Abstract
Chemotherapeutic agents are widely used to treat patients with systemic cancer. The efficacy of these therapies is undermined by their adverse side-effect profiles such as cognitive deficits that have a negative impact on the quality of life of cancer survivors. Cognitive side effects occur across a variety of domains, including memory, executive function, and processing speed. Such impairments are exacerbated under cognitive challenges and a subgroup of patients experience long-term impairments. Episodic memory in rats can be examined using a source memory task. In the current study, rats received paclitaxel, a taxane-derived chemotherapeutic agent, and learning and memory functioning was examined using the source memory task. Treatment with paclitaxel did not impair spatial and episodic memory, and paclitaxel treated rats were not more susceptible to cognitive challenges. Under conditions in which memory was not impaired, paclitaxel treatment impaired learning of new rules, documenting a decreased sensitivity to changes in experimental contingencies. These findings provide new information on the nature of cancer chemotherapy-induced cognitive impairments, particularly regarding the incongruent vulnerability of episodic memory and new learning following treatment with paclitaxel.
Keywords: episodic memory, source memory, spatial memory, learning, paclitaxel, rats
1. Introduction
Chemotherapeutic agents are widely used to treat patients with systemic cancer. However, the efficacy of these therapies is undermined by their adverse side-effect profiles such as cognitive deficits that have a negative impact on the quality of life of cancer survivors. Subtle cognitive side effects have been observed in a substantial number of patients, with incidence ranging from 15–80% of patients receiving chemotherapy [1]. Commonly reported domains of cognition affected by chemotherapy treatment include memory, executive function, and processing speed [2, 3]. While recovery of cognitive function is observed, a subgroup of patients experience long-term impairments that can be observed up to one year following chemotherapy treatment, severely impacting patients’ abilities to return to academic, occupational, or social activities [1, 4].
This family of symptoms suggests a variety of mechanisms targeted by chemotherapeutics. The development of rodent models of chemotherapy-induced cognitive impairments has helped to elucidate the driving factors, which are not limited to, but include, decreased neurogenesis, neurotoxic effects of inflammation and cytokine deregulation, oestrogen or testosterone reduction, and genetic variation [2]. These cellular and morphological changes following chemotherapy treatment are correlated with impairments seen in learning and memory [5, 6] but are uncorrelated with other memory assessments [7]. With the exception of a body of work by Winocur and colleagues [6, 8], the predominant measure of cognitive impairment in rodent models is spatial learning and memory as measured through tasks that involve hippocampal and frontal network systems such as Morris water maze learning and memory, novel location recognition, and non-matching to sample [1, 5, 8–11].
Paclitaxel and docetaxel, both representing the taxane family of drugs, are widely used in the treatment of ovarian cancer, breast cancer, lung cancer, and pancreatic cancer. Suppression of cell growth is achieved through the polymerization of microtubules by binding to the beta subunit of tubulin. Paclitaxel leads to symptoms of peripheral neuropathy in animal models. Additionally, acute encephalopathy in patients that have received paclitaxel has been documented [12–14]. Self-reported cognitive symptoms (i.e. confusion, word finding difficulty, and behavioral changes) have been reported with an onset as short as 5 hours and a duration lasting as long as 6 months post paclitaxel administration [12]. In a clinical analysis of cognitive functioning, mood, and quality of life of breast cancer patients receiving paclitaxel, the CNS risk of paclitaxel was confirmed; patients receiving a combination of paclitaxel, 5-fluorouracik, Adriamycin, and cyclophosphamide showed deficits in learning and memory up to a year following the termination of treatment [15]. Additionally, rodents treated with paclitaxel or docetaxel show impairments in learning and memory [10, 11, 16]. Despite paclitaxel’s low rate of CNS penetration, paclitaxel-related changes in morphology and cognition suggest detrimental impact of the treatment of paclitaxel and other taxanes on cognitive functioning.
Efforts of researchers to model chemotherapy-induced cognitive impairments have been undermined by inconsistent and conflicting results. This field of research is further hindered by the narrow range of tasks used to document impairments in rodents despite the wide range in symptoms observed in human patients. Tasks utilizing measures of spatial memory consistently document a chemotherapy-induced impairment in learning and memory. However, the pattern of development is often subtle, incongruent, and transient. Chemotherapy-treated rodents show incongruent rates of recovery with hippocampal-based tasks showing long-term impairments while frontal-lobe based tasks show recovery of function [8]. This acute and transient effect is also observed in learning, wherein chemotherapy affects the rate of learning, but may not be altogether debilitating. The subtlety and variety of effects observed highlights the need for the inclusion of tasks that are sensitive to subtle changes in cognition and encompass the range of impairments observed in human patients. There have been promising efforts to elucidate these subtle changes through the use of cognitive challenges. Studies of patients treated with chemotherapy suggest that an attenuation of cognitive impairments may be observed in patients after introduction of an increased memory load [17]. Additionally, damage to the hippocampus caused rats to be more susceptible to high-interference conditions [18], an effect that was replicated in a rat model of chemotherapy-induced hippocampal neuropathy [6].
One potential means to study cognitive impairment following chemotherapy treatment in an animal model is the source memory task, a radial arm maze procedure that requires the animal to remember the source of information. Source memory is a key feature of episodic memory in humans and rodents. Impairments in source memory are a characteristic of key disorders in memory, such as Alzheimer’s disease [19], mild cognitive impairment [20], amnesia [21], and normal aging [22]. We have previously shown that the source memory task allows for the independent measure of spatial and episodic memory and rule learning [23–26]. Furthermore, this task is hippocampal-dependent [25], and is sensitive to pharmacologically-induced episodic-memory impairments that are not detected in a spatial-memory task [23]. In the source memory task, rats are presented with the opportunity to encode multiple features of an event, including what-where-source information: what (food flavor), where (maze location), and source (self-generated food seeking/running to the food site; or experimenter-generated food seeking/placement by the experimenter at the food site). A replenishment rule dictates whether food locations will provide additional food in the future. In our approach, one randomly selected location each session, is designated as the replenishment location, and a second randomly selected location is designated as the non-replenishment location. Accurate memory of these episodic features allows rats to predict the replenishment of a favorable food reward, whereas the absence of source memory would result in the inability to predict the replenishment. Our index of source memory is a higher probability of revisiting the replenishment location relative to the non-replenishment location. Chemotherapy-induced episodic memory impairment has not been investigated in rodents but has been reported as an observed impairment following chemotherapy in humans [27]. By developing an animal model of chemotherapy using source memory, the mechanism underlying episodic memory impairment may be explored.
The present study thus examined the effects of the chemotherapeutic agent paclitaxel on the ability of rats to learn and remember events. Rats trained in the source memory task were treated with paclitaxel or vehicle and memory function was examined using the source memory task, which allows for concurrent examination of both spatial and source memory (described in Experiment 1). A lack of observed impairment in the source memory task does not definitively support the conclusion that episodic and spatial memory are not vulnerable to chemotherapy. As mentioned previously, acute impairments following chemotherapy treatment may be detectable in some experimental conditions, while absent in others. One method of revealing subtle impairments is through the introduction of a cognitive challenge; a cognitive challenge is the introduction of increased demand on cognitive processes. If treatment with paclitaxel impairs episodic memory, introducing a cognitive challenge is an approach to revealing the putative impairment. Methods by which a cognitive challenge can be implemented within the source memory task include increasing the time in which rats must retain the source and spatial information and through the development of proactive interference.
Lastly, extensive research in human patients receiving chemotherapy has revealed that a number of domains of cognition are impaired, while others are spared. Learning is one domain of cognition that is impaired. Rule learning is a feature embedded within the source memory task. Rats predict replenishment and non-replenishment locations based on a replenishment rule. A learning task can be implemented by reversing the replenishment rule, requiring rats to re-learn which items of source information predict replenishment. Learning is indexed through the rate at which rats are able to change their behavior when the experimental contingencies are changed.
