg) were injected (ip.) 1x per day for 3d prior to carrageenan induced hairy hindpaw skin inflammation at P7 or P14. Alternatively, a single injection of the highest dose (0.5mg/kg) was given at the same time as peripheral inflammation in other cohorts. In regards to the 3d pretreatment strategy, mechanical hypersensitivity (A) observed during inflammation is blocked at the higher doses of GH, as was heat hypersensitivity (B). Similar results were also found in mice at P14 for both mechanical and heat hypersensitivity (C, D). A single dose of 0.5mg/kg GH delivered at the time of inflammation however was insufficient to fully block mechanical (E) hypersensitivity found during carrageenan induced inflammation at P7. The single dose of GH at P7 was sufficient however to partially reduce heat hypersensitivity at P7 (F). No effects of single dose GH were found in mice inflamed/treated at P14 in regards to the observed mechanical (G) or heat, (H) hypersensitivity. *p<0.05 vs.baseline; #p<0.05 vs. baseline and carrageenan alone; One-way ANOVA/Holm Sidak post hoc.
Supplementary Figure 3: Comparison of the effects of GH treatment on baseline mechanical and heat responsiveness in neonates at P7 or P14.
GH treatment in un-inflamed mice at P7 reduces the baseline paw withdrawal threshold (in grams (g)) to mechanical stimulation of the hairy hindpaw skin compared to untreated naïve mice (A). Heat withdrawal latencies (in seconds (s)) were also increased by GH at this neonatal age when specifically assessing 45°C withdrawal latencies (B). GH treatment however in P14 mice did not alter baseline mechanical thresholds (C) or heat withdrawal latencies to 50°C water (D). *p<0.05 vs. naïve; ANOVA/Holm-Sidak post hoc.
Supplementary Figure 4: Effects of GH pretreatment on cutaneous GH levels and behavioral responsiveness in P7 neonates with hairy hindpaw skin inflammation.
A: Relative to naïve skin, the levels of GH protein detected in the hairy hindpaw skin is reduced 36.5% (±10%) 1d after cutaneous inflammation at P7. Levels return to that detected in naïve mice by 3d. GH pretreatment in inflamed P7 neonates completely blocked the reduction in GH levels induced by inflammation at 1d. No differences in GH protein were detected in mice with GH pretreatment and cutaneous inflammation at 3d relative to naïves. These levels were similar to that observed at the 1d time point in GH treated mice with P7 inflammation. B: Analysis of the forepaw (FP) or back skin of naïve mice or skin from these same areas obtained from mice with hairy hindpaw skin inflammation revealed no differences in GH protein levels. C: P7 neonates with cutaneous inflammation show significantly reduced mechanical withdrawal thresholds relative to their baseline at 1d and this is restored by 3d. GH pretreatment completely blocked mechanical hypersensitivity induced by carrageenan injection at 1d and this was also maintained throughout testing at 3d and 7d post injury in these cohorts. D: Heat withdrawal latencies to 45°C water are reduced by P7 inflammation at 1d and 3d and this returns to baseline by 7d post injury. GH pretreatment completely blocked this effect of carrageenan on heat responsiveness at all time points tested. GH treated mice with hairy skin inflammation did not vary from their baseline values for mechanical or heat sensitivity at any time point. *p<0.05 vs. naïve or baseline. One-way ANOVA with Tukey’s or Holm Sidak post hoc tests.
Supplementary Figure 5: Growth hormone (GH) pretreatment blocks hypersensitivity of “A”-fiber sensory neurons to mechanical and thermal stimuli during cutaneous inflammation initiated at P7 as assessed with ex vivo recording.
A: At P7, carrageenan injection into the hairy hindpaw skin was found to increase the firing of mechanically sensitive and sometimes heat sensitive A-fiber nociceptors (A-high threshold mechanoreceptor: A-HTMR) one day post inflammation; however, this was completely blocked by GH pretreatment. In fact, responses of A-HTMRs to mechanical stimuli did not change relative to naives at any time point post inflammation. B, C: Similar results on firing rate (B) and mean peak instantaneous frequencies (C) to heat were found in these cell types at P7 when combining data from both time points (1d and 3d). Examples of corresponding responses to various stimuli are presented with each panel(s). VF= von Frey filament (peak response for cell). *p<0.05 vs. naïve; # p <0.07 vs. naive; One-way ANOVA on Ranks/Dunn’s post hoc.
Supplementary Figure 6: Effects of GH pretreatment on body weight, temperature, paw edema and mRNA expression of select cytokines and growth factors in skin post inflammation at P7.
