Abstract
α2A adrenergic receptor (α2A-AR) activation has been shown in animal models to play an important role in regulating the balance of acute pain inhibition vs. facilitation after both physical and psychological stress. To our knowledge the influence of genetic variants in the gene encoding α2A-AR, ADRA2A, on acute pain outcomes in humans experiencing traumatic stress has not been assessed. In this study, we tested whether a genetic variant in the 3′UTR of ADRA2A, rs3750625, is associated with acute musculoskeletal pain (MSP) severity following motor vehicle collision (MVC, n = 948) and sexual assault (n = 84), and whether this influence was affected by stress severity. We evaluated rs3750625 because it is located in the seed binding region of miR-34a, a microRNA (miRNA) known to regulate pain and stress responses. In both cohorts, the minor allele at rs3750625 was associated with increased MSP in distressed individuals (stress*rs3750625 p = 0.043 for MVC cohort and p = 0.007 for sexual assault cohort). We further found that (1) miR-34a binds the 3′UTR of ADRA2A, (2) the amount of repression is greater when the minor (risk) allele is present, (3) miR-34a in the IMR-32 adrenergic neuroblastoma cell line affects ADRA2A expression, (4) miR-34a and ADRA2A are expressed in tissues known to play a role in pain and stress, (5) following forced swim stress exposure, rat peripheral nerve tissue expression changes are consistent with miR-34a regulation of ADRA2A. Together these results suggest that ADRA2A rs3750625 contributes to post-stress MSP severity by modulating miR-34a regulation.
Keywords: Motor vehicle collision, sexual assault, stress, trauma, Musculoskeletal pain, Polymorphism, Single Nucleotide, microRNA, miR-34a, Adrenergic receptor
Introduction
Exposure to traumatic stress is, unfortunately, common in life.[42] For example, in America each year more than eleven million individuals experience a motor vehicle collision (MVC) and nearly 700,000 women are sexually assaulted.[1; 49] The precise molecular mechanisms influencing acute MSP severity after stressful events such as MVC and sexual assault remain poorly understood.
α2A adrenergic receptor (α2A-AR) activation has been shown to influence pain outcomes in both pre-clinical and human studies.1-10 These data include animal model data demonstrating that α2A-AR activation plays an important role in regulating the balance of acute pain inhibition vs. facilitation after both physical and psychological stress.[19] However, to our knowledge the influence of α2A-AR activation on acute MSP outcomes in humans experiencing traumatic stress has not been assessed. One way to assess the potential influence of α2A-AR activation on acute MSP in trauma-exposed individuals is to evaluate for associations between genetic variants influencing α2A-AR function and acute MSP outcomes. If α2A-AR activation influences acute MSP severity in humans experiencing traumatic stress, then inherited differences in the gene for α2A-AR, ADRA2A, should be associated with individual differences in acute MSP severity. Few studies have examined associations between ADRA2A polymorphisms and responses to stress generally or pain outcomes specifically. One 2004 study of healthy volunteers found that a polymorphism in the 3′UTR (3′untranslated region) of ADRA2A modulates autonomic responses to physiological and environmental stress,[23] and another study of healthy volunteers found that ADRA2A polymorphisms predict evoked pain responses.[32] To our knowledge, no prospective cohort studies have evaluated associations between ADRA2A genetic variants and clinical acute MSP outcomes following trauma/stress exposure.
In this study, we assessed the association between a single nucleotide polymorphism (SNP) in the 3′UTR of ADRA2A, rs3750625, and acute MSP outcomes among individuals experiencing MVC and sexual assault. We evaluated this SNP because bioinformatics analyses indicate that it is in the seed binding region of miR-34a, a miRNA known to affect pain and stress responses.[27; 60] Increasing evidence indicates that one common mechanism by which genetic polymorphisms influence cellular function is by altering miRNA seed binding regions, thus altering the ability of miRNA to bind and regulate mRNA and the amount of protein product produced.[2] When assessing the association between rs3750625 and acute MSP outcomes, we included potential gene variant × sex and gene variant × stress interactions, because genetic influences on MSP after stress exposure are often sex and/or stress severity dependent (e.g.,[8; 39; 58]). We then experimentally tested whether the allele present at rs3750625 affects miR-34a binding to the 3′UTR of ADRA2A, and whether miR-34a and ADRA2A expression levels change in key tissues involved in stress-induced hyperalgesia in an animal model of stress exposure.
Methods
Motor Vehicle Collision Cohort
Study design and population
The methods of the MVC study have been described.[45] In brief, individuals ≥ 18 and ≤ 65 years of age presenting to one of eight EDs in four no-fault insurance states within 24 hours of MVC who did not have fracture or other injury requiring hospital admission were enrolled. Patients who were not alert and oriented were excluded, as were pregnant patients, prisoners, patients unable to read and understand English, patients taking a β-adrenoreceptor antagonist, or patients taking opioids above a total daily dose of 30 mg of oral morphine or equivalent. In addition, enrollment was limited to non-Hispanic whites (the most common ethnicity at study sites). Informed consent was obtained from all participants and Institutional Review Board (IRB) approval was obtained at all study sites.
DNA collection and genotyping
Study personnel collected blood samples at the time of enrollment using PAXgene DNA tubes. Following DNA purification (PAXgene blood DNA kit, QIAGEN), genotyping using the Sequenom platform was performed at rs3750625. As described above, this SNP was chosen for analysis based on its location in a regulatory region of the pain and stress-associated gene, ADRA2A, and because bioinformatics analyses suggest that this allele might affect miR-34a binding.([12; 37; 50; 62]) Two Hapmap samples and two repeat samples were included in each genotyping batch of 96 samples to ensure genotyping accuracy and reliability. rs3750625 was in Hardy-Weinberg equilibrium (p > .05), and repeat genotyping demonstrated greater than 98% call agreement.
Assessments
Acute MSP severity was assessed in the ED using a verbal 0-10 numeric rating scale (NRS).[9] Distress in the ED was measured using the Peritraumatic distress inventory.[11] A validated cut-off of 23 was used to identify those with substantial distress.[41]
Sexual Assault Cohort
Study design and population
The methods of the sexual assault study have been reported.[4; 38] In brief, women 18 years of age or older who presented to one of ten Sexual Assault Nurse Examiner (SANE) programs in four states for medical care within 72 hours of sexual assault were recruited. Women unable to give informed consent (e.g., due to intoxication) were excluded, as were women who were hospitalized after sexual assault, lived with their assailant, were prisoners, were pregnant, did not have a telephone, and/or did not live within driving distance for follow-up interviews. Institutional Review Board (IRB) approval was obtained at all study sites and all study participants provided written informed consent.
DNA collection and genotyping
Saliva specimens were obtained at one week follow-up evaluations using Oragene DNA Self-Collection Kits. Following DNA purification, genotyping was performed within the same batches as MVC cohort samples. Hapmap samples and two repeat samples were included in each genotyping batch to ensure genotypic accuracy and reliability; repeated genotyping demonstrated greater than 98% call agreement, and rs3750625 was in Hardy-Weinberg equilibrium (p > .05).
Assessments
Acute MSP severity was assessed in the ED using a verbal 0-10 NRS. One week after the assault, sexual assault survivors completed an in-person computerized self-report questionnaire which included an assessment of the individual’s distress levels at the time of assault using the Acute Stress Disorder (ASD) questionnaire.[13] The ASD is a 19-item questionnaire assessing feelings that were experienced during or immediately after the assault. Each item on the questionnaire was evaluated via numeric rating scale from 0 (no distress) to 5 (high distress). A cut-off of 56 for substantial acute stress (ASD scale) has been reported previously.[13] However, over 90% of the participants in the sexual assault cohort reported distress levels above this threshold, thus we used the median score of 77 to distinguish more vs. less distressed sexual assault survivors.