2. Experiment 1
2.1. Methods and materials
2.1.1. Subjects
Twenty male Long Evans rats (398 g and 8 months of age at the onset of paclitaxel treatment; acquired from Envigo, Indianapolis, IN) were used in these experiments. Rats were experimentally naive prior to this work and were individually housed with light onset and offset in the colony at 7 a.m. and 7 p.m. EST, respectively. Two rats failed to revisit the replenishment location or failed to avoid visited spatial memory locations at initial stages of training and were excluded from the study. The rats received 45-mg chow and chocolate pellets (F0165 and F0299, respectively; Bio-Serv, Frenchtown, NJ) during experimental sessions and 15 g/day of 5012-Rat-Diet (PMI Nutrition International, St. Louis, MO) after completing each session. Water was available ad lib, except when the rat was in the maze.
2.1.2. Drug preparation and administration
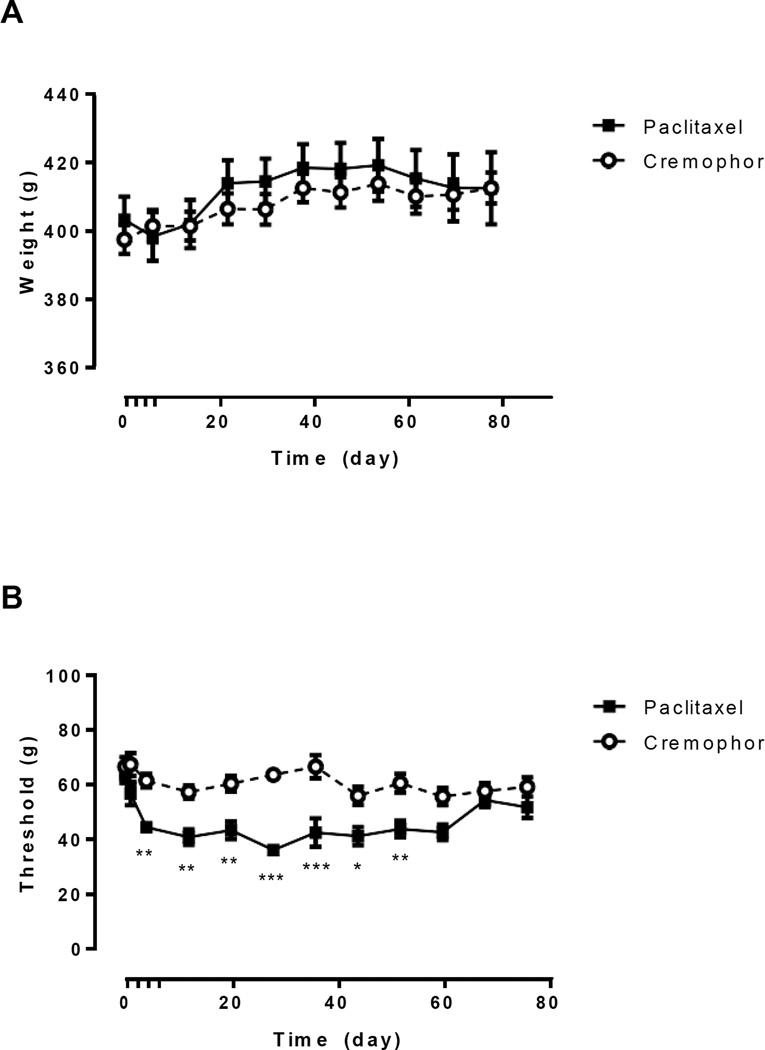
Paclitaxel was purchased from Tecoland Corporation (Irvine, CA). Paclitaxel was dissolved in a cremophor-based vehicle (1:1:18 ratio of Cremophor EL/ethanol/saline). Rats received four once daily intraperitoneal (i.p.) injections of either paclitaxel (2 mg/kg/day i.p) or cremophor vehicle (1 ml/kg/day i.p.), administered on alternate days (days 0, 2, 4, and 6). Rats were assigned to either the paclitaxel or cremophor vehicle treatment group via a randomized block design based on baseline performance in the source memory task. Rats treated with paclitaxel showed no loss in weight compared to rats treated with cremophor (Figure 1A; F(1,140) = 0.20, p = .660).
Figure 1.
Treatment with the chemotherapeutic agent paclitaxel resulted in a decrease in mechanical withdrawal thresholds while no changes in weight were observed. (A) Rats did not differ in weight based on chemotherapy-treatment group. (B) Paclitaxel-treated rats showed a decrease in mechanical withdrawal thresholds compared to cremophor-treated controls. No difference between groups was observed starting 60 days following the initiation of paclitaxel treatment. Vertical dashes on the x-axis represent drug treatment on days 0, 2, 4, and 6. *p < 0.05, **p < 0.01, ***p < 0.001. Data are shown as means ± SEM.
2.1.3. Mechanical withdrawal thresholds
Mechanical withdrawal thresholds were assessed using a digital Electrovonfrey Anesthesiometer (IITC model Alemo 2390-5; Woodland Hills, CA) equipped with a rigid tip; the anesthesiometer measures the minimum force (in grams) required to elicit paw withdrawal. Rats were individually placed underneath inverted plastic cages on an elevated mesh platform and allowed 30 minutes to habituate prior to testing. Approximately 10 minute interstimulus intervals occurred between tests. Paclitaxel treated rats showed a decrease in mechanical withdrawal thresholds compared to experimental controls (Figure 1B; F(1,324) = 129.60, p < .001). There was a significant interaction between time and treatment group (F(21,324) = 2.33, p = .001).
2.1.4. Apparatus
All behavioral procedures took place in an 8-arm radial maze (described in Babb and Crystal [28]). The maze consisted of a central hub, eight runways, guillotine doors, and a food trough and pellet dispenser at the distal end of each arm. Experimental events, movement of guillotine doors, activation of food dispensers, and interruption of photobeams, were controlled by a computer. Data were recorded (10-ms resolution) with MED-PC software (version 4.1). Chocolate- and chow-flavored pellets were placed outside each runway in order to keep food odors constant throughout all parts of the experiment. The maze was cleaned with 2% chlorohexide prior to placing each rat in the maze.
2.1.5. Behavioral Procedure
To evaluate episodic and spatial memory impairment, rats were trained on the source memory task prior to chemotherapy treatment. The source memory task requires rats to attend to three key features: what (food flavor), where (location in the maze), and source (the origin of the information). Rats were taught that whether or not a distinctive food item (i.e., chocolate flavored pellets) replenished was based on the method by which the rat encountered the item (i.e., the source information). Rats discovered chocolate either by running to the location (i.e., self-generated food seeking) or by being placed at the chocolate location (i.e., experimenter-generated food seeking). The chocolate location that was found through self-generated food seeking replenished, whereas the chocolate location found through placement by an experimenter did not replenish. Other locations were baited with standard chow-flavored food, which never replenished.
Successful source memory retention allows rats to correctly predict the replenishing chocolate location while avoiding the non-replenishing chocolate location. Rats that are able to remember the source information will return to the replenishing location (R; i.e., self-generated chocolate location) to receive additional rewards and avoid the non-replenishing location (NR; i.e., experimenter-generated location). Source memory is indexed by the difference between the return rates to the two chocolate locations. Therefore, rats trained in the source memory procedure show a preference for the replenishing location, documented as a positive R-NR score (difference in the probabilities of returning to the replenishing and non-replenishing locations).
If episodic memory is impaired, rats may be unable to remember the source information, in which case there would be equivalent preference for the two chocolate locations. This source memory impairment is documented as an equal return rate to both locations resulting in an R-NR score of 0. Additionally, spatial memory is an aspect of the source memory task. Spatial memory is independently measured as the correct visits to unvisited locations containing a chow reward. Selective elimination of source memory is documented as a high success rate to spatial locations in the midst of no observed preference for either chocolate location.