A, B: GH pretreatment had no overt effects on body weight (A) or body temperature (B) post treatment (1–3d) in mice with inflammation at P7. Although mice with inflammation only showed a slight increase in body weight from carrageenan injection relative to naïve animals, this was not found to be different than mice treated with GH (p>0.05). GH treated mice with inflammation were also not found to be different than naives at P7. Although no effects of inflammation were found on body temperature at P7, pretreatment of mice with GH showed a slight increase in body temperature that was different than naives, but not different than mice with carrageenan injection alone. * p<0.05 vs. naïve but not Carr. only; #p<0.05 vs. naïve, but not Carr.+GH treated mice; n=4–14/group; One-way ANOVA/Holm Sidak post hoc. C: Carrageenan induced paw edema was unaffected by GH pretreatment. Values presented represent the paw volume ratio between ipsilateral and contralateral hindpaws. D: Of the cytokines and growth factors found to be detected in skin of neonates after inflammation, the carrageenan induced upregulation of interleukin 1β (IL1β) and glial cell line-derived neurotrophic factor (GDNF) were not altered by GH pretreatment. Although each group showed enhanced expression in skin relative to naives, this increase in gene expression was not found to be different from each other. No changes in nerve growth factor (NGF) or tumor necrosis factor α (TNFα) were found in the skin in any group relative to naïves. n=3–4; One-way ANOVA/Tukey’s post hoc. Values are presented as a percent change from naïve.
Supplementary Figure 7: Behavioral effects of siRNA (siCON) injection on mechanical and heat withdrawal responses in P7 or P14 neonates. A: Injection of non-targeting control siRNAs (siCON) into un-inflamed mice (no carrageenan) slightly elevated mechanical withdrawal thresholds to von Frey filament stimulation of the hairy skin at P7, but this subtle increase in mechanical thresholds did not reach statistical significance at P14. B: No effects of siRNA injection were detected in regards to heat withdrawal latencies to 45°C (P7) or 50°C (P14) water relative to their baseline measurements at either age tested. *p<0.05 and #p<0.07 vs. baseline; One-way ANOVA with Holm-Sidak post hoc.
Supplementary Figure 8: Effects of GH on IGFr1/IGF-1 protein levels and consequences of nerve specific inhibition of IGFr1 on mechanical and heat responsiveness during P7 cutaneous inflammation.
A: Western blot results show that at P7, hairy hindpaw skin inflammation induces a significant increase in IGFr1 protein in the DRGs that can be inhibited by GH pretreatment. Neither carrageenan injection nor GH pretreatment in P7 inflamed neonates alters the levels of IGF-1 protein in the skin. C: Saphenous nerve injection of siRNAs targeting IGFr1 (siIGFr1) successfully blocks the increase in IGFr1 expression found in the DRGs of mice with saphenous nerve injection of control, non-targeting siRNAs (siCON) and hairy skin carrageenan injection at P7. A-C: Example western blots of IGFr1, IGF-1 and GAPDH are provided from each condition (n=3–4/group). D: siIGFr1 injection completely prevented the carrageenan (Carr.) induced decrease in ipsilateral mechanical paw withdrawal thresholds 1d after cutaneous inflammation (siCON+Carr). siIGFr1 injection also inhibited the inflammation-induced reduction in heat withdrawal latency at 45°C (E). F-G: siIGFr1 (n=4 (mechanical) and n=3 (heat)) reversed the inflammation (siCON+Carr) induced increase in A-fiber high threshold mechanoreceptor (A-HTMR) mechanical (n=28) firing rates (F), heat (n=9) firing rates (G) and mean peak instantaneous frequencies (IF) to heat (H) 1d after carrageenan injection into the hairy hindpaw skin. (naïve: n=7, mechanical; n=2, heat). *p<0.05 vs. naïve or baseline; #p>0.05 vs. baseline and siCON; ##p < 0.069 vs. siIGFr1+Carr. A-E: One-way ANOVA with Tukey’s or Holm-Sidak post hoc; F-G: Kruskal Wallis with Dunn’s post hoc.
Acknowledgments
This work was supported by NIH/NICHD (R03HD077483-01; MPJ), Rita Allen Foundation/American Pain Society (MPJ), the Board of Trustees at Cincinnati Children’s Hospital Medical Center (CCHMC) and the Department of Anesthesia at CCHMC. We would also like to thank Dr. Mark Baccei for helpful comments on the manuscript.