Statistical analyses
Sociodemographic characteristics of the sample were summarized using standard descriptive statistics. General linear models were used to evaluate the association between rs3750625 with acute MSP outcomes and for potential sex × rs3750625 and stress × rs3750625 interactions, adjusting for potential confounding by age and study site. Sex and stress-dependent effects were evaluated because of increasing evidence that such interactions are frequently present (e.g.,[8; 39; 58]) and because such effects have been found in ADRA2A previously.[19; 23] A dominant genetic model (homozygous for major allele vs. other) was used in all analyses due to the low minor allele frequency (MAF = 0.05) for rs3750625 in European Americans. Mean levels of MSP in distressed and non-distressed individuals homozygous for the major allele (CC) or individuals heterozygous or homozygous for the minor allele (CA/AA) were obtained from general linear models. All statistical analyses were completed using SPSS software (v.23; SPSS Inc, Chicago, IL).
Bioinformatics analyses
The miRdSNP online algorithm (http://mirdsnp.ccr.buffalo.edu/search.php) was used to identify whether rs3750625 was predicted to affect microRNA (miRNA) binding.[12] The UCSC genome browser (https://genome.ucsc.edu/) was used to determine species conservation.[28] RNA hybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/) was used to predict the secondary structure of the miR-34a-ADRA2A binding event.[33]
Constructs
Lentiviral constructs containing either a firefly luciferase gene (pL-SV40-GL3) or a renilla luciferase gene (pL-SV40-RLUC) were used for dual luciferase assays.[26] The 3′UTR of ADRA2A was amplified from human genomic DNA using primers 1-F and 1-R (Supplementary Table 1). The resullting 1360bp product was cloned downstream of the firefly luciferase gene in pL-SV40-GL3 using XhoI and EcoRI restriction enzyme sites. This newly created construct, pL-GL3-ADRA2A-maj, was sequenced to confirm the major allele, C, at position rs3750625 in the ADRA2A 3′UTR, and was then used as template to mutate the major allele to the minor allele, A, using the QuickChange II Site-Directed Mutagenesis Kit (Promega) and primers 2-F and 2-R (Supplementary Table 1). The construct containing the minor allele was named pL-GL3-ADRA2A-min.
Seed region and compensatory region mutations were made in the ADRA2A 3′UTR using two-step PCR. pL-GL3-ADRA2A-maj was used as the parent construct, and primers containing mutant sequence at either the seed region or compensatory site were used to create constructs pL-GL3-ADRA2A-seed (Primers 1-F, 1-R, 7-F, and 7-R, Supplementary Table 1) and pL-GL3-ADRA2A-comp (Primers 1-F, 1-R, 6-F, 6-R, Supplementary Table 1), respectively. The pcDNA-based miR-34a expression construct (pC-34a) was generously donated by Moshe Oren (The Weizmann Institute of Science).
pLCE-s34 and associated controls, pLCE and pLCE sCXCR4 were generously donated from the Luftig Lab (Duke University). Generation of these constructs and the efficacy with which pLCE-s34 knocks down miR-34a expression has been previously descibed.[24]
Cell culture and generation of neuroblastoma cells stably knocked down for miR-34a expression
HEK293T cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS) and 1% gentamicin. IMR-32 cells are human adrenergic neuroblastoma cells with high levels of the catecholamine precursor, tyrosine hydroxylase.[61] They were cultured in Eagle’s Minimum Essential Medium (EMEM) with 10% FBS and 1% gentamicin. All cells were incubated at 37°C, 5% CO2.
To address whether miR-34a plays a significant role in regulating endogenous levels of ADRA2A, we suppressed miR-34a activity in the IMR-32 neuroblastoma cell line. miR-34a activity was inhibited by transduction of these cells with a GFP-based lentiviral miRNA sponge specific for miR-34a (s34). miRNA sponges represent a well-established technique [21; 36; 59] for specifically and stably blocking the activity of an miRNA through overexpression of an mRNA containing the GFP indicator gene linked to multiple (in this case, four) copies of an incompletely complementary artifical target site for the miRNA of interest. This particular miR-34a sponge has previously been shown to result in robust knockdown of miR-34a.[24]
We also separately transduced a previously described [26] control lentiviral miRNA sponge vector (sCXCR4) which expresses a sponge specific for a small interfering RNA that inhibits CXCR4 mRNA expression. The second control vector represents the parental lentiviral vector expressing only GFP (pLCE). Viral transductants for pLCE-s34 were produced in HEK293T cells using a third generation lentiviral packaging system [20] consisting of pMD2-VSVG, pRSV-REV, pMDLgp, and either pLCE-s34 (‘s34’), pLCE (GFP control), or PLCE-sCXCR4 (‘sCXCR4’, a control sponge). HEK293T cells were transfected using Fugene 6 Reagent (Promega). 48 hours after transfection, viral media was collected, filtered, and concentrated using Amicon Ultra centrifugal columns, then added to the culture medium atop IMR-32 cells, and integration of sponge sequence was monitored by GFP expression. Cells with the top 30% mean GFP fluorescence were sorted using FACS analysis (ARIA II) and grown in culture until sufficient numbers of cells were available for RNA isolation via TRIzol (Life Technologies) or for protein isolation via RIPA buffer (Pierce).
Dual luciferase assays
Binding of miR-34a to ADRA2A was assessed using the above described FLUC and RLUC-based indicator vectors, pL-SV40-GL3 and pL-SV40-RLUC. HEK293T cells were co-transfected with pC-34a, pL-SV40-RLUC, and either pL-GL3-ADRA2A-maj or pL-GL3-ADRA2A-min using Fugene 6 Transfection Reagent (Promega). To asses dose-dependent binding, 20fmol, 60fmol, and 120fmol of pC-34a were used. For seed vs compensatory binding experiments, 150fmol of pC-34 was used.
Seventy-two hours after transfection, cells were collected and lysed. RLUC and FLUC levels were measured on a SpectraMax microplate reader (Molecular Devices) using substrates from a dual luciferase reporter assay kit as described (Promega); FLUC values were normalized to RLUC values.
RT-qPCR and Western blotting
miRNA expression was measured by stem-loop reverse transcription- quantitative PCR (RT-qPCR). Total RNA was prepared with TRIzol reagent according to the manufacturer’s instructions and 10 ng of total RNA per reverse transcription reaction was used. Primers for RT and probes for quantitative PCR (TaqMan) were ordered from Life Technologies and used as directed. miR-34a expression levels (microRNA assay number 000426; Life Technologies) were normalized to RNU48 (human samples) or U87 (rat samples) (assay numbers 001006 and 001712, respectively; Life Technologies).
For analysis of ADRA2A mRNA expression, RNA was first treated with DNaseI (New England Biolabs) to remove genomic DNA. Random primers were used for reverse transcription as described (High Capacity Reverse Transcription Kit, Life Technologies) and SYBR reagents were used for qPCR with transcript specific primers as instructed (Life Technologies). Primer sequences for detecting ADRA2A mRNA in humans and rats are shown in Supplementary Table 1. Endogenous control primers were designed against B2M (human) and Beta actin (Rat); their sequences are also shown in Supplementary Table 1.
To compare α2A-AR protein levels after miR-34a knock-down, protein was isolated from cells using RIPA buffer (ThermoFisher Scientific) and protease inhibitors (ThermoFisher Scientific). Total protein concentrations were measured with a BCA Protein Assay (Pierce) and equal amounts of protein (26μg per lane) were loaded onto a 4-12% Bis-Tris gel (LifeTechnologies) and analyzed by Western blotting. Primary rabbit anti-ADRA2A was from Boster Antibody and ELISA Experts (PA2197), and primary rabbit anti-β-actin was from Cell Signaling Technology (13E5, #4970). Secondary anti-rabbit IgG HRP was from Cell Signaling Technology (#7074). Signals were developed using ECL Western Blotting Substrate (Pierce) and in accordance with product literature, bands corresponding to ~50kDa were identified as ADRA2A.