2.1.6. Preliminary training
Preliminary training for the source memory task consisted of three stages: maze familiarization, 8-arm radial arm maze training, and two-phase training. In the maze familiarization phase, rats were habituated to the maze. In each daily session, two arms were randomly assigned as the chocolate-flavored pellet locations; the remaining 6 locations contained chow-flavored pellets. Chocolate- or chow-flavored pellets (consistent with pellet flavor assignment) were placed along each arm and in the food trough during 5 daily 10-minute familiarization sessions. During daily sessions, each rat was placed individually in the central hub of the maze. Placement in the maze hub was in a pseudo random direction. Following a 30-second habituation period, all eight doors opened and the rats were allowed to explore and eat pellets for 10 minutes.
The 8-arm radial arm maze training occurred in 10 daily sessions. In each session, rats were given access to all eight locations. Two of the eight locations provided 3 chocolate pellets; the remaining six locations provided 1 chow pellet. Chocolate locations provided additional helpings of food upon subsequent visits for a total of five visits to each chocolate location. Revisits to chow locations did not provide additional helpings of food. A visit to an arm following depletion of chow was counted as an error. The trial was terminated once the rat visited all eight arms or 15 minutes had elapsed.
Two-phase training occurred in 23 daily sessions. Each session was comprised of two phases, or opportunities, to forage for food: a study and a test phase. During the study phase, rats were given access to four out of eight randomly selected locations. Two of the four locations provided three chocolate pellets, while the remaining two locations provided a chow pellet. The study phase was terminated once the rat visited all four arms or 15 minutes had elapsed. The rat was then returned to its holding cage and the maze was cleaned. Following a retention interval of approximately 5 minutes the rat was returned to the maze hub. The test phase was initiated by the opening of all eight doors. In the test phase both chocolate locations provided additional helpings of chocolate (replenishment) and the remaining previously unvisited locations provided chow. Rats could return to each chocolate location for a total of five visits to receive additional helpings of chocolate. Chow locations did not replenish. The test phase was terminated after both chocolate locations and all four previously unvisited chow locations were visited or 15 minutes had elapsed.
2.1.7. Source memory task
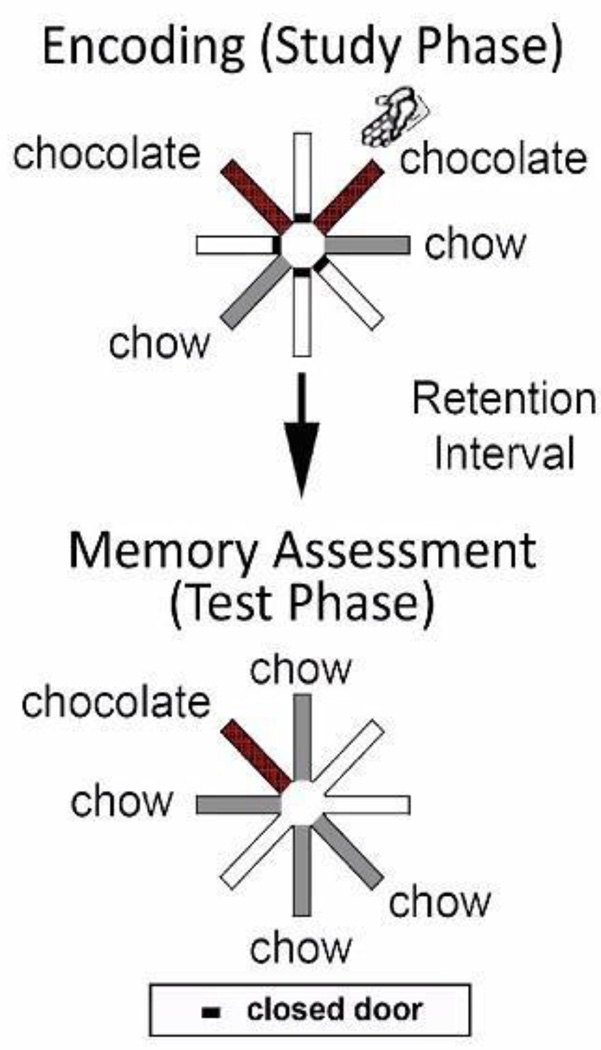
The source memory task was comprised of two phases, or opportunities, to forage for food (for 60 sessions prior to treatment with paclitaxel or cremophor): a study and a test phase (Figure 2). In the study phase, the rats were given access to four out of eight arms. Two of the four arms provided 3 chocolate pellets; the remaining two locations provided 1 chow pellet. During the study phase, the locations did not replenish food on subsequent revisits. Controlled access to individual arms allowed the experimenter to place the rat in one of the designated chocolate arms (randomly selected); the rat was placed at the distal end of the arm near the food trough and facing the trough and dispenser. Therefore, in the study phase, each rat visited one chocolate location by running down the runway (self-generated food seeking) and one chocolate location by being placed by the experimenter in the arm (experimenter-generated food seeking); the order was randomly determined. The study phase ended when food was dispensed at all four accessible locations. The rat was removed from the maze and placed in the home cage for a retention interval of 5 min. The rat was returned to the central hub and all 8 arms opened to begin the test phase (second helpings). In the test phase, 1 chow pellet was provided at each of the four previously unvisited arms, and 3 chocolate pellets were provided at the self-generated chocolate location (replenishment). The experimenter-generated chocolate location did not replenish (nonreplenishment). Rats could return to the replenishing chocolate location for a total of five visits to receive additional helpings of chocolate. Chow locations did not replenish. The test phase was terminated after the replenishing chocolate location and all four previously unvisited chow locations were visited or 15 minutes had elapsed.
Figure 2.
A schematic of the maze. In the study phase, two randomly selected locations provide chocolate (shown in red) – one encountered via self-exploration (self-generated chocolate location) and the other encountered through placement by the experimenter (experimenter-generated location; indicated by the hand icon). The self-generated chocolate location replenished after a retention interval, whereas the experimenter-generated chocolate location did not replenish. Other locations were baited with standard chow-flavored food (shown in grey), which never replenished.
2.1.8. Data Analysis
The probability of revisiting chocolate was calculated as reported in our previous work [26], as follows: The probability of visiting a chocolate location was calculated as at least one visit to the chocolate location for the first five choices in the test phase (1 if at least one visit occurred; 0 otherwise); the probability expected by chance (i.e. random arm entries) is 0.487 (calculated with geometric distribution); this probability was calculated separately for the replenishing and non-replenishing chocolate locations. Source memory was measured as the difference between the probabilities of a rat revisiting the replenishing (R) and non-replenishing (NR) locations. For estimates of accuracy in spatial working memory (i.e., avoiding chow-flavored locations), a correct visit was defined as visiting an arm that was baited with chow in the test phase, and the analysis of the first four choices was restricted to the six non-chocolate arms; accuracy expected by chance (i.e., random arm entries) is 0.518. Memory impairment was analyzed by a mixed-design ANOVA followed by an LSD post-hoc test, where applicable. All statistical analysis was performed using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA). Statistical tests were considered significant at alpha level of p = 0.05.
2.2. Results
Source and spatial memory was examined during two treatment conditions: prior to treatment (baseline) and following treatment (post-treatment). Baseline performance in the source memory test was collected over the week prior to chemotherapy treatment. Two rats did not meet criteria (preference for the replenishing chocolate location during the test phase; R-NR ≤ 0.30) and were excluded from the remainder of the study. Prior to chemotherapy treatment, both experimental groups (n = 9 for each) showed preference (positive R-NR score) for the replenishing location (Paclitaxel: mean = 0.80, SD = 0.12; Cremophor: 0.81, SD = 0.07), and performed above chance on measures of spatial memory (Paclitaxel: mean = 0.80, SD = 0.12; Cremophor: 0.81, SD = 0.07). Post-treatment memory assessment began on the week following chemotherapy treatment. Individual performance during the post-treatment condition was averaged over 10 daily sessions.