Footnotes
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Literature Cited
- 1.Beland BFM. Influence of peripheral inflammation on the postnatal maturation of primary sensory neuron phenotype in rats. J Pain. 2001;2:36–45. doi: 10.1054/jpai.2001.17697. [DOI] [PubMed] [Google Scholar]
- 2.Bennett R. Growth hormone in musculoskeletal pain states. Curr Rheumatol Rep. 2004;6:266–273. doi: 10.1007/s11926-004-0034-z. [DOI] [PubMed] [Google Scholar]
- 3.Bennett RM. Disordered growth hormone secretion in fibromyalgia: a review of recent findings and a hypothesized etiology. [Accessed 28 Jul 2016];Zeitschrift für Rheumatol. 1998 57(Suppl 2):72–6. doi: 10.1007/s003930050240. Available: http://www.ncbi.nlm.nih.gov/pubmed/10025088. [DOI] [PubMed] [Google Scholar]
- 4.Bjersing JL, Dehlin M, Erlandsson M, Bokarewa MI, Mannerkorpi K. Changes in pain and insulin-like growth factor 1 in fibromyalgia during exercise: the involvement of cerebrospinal inflammatory factors and neuropeptides. Arthritis Res Ther. 2012;14:R162. doi: 10.1186/ar3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 2008;56:791–800. doi: 10.1002/glia.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong PKK, Jung RT, Scrimgeour CM, Rennie MJ, Paterson CR. Energy expenditure and body composition in growth hormone deficient adults on exogenous growth hormone. Clin Endocrinol (Oxf) 1994;40:103–110. doi: 10.1111/j.1365-2265.1994.tb02451.x. [DOI] [PubMed] [Google Scholar]
- 7.Cimaz R, Rusconi R, Fossali E, Careddu P. Unexpected healing of cutaneous ulcers in a short child. Lancet (London, England) 2001;358:211–2. doi: 10.1016/S0140-6736(01)05413-7. [DOI] [PubMed] [Google Scholar]
- 8.Craner MJ, Klein JP, Black Ja, Waxman SG. Preferential expression of IGF-I in small DRG neurons and down-regulation following injury. Neuroreport. 2002;13:1649–52. doi: 10.1097/00001756-200209160-00016. [DOI] [PubMed] [Google Scholar]
- 9.Cuatrecasas G, Alegre C, Casanueva FF. GH/IGF1 axis disturbances in the fibromyalgia syndrome: is there a rationale for GH treatment? Pituitary. 2014;17:277–83. doi: 10.1007/s11102-013-0486-0. [DOI] [PubMed] [Google Scholar]
- 10.Cuatrecasas G, Gonzalez MJ, Alegre C, Sesmilo G, Fernandez-Solà J, Casanueva FF, Garcia-Fructuoso F, Poca-Dias V, Izquierdo JP, Puig-Domingo M. High prevalence of growth hormone deficiency in severe fibromyalgia syndromes. J Clin Endocrinol Metab. 2010;95:4331–7. doi: 10.1210/jc.2010-0061. [DOI] [PubMed] [Google Scholar]
- 11.Elitt CM, McIlwrath SL, Lawson JJ, Malin Sa, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farris GM, Miller GK, Wollenberg GK, Molon-Noblot S, Chan C, Prahalada S. Recombinant rat and mouse growth hormones: risk assessment of carcinogenic potential in 2-year bioassays in rats and mice. Toxicol Sci. 2007;97:548–61. doi: 10.1093/toxsci/kfm059. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald M, Beggs S. Book Review: The Neurobiology of Pain: Developmental Aspects. Neurosci. 2001;7:246–257. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- 14.Fred, Simon Targeting p38 Mitogen-activated Protein Kinase to Reduce the Impact of Neonatal Microglial Priming on Incision-induced Hyperalgesia in the Adult Rat. Anesthesiology. 2015;122:1377–1390. doi: 10.1097/ALN.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia JM, Cata JP, Dougherty PM, Smith RG. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology. 2008;149:455–60. doi: 10.1210/en.2007-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartner MH, Benson JD, Caldwell MD. Insulin-like growth factors I and II expression in the healing wound. J Surg Res. 1992;52:389–394. doi: 10.1016/0022-4804(92)90121-f. [DOI] [PubMed] [Google Scholar]
- 17.Goodrich CA. Measurement of body temperature in neonatal mice. J Appl Physiol. 1977;43:1102–5. doi: 10.1152/jappl.1977.43.6.1102. Available: http://www.ncbi.nlm.nih.gov/pubmed/606696. [DOI] [PubMed] [Google Scholar]