Animal studies
The US Army Institute of Surgical Research Institutional Animal Care and Use Committee approved the described animal study, which was conducted in compliance with the Animal Welfare Act, Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals and conformed to federal guidelines. All efforts were made to minimize the number of animals used in these experiments and to minimize potential suffering.
Six adult (200-225g) male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were pair housed in a 12:12 hour light: dark cycle with ad libitum access to food and water. To induce stress, rats were individually placed in cylindrical polyethylene containers measuring 30-cm in diameter and 50-cm in height and containing 20-cm of room temperature water for 20 minutes, as previously described.[46] This was sufficient to force the rats to swim, as they were unable to reach the bottom of the cylinder. Baseline (no stress) subjects were placed in empty cylinders. Swim sessions were performed once daily for three consecutive days. All rats were towel dried and returned to clean cages following each session, and were observed for behavioral signs of distress.
Twenty-four hours following stress/sham exposure, rats were given an overdose of pentobarbital sodium. The following tissues were collected: adrenal glands, peripheral nerves, and dorsal root ganglion from L2-L5. Each tissue was isolated and immediately stored in RNA later (ThermoFisher) at −80°C. RNA was isolated using Trizol and RNA concentration was measured using a NanoDrop 2000 (Thermo Scientific).
Results
Cohort characteristics
Characteristics of study participants in the initial cohort (MVC, n = 948) and replication cohort (sexual assault, n = 84) are shown in Table 1. In the MVC cohort all participants were European American and the majority of the participants were women. For the sexual assault cohort, the majority of the participants were European American and all study participants were women. In general, individuals experiencing MVC were older than sexual assault participants, reported less overall MSP in the ED, and had less distress at the time of trauma.
Table 1.
Baseline characteristics of study participants
| Characteristic | MVC | SA |
|---|---|---|
| Enrolled, n | 948 | 84 |
| Age, years, mean (SD) | 36 (13) | 26 (8) |
| Females, n(%) | 575 (61) | 84 (100) |
| Ethnicity | ||
| European American | 948 (100) | 54 (64) |
| African American | - | 30 (36) |
| Education, n(%) | ||
| 8-11 years | 42 (4) | 11 (13) |
| HS | 184 (19) | 16 (19) |
| Post-HS training (not college) | 57 (6) | 1 (1) |
| Some college | 311 (33) | 43 (51) |
| College | 237 (25) | 9 (11) |
| Post-college | 113 (12) | 3 (4) |
| Overall ED pain, 0-10 NRS, mean(SD) | 5.5 (2.4) | 6.8 (2.9) |
| Distress in the early aftermath of trauma, PDI scale (MVC) and ASD (SA), median |
18 | 77 |
MVC = motor vehicle collision, SA = sexual assault, SD = standard deviation, HS = high-school, ED = emergency department, NRS = numeric rating scale, PDI = peritraumatic distress inventory, ASD = acute stress disorder scale
ADRA2A rs3750625 is located within the seed region of a predicted binding site for miR-34a
Based on computational data from the online algorithm, miRdSNP, ADRA2A rs3750625 is located in the seed binding region for miR-34a.[12] The minor allele, A, at ADRA2A rs3750625 is predicted to create an A-U base-pair between miR-34a and ADRA2A, increasing seed region binding affinity between miR-34a and ADRA2A. This position is unpaired if miR-34a binds the ADRA2A major allele (C, Figure 1A). Examination of the full miR-34a binding region of ADRA2A revealed that this region in general, and the seed region in particular, is highly conserved across mammalian species, suggesting that this is an important miRNA binding site (Figure 1B).
Figure 1.
rs3750625 is in the seed binding region of miR-34a (A) rs3750625 (black arrow, bolded nucleotides) maps within a potential seed binding site for miR-34a. Predicted base pairing between miR-34a and the ADRA2A 3′UTR with either the major or minor allele is indicated by a solid vertical line (Watson-Crick) or by a gray dot (wobble). The seed region of miR-34a (nucleotides 2-8) is underlined. (B) Nucleotide conservation amongst 7 mammals in the miR-34a binding region of ADRA2A. The arrow indicates the location of rs3750625. Conserved nucleotides are greyed.
Acute MSP following MVC and sexual assault
In initial general linear models, the influence of ADRA2A rs3750625 on acute MSP severity after both MVC and sexual assault depended on the level of peritraumatic stress (Table 2). Because of this interaction, the influence of ADRA2A rs3750625 was subsequently evaluated among individuals with higher and lower levels of peritraumatic stress separately.
TABLE 2.
General linear model examining the relationship between candidate predictors and overall acute pain severity following Motor Vehicle Collision (n = 922) and sexual assault (n = 80).
| Motor Vehicle Collision | Sexual Assault | |||
|---|---|---|---|---|
|
| ||||
| Variablea | F | p value | F | p value |
| Age | 3.83 | 0.051 | <0.001 | 0.999 |
| Sex | 0.037 | 0.947 | N/A | N/A |
| Peritraumatic distressb | 34.19 | <0.001 | 0.011 | 0.918 |
| rs3750625c | 3.224 | 0.073 | 5.432 | 0.007 |
| rs3750625 * sex | 0.102 | 0.750 | N/A | N/A |
| rs3750625 * distress | 4.103 | 0.043 | 5.467 | 0.007 |
study site was also included as a categorical variable
For the MVC cohort distress was assessed in the Emergency Department using the Peritraumatic Distress Inventory[11], For the sexual assault cohort distress was assessed using the Acute Stress Disorder Questionnaire[13].
Dominant genetic model used for rs3750625
ADRA2A rs3750625 influences acute MSP severity among individuals with higher levels of peritraumatic distress
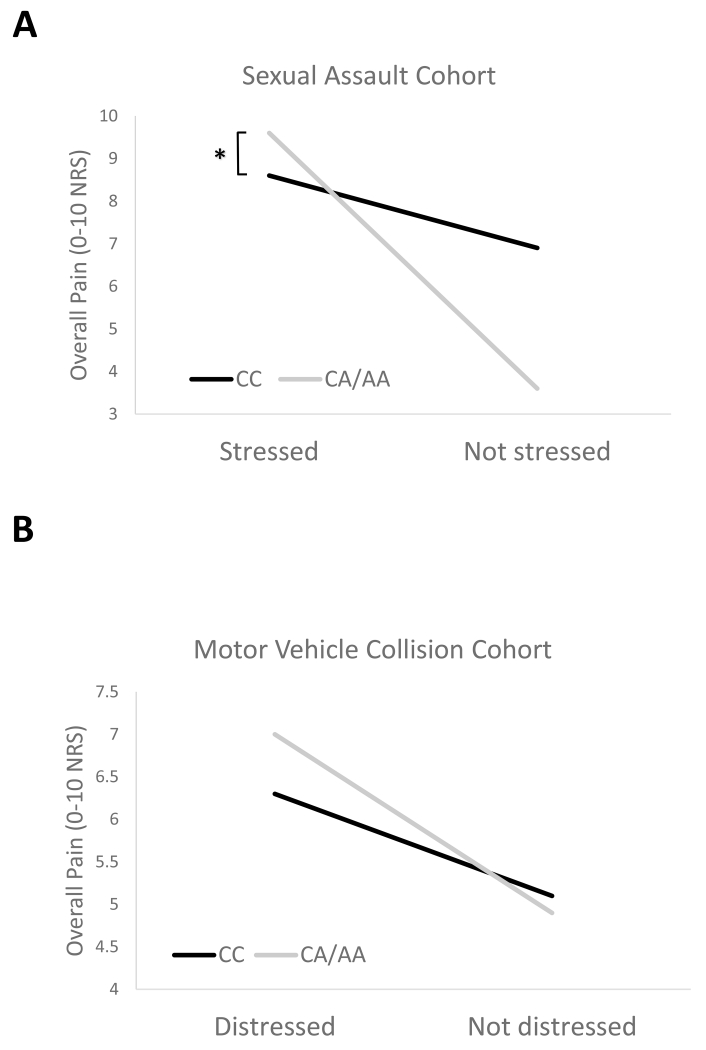
Individuals who reported higher levels of peritraumatic stress during and after sexual assault and had one or more copies of the ADRA2A rs3750625 minor allele (CA/AA) experienced more severe acute MSP following sexual assault than individuals homozygous for the major allele (CC = 9.3 vs CA/AA = 8.4, p = 0.020, Figure 2). The same direction of effect and very similar effect size was observed in the MVC cohort among those who reported higher levels of peritraumatic stress during and after the MVC, although this difference did not reach statistical significance (CC = 7.1 vs CA/AA = 6.3, p = 0.120, Figure 2).