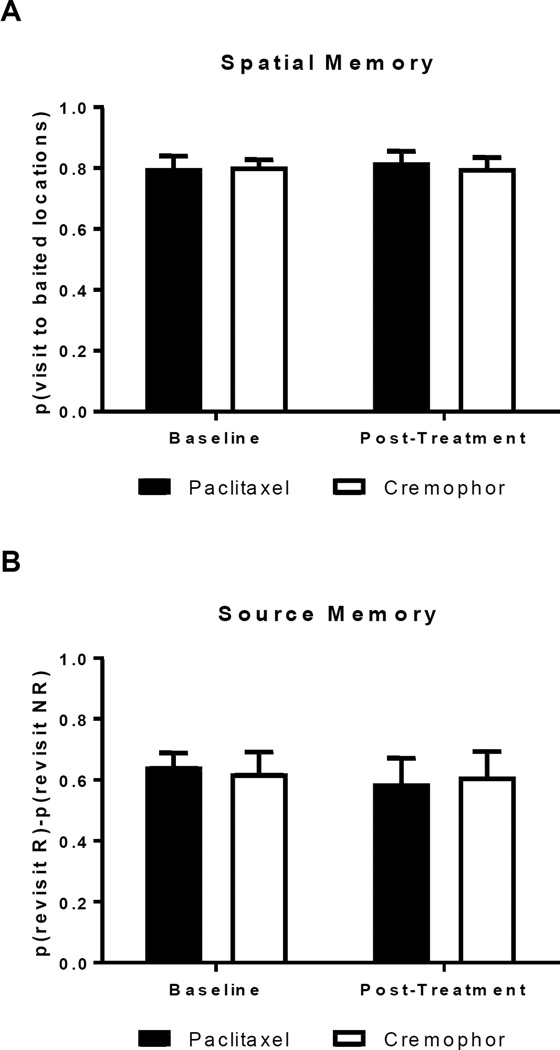
Figure 3A depicts spatial memory performance at chow locations for rats treated with paclitaxel or cremophor in baseline and post-treatment conditions. Rats treated with paclitaxel were not impaired in spatial memory following chemotherapy treatment. These findings were confirmed by a 2 (baseline, post-treatment) × 2 (paclitaxel, cremophor) ANOVA on measures of spatial memory. No effect of chemotherapy group was observed (F(1,16) = 1.46, p = .245), and rats did not decline from baseline following treatment (F(1,16) = 0.06, p = .807). Additionally, no interaction between treatment condition and chemotherapy group was observed F(1,16) = 0.61, p = .446). Figure 3B shows source memory performance for rats treated with paclitaxel and cremophor in baseline and post-treatment conditions. Rats treated with paclitaxel did not show source memory impairment following chemotherapy treatment. These findings were confirmed by a 2 (baseline, post-treatment) × 2 (paclitaxel, cremophor) ANOVA on measures of source memory. No effect of chemotherapy was observed (F(1,16) = 0.00, p = 1.00), and rats did not decline from baseline following treatment (F(1,16) = 0.23, p = .638). Additionally, no interaction between treatment condition and chemotherapy group was observed (F(1,16) = 0.00, p = .753). The same conclusions were obtained when examining post-treatment performance when averaged by week.
Figure 3.
Spatial and source memory were not impaired following treatment with the chemotherapeutic agent paclitaxel. (A) High spatial memory performance is represented by a high revisit rate to previously unvisited chow locations. (B) Positive source memory scores document that rats preferentially revisit the chocolate location that is about to replenish (R), while avoiding the non-replenishing (NR) chocolate location. Data are shown as mean + SEM.
2.3. Discussion
Following treatment with either the chemotherapeutic agent paclitaxel or cremophor rats were assessed on spatial and source memory. Performance in the source memory task was compared to pre-treatment baselines. Treatment with the chemotherapeutic agent paclitaxel did not impair source or spatial memory during the first two weeks following treatment. Rats performed at baseline levels on spatial memory and showed strong preference for the replenishing chocolate location, indicating intact source memory retention.
3. Experiment 2
Memory impairments following treatment with a chemotherapeutic agent are often subtle or detectable only under experimental challenges; nonexistent or small impairments in cognitions as a function of chemotherapy treatment in some experimental conditions are detectable or exacerbated under other, more demanding, conditions [6, 11, 17]. Because chemotherapy treatment did not appear to produce an impairment in memory in the source memory task in Experiment 1, in Experiments 2 and 3 we introduced more demanding conditions to more fully characterize the impact of chemotherapy on memory.
An impact of a moderate retention interval on source memory was assessed during Week 3. We have previously shown that source memory survives unusually long retention intervals (7–14 days) [24–26]. In contrast, spatial memory decays within 24 hours of encoding; rats tested with a moderate retention interval of 6-hours show attenuation, but not complete elimination, of spatial memory [29]. In the source memory task, a retention interval of approximately 5 minutes occurred between encoding (study) and memory assessment (test) phases in Experiment 1. In Experiment 2, the retention interval was approximately 6 hours.
3.1. Methods and materials
3.1.1. Subjects
The subjects were the same as those used in Experiment 1.
3.1.2. Apparatus
The apparatus was identical to that used in Experiment 1.
3.1.3. Behavioral Procedure
Experiment 2 began on Week 3 following chemotherapy treatment. The source memory task used in Experiment 2 was identical to the one used in Experiment 1 with the following exception: the retention interval was approximately 6 hours. Following the study phase, the rat was returned to its cage and housed in an adjacent room. At the culmination of the 6-hour retention interval, the rat was returned to the testing room and the test phase was initiated by placing the rat in the maze hub.
3.1.4. Data Analysis
Statistical analysis of spatial and source memory was identical to that in Experiment 1.
3.2. Results
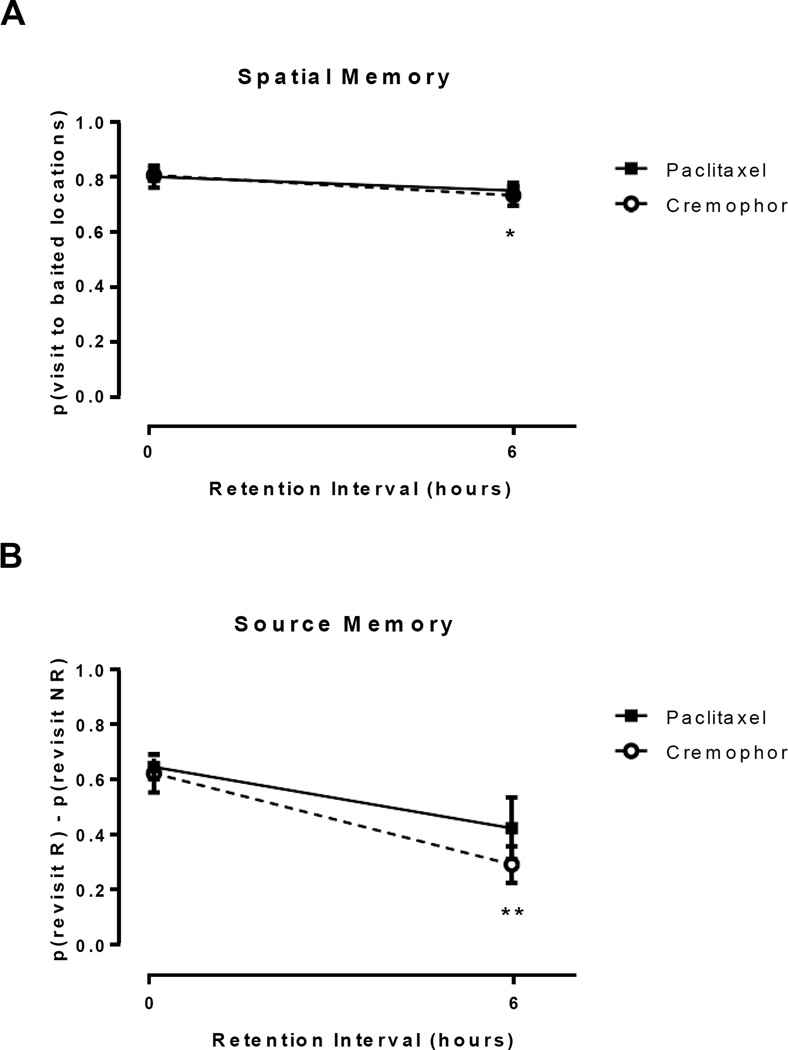
Figure 4A depicts spatial memory performance of rats treated with paclitaxel and cremophor after a retention intervals of 5 minutes and 6 hours. Rats tested after a 6-hour retention interval showed a decrease in performance with no effect of chemotherapy treatment. These findings were confirmed by a 2 (5-minute retention interval, 6-hour retention interval) × 2 (paclitaxel, cremophor) ANOVA on spatial memory. When assessed on spatial memory following a 6-hour retention interval, rats made more errors than when assessed following a 5-minute retention interval (F(1,16) = 5.23, p = .036). For spatial memory, no effect of chemotherapy group was observed (F(1,16) = 0.2, p = .886) and there was no interaction between chemotherapy group and retention interval (F(1,16) = 0.17, p = .683). Additionally, source memory was impaired following an increase in retention interval, with no observable effect of chemotherapy treatment (Figure 4B). These findings were confirmed by a 2 (5-minute retention interval, 6-hour retention interval) × 2 (paclitaxel, cremophor) ANOVA on source memory. There was a significant decrease in accuracy of source memory as retention interval was increased (F(1,16) = 8.23, p = .009). For source memory, no effect of chemotherapy group was observed (F(1,16) = 0.55, p = .468) and there was no interaction between chemotherapy group and retention interval (F(1,16) = 1.87, p = .191).
Figure 4.
A moderate retention interval did not result in accentuation of chemotherapy-induced source and spatial memory performance. (A) High spatial memory performance is represented by a high revisit rate to previously unvisited chow locations. Spatial memory was impaired following a retention interval of 6 hours. (B) Positive source memory scores document that rats preferentially revisit the chocolate location that is about to replenish (R) while avoiding the non-replenishing (NR) chocolate location. Source memory was impaired following a retention interval of 6 hours. *p < 0.05, **p < 0.01. Data are shown as means ± SEM.
3.3. Discussion
In Experiment 1, no effect of chemotherapy treatment was observed in either spatial or source memory. Therefore, the retention interval prior to memory assessment was increased to 6 hours in Experiment 2, which introduced a more demanding spatial working memory load. A moderate retention interval of 6 hours impaired, but did not eliminate, spatial and source memory in the source memory task. Following a 6-hour retention interval, rats continued to perform above chance on spatial memory, and showed a preference for the replenishing chocolate location. The decline in accuracy for the source memory measures was unexpected, due to previous findings in our lab documenting unimpaired source memory following retention intervals of at least 7 days [24–26]. In the current study, this extension was the first time that the rats were introduced to a long retention interval, which may have caused decrement due to the expectation that leaving the testing room signaled that the session was over. Despite the cognitive challenge that the moderate retention interval imposed (as documented by the decline in accuracy), we did not observe any increased susceptibility to cognitive challenge for rats treated with paclitaxel.
4. Experiment 3
Proactive interference is the effect of previously learned material hindering retention of subsequent learning of similar information. Roberts and Dale [30] characterized this effect on spatial memory in rats; rats were presented with opportunities to explore a maze in succession. The memory of past exploration impaired memory of subsequent exploration of the maze, resulting in an increase in spatial memory errors as the number of successive sessions increased. This increase in errors is due to confusion of similar stimuli presented prior to terminal memory encoding and assessment, a finding that has been replicated [31, 32]. Interference is effective in accentuating hippocampal-related cognitive impairments, including in rodents treated with a chemotherapeutic agent [6, 18]. We tested the hypothesis that chemotherapy-treated rats have increased susceptibility to proactive interference. We implemented proactive interference by conducting two successive source memory sessions (i.e., maze encoding session 1 and memory assessment session 1, followed by a new trial: maze encoding session 1 and memory assessment session 2). If rats are impaired due to a proactive interference manipulation, accuracy in measures of spatial or source memory will be impaired during the second session of the day relative to performance in the first session of the day. Performance during the proactive interference trials was also compared to baseline performance (from Experiment 1) to assess generalized impairment.
4.1. Methods and materials
4.1.1. Subjects
The subjects were the same as those used in Experiments 1 and 2.
4.1.2. Apparatus
The apparatus was identical to that used in Experiment 1.
4.1.3. Behavioral Procedure
Proactive interference trials occurred during Weeks 4 and 6. The source memory task was identical to the source memory task used in Experiment 1. Each daily proactive interference trial consisted of two source memory sessions (i.e., maze encoding session 1 and memory assessment session 1, followed by a new trial: maze encoding session 1 and memory assessment session 2). Upon conclusion of Session 1, rats were returned to their home cage and the maze was cleaned. Chocolate and chow locations were randomly and independently assigned for the first session of the day (Sessions 1) and the second session of the day (Session 2). Rats were returned to the maze hub after an inter-session interval of approximately 5 minutes.
4.1.4. Data Analysis
Statistical analysis of spatial and source memory were identical to that in Experiment 1.
4.2. Results
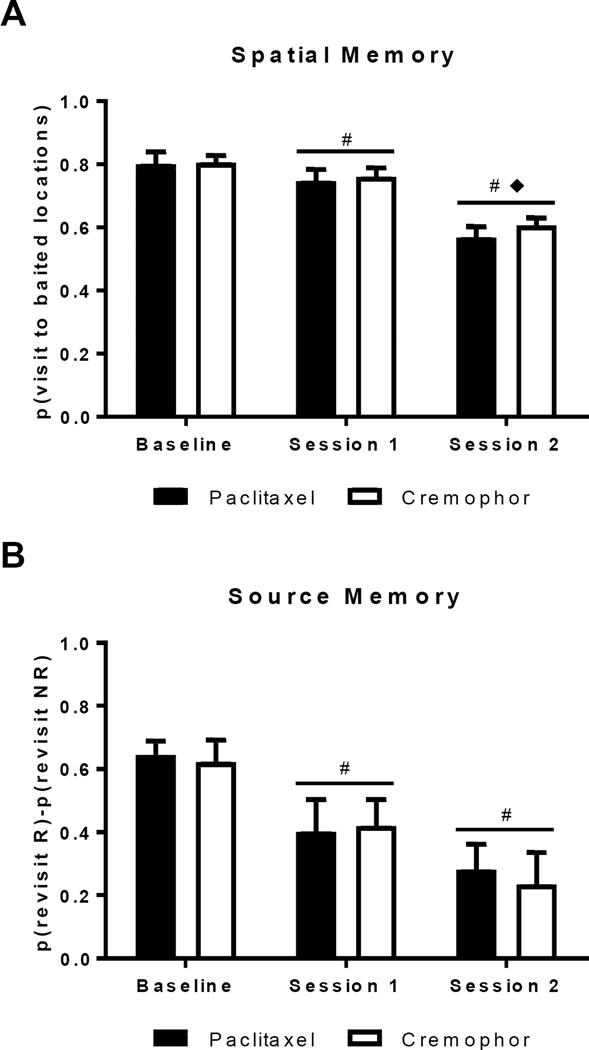
Figure 5A describes the spatial memory for rats treated with paclitaxel and cremophor during baseline, the first session of the day (Session 1), and the second session of the day (Session 2). Successive testing in the source memory task resulted in increased errors during the second session of the day as well as inducing general impairment in both the first and second session of the day. These results were confirmed by a 2 (paclitaxel, cremophor) × 3 (baseline, Session 1, Session 2) ANOVA for spatial memory. There was a significant effect of session type (F(2,32) = 32.44, p < .001). No effect of chemotherapy group was observed (F(1,16) = 0.40, p = .538) and there was no interaction between chemotherapy group and session type (F(2,32) = 0.20, p = .819). Post-hoc tests reveal that spatial memory during the second session of the day was impaired compared to baseline (p < .001) and the first session of the day (p < .001), and that performance was additionally impaired during the first session of the day compared to baseline (p = .036).
Figure 5.
Source and spatial memory impairment was observed following the introduction of proactive interference trials. Baseline performance was compared to performance during proactive interference trials, which is presented as first (Session 1) and second (Session 2) of the day. (A) High spatial memory was documented by a high visit rate to baited locations. Impaired spatial memory performance was documented in the second session of the day. Additionally, the proactive interference trials led to a global impairment in spatial memory across both sessions of the day; rats had poor spatial memory in both the first and second session of the day compared to baseline performance. (B) Positive source memory scores document that rats preferentially revisit the chocolate location that is about to replenish (R) while avoiding the non-replenishing (NR) chocolate location. Global impairment to source memory was documented during the proactive interference task. # R-NR scores significantly below baseline, ◆ R-NR scores significantly below Session 1. Data are shown as means + SEM.
Figure 5B describes source memory for rats treated with paclitaxel and cremophor during baseline, the first session of the day (Session 1), and the second session of the day (Session 2). Successive testing in the source memory task resulted in general impairment of source memory during both the first and second session of the day, with no effect of chemotherapy treatment. These results were confirmed by a 2 (paclitaxel, cremophor) × 3 (baseline, Session 1, Session 2) ANOVA for source memory. There was a significant effect of session type (F(2,32) = 11.80, p < .001). No effect of chemotherapy group was observed (F(1,16) = 0.05, p = .828) and there was no interaction between chemotherapy group and session type (F(2,32) = 0.09, p = .915). Post-hoc tests reveal that rats had more errors in source memory during the first (p = .012) and second session of the day (p < .001) compared to baseline performance; there was no observable difference between source memory performance during the first and second sessions of the day (p = .099).
4.3. Discussion
Further characterization of potential source memory and spatial memory impairments following chemotherapy was achieved through the proactive interference task. An effect of proactive interference was observed in spatial memory when two consecutive sessions were presented each day. Rats had impaired memory for spatial information during the second session of the day, indicating that the similar, previously encoded spatial information (from Session 1) impaired subsequent performance (in Session 2). The proactive interference manipulation led to global impairment in both source and spatial memory, likely due to day to day confusion; in both daily sessions during the proactive interference trials, rats performed significantly below baseline performance. In Experiment 1, source and spatial memory were not impaired following treatment with paclitaxel. This observed lack of chemotherapy-induced impairment was further investigated in Experiments 2 and 3. By increasing the processing demands, potentially subtle impairments or vulnerabilities to cognitive challenges were explored. The cognitive challenges used in these experiments produced overall impairments, but increased vulnerability to impairment was not observed in the paclitaxel-treated rats.
5. Experiment 4
In a mouse model of chemotherapy-induced neuropathy, cognitive functioning showed long-lasting impairments in hippocampus-sensitive memory tests and discrimination learning while recovery was observed in frontal lobe-sensitive tasks [8]. The differentiation in observed impairments suggest that chemotherapy may have differing effects on various cognitive functions in both humans and rodents. It is possible that, despite a lack of impairment in episodic and spatial memory, paclitaxel may impair learning of a new rule. Rule learning is an aspect embedded within the source memory task; rats discriminate between the two chocolate locations based on the replenishment rule (i.e. experimenter-placement at a chocolate location predicts nonreplenishment of chocolate). In this experiment we reversed the replenishment rule, at which point the experimenter-generated location now replenished during the test phase while the self-generated location now did not replenish. Following the rule reversal, positive R-NR scores represent preference for the experimenter-generated location while negative scores represent preference for the self-generated location.
Prior to the reversal learning task, rats returned with high probability to the replenishing location while avoiding the non-replenishing location. This difference in return rates, as an index of source memory, resulted in a positive R-NR score of approximately .64. Insensitivity to new experimental contingencies would produce perseveration of behavior (i.e., continued preference for the non-replenishing location, despite the fact that the previously replenishing location is now non-replenishing). If rats do not change their preference despite the reversal in replenishment contingencies, then rats should not change their return rates to either chocolate location, resulting in a negative R-NR score of approximately −.64. Alternatively, rats sensitive to changes in experimental contingencies will show a rapid change in preference for the now replenishing location and non-replenishing locations (e.g., lack of preference for either chocolate location). Upon initial detection of changes in experimental contingencies (i.e., nonreplenishment of the self-generated chocolate location), rats that do not perseverate will change behavior within the initial sessions, resulting in a visit to both chocolate locations. This lack of preference for locations would be documented by an R-NR score of 0. As rats learn the new experimental contingencies, the probability that rats return to the replenishing location (i.e., experimenter-generated location) will increase while the probability that rats return to the non-replenishing location (i.e., self-generated location) will decrease. Learning of the new replenishment rule is indexed by a preference for the new replenishing chocolate location (i.e., R-NR > 0).
5.1. Methods and materials
5.1.1. Subjects
The subjects were the same as those used in Experiments 1, 2, and 3 with the following exception. Three additional rats (one from paclitaxel and two from cremophor groups) failed to revisit the replenishment location or failed to avoid visited chow locations during inter-experimental memory assessments and were excluded from Experiment 4.
5.1.2. Apparatus
The apparatus was identical to that used in Experiment 1.
5.1.3. Behavioral Procedure
The source memory task used in Experiment 4 was identical to that of Experiment 1, with the following exception of the replenishment rule. In Experiment 4, the replenishment rule was reversed; during the test phase, the experimenter-generated location from the study phase replenished in the test phase whereas the self-generated location from the study phase did not replenish in the test phase. Following the rule reversal, negative R-NR scores represent preference for the self-generated location (i.e., perseveration), whereas positive R-NR scores represent preference for the experimenter-generated location. The reversal learning task began on Week 8. The reversal learning task contained 18 daily sessions.
5.1.4. Data Analysis
Data analysis was identical to that performed in Experiment 1. Perseveration in learning following the rule reversal was analyzed through a within-subjects t-test, and identified as an R-NR score significantly below 0. Initial performance of source and spatial memory was averaged over the first two sessions; terminal performance of source and spatial memory was averaged over the final two sessions.
5.2. Results
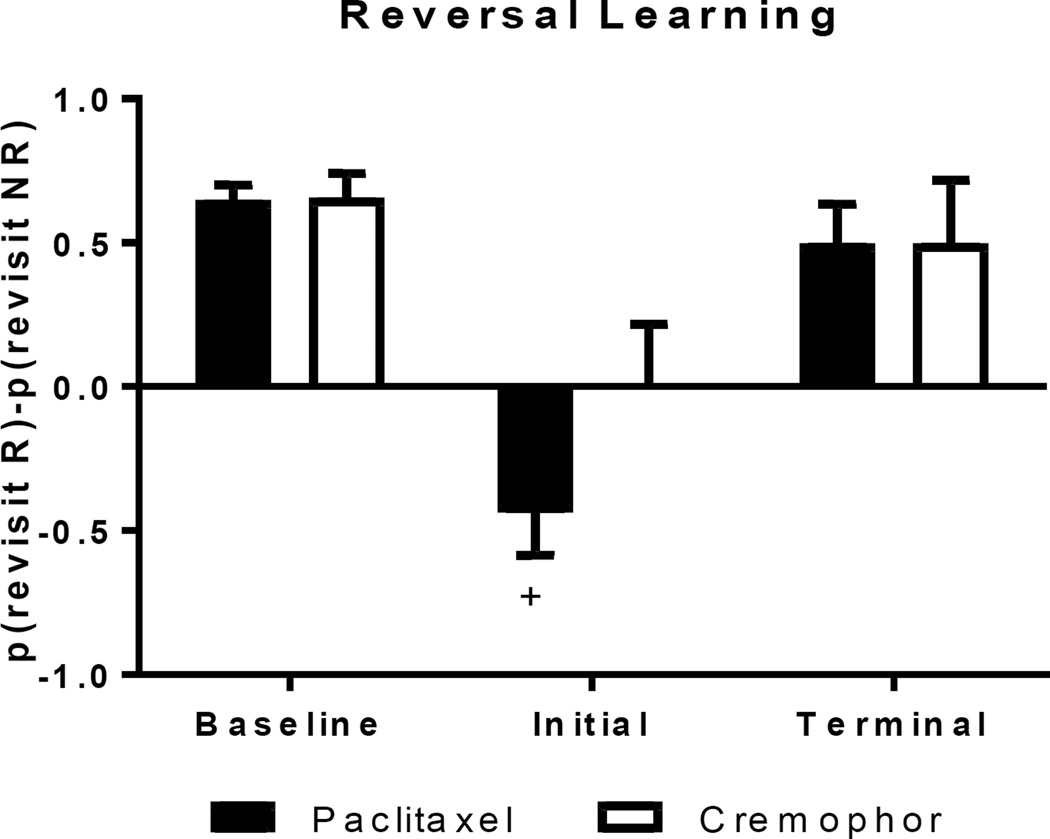
An index of rats (paclitaxel group: n = 8; cremophor group: n = 7) preference for the replenishing chocolate location during the initial (first two sessions following reversal of the replenishment rule) and terminal sessions (final two sessions) compared to baseline performance is described in Figure 6. Perseveration was measured as preference for the nonreplenshing location (the difference between the probability of visiting the replenishing and non-replenishing location (R-NR score) significantly below 0). The paclitaxel treated rats showed perseveration during the initial sessions following reversal, while the cremophor group showed no preference for either chocolate location. These findings were supported by a 2 (paclitaxel, cremophor) × 3 (baseline, initial, terminal) ANOVA for source memory. There was a significant effect of time point (F(2,26) = 19.66, p < .001); post hoc LSD tests document this effect as an decrease in source memory accuracy during the initial sessions, compared to the baseline (p < .001) and terminal sessions (p = .003). No effect of treatment (F(1,13) = 1.29, p = .277) or interaction between treatment and time point (F(2,26) = 1.46, p = .250) was observed. Planned comparisons examined R-NR scores for rats treated with paclitaxel and cremophor during the initial sessions. Rats treated with paclitaxel had R-NR scores that were significantly below zero (Mean = −0.44, SD = 0.15; t(7) = 2.97, p = 0.02), while cremophor-treated rats showed no preference for the replenishing or non-replenishing locations (Mean = 0.00, SD 0.22; t(7) = 0.00, p = 1.00).
Figure 6.
Rats treated with the chemotherapeutic agent paclitaxel perseverated while experimental controls changed their behavior in response to changes in experimental contingencies during the initial sessions following reversal of the replenishment rule. Positive source memory scores document that rats preferentially revisit the chocolate location that is about to replenish (R) while avoiding the non-replenishing (NR) chocolate location. Following reversal of the replenishment rule (Initial and Terminal), negative scores document perseveration, whereas positive scores document learning. A cross (+) indicates source memory scores significantly below zero. Data are shown as means + SEM.
5.3. Discussion
In the current experiment, we explored the potential vulnerabilities of learning to treatment with chemotherapy. Beginning in the 8th week following chemotherapy treatment, the replenishment contingencies in the source memory task were reversed. This reversal required rats to learn the new experimental contingencies in order to efficiently receive a preferred food reward. Lack of sensitivity to changes in the experimental contingencies results in perseveration of behavior, which is observed in the current study as continued preference for the now non-replenishing location. During the initial sessions following the reversal of the replenishment rule paclitaxel-treated rats perseverated; due to a lack of sensitivity for changes in experimental contingencies, rats treated with paclitaxel returned to the non-replenishing location and avoided the replenishing location. In contrast, the cremophor group detected changes in the experimental contingencies and began to change their behavior accordingly, resulting in an initial R-NR score of 0 (a score of 0 means that, at this point, they expressed no preference between R and NR conditions). Following the initial sessions, the rats treated with paclitaxel demonstrated the ability to learn the new rule at the same rate as the experimental controls. By the terminal sessions (18 sessions following the rule reversal) both groups preferred the replenishing location. Because all rats received a reversal of the initial replenishment/nonreplenishment rule, it is not known if the observed impairment is restricted to the rule change used in the current experiment.
6. General Discussion
This is the first evaluation of the impact of chemotherapy using a paradigm that taps into episodic memory function in rodents (i.e., source memory). Following treatment with the chemotherapeutic agent paclitaxel, rats were assessed on spatial and source memory. In Experiment 1, no impairments in source or spatial memory were observed, suggesting that these domains of memory are not impaired through this paclitaxel regime. In order to elucidate subtle changes in cognition, we introduced cognitive challenges expected to preferentially disrupt performance in rodents treated with paclitaxel. Cognitive challenges were implemented through the introduction of an increased retention interval (Experiment 2) and proactive interference (Experiment 3). A moderate retention interval impaired, but did not completely eliminate, spatial and source memory. In addition, successive testing using the source memory test hindered memory of spatial information acquired during the second session of the day, and testing with two sessions per day caused global impairment in both source and spatial memory. There is evidence that cognitive challenges, such as the ones used in this study, accentuate impairments associated with hippocampal inactivation and decreased hippocampal neurogenesis [6, 17, 18]. While the moderate retention interval and proactive interference tasks introduced a cognitive challenge, increased susceptibility to impairment was not observed in the paclitaxel-treated rats in the present study. These findings expand our knowledge of the effects of paclitaxel on episodic memory and spatial memory.
To further characterize the effects of paclitaxel on cognition, in Experiment 4 a reversal learning task was used to assess the ability to learn new rules. Embedded within the source memory task is a learned replenishment rule (i.e. replenishment of a favored food item is contingent on the method by which a rat initially found the location). By reversing the replenishment rule, rats must be sensitive to the change in experimental contingencies and change their behavior accordingly in order to efficiently receive a food reward. In the reversal learning task, learning is indexed as an increase in return rates to the new replenishing location while avoiding the old replenishing location. Lack of sensitivity to these changes results in perseveration of behavior, which is continuation of a preference for the now non-replenishing location. Under conditions in which episodic memory was not impaired, rats treated with paclitaxel showed impaired rule learning; rats treated with paclitaxel were not sensitive to changes in experimental contingencies and were slower to change their behavior than the cremophor group. This observed impairment in learning was confined to initial sensitivity to changes in the experimental contingencies. Following the initial sessions, paclitaxel rats displayed the ability to learn the new rule by increasing revisit rates to the replenishing locations and learning to avoid non-replenishing locations based on the new replenishment rule.
The lack of observed impairment in memory in Experiments 1–3 could potentially be attributed to the protective effect of extensive training and daily enrichment. Environmental enrichment is linked to increased hippocampal neurogenesis and is protective against chemotherapy-induced decreased neurogenesis and cognitive impairment [33, 34]. Rats used in the current study received extensive training prior to chemotherapy treatment, which spanned over five months and comprised of five daily sessions per week. During this training period, rats received handling and experience in rule learning and memory evaluation. It is plausible that this training and testing regime acts as a protective enrichment against decreased neurogenesis, however this remains to be investigated.
Our data provide new information on the nature of cancer chemotherapy-induced cognitive impairments. Impairments in episodic memory have been linked to cognitive impairments associated with chemotherapy treatment, or “chemo fog,” [27]. It should be noted, however, that the putative episodic memory impairment in people examined subjective reports and verbal and visual memory, which share aspects of, but may not directly measure episodic memory impairment [35, 36]. The present study represents the first evaluation of the nature of episodic memory impairment in a rodent model of chemotherapy-induced cognitive impairment. Episodic memory was not found to be vulnerable to paclitaxel treatment. While no impairments in episodic memory were observed, even under conditions of increased cognitive load, rats treated with paclitaxel showed impairment in a reversal learning task. Specifically, paclitaxel treated rats were unable to rapidly adapt to new experimental contingencies.
In summary, the present results show that not all forms of cognition are affected by the chemotherapeutic drug paclitaxel. In the absence of episodic memory impairment following chemotherapy-treatment, paclitaxel treatment leads to impairment in detection of changes in experimental contingencies necessary for rapid learning. Taken together, these results provide insight into the differential effects of cancer chemotherapies and support future exploration of selectively spared and impaired processes to gain insight into the nature of chemotherapy’s impact on cognition.
Highlights.
Treatment with paclitaxel did not impair spatial and episodic memory
Paclitaxel treated rats were not more susceptible to cognitive challenges
Paclitaxel impaired learning of new rules
Paclitaxel decreased sensitivity to changes in experimental contingencies
Acknowledgments
This work was supported by R21AG044530 (to JDC), R21AG051753 (to JDC), R01CA200417 (to AGH), R21DA037673 (to AGH). AES and RAS were supported by the Harlan Scholars Program. RAS was supported by T32DA024628. All procedures were approved by the Bloomington Institutional Animal care and Use Committee at Indiana University and followed national guidelines. We thank Wan-Hung Lee for her assistance with training in drug preparation and Hailey Meyer for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding and Disclosure
The authors declare no conflict of interest.
References
- 1.Seigers R, Schagen SB, Van Tellingen O, Dietrich J. Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging Behav. 2013;7(4):453–459. doi: 10.1007/s11682-013-9250-3. [DOI] [PubMed] [Google Scholar]
- 2.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12(3):267–275. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 4.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 5.Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. Bmc Neurosci. 2011;12 doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winocur G, Wojtowicz JM, Tannock IF. Memory loss in chemotherapy-treated rats is exacerbated in high-interference conditions and related to suppression of hippocampal neurogenesis. Behav Brain Res. 2015;281:239–244. doi: 10.1016/j.bbr.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Boyette-Davis JA, Fuchs PN. Differential effects of paclitaxel treatment on cognitive functioning and mechanical sensitivity. Neuroscience Letters. 2009;453(3):170–174. doi: 10.1016/j.neulet.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Winocur G, Henkelman M, Wojtowicz JM, Zhang H, Binns MA, Tannock IF. The effects of chemotherapy on cognitive function in a mouse model: a prospective study. Clin Cancer Res. 2012;18(11):3112–3121. doi: 10.1158/1078-0432.CCR-12-0060. [DOI] [PubMed] [Google Scholar]
- 9.Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35(3):729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Callaghan CK, O'Mara SM. Long-term cognitive dysfunction in the rat following docetaxel treatment is ameliorated by the phosphodiesterase-4 inhibitor, rolipram. Behav Brain Res. 2015;290:84–89. doi: 10.1016/j.bbr.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Fardell JE, Vardy J, Johnston IN. The short and long term effects of docetaxel chemotherapy on rodent object recognition and spatial reference memory. Life Sci. 2013;93(17):596–604. doi: 10.1016/j.lfs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Muallaoglu S, Kocer M, Guler N. Acute transient encephalopathy after weekly paclitaxel infusion. Med Oncol. 2012;29(2):1297–1299. doi: 10.1007/s12032-011-9956-2. [DOI] [PubMed] [Google Scholar]
- 13.Perry JR, Warner E. Transient encephalopathy after paclitaxel (Taxol) infusion. Neurology. 1996;46(6):1596–1599. doi: 10.1212/wnl.46.6.1596. [DOI] [PubMed] [Google Scholar]
- 14.Ziske CG, Schottker B, Gorschluter M, Mey U, Kleinschmidt R, Schlegel U, Sauerbruch T, Schmidt-Wolf IG. Acute transient encephalopathy after paclitaxel infusion: report of three cases. Ann Oncol. 2002;13(4):629–631. doi: 10.1093/annonc/mdf025. [DOI] [PubMed] [Google Scholar]
- 15.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 16.Fardell JE, Zhang J, De Souza R, Vardy J, Johnston I, Allen C, Henderson J, Piquette-Miller M. The impact of sustained and intermittent docetaxel chemotherapy regimens on cognition and neural morphology in healthy mice. Psychopharmacology (Berl) 2014;231(5):841–852. doi: 10.1007/s00213-013-3301-8. [DOI] [PubMed] [Google Scholar]
- 17.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winocur G. Effects of interference on discrimination learning and recall by rats with hippocampal lesions. Physiol Behav. 1979;22(2):339–345. doi: 10.1016/0031-9384(79)90096-9. [DOI] [PubMed] [Google Scholar]
- 19.Gallo DA, Chen JM, Wiseman AL, Schacter DL, Budson AE. Retrieval monitoring and anosognosia in Alzheimer's disease. Neuropsychology. 2007;21(5):559–568. doi: 10.1037/0894-4105.21.5.559. [DOI] [PubMed] [Google Scholar]
- 20.Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing Multiple Memory Deficits and Their Relation to Everyday Functioning in Individuals With Mild Cognitive Impairment. Neuropsychology. 2009;23(2):168–177. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- 21.Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewert JB, Stark CEL, Hopkins RO, Squire LR. Item memory, source memory, and the medial temporal lobe: Concordant findings from fMRI and memory-impaired patients. P Natl Acad Sci USA. 2006;103(24):9351–9356. doi: 10.1073/pnas.0602716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schacter DL, Kaszniak AW, Kihlstrom JF, Valdiserri M. The Relation between Source Memory and Aging. Psychol Aging. 1991;6(4):559–568. doi: 10.1037//0882-7974.6.4.559. [DOI] [PubMed] [Google Scholar]
- 23.Smith AE, Xu Z, Lai YY, Kulkarni PM, Thakur GA, Hohmann AG, Crystal JD. Source memory in rats is impaired by an NMDA receptor antagonist but not by PSD95-nNOS protein-protein interaction inhibitors. Behav Brain Res. 2016;305:23–29. doi: 10.1016/j.bbr.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crystal JD, Alford WT. Validation of a rodent model of source memory. Biol Letters. 2014;10(3) doi: 10.1098/rsbl.2014.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crystal JD, Alford WT, Zhou W, Hohmann AG. Source memory in the rat. Curr Biol. 2013;23(5):387–391. doi: 10.1016/j.cub.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crystal JD, Smith AE. Binding of episodic memories in the rat. Curr Biol. 2014;24(24):2957–2961. doi: 10.1016/j.cub.2014.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2012;227(2):376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babb SJ, Crystal JD. Discrimination of what, when, and where: Implications for episodic-like memory in rats. Learn Motiv. 2005;36(2):177–189. [Google Scholar]
- 29.Beatty WW, Shavalia DA. Spatial memory in rats: time course of working memory and effect of anesthetics. Behav Neural Biol. 1980;28(4):454–462. doi: 10.1016/s0163-1047(80)91806-3. [DOI] [PubMed] [Google Scholar]
- 30.Roberts WA, Dale RHI. Remembrance of Places Lasts - Proactive-Inhibition and Patterns of Choice in Rat Spatial Memory. Learn Motiv. 1981;12(3):261–281. [Google Scholar]
- 31.Bratch A, Kann S, Cain JA, Wu JE, Rivera-Reyes N, Dalecki S, Arman D, Dunn A, Cooper S, Corbin HE, Doyle AR, Pizzo MJ, Smith AE, Crystal JD. Working Memory Systems in the Rat. Curr Biol. 2016;26(3):351–355. doi: 10.1016/j.cub.2015.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright AA. An experimental analysis of memory processing. J Exp Anal Behav. 2007;88(3):405–433. doi: 10.1901/jeab.2007.88-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winocur G, Wojtowicz JM, Merkley CM, Tannock IF. Environmental enrichment protects against cognitive impairment following chemotherapy in an animal model. Behav Neurosci. 2016;130(4):428–436. doi: 10.1037/bne0000155. [DOI] [PubMed] [Google Scholar]
- 34.Winocur G, Wojtowicz JM, Huang J, Tannock IF. Physical exercise prevents suppression of hippocampal neurogenesis and reduces cognitive impairment in chemotherapy-treated rats. Psychopharmacology. 2014;231(11):2311–2320. doi: 10.1007/s00213-013-3394-0. [DOI] [PubMed] [Google Scholar]
- 35.Lee PW, Hung BK, Woo EK, Tai PT, Choi DT. Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry. 1989;52(4):488–492. doi: 10.1136/jnnp.52.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer JH, Crowe AB, Larson DA, Sneed PK, Gutin PH, McDermott MW, Prados MD. Neuropsychological sequelae of medulloblastoma in adults. Int J Radiat Oncol Biol Phys. 1997;38(1):21–26. doi: 10.1016/s0360-3016(96)00592-5. [DOI] [PubMed] [Google Scholar]