- 18.Herndon DN, Hawkins HK, Nguyen TT, Pierre E, Cox R, Barrow RE. Characterization of growth hormone enhanced donor site healing in patients with large cutaneous burns. Ann Surg. 1995;221:649–56. doi: 10.1097/00000658-199506000-00004. discussion 656–9. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1234688&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience. 2006;143:501–514. doi: 10.1016/j.neuroscience.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jankowski MP, Lawson JJ, McIlwrath SL, Rau KK, Anderson CE, Albers KM, Koerber HR. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci. 2009;29:1636–47. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jankowski MP, Rau KK, Soneji DJ, Anderson CE, Koerber HR. Enhanced artemin/GFRα3 levels regulate mechanically insensitive, heat-sensitive C-fiber recruitment after axotomy and regeneration. J Neurosci. 2010;30:16272–16283. doi: 10.1523/JNEUROSCI.2195-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankowski MP, Rau KK, Soneji DJ, Ekmann KM, Anderson CE, Molliver DC, Koerber HR. Purinergic receptor P2Y1 regulates polymodal C-fiber thermal thresholds and sensory neuron phenotypic switching during peripheral inflammation. Pain. 2012;153:410–419. doi: 10.1016/j.pain.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jankowski MP, Ross JL, Weber JD, Lee FB, Shank AT, Hudgins RC. Age-dependent sensitization of cutaneous nociceptors during developmental inflammation. Mol Pain. 2014;10:34. doi: 10.1186/1744-8069-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings E, Fitzgerald M. Postnatal changes in responses of rat dorsal horn cells to afferent stimulation: a fibre-induced sensitization. J Physiol. 1998;509(Pt 3):859–68. doi: 10.1111/j.1469-7793.1998.859bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kastrup Y, Le Greves M, Nyberg F, Blomqvist A. Distribution of growth hormone receptor mRNA in the brain and spinal cord of the rat. Neuroscience. 2005;130:419–25. doi: 10.1016/j.neuroscience.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Koch SC, Tochiki KK, Hirschberg S, Fitzgerald M. C-fiber activity-dependent maturation of glycinergic inhibition in the spinal dorsal horn of the postnatal rat. Proc Natl Acad Sci. 2012;109:12201–12206. doi: 10.1073/pnas.1118960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koltzenburg M, Stucky CL, Lewin GR. Receptive Properties of Mouse Sensory Neurons Innervating Hairy Skin. [Accessed 22 Oct 2015];J Neurophysiol. 1997 78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. Available: http://jn.physiology.org/content/78/4/1841.short. [DOI] [PubMed] [Google Scholar]
- 29.Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. [Accessed 9 Jun 2016];J Neurophysiol. 1992 68:581–595. doi: 10.1152/jn.1992.68.2.581. Available: http://jn.physiology.org/content/68/2/581.short. [DOI] [PubMed] [Google Scholar]
- 30.Kurimoto K, Yabuta Y, Ohinata Y, Ono Y, Uno KD, Yamada RG, Ueda HR, Saitou M. An improved single-cell cDNA amplification method for efficient high-density oligonucleotide microarray analysis. Nucleic Acids Res. 2006;34:e42. doi: 10.1093/nar/gkl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landis CA, Lentz MJ, Rothermel J, Riffle SC, Chapman D, Buchwald D, Shaver JL. Decreased nocturnal levels of prolactin and growth hormone in women with fibromyalgia. J Clin Endocrinol Metab. 2001;86:1672–8. doi: 10.1210/jcem.86.4.7427. [DOI] [PubMed] [Google Scholar]
- 32.Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord. 2007;7:225–235. doi: 10.1007/s11154-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 33.Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 Unlike TRPV2 Is Restricted to a Subset of Mechanically Insensitive Cutaneous Nociceptors Responding to Heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leal-Cerro A, Povedano J, Astorga R, Gonzalez M, Silva H, Garcia-Pesquera F, Casanueva FF, Dieguez C. The growth hormone (GH)-releasing hormone-GH-insulin-like growth factor-1 axis in patients with fibromyalgia syndrome. J Clin Endocrinol Metab. 1999;84:3378–81. doi: 10.1210/jcem.84.9.5982. [DOI] [PubMed] [Google Scholar]
- 35.Lee S-M, Vasishtha M, Prywes R. Activation and repression of cellular immediate early genes by serum response factor cofactors. J Biol Chem. 2010;285:22036–22049. doi: 10.1074/jbc.M110.108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Baccei ML. Excitatory synapses in the rat superficial dorsal horn are strengthened following peripheral inflammation during early postnatal development. Pain. 2009;143:56–64. doi: 10.1016/j.pain.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Baccei ML. Pacemaker neurons within newborn spinal pain circuits. J Neurosci. 2011;31:9010–9022. doi: 10.1523/JNEUROSCI.6555-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Eisenach JC. α 2A-Adrenoceptor Stimulation Reduces Capsaicin-Induced Glutamate Release from Spinal Cord Synaptosomes. 2001;299:939–944. [PubMed] [Google Scholar]