Figure 2.
Acute MSP following trauma is more severe in individuals with the minor allele at ADRA2A rs3750625 who also reported stress in the early aftermath of trauma exposure. (A) Following sexual assault, MSP severity was higher in stressed individuals with at least one copy of the ADRA2A rs3750625 minor allele (CA/AA) versus individuals with the major allele (CC), *p = 0.020. Non-stressed individuals with at least one copy of the ADRA2A rs3750625 minor allele reported lower MSP severity, p = 0.116. A stress cut-off of 77 was used to distinguish stressed from non-stressed individuals (ASD scale, median) (B) A similar crossover interaction was observed following motor vehicle collision. A distress cut off of 23 was used to distinguish distressed from non-distressed individuals (PDI scale, previously validated cut-off[41]).
miR-34a binds to the 3′UTR of ADRA2A
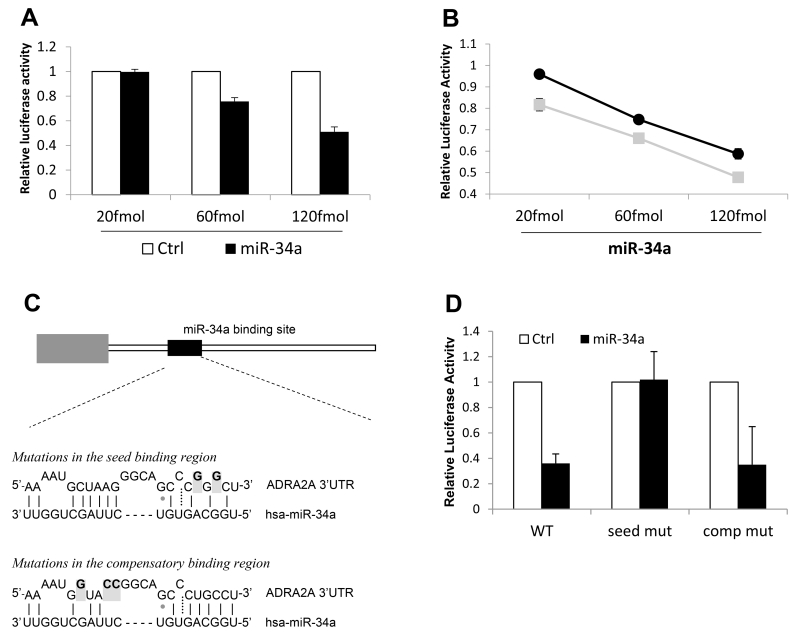
To experimentally test whether miR-34a binds to the 3′UTR of ADRA2A, we cloned the full ADRA2A 3′UTR downstream of a firefly luciferase reporter gene and performed dual luciferase reporter assays.[26] Somewhat surprisingly, given imperfect predicted seed binding, miR-34a binding to the 3′UTR of ADRA2A caused a dose-dependent decrease in luciferase production with the major allele (Figure 3A).
Figure 3.
Characterization and functional assessment of miR-34a binding to the 3′UTR of ADRA2A. (A) miR-34a binding to the 3′UTR of ADRA2A containing the major allele at rs3750625. Relative luciferase activity (y axis) indicates miR-34a binding to ADRA2A (black bars) in a dose-dependent manner. Transfection of a control miRNA was used to determine background luciferase activity (open bars). Each experiment was repeated three times in triplicate, error bars show standard error of the mean. (B) Comparison of miR-34a binding to the 3′UTR of ADRA2A with the minor allele (grey line, grey squares) versus the major allele (black line, black circles) at rs3750625. Error bars show standard error of the mean based on three experimental replicates. Differences in luciferase activity between the major and minor alleles was significant at all three concentrations of miRNA added. **p < 0.005 (Mann-Whitney U test). (C) Relative location of the miR-34a binding site in the context of the 1.4kb ADRA2A 3′UTR. Mutations that destabilize base pairing (greyed and bolded nucleotides) were made in either the seed binding region or the compensatory binding region of pL-GL3-ADRA2A-maj. (D) Relative luciferase activity was measured for miR-34a binding (150fmol) to the ADRA2A 3′UTR with no mutations (WT), with mutations in the seed region (seed mut) or with mutations in the compensatory region (comp mut). Results are the average of three experiments performed in triplicate. Error bars show standard deviation.
The ADRA2A rs3750625 allele affects miR-34a binding in vitro
We next compared binding of miR-34a to the 3′UTR of ADRA2A in the presence of the minor allele. Consistent with miRdSNP prediction of increased base pairing of miR-34a to the ADRA2A 3′UTR seed region in the presence of the minor allele (Figure 1A), decreased luciferase production was observed in the presence of the minor allele vs the major allele at all concentrations of miR-34a (Figure 3B).
The seed region of the miR-34a binding site in the 3′UTR of ADRA2A confers the majority of miR-34a binding specificity in vitro
Because we observed binding of miR-34a to the 3′UTR of ADRA2A with the major allele present, which is not predicted to form perfect seed pairing (suggesting a possible 3′compensatory binding mechanism [5]), we made mutations in both the seed region and the compensatory region of the ADRA2A 3′UTR and tested miR-34a binding (Figure 3C). Dual luciferase reporter assays demonstrated that the majority of binding between miR-34a and the ADRA2A 3′UTR is conferred through the seed sequence (Figure 3D), and that this binding site is the only miR-34a site in the ADRA2A 3′UTR. (No binding was observed when this seed site was mutated).
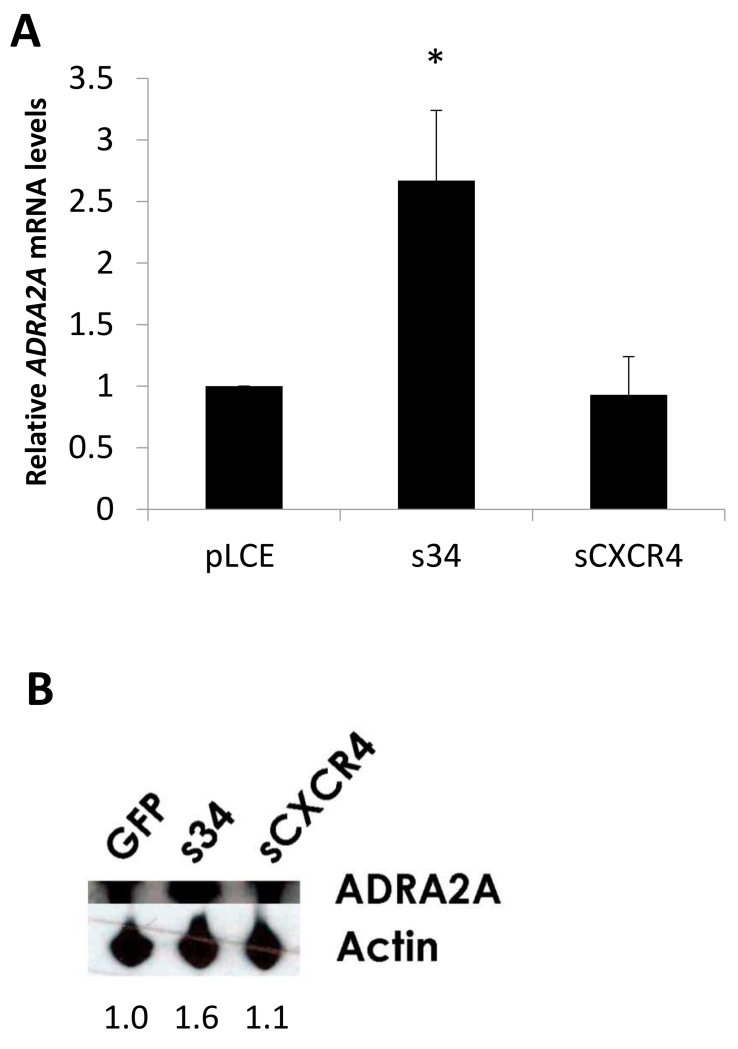
miR-34a regulates endogenous expression of ADRA2A in cell culture
ADRA2A mRNA and protein levels were assayed in an IMR-32 neuroblastoma cell line stably expressing a miR-34a sponge. (IMR-32 cells exhibit adrenergic neurotransmitter properties [47; 61] and express both miR-34a and ADRA2A (Supplementary Figure 1).) ADRA2A mRNA levels (measured via RT-qPCR) increased by 2.5 fold in the absence of miR-34a (Figure 4A, p <0.05, Mann Whitney U test). Consistent with this result, using Western Blotting, we also detected an increase in the level of ADRA2A protein in the presence of the miR-34a sponge, as compared to pLCE and sCXCR4 controls (Figure 4B).
Figure 4.
miR-34a regulates ADRA2A expression in neuroblastoma cells. miR-34a was knocked down in the IMR-32 adrenergic neuroblastoma cell line using a sponge construct, pLCE-s34. (A) ADRA2A mRNA expression was measured by RT-qPCR of RNA isolated from IMR-32 cells expressing pLCE-s34 (s34). Levels of ADRA2A mRNA expression in s34 were calculated relative to IMR-32 cells expressing empty vector (pLCE) or control sponge (sCXCR4). Quantification of mRNA was performed three times in duplicate. Error bars show standard deviation. * p < 0.05 (Mann-Whitney U test). (B) ADRA2A protein levels from cell lysates of sponge (and control) expressing IMR-32 cells were measured by Western Blotting. Quantification of band intensity was calculated using band intensity measurements from the NIH image analysis program, Image J.
miR-34a and ADRA2A are co-expressed in rat tissues relevant to pain and stress and their expression levels change in response to forced swim stress exposure
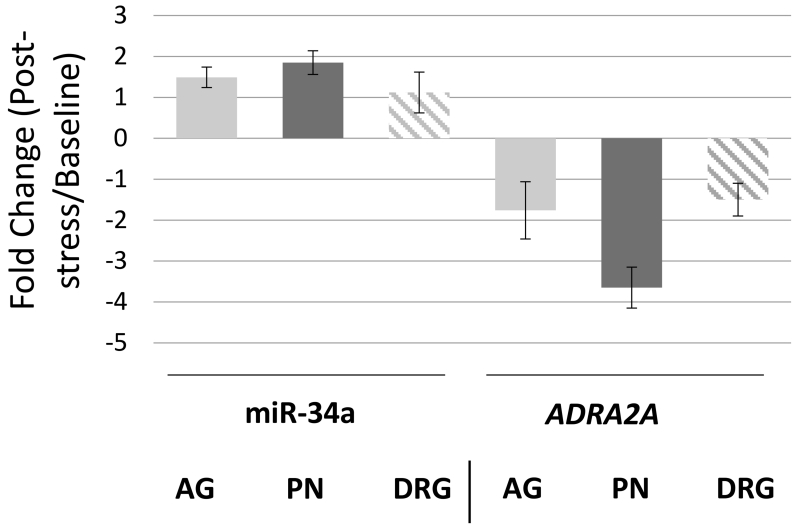
For regulation to occur in vivo, miR-34a and ADRA2A must both be expressed in stress-relevant tissues. Previous animal model studies have shown that pain development following stress exposure is influenced by the release of catecholamines from the adrenal gland that then sensitize peripheral nociceptors.[19; 29] We therefore examined miR-34a and ADRA2A mRNA expression in the adrenal gland, peripheral nerve, and dorsal root ganglion (DRG), both before and after forced swim stress.
Peripheral nerve and DRG
Consistent with previous reports, ADRA2A mRNA and miR-34a were both expressed in the DRG [3; 25; 55; 56] and peripheral nerve tissue, with higher levels of each RNA in the DRG (Supplementary Figure 2). Following forced swim stress exposure, ADRA2A mRNA levels decreased in the peripheral nerve (Figure 5). In contrast, in peripheral nerve tissues miR-34a expression levels increased. No change in ADRA2A mRNA or miR-34a expression was detected in the DRG.
Figure 5.
Comparison of miR-34a and ADRA2A mRNA expression levels in Sprague-Dawley rats in response to forced swim stress exposure in the following tissues: Adrenal Gland (AG), Peripheral Nerve (PN), and Dorsal Root Ganglion (DRG). RNA was isolated from rat tissues at baseline (no stress exposure) and from rat tissues after exposure to 30 minutes of repeated forced swim stress on three consecutive days. RT-qPCR was used for miR-34a and ADRA2A mRNA quantification. Fold change was calculated from a minimum of three independent experiments performed in duplicate. Error bars represent standard deviations.
Adrenal gland
ADRA2A and miR-34a were also expressed in the adrenal gland, at lower levels than in the peripheral nerve and DRG (Supplementary Figure 2). Swim stress exposure had little to no effect on adrenal gland ADRA2A or miR-34a expression levels (Figure 5).
Discussion
To our knowledge, the present report is the first to provide evidence that acute MSP severity following traumatic stress exposure is influenced by microRNA regulation of the expression of an important stress/pain gene (i.e., ADRA2A). In addition, our results indicate that a specific SNP in the ADRA2A gene nucleotide sequence, where miR-34a binds, affects acute MSP variability. Specifically, our results suggest that distressed individuals with the rs3750625 minor allele experience an approximately 1 point increase (on a 0-10 NRS scale) in acute MSP after MVC trauma or sexual assault, due to the fact that the 3′UTR of the ADRA2A gene is bound more efficiently by miR-34a when the minor allele is present. Importantly, the influence of the rs3750625 allele was stress dependent. This finding is consistent with the known regulation of miR-34a by stress,[18; 27] and suggests that activation of the same physiologic systems which result in subjective symptoms of stress/distress also increase the expression of miR-34a and enhance the impact of the rs3750625 allele on the cell physiology of stress-relevant tissues and resulting phenotype. These data also provide an example of the important influence of stress-induced hyperalgesia on clinical pain outcomes after trauma exposure, and provide data supporting a physiologic mechanism by which increased stress causes increased acute MSP, distinct from traditionally hypothesized mechanisms such as muscle tension.[43]
Donello et al previously showed that, in the absence of ADRA2A, animals exhibit acute hyperalgesia (vs analgesia) following exposure to sound and footshock stressors.[19] Our results showing that trauma-exposed individuals with the minor allele at rs3750625 have increased miR-34a binding to ADRA2A, reduced ADRA2A transcript, and increased acute MSP are consistent with these pre-clinical data. In addition, data from Donello et al, together with data from Levine and collegues, indicate that low levels of peripherally located α2A-AR receptors lead to reduced feedback inhibition of norepinephrine release in response to stress.[19; 30] Norepinephrine can sensitize sensory afferents, thus high levels of this neurotransmitter (due to low levels of α2A-AR) result in increased hyperalgesia.[31] Our data indicate that expression levels of ADRA2A and miR-34a RNAs change in peripheral nerves in response to stress. If miR-34a binds ADRA2A more efficiently in individuals with the risk allele, then reduced levels of α2A-AR in such individuals would be anticipated to result in higher circulating levels of norepinephrine and greater afferent sensitization. Future studies measuring α2A-AR gene expression and circulating levels of catecholamines in trauma-exposed individuals with the major vs. minor allele could test this hypothesis.
Our results and those from other groups suggest that miR-34a regulation of ADRA2A is likely to be tissue-specific. In our study, we assayed three stress-sensitive rat tissues (the adrenal gland, DRG, and peripheral nerves) for biologically relevant overlapping expression of miR-34a and ADRA2A mRNA and for expression level changes following stress exposure. Though the two RNAs were co-located in all three tissues, change in expression following stress exposure was only consistent with direct miR-34a regulation of ADRA2A in the peripheral nerves. These data are consistent with previous reports, which have also suggested that miR-34a and ADRA2A expression changes following stress exposure are tissue (and perhaps stress) specific. For example, ADRA2A expression in the locus corelus of Wistar and Wistar-Kyoto rats exhibited no change following acute restraint stress,[51] whereas ADRA2A was significantly downregulated in the bed nucleus of the stria terminalis in response to repeated footshock stressors.[34] The fact that miR-34a and ADRA2A interaction is likely tissue-specific suggests important implications regarding potential therapeutic targeting of miRNA, since miR-34a has also been shown to play roles in tumorigenesis and cardiac function.[6; 7; 17; 24; 48; 53; 57] In other words, to avoid off-target effects, such treatment would likely need to be specifically targeted to pain-relevant tissues. Of note, in addition to ADRA2A, predicted or experimentally validated targets of miR-34a and/or miR-449 (a member of the same miRNA family) include gene transcripts such as COMT, CRHR1, SLC6A1, NOTCH1, and ADCY5.[14; 18; 40; 52] The effect of miR-34a/miR-449 on these transcripts, and/or polymorphisms in these genes affecting miRNA binding, may also influence acute or persistent MSP outcomes after trauma exposure. Therefore, future studies examining more general transcriptional changes in miR-34a-influenced genes following different types of stress and/or pain exposures are warranted.
We focused on rs3750625 in ADRA2A because it was predicted to affect a miR-34a binding site by the miRdSNP online database. Interestingly, however, because there is not perfect complimentarity between the seed region of ADRA2A with the major allele and miR-34a, other miRNA target prediction algorithms such as TargetScan and DIANA MicroT-CDS were not able to predict a binding event at this location. Therefore, our approach enabled us to uncover a miRNA binding event that would not have been discovered using traditional algorithm-based miRNA target identification techiques. Additionally, because of the general importance of seed binding for miRNA-mRNA interactions,[35] we hypothesized that we would detect less binding with the major allele than we observed. This observed binding led us to test whether miR-34a bound non-canonically, ie via the compensatory region.[5] Surprisingly, we found that the binding activity was in fact conferred through the seed region, providing evidence that a seed region with a single mismatch can still confer binding. Despite the evident importance of seed region binding in the present study, many non-seed region target sites (with a wobble base pair or bulge in the seed) have been identified and are of high biologic relevance.[10; 16; 22]
Our data is interesting in that it might shed light on a finding from a paper published in 2004 showing that a genetic association in the 3′UTR of ADRA2A is associated with physiological reponses to stress.[23] In this manuscript, the authors showed that the minor allele of a polymorphism in a DraI site caused rapid degradation of the mRNA. Upon examination of the ADRA2A 3′UTR, there are four DraI sites; the functional polymorphism examined in the 2004 manuscript encompasses rs553668 and is located 22nt upstream of rs3750625. Although this site is immediately outside of the miR-34a binding region, it is still possible that the allele affects miR-34a binding through indirect mechanisms such as RNA folding or protein binding.[2] Because miRNA were not widely studied until the early part of the millenium, it is likely that the authors of this paper did not consider potential effects of miRNA.
Some limitations should be considered when interpreting the results of this study. First, we focused on a single polymorphism instead of analyzing SNP association with acute pain outcomes using previously established haplotypes.[54] It is therefore possible that other polymorphisms in LD with rs3750625 such as rs1800763 also serve as functional polymorphisms that work cooperatively with rs3750625-mediating miRNA binding changes to affect ADRA2A mRNA levels. Second, our genetic association results were not as significant in our large MVC cohort as they were in the smaller sexual assault cohort. This could be due to the relative frequency of the minor allele in European Americans (5% in the MVC cohort) vs in African Americans (~15%). Thus in the sexual assault cohort, which is over one-third African American (n = 30, 36%), we might have had more statistical power to detect differences. Third, because miR-34a and ADRA2A are predominately expressed in tissues that are not feasible to collect in a live human cohort, we were not able to examine whether levels of these RNA differ in individuals with the major vs minor allele. However, in future studies, it would be interesting to measure plasma catecholamine levels (with the caveat that quantification is difficult and not always reliable[15; 44]) in these individuals. Fourth, animal and cell culture experiments included in this study used a single animal line, Sprague Dawley rats, and a single cell culture line, IMR-32 neuroblastoma cells. Future studies should evaluate experimental results in additional animal and cell lines. Finally, we did not directly measure the effects of miR-34a on MSP severity following stress exposure in animals. It would be interesting to examine acute hyperalgesia following stress exposure in animals that do not express miR-34a.
Supplementary Material
Supplementary Figure 1. Relative expression levels of miR-34a and ADRA2A RNA in two adrenergic neuroblastoma cell lines, IMR-32 and SH-SY5Y
Supplementary Figure 2. miR-34a and ADRA2A mRNA are co-expressed in rat tissues relevant to stress-induced pain development. (A and C) Cycle threshold values for detection of miR-34a or ADRA2A mRNA (as indicated) in the adrenal gland (AG), peripheral nerve (PN), and dorsal root ganglion (DRG) of rats at baseline using TaqMan RT-qPCR. Values are the average of three independent experiments performed in duplicate or triplicate from six animals. Error bars represent standard deviation. The higher the cycle threshold, the lower the expression level. (B and D) Fold difference in expression of miR-34a expression in PN and DRG relative to AG expression at baseline.
Summary.
ADRA2A variant rs3750625 alters a miR-34a binding site and is associated with acute pain severity following motor vehicle collision and sexual assault traumas.
Acknowledgements
We would like to thank the participants for taking part in this study.
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR0563282. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding was also provided by The Mayday Fund and The American Pain Society via a Future Leaders in Pain grant, and by the United States Army Medical Research and Materiel Command Combat Casualty Care Research and the Clinical and Rehabilitative Medicine Research programs. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Scientific Meeting Presentation: American Pain Society Meeting 2014, Tampa FL
References
- [1].U.S. Census Bureau . Statistical Abstract of the United States: 2012. Washington, DC: 2011. [Google Scholar]
- [2].Arnold M, Ellwanger DC, Hartsperger ML, Pfeufer A. [Google Scholar]
- [3].Bak M, Silahtaroglu A, Moller M, Christensen M, Rath MF, Skryabin B, Tommerup N, Kauppinen S. MicroRNA expression in the adult mouse central nervous system. Rna. 2008;14(3):432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ballina LE, Ulirsch JC, Soward AC, Rossi C, Rotolo S, Linnstaedt SD, Heafner T, Foley KA, Batts J, Collette R, Holbrook D, Zelman S, McLean SA. mu-Opioid receptor gene A118G polymorphism predicts pain recovery after sexual assault. J Pain. 2013;14(2):165–171. doi: 10.1016/j.jpain.2012.10.013. [DOI] [PubMed] [Google Scholar]
- [5].Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bernardo BC, Gao X-M, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, Gregorevic P, Obad S, Kauppinen S, Du X-J. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proceedings of the National Academy of Sciences. 2012;109(43):17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495(7439):107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- [8].Bortsov AV, Diatchenko L, McLean SA. Complex multilocus effects of catechol-O-methyltransferase haplotypes predict pain and pain interference 6 weeks after motor vehicle collision. Neuromolecular medicine. 2014;16(1):83–93. doi: 10.1007/s12017-013-8255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bortsov AV, Smith JE, Diatchenko L, Soward AC, Ulirsch JC, Rossi C, Swor RA, Hauda WE, Peak DA, Jones JS, Holbrook D, Rathlev NK, Foley KA, Lee DC, Collette R, Domeier RM, Hendry PL, McLean SA. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. Pain. 2013 doi: 10.1016/j.pain.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brodersen P, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nature reviews Molecular cell biology. 2009;10(2):141–148. doi: 10.1038/nrm2619. [DOI] [PubMed] [Google Scholar]
- [11].Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, Fagan J, Marmar CR. The Peritraumatic Distress Inventory: a proposed measure of PTSD criterion A2. The American journal of psychiatry. 2001;158(9):1480–1485. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- [12].Bruno AE, Li L, Kalabus JL, Pan Y, Yu A, Hu Z. miRdSNP: a database of disease-associated SNPs and microRNA target sites on 3′UTRs of human genes. BMC genomics. 2012;13:44. doi: 10.1186/1471-2164-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bryant RA, Moulds ML, Guthrie RM. Acute Stress Disorder Scale: a self-report measure of acute stress disorder. Psychological Assessment. 2000;12(1):61. [PubMed] [Google Scholar]
- [14].Capuano M, Iaffaldano L, Tinto N, Montanaro D, Capobianco V, Izzo V, Tucci F, Troncone G, Greco L, Sacchetti L. MicroRNA-449a overexpression, reduced NOTCH1 signals and scarce goblet cells characterize the small intestine of celiac patients. PloS one. 2011;6(12):e29094. doi: 10.1371/journal.pone.0029094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Christensen NJ. The biochemical assessment of sympathoadrenal activity in man. Clinical Autonomic Research. 1991;1(2):167–172. doi: 10.1007/BF01826215. [DOI] [PubMed] [Google Scholar]
- [16].Clark PM, Loher P, Quann K, Brody J, Londin ER, Rigoutsos I. Argonaute CLIP-Seq reveals miRNA targetome diversity across tissue types. Scientific reports. 2014:4. doi: 10.1038/srep05947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Concepcion CP, Han Y-C, Mu P, Bonetti C, Yao E, D’andrea A, Vidigal JA, Maughan WP, Ogrodowski P, Ventura A. Intact p53-dependent responses in miR-34–deficient mice. PLoS Genet. 2012;8(7):e1002797. doi: 10.1371/journal.pgen.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dias BG, Goodman JV, Ahluwalia R, Easton AE, Andero R, Ressler KJ. Amygdala-dependent fear memory consolidation via miR-34a and notch signaling. Neuron. 2014;83(4):906–918. doi: 10.1016/j.neuron.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Donello JE, Guan Y, Tian M, Cheevers CV, Alcantara M, Cabrera S, Raja SN, Gil DW. A peripheral adrenoceptor-mediated sympathetic mechanism can transform stress-induced analgesia into hyperalgesia. The Journal of the American Society of Anesthesiologists. 2011;114(6):1403–1416. doi: 10.1097/ALN.0b013e31821c3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. Journal of virology. 1998;72(11):8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nature methods. 2007;4(9):721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Elefant N, Altuvia Y, Margalit H. A wide repertoire of miRNA binding sites: prediction and functional implications. Bioinformatics. 2011;27(22):3093–3101. doi: 10.1093/bioinformatics/btr534. [DOI] [PubMed] [Google Scholar]
- [23].Finley JC, Jr., O’Leary M, Wester D, MacKenzie S, Shepard N, Farrow S, Lockette W. A genetic polymorphism of the alpha2-adrenergic receptor increases autonomic responses to stress. Journal of applied physiology. 2004;96(6):2231–2239. doi: 10.1152/japplphysiol.00527.2003. [DOI] [PubMed] [Google Scholar]
- [24].Forte E, Salinas RE, Chang C, Zhou T, Linnstaedt SD, Gottwein E, Jacobs C, Jima D, Li QJ, Dave SS, Luftig MA. The Epstein-Barr virus (EBV)-induced tumor suppressor microRNA MiR-34a is growth promoting in EBV-infected B cells. J Virol. 2012;86(12):6889–6898. doi: 10.1128/JVI.07056-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gold MS, Dastmalchi S, Levine JD. Alpha 2-adrenergic receptor subtypes in rat dorsal root and superior cervical ganglion neurons. Pain. 1997;69(1-2):179–190. doi: 10.1016/s0304-3959(96)03218-6. [DOI] [PubMed] [Google Scholar]
- [26].Gottwein E, Cullen BR. A human herpesvirus microRNA inhibits p21 expression and attenuates p21-mediated cell cycle arrest. Journal of virology. 2010;84(10):5229–5237. doi: 10.1128/JVI.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, Hornstein E, Chen A. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(40):14191–14203. doi: 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome research. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Khasar SG, Dina OA, Green PG, Levine JD. Sound Stress–Induced Long-Term Enhancement of Mechanical Hyperalgesia in Rats Is Maintained by Sympathoadrenal Catecholamines. The Journal of Pain. 2009;10(10):1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Khasar SG, McCarter G, Levine JD. Epinephrine produces a β-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. Journal of neurophysiology. 1999;81(3):1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- [32].Kohli U, Muszkat M, Sofowora GG, Harris PA, Friedman EA, Dupont WD, Scheinin M, Wood AJ, Stein CM, Kurnik D. Effects of variation in the human alpha2A- and alpha2C-adrenoceptor genes on cognitive tasks and pain perception. Eur J Pain. 2010;14(2):154–159. doi: 10.1016/j.ejpain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kruger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic acids research. 2006;34(Web Server issue):W451–454. doi: 10.1093/nar/gkl243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lebow M, Neufeld-Cohen A, Kuperman Y, Tsoory M, Gil S, Chen A. Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(20):6906–6916. doi: 10.1523/JNEUROSCI.4012-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- [36].Linnstaedt SD, Gottwein E, Skalsky RL, Luftig MA, Cullen BR. Virally induced cellular microRNA miR-155 plays a key role in B-cell immortalization by Epstein-Barr virus. J Virol. 2010;84(22):11670–11678. doi: 10.1128/JVI.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McCracken JT, Badashova KK, Posey DJ, Aman MG, Scahill L, Tierney E, Arnold LE, Vitiello B, Whelan F, Chuang SZ, Davies M, Shah B, McDougle CJ, Nurmi EL. Positive effects of methylphenidate on hyperactivity are moderated by monoaminergic gene variants in children with autism spectrum disorders. The pharmacogenomics journal. 2014;14(3):295–302. doi: 10.1038/tpj.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McLean SA, Soward AC, Ballina LE, Rossi C, Rotolo S, Wheeler R, Foley KA, Batts J, Casto T, Collette R, Holbrook D, Goodman E, Rauch SA, Liberzon I. Acute severe pain is a common consequence of sexual assault. J Pain. 2012;13(8):736–741. doi: 10.1016/j.jpain.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Meloto CB, Bortsov A, Bair E, Helgeson E, Ostrom C, Smith S, Dubner R, Slade GD, Fillingim RB, Greenspan JD, Ohrbach R, Maixner W, McLean S, Diatchenko L. Modification of COMT-dependent pain sensitivity by psychological stress and sex. Pain. 2015 doi: 10.1097/j.pain.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nemoto T, Mano A, Shibasaki T. miR-449a contributes to glucocorticoid-induced CRF-R1 downregulation in the pituitary during stress. Molecular Endocrinology. 2013;27(10):1593–1602. doi: 10.1210/me.2012-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nishi D, Matsuoka Y, Yonemoto N, Noguchi H, Kim Y, Kanba S. Peritraumatic Distress Inventory as a predictor of post-traumatic stress disorder after a severe motor vehicle accident. Psychiatry and clinical neurosciences. 2010;64(2):149–156. doi: 10.1111/j.1440-1819.2010.02065.x. [DOI] [PubMed] [Google Scholar]
- [42].Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. Journal of consulting and clinical psychology. 1992;60(3):409. doi: 10.1037//0022-006x.60.3.409. [DOI] [PubMed] [Google Scholar]
- [43].Norton PJ, Asmundson GJ. Amending the fear-avoidance model of chronic pain: what is the role of physiological arousal? Behavior Therapy. 2003;34:17–30. [Google Scholar]
- [44].Peaston RT, Weinkove C. Measurement of catecholamines and their metabolites. Annals of clinical biochemistry. 2004;41(1):17–38. doi: 10.1258/000456304322664663. [DOI] [PubMed] [Google Scholar]
- [45].Platts-Mills TFBL, Bortsov AV, Soward A, Swor RA, Jones JS, Lee DC, Peak DA, Domeier RM, Rathlev NK, Hendry PL, McLean SA. Using emergency department-based inception cohorts to determine genetic characteristics associated with long term patient outcomes after motor vehicle collision: methodology of the CRASH study. BMC Emerg Med. 2011;11(14) doi: 10.1186/1471-227X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacology, Biochemistry, and Behavior. 2000;67(3):449–458. doi: 10.1016/s0091-3057(00)00374-9. [DOI] [PubMed] [Google Scholar]
- [47].Rabinovsky ED, Le WD, McManaman JL. Differential effects of neurotrophic factors on neurotransmitter development in the IMR-32 human neuroblastoma cell line. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12(1):171–179. doi: 10.1523/JNEUROSCI.12-01-00171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Molecular cell. 2007;26(5):731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- [49].Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- [50].Rubin DH, Althoff RR, Ehli EA, Davies GE, Rettew DC, Crehan ET, Walkup JT, Hudziak JJ. Candidate gene associations with withdrawn behavior. Journal of child psychology and psychiatry, and allied disciplines. 2013;54(12):1337–1345. doi: 10.1111/jcpp.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sands S, Strong R, Corbitt J, Morilak D. Effects of acute restraint stress on tyrosine hydroxylase mRNA expression in locus coeruleus of Wistar and Wistar-Kyoto rats. Brain research Molecular brain research. 2000;75(1):1. doi: 10.1016/s0169-328x(99)00255-7. [DOI] [PubMed] [Google Scholar]
- [52].Shenoda BB, Alexander GM, Ajit SK. Hsa-miR-34a mediated repression of corticotrophin releasing hormone receptor 1 regulates pro-opiomelanocortin expression in patients with complex regional pain syndrome. Journal of Translational Medicine. 2016;14(1):1. doi: 10.1186/s12967-016-0820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Silber J, Jacobsen A, Ozawa T, Harinath G, Pedraza A, Sander C, Holland EC, Huse JT. miR-34a repression in proneural malignant gliomas upregulates expression of its target PDGFRA and promotes tumorigenesis. PLoS One. 2012;7(3):e33844. doi: 10.1371/journal.pone.0033844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Small KM, Brown KM, Seman CA, Theiss CT, Liggett SB. Complex haplotypes derived from noncoding polymorphisms of the intronless α2A-adrenergic gene diversify receptor expression. Proceedings of the National Academy of Sciences. 2006;103(14):5472–5477. doi: 10.1073/pnas.0601345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(15):5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Stone LS, Vulchanova L, Riedl MS, Wang J, Williams FG, Wilcox GL, Elde R. Effects of peripheral nerve injury on alpha-2A and alpha-2C adrenergic receptor immunoreactivity in the rat spinal cord. Neuroscience. 1999;93(4):1399–1407. doi: 10.1016/s0306-4522(99)00209-2. [DOI] [PubMed] [Google Scholar]
- [57].Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proceedings of the National Academy of Sciences. 2007;104(39):15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ulirsch JC, Weaver MA, Bortsov AV, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, McLean SA. No man is an island: living in a disadvantaged neighborhood influences chronic pain development after motor vehicle collision. Pain. 2014;155(10):2116–2123. doi: 10.1016/j.pain.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Valastyan S, Weinberg RA. Assaying microRNA loss-of-function phenotypes in mammalian cells: emerging tools and their potential therapeutic utility. RNA biology. 2009;6(5):541–545. doi: 10.4161/rna.6.5.10081. [DOI] [PubMed] [Google Scholar]
- [60].von Schack D, Agostino MJ, Murray BS, Li Y, Reddy PS, Chen J, Choe SE, Strassle BW, Li C, Bates B, Zhang L, Hu H, Kotnis S, Bingham B, Liu W, Whiteside GT, Samad TA, Kennedy JD, Ajit SK. Dynamic changes in the microRNA expression profile reveal multiple regulatory mechanisms in the spinal nerve ligation model of neuropathic pain. PloS one. 2011;6(3):e17670. doi: 10.1371/journal.pone.0017670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].West GJ, Uki J, Herschman HR, Seeger RC. Adrenergic, cholinergic, and inactive human neuroblastoma cell lines with the action-potential Na+ ionophore. Cancer research. 1977;37(5):1372–1376. [PubMed] [Google Scholar]
- [62].Yang L, Qian Q, Liu L, Li H, Faraone SV, Wang Y. Adrenergic neurotransmitter system transporter and receptor genes associated with atomoxetine response in attention-deficit hyperactivity disorder children. Journal of neural transmission. 2013;120(7):1127–1133. doi: 10.1007/s00702-012-0955-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Relative expression levels of miR-34a and ADRA2A RNA in two adrenergic neuroblastoma cell lines, IMR-32 and SH-SY5Y
Supplementary Figure 2. miR-34a and ADRA2A mRNA are co-expressed in rat tissues relevant to stress-induced pain development. (A and C) Cycle threshold values for detection of miR-34a or ADRA2A mRNA (as indicated) in the adrenal gland (AG), peripheral nerve (PN), and dorsal root ganglion (DRG) of rats at baseline using TaqMan RT-qPCR. Values are the average of three independent experiments performed in duplicate or triplicate from six animals. Error bars represent standard deviation. The higher the cycle threshold, the lower the expression level. (B and D) Fold difference in expression of miR-34a expression in PN and DRG relative to AG expression at baseline.