Abstract
The brain could be exposed to irradiation as part of a nuclear accident, radiological terrorism (dirty bomb scenario) or a medical radiological procedure. In the context of accidents or terrorism, there is considerable interest in compounds that can mitigate radiation-induced injury when treatment is initiated a day or more after the radiation exposure. As it will be challenging to determine the radiation exposure an individual has received within a relatively short time frame, it is also critical that the mitigating agent does not negatively affect individuals, including emergency workers, who might be treated, but who were not exposed. Alterations in hippocampus-dependent cognition often characterize radiation-induced cognitive injury. The catalytic ROS scavenger EUK-207 is a member of the class of metal-containing salen manganese (Mn) complexes that suppress oxidative stress, including in the mitochondria, and have been shown to mitigate radiation dermatitis, promote wound healing in irradiated skin, and mitigate vascular injuries in irradiated lungs. As the effects of EUK-207 against radiation injury in the brain are not known, we assessed the effects of EUK-207 on sham-irradiated animals and the ability of EUK-207 to mitigate radiation-induced cognitive injury. The day following irradiation or sham-irradiation, the mice started to receive EUK-207 and were cognitively tested 3 months following exposure. Mice irradiated at a dose of 15 Gy showed cognitive impairments in the water maze probe trial. EUK-207 mitigated these impairments while not affecting cognitive performance of sham-irradiated mice in the water maze probe trial. Thus, EUK-207 has attractive properties and should be considered an ideal candidate to mitigate radiation-induced cognitive injury.
1. Introduction
Brain irradiation, such as used to treat brain tumors, is associated with hippocampus-dependent cognitive impairments [1], including deficits in spatial information processing; and their severity might depend on the dose delivered to the medial temporal lobes, the site of the hippocampus [1]. In agreement with these human data, mice exposed to brain radiation show deficits in hippocampus-dependent spatial memory [2-5]. The brain could also be exposed to irradiation as part of a nuclear accident, radiological terrorism or medical radiological procedures. In the context of the first two scenarios, there is considerable interest in compounds that can mitigate radiation-induced injury when treatment is initiated a day after the radiation exposure. As it will be challenging to quickly determine the radiation exposure an individual has received within a relatively short time frame, it is also critical that the mitigating agent does not negatively affect individuals who might be treated but who were not exposed [6-9].
The detrimental effects of ionizing irradiation might involve oxidative stress and the formation of reactive oxygen species (ROS) [10, 11], and thus the role of ROS in effects of irradiation on the brain have been studied [12-15]. However, ROS also play a positive role in learning and memory [16-19]. Therefore, antioxidants might have opposing effects on cognitive performance in sham-irradiated and irradiated mice. For example, while the antioxidant α-lipoic acid prevented 56Fe-irradiation induced hippocampus- and cortex-dependent impairments in the water maze, it induced impairments in object recognition and cued fear memory in sham-irradiated mice [20].
Antioxidant approaches, including compounds that scavenge ROS, have been assessed for mitigation of radiation injury [21-24]. The synthetic SOD/catalase mimetic EUK-207 is a member of the class of metal-containing salen manganese (Mn) complexes [25]. EUK-207 suppresses oxidative stress, including in the mitochondria [26, 27] and has been used successfully to mitigate radiation dermatitis, promote wound healing in irradiated skin [28], and mitigate vascular injuries in irradiated lungs [29] (for a review, see Doctrow et al. [30, 31]). While EUK-207 suppresses age-related cognitive impairment in mice [32, 33] and salen Mn complexes exhibit numerous other neuroprotective effects in vivo [25, 34, 35], the effects of EUK-207 on radiation injury in the brain are not known. Therefore, in this proof-of-principle study, we assessed the effects of EUK-207 in sham-irradiated animals and the ability of EUK-207 to mitigate radiation-induced cognitive injury.
2. Materials and Methods
2.1. Mice
Two-month-old C57Bl6/J male mice (n = 48) purchased from Jackson Labs were used for the current study. The mice were kept on a 12:12 hr light-dark schedule (lights on at 6AM) with lab chow (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St. Louis, MO) and water given ad libitum, unless indicated otherwise. All procedures were according to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of the Oregon Health and Science University (OHSU). OHSU has an Association for the Assessment and Accreditation of Laboratory Animal Care approved animal facility.
2.2. Irradiation and Treatments
Following i.p. anesthesia (ketamine (Sigma), 80 mg/kg and xylazine (Sigma), 20 mg/kg), mice were sham-irradiated (n = 16) or irradiated at 10 Gy (n = 16) or 15 Gy (n = 16) in a Mark 1 Cesium Irradiator (Shepherd and Associates, San Fernando, CA). The cerebellum, eyes, and body were shielded with lead. For the treatment study (n = 32), twenty hours following sham-irradiation or irradiation, the mice were anesthetized as described above and received a subcutaneously implanted Alzet minipump containing either vehicle (water) or EUK-207 (0.2 mg/kg/day). Mice received new pumps every month, as recommended by the manufacturer, and were tested for cognitive performance in the water maze and fear conditioning tests, as described below, three months after sham-irradiation or irradiation. Mice were housed singly starting 3 days prior to cognitive testing.
2.3. Cognitive testing
The person testing the mice was blinded to their treatments. In week 1, the mice were tested for spatial learning and memory in the water maze. In week 2, the mice were tested for contextual and cued fear conditioning.
2.3.1. Water maze
The water maze test was used to assess hippocampus-dependent spatial learning and memory. A circular pool (diameter 140 cm) was filled with opaque water (24°C) and mice were trained to locate a submerged platform. To determine if irradiation affected the ability to swim or learn the water maze task, mice were first trained to locate a clearly marked platform (visible platform, Days 1 and 2). Mice were subsequently trained to locate the platform when it was hidden beneath the surface of opaque water (Days 3–5). Training during the hidden platform sessions (acquisition) required the mice to learn the location of the hidden platform based on extra-maze cues. For both visible and hidden sessions, there were two daily sessions, morning and afternoon, which were 2 h apart. Each session consisted of three trials (with 10-min inter-trial intervals). A trial ended when the mice located the platform. Mice that failed to locate the platform within 60 s were led to the platform by placing a finger in front of their swim path. Mice were taken out of the pool after they were physically on the platform for a minimum of 3 s. During visible platform training, the platform was moved to a different quadrant of the pool for each session. For the hidden platform training, the platform location was kept constant. Mice were placed into the water facing the edge of the pool in one of nine randomized locations. The start location was changed for each trial. The swimming patterns of the mice were recorded with the Noldus Ethovision video tracking system (Ethovision XT, Noldus Information Technology, Wageningen, Netherlands) set at six samples/s. The cumulative distance to the platform was used as a measure of performance for the visible and hidden sessions. So, mice that swim closer to the platform will have a lower cumulative distance to the platform measure. Because swim speeds can influence the time it takes to reach the platform, they were also analyzed to assess if there were genotype or treatment differences in this measure.
Subsequently, cognitive performance was analyzed in a probe trial (platform removed) conducted 1 h after the last hidden trial of each mouse. The time spent in the target quadrant, the quadrant where the platform was previously located during hidden platform training, was compared to the time spent in the three non-target quadrants. For the probe trials, mice were placed into the water in the quadrant opposite from the target quadrant.
2.3.2. Fear conditioning
Hippocampal function was also assessed using the contextual fear conditioning task. In this task, mice learned to associate the environmental context (fear conditioning chamber) with a mild foot shock (unconditioned stimulus, US). The mild foot shock was also paired with a tone (conditioned stimulus, CS) to allow assessment of cued fear conditioning, which is hippocampus-independent. When mice were re-exposed to the context or the tone, conditioned fear resulted in freezing behavior. Mice displayed this conditioned fear by ceasing all movement except for respiration (i.e., freezing). On Day 1, each mouse was placed in a fear conditioning chamber (Kinder Scientific, Poway, CA) and allowed to explore for 2 min before delivery of a 30-s tone (80 db) which was immediately followed by a 2-s foot shock (0.6 mA). Two minutes later, a second CS-US pair was delivered. On Day 2 each mouse was first placed in the fear conditioning chamber containing the exact same context, but there was no administration of a tone or foot shock. Freezing was analyzed for 3 min. One hour later, the mice were placed in a new context (containing a different odor, cleaning solution, floor texture, walls and shape) where they were allowed to explore for 3 min before being re-exposed to the fear conditioning tone and freezing was assessed for an additional 3 min. Freezing was measured using a Noldus Ethovision video tracking system.
2.4. 3-nitro tyrosine (NT) immunohistochemistry
Coronal sections (30 μm) of relevant brain regions of two mice of four experimental groups (sham-irradiation, vehicle-treated; sham-irradiation, EUK-207 treated; 15 Gy irradiation, vehicle-treated; 15 Gy irradiation, EUK-207 treated), guided by the Paxinos mouse brain atlas, were generated from the fixed frozen brains of the mice using a cryostat (Microm). Unfortunately, due to sample loss during tissue shipment, the brains of all mice could not be processed for immunohistochemistry. Sections from remaining (n = 2 mice per group) samples (10 μm) were serially mounted on Superfrost microscope slides (Fisher Scientific) and stored at −80 °C until shipment to the laboratory of Dr. Doctrow at Boston University to assess immunohistochemical staining for 3-NT, a marker of oxidative injury indicating peroxynitrite modified proteins [36, 37]. Slides were allowed to air dry for 10 minutes at room temperature and then placed on a slide warmer for 20-45 minutes. Sections were fixed for 10 min in ice-cold acetone: ethanol (3:1), and then washed once with 0.2% Triton X-100 in PBS for 10 min and twice with H2O for 5 min. Antigen retrieval was performed by incubation in hot 10mM citric acid, pH 6.0 for 10 min. Then sections were incubated in 3% H2O2 for 10 min to block endogenous peroxidases. Following a 5 min PBS rinse, sections were blocked with a mixture of 5% normal goat serum (Vector Labs), 1% BSA (Sigma) in PBS for 2 h. Sections were then incubated for 48 h at 4°C in a humidifying chamber with anti-3-nitrotyrosine antibody (Millipore, #06-284), 1:200 in 1% BSA in PBS. Negative control was 1% BSA in PBS only. This antibody could also be blocked by pre-incubation with antigen (not shown). After 2 × 5 min rinses with PBS, the sections were incubated for 4 h with biotinylated goat anti-rabbit IgG (1:200; Vector Labs, PK-4001). After 2 × 5 min rinses with PBS, the sections were incubated for 30 min with ABC reagent prepared as described by the manufacturer (Vector Labs, PK-4001). After a 5-min rinse with PBS, sections were incubated with DAB substrate for 2-10 min, as described by the manufacturer (Vector Labs, SK-4100). Sections were then counterstained with Ehrlich's hematoxylin and mounted.
2.5. Statistical Analysis
Differences among means were evaluated by ANOVA, followed by Tukey-Kramer post hoc test if indicated. All statistical analyses were performed using SPSS (Chicago, IL) and GraphPad Prism (San Diego, CA) software. The visible and hidden platform water maze learning curves and freezing prior to and during the tone in the cued fear memory test were analyzed using repeated-measures ANOVA with radiation and drug treatment as between group factors. The probe trial data of each experimental group were analyzed using a one-way ANOVA and if significant, using multiple comparisons to compare time spent in the target quadrant with than with any of the three non-Target quadrant. Percent freezing in the contextual fear memory test and the difference in freezing during the tone with that prior to the tone in the cued fear memory test was analyzed by ANOVA using radiation and treatment as between group factors. All figures were generated using GraphPad Prism software. Data are expressed as mean ± SEM. The null hypothesis was rejected at the 0.05 level for all analyses.
3. Results
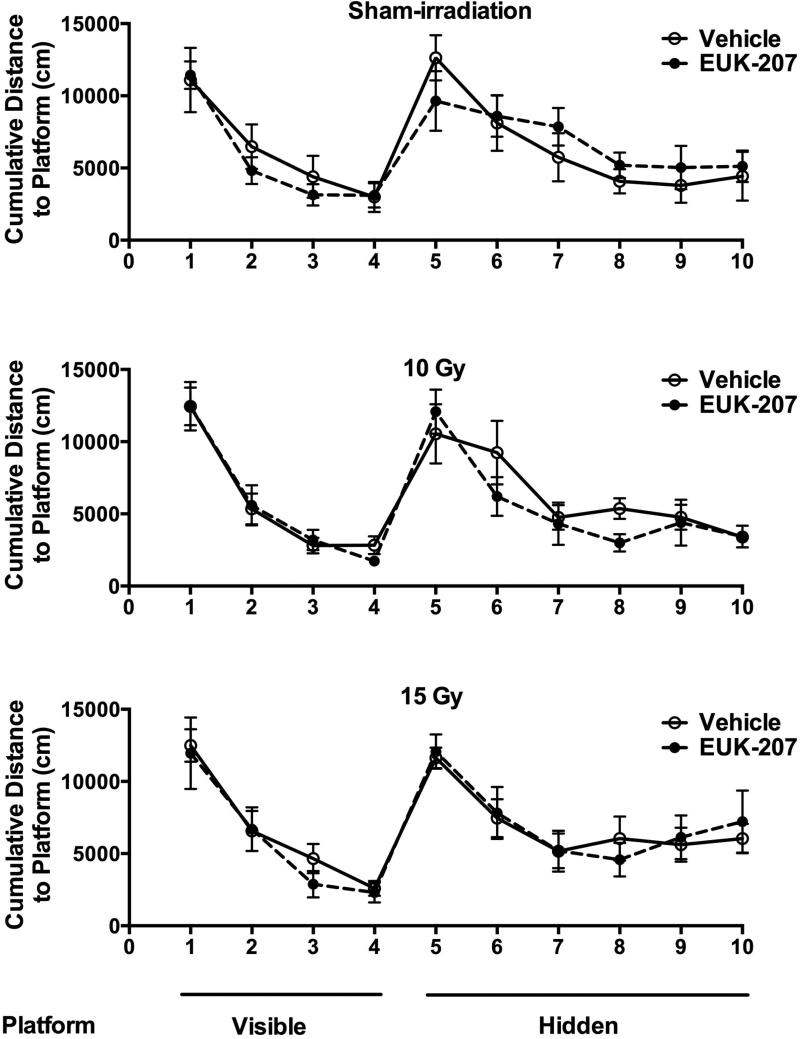