- 39.De Lima J, Alvares D, Hatch DJ, Fitzgerald M. Sensory hyperinnervation after neonatal skin wounding: effect of bupivacaine sciatic nerve block. Br J Anaesth. 1999;83:662–664. doi: 10.1093/bja/83.4.662. [DOI] [PubMed] [Google Scholar]
- 40.Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–56. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A Hierarchical NGF Signaling Cascade Controls Ret-Dependent and Ret-Independent Events during Development of Nonpeptidergic DRG Neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 42.Malemud CJ. The basis for medical therapy of fibromyalgia with growth hormone. Pain. 2012;153:1342–1343. doi: 10.1016/j.pain.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 43.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–60. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 44.Marsh D, Dickenson A, Hatch D, Fitzgerald M. Epidural opioid analgesia in infant rats II: responses to carrageenan and capsaicin. Pain. 1999;82:33–38. doi: 10.1016/S0304-3959(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 45.Mearow KM, Kril Y, Diamond J. Increased NGF mRNA expression in denervated rat skin. [Accessed 23 Oct 2015];Neuroreport. 1993 4:351–4. doi: 10.1097/00001756-199304000-00002. Available: http://www.ncbi.nlm.nih.gov/pubmed/8499587. [DOI] [PubMed] [Google Scholar]
- 46.Miura M, Sasaki M, Mizukoshi K, Shibasaki M, Izumi Y, Shimosato G, Amaya F. Peripheral sensitization caused by insulin-like growth factor 1 contributes to pain hypersensitivity after tissue injury. Pain. 2011;152:888–895. doi: 10.1016/j.pain.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Molliver D, Wright D, Leitner M, Parsadanian AS, Doster K, Wen D, Yan Q, Snider W. IB4-Binding DRG Neurons Switch from NGF to GDNF Dependence in Early Postnatal Life. Neuron. 1997;19:849–861. doi: 10.1016/S0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 48.Molliver DC, Rau KK, McIlwrath SL, Jankowski MP, Koerber HR. The ADP receptor P2Y1 is necessary for normal thermal sensitivity in cutaneous polymodal nociceptors. Mol Pain. 2011;7:13. doi: 10.1186/1744-8069-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nathan a, Rose JB, Guite JW, Hehir D, Milovcich K. Primary Erythromelalgia in a Child Responding to Intravenous Lidocaine and Oral Mexiletine Treatment. Pediatrics. 2005;115:e504–e507. doi: 10.1542/peds.2004-1395. [DOI] [PubMed] [Google Scholar]
- 50.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol. 2002;87:721–731. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 51.Pu SF, Zhuang HX, Marsh DJ, Ishii DN. Insulin-like growth factor-II increases and IGF is required for postnatal rat spinal motoneuron survival following sciatic nerve axotomy. J Neurosci Res. 1999;55:9–16. doi: 10.1002/(SICI)1097-4547(19990101)55:1<9::AID-JNR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds ML, Ward A, Graham CF, Coggeshall RFM. Decreased skin sensory innervation in transgenic mice overexpressing insulin-like growth factor-II. Neuroscience. 1997;79:789–797. doi: 10.1016/s0306-4522(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 53.Rhodin a, von EHREN M, Skottheim B, Grönbladh a, Ortiz-Nieto F, Raininko R, Gordh T, Nyberg F. Recombinant human growth hormone improves cognitive capacity in a pain patient exposed to chronic opioids. Acta Anaesthesiol Scand. 2014;58:759–765. doi: 10.1111/aas.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ririe DG, Vernon TL, Tobin JR, Eisenach JC. Age-dependent Responses to Thermal Hyperalgesia and Mechanical Allodynia in a Rat Model of Acute Postoperative Pain. Anesthesiology. 2003;99:443–448. doi: 10.1097/00000542-200308000-00027. [DOI] [PubMed] [Google Scholar]
- 55.Ritter aM, Lewin GR, Kremer NE, Mendell LM. Requirement for nerve growth factor in the development of myelinated nociceptors in vivo. Nature. 1991;350:500–502. doi: 10.1038/350500a0. [DOI] [PubMed] [Google Scholar]
- 56.Ritter aM, Woodbury CJ, Albers K, Davis BM, Koerber HR. Maturation of cutaneous sensory neurons from normal and NGF-overexpressing mice. J Neurophysiol. 2000;83:1722–1732. doi: 10.1152/jn.2000.83.3.1722. Available: http://www.ncbi.nlm.nih.gov/pubmed/10712492. [DOI] [PubMed] [Google Scholar]
- 57.Rosenfeld RG, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res. 2009;71:36–40. doi: 10.1159/000192434. [DOI] [PubMed] [Google Scholar]
- 58.Ross JL, Queme LQ, Cohen ER, Green KJ, Lu P, Shank AT, An S, Hudgins RC, JM Muscle IL1β drives ischemic myalgia via ASIC3-mediated sensory neuron sensitization. J Neurosci. 2016 doi: 10.1523/JNEUROSCI.4582-15.2016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross JL, Queme LF, Shank AT, Hudgins RC, Jankowski MP. Sensitization of group III and IV muscle afferents in the mouse after ischemia and reperfusion injury. J Pain. 2014;15:1257–70. doi: 10.1016/j.jpain.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salomon F, Cuneo RC, Hesp R, Sönksen PH. [Accessed 9 Jun 2016];The Effects of Treatment with Recombinant Human Growth Hormone on Body Composition and Metabolism in Adults with Growth Hormone Deficiency. 2010 doi: 10.1056/NEJM198912283212605. Available: http://www.nejm.org/doi/full/10.1056/NEJM198912283212605. [DOI] [PubMed]
- 61.Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005;132:193–201. doi: 10.1016/j.neuroscience.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 62.Sonntag WE, Csiszar A, De Cabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: Progress and controversies. Journals Gerontol - Ser A Biol Sci Med Sci. 2012;67A:587–598. doi: 10.1093/gerona/gls115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Souza FM, Collett-Solberg PF. Adverse effects of growth hormone replacement therapy in children. Arq Bras Endocrinol Metabol. 2011;55:559–565. doi: 10.1590/S0004-27302011000800009. [DOI] [PubMed] [Google Scholar]
- 64.Talhouk RS, Saadé NE, Mouneimne G, Masaad Ca, Safieh-Garabedian B. Growth hormone releasing hormone reverses endotoxin-induced localized inflammatory hyperalgesia without reducing the upregulated cytokines, nerve growth factor and gelatinase activity. Prog Neuro-Psychopharmacology Biol Psychiatry. 2004;28:625–631. doi: 10.1016/j.pnpbp.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Torsney C, Fitzgerald M. Age-dependent effects of peripheral inflammation on the electrophysiological properties of neonatal rat dorsal horn neurons. [Accessed 23 Oct 2015];J Neurophysiol. 2002 87:1311–7. doi: 10.1152/jn.00462.2001. Available: http://jn.physiology.org/content/87/3/1311.abstract. [DOI] [PubMed] [Google Scholar]
- 66.Valdés Ja, Flores S, Fuentes EN, Osorio-Fuentealba C, Jaimovich E, Molina A, Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K, Zheng W-H, Quirion R. IGF-1 induces IP3-dependent calcium signal involved in the regulation of myostatin gene expression mediated by NFAT during myoblast differentiation. BMC Neurosci. 2013;56:791–800. doi: 10.1002/jcp.24298. [DOI] [PubMed] [Google Scholar]
- 67.Varco-Merth B, Rotwein P. Differential effects of STAT proteins on growth hormone-mediated IGF-I gene expression. Am J Physiol Endocrinol Metab. 2014;307:E847–55. doi: 10.1152/ajpendo.00324.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker SM, Beggs S, Baccei ML. Persistent changes in peripheral and spinal nociceptive processing after early tissue injury. Exp Neurol. 2016 doi: 10.1016/j.expneurol.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105:185–195. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 70.Waters MJ, Hoang HN, Fairlie DP, Pelekanos Ra, Brown RJ. New insights into growth hormone action. J Mol Endocrinol. 2006;36:1–7. doi: 10.1677/jme.1.01933. [DOI] [PubMed] [Google Scholar]
- 71.Ye Y, Woodbury CJ. Early postnatal loss of heat sensitivity among cutaneous myelinated nociceptors in Swiss-Webster mice. J Neurophysiol. 2010;103:1385–1396. doi: 10.1152/jn.00472.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu Z-B, Dong X-Y, Han S-P, Chen Y-L, Qiu Y-F, Sha L, Sun Q, Guo X-R. Transcutaneous bilirubin nomogram for predicting neonatal hyperbilirubinemia in healthy term and late-preterm Chinese infants. Eur J Pediatr. 2011;170:185–91. doi: 10.1007/s00431-010-1281-9. [DOI] [PubMed] [Google Scholar]
- 73.Zhang C, Li G, Liang S, Xu C, Zhu G, Wang Y, Zhang A, Wan F. Myocardial ischemic nociceptive signaling mediated by P2X3 receptor in rat stellate ganglion neurons. Brain Res Bull. 2008;75:77–82. doi: 10.1016/j.brainresbull.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Qin W, Qian Z, Liu X, Wang H, Gong S, Sun Y-G, Snutch TP, Jiang X, Tao J. Peripheral pain is enhanced by insulin-like growth factor 1 through a G protein-mediated stimulation of T-type calcium channels. Sci Signal. 2014;7:ra94–ra94. doi: 10.1126/scisignal.2005283. [DOI] [PubMed] [Google Scholar]
- 75.Zheng W-H, Quirion R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 2006;7:51. doi: 10.1186/1471-2202-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Antibody controls for use of growth hormone (GH) antibody in western blotting and effects of GH treatment on cutaneous GH levels in naïve neonates.
A: In normal skin at P7 or P14, GH protein is detected at the predicted weight around 22kD using a GH antibody. Negative control experiments show that without primary antibody incubation, no bands are detected for GH at 22kD while GAPDH protein is readily detected in these samples. Processing blots with both the GH antibody and a GH blocking peptide also eliminates band detection at 22kD while GAPDH is still detected. B: Treating naïve neonates with GH for three days prior to analysis at 24hrs after the final dose, does not significantly alter the levels of cutaneous GH detected in either P7 or P14 mice. One-way ANOVA with Tukey’s post hoc test.
Supplementary Figure 2: Dose response analysis of the effectiveness of growth hormone (GH) treatment on mechanical and thermal sensitivity 1d post inflammation at P7 or P14.
Three different doses of GH (0.1, 0.25 and 0.5mg/kg) were injected (ip.) 1x per day for 3d prior to carrageenan induced hairy hindpaw skin inflammation at P7 or P14. Alternatively, a single injection of the highest dose (0.5mg/kg) was given at the same time as peripheral inflammation in other cohorts. In regards to the 3d pretreatment strategy, mechanical hypersensitivity (A) observed during inflammation is blocked at the higher doses of GH, as was heat hypersensitivity (B). Similar results were also found in mice at P14 for both mechanical and heat hypersensitivity (C, D). A single dose of 0.5mg/kg GH delivered at the time of inflammation however was insufficient to fully block mechanical (E) hypersensitivity found during carrageenan induced inflammation at P7. The single dose of GH at P7 was sufficient however to partially reduce heat hypersensitivity at P7 (F). No effects of single dose GH were found in mice inflamed/treated at P14 in regards to the observed mechanical (G) or heat, (H) hypersensitivity. *p<0.05 vs.baseline; #p<0.05 vs. baseline and carrageenan alone; One-way ANOVA/Holm Sidak post hoc.
Supplementary Figure 3: Comparison of the effects of GH treatment on baseline mechanical and heat responsiveness in neonates at P7 or P14.
GH treatment in un-inflamed mice at P7 reduces the baseline paw withdrawal threshold (in grams (g)) to mechanical stimulation of the hairy hindpaw skin compared to untreated naïve mice (A). Heat withdrawal latencies (in seconds (s)) were also increased by GH at this neonatal age when specifically assessing 45°C withdrawal latencies (B). GH treatment however in P14 mice did not alter baseline mechanical thresholds (C) or heat withdrawal latencies to 50°C water (D). *p<0.05 vs. naïve; ANOVA/Holm-Sidak post hoc.
Supplementary Figure 4: Effects of GH pretreatment on cutaneous GH levels and behavioral responsiveness in P7 neonates with hairy hindpaw skin inflammation.
A: Relative to naïve skin, the levels of GH protein detected in the hairy hindpaw skin is reduced 36.5% (±10%) 1d after cutaneous inflammation at P7. Levels return to that detected in naïve mice by 3d. GH pretreatment in inflamed P7 neonates completely blocked the reduction in GH levels induced by inflammation at 1d. No differences in GH protein were detected in mice with GH pretreatment and cutaneous inflammation at 3d relative to naïves. These levels were similar to that observed at the 1d time point in GH treated mice with P7 inflammation. B: Analysis of the forepaw (FP) or back skin of naïve mice or skin from these same areas obtained from mice with hairy hindpaw skin inflammation revealed no differences in GH protein levels. C: P7 neonates with cutaneous inflammation show significantly reduced mechanical withdrawal thresholds relative to their baseline at 1d and this is restored by 3d. GH pretreatment completely blocked mechanical hypersensitivity induced by carrageenan injection at 1d and this was also maintained throughout testing at 3d and 7d post injury in these cohorts. D: Heat withdrawal latencies to 45°C water are reduced by P7 inflammation at 1d and 3d and this returns to baseline by 7d post injury. GH pretreatment completely blocked this effect of carrageenan on heat responsiveness at all time points tested. GH treated mice with hairy skin inflammation did not vary from their baseline values for mechanical or heat sensitivity at any time point. *p<0.05 vs. naïve or baseline. One-way ANOVA with Tukey’s or Holm Sidak post hoc tests.
Supplementary Figure 5: Growth hormone (GH) pretreatment blocks hypersensitivity of “A”-fiber sensory neurons to mechanical and thermal stimuli during cutaneous inflammation initiated at P7 as assessed with ex vivo recording.
A: At P7, carrageenan injection into the hairy hindpaw skin was found to increase the firing of mechanically sensitive and sometimes heat sensitive A-fiber nociceptors (A-high threshold mechanoreceptor: A-HTMR) one day post inflammation; however, this was completely blocked by GH pretreatment. In fact, responses of A-HTMRs to mechanical stimuli did not change relative to naives at any time point post inflammation. B, C: Similar results on firing rate (B) and mean peak instantaneous frequencies (C) to heat were found in these cell types at P7 when combining data from both time points (1d and 3d). Examples of corresponding responses to various stimuli are presented with each panel(s). VF= von Frey filament (peak response for cell). *p<0.05 vs. naïve; # p <0.07 vs. naive; One-way ANOVA on Ranks/Dunn’s post hoc.
Supplementary Figure 6: Effects of GH pretreatment on body weight, temperature, paw edema and mRNA expression of select cytokines and growth factors in skin post inflammation at P7.
A, B: GH pretreatment had no overt effects on body weight (A) or body temperature (B) post treatment (1–3d) in mice with inflammation at P7. Although mice with inflammation only showed a slight increase in body weight from carrageenan injection relative to naïve animals, this was not found to be different than mice treated with GH (p>0.05). GH treated mice with inflammation were also not found to be different than naives at P7. Although no effects of inflammation were found on body temperature at P7, pretreatment of mice with GH showed a slight increase in body temperature that was different than naives, but not different than mice with carrageenan injection alone. * p<0.05 vs. naïve but not Carr. only; #p<0.05 vs. naïve, but not Carr.+GH treated mice; n=4–14/group; One-way ANOVA/Holm Sidak post hoc. C: Carrageenan induced paw edema was unaffected by GH pretreatment. Values presented represent the paw volume ratio between ipsilateral and contralateral hindpaws. D: Of the cytokines and growth factors found to be detected in skin of neonates after inflammation, the carrageenan induced upregulation of interleukin 1β (IL1β) and glial cell line-derived neurotrophic factor (GDNF) were not altered by GH pretreatment. Although each group showed enhanced expression in skin relative to naives, this increase in gene expression was not found to be different from each other. No changes in nerve growth factor (NGF) or tumor necrosis factor α (TNFα) were found in the skin in any group relative to naïves. n=3–4; One-way ANOVA/Tukey’s post hoc. Values are presented as a percent change from naïve.
Supplementary Figure 7: Behavioral effects of siRNA (siCON) injection on mechanical and heat withdrawal responses in P7 or P14 neonates. A: Injection of non-targeting control siRNAs (siCON) into un-inflamed mice (no carrageenan) slightly elevated mechanical withdrawal thresholds to von Frey filament stimulation of the hairy skin at P7, but this subtle increase in mechanical thresholds did not reach statistical significance at P14. B: No effects of siRNA injection were detected in regards to heat withdrawal latencies to 45°C (P7) or 50°C (P14) water relative to their baseline measurements at either age tested. *p<0.05 and #p<0.07 vs. baseline; One-way ANOVA with Holm-Sidak post hoc.
Supplementary Figure 8: Effects of GH on IGFr1/IGF-1 protein levels and consequences of nerve specific inhibition of IGFr1 on mechanical and heat responsiveness during P7 cutaneous inflammation.
A: Western blot results show that at P7, hairy hindpaw skin inflammation induces a significant increase in IGFr1 protein in the DRGs that can be inhibited by GH pretreatment. Neither carrageenan injection nor GH pretreatment in P7 inflamed neonates alters the levels of IGF-1 protein in the skin. C: Saphenous nerve injection of siRNAs targeting IGFr1 (siIGFr1) successfully blocks the increase in IGFr1 expression found in the DRGs of mice with saphenous nerve injection of control, non-targeting siRNAs (siCON) and hairy skin carrageenan injection at P7. A-C: Example western blots of IGFr1, IGF-1 and GAPDH are provided from each condition (n=3–4/group). D: siIGFr1 injection completely prevented the carrageenan (Carr.) induced decrease in ipsilateral mechanical paw withdrawal thresholds 1d after cutaneous inflammation (siCON+Carr). siIGFr1 injection also inhibited the inflammation-induced reduction in heat withdrawal latency at 45°C (E). F-G: siIGFr1 (n=4 (mechanical) and n=3 (heat)) reversed the inflammation (siCON+Carr) induced increase in A-fiber high threshold mechanoreceptor (A-HTMR) mechanical (n=28) firing rates (F), heat (n=9) firing rates (G) and mean peak instantaneous frequencies (IF) to heat (H) 1d after carrageenan injection into the hairy hindpaw skin. (naïve: n=7, mechanical; n=2, heat). *p<0.05 vs. naïve or baseline; #p>0.05 vs. baseline and siCON; ##p < 0.069 vs. siIGFr1+Carr. A-E: One-way ANOVA with Tukey’s or Holm-Sidak post hoc; F-G: Kruskal Wallis with Dunn’s post hoc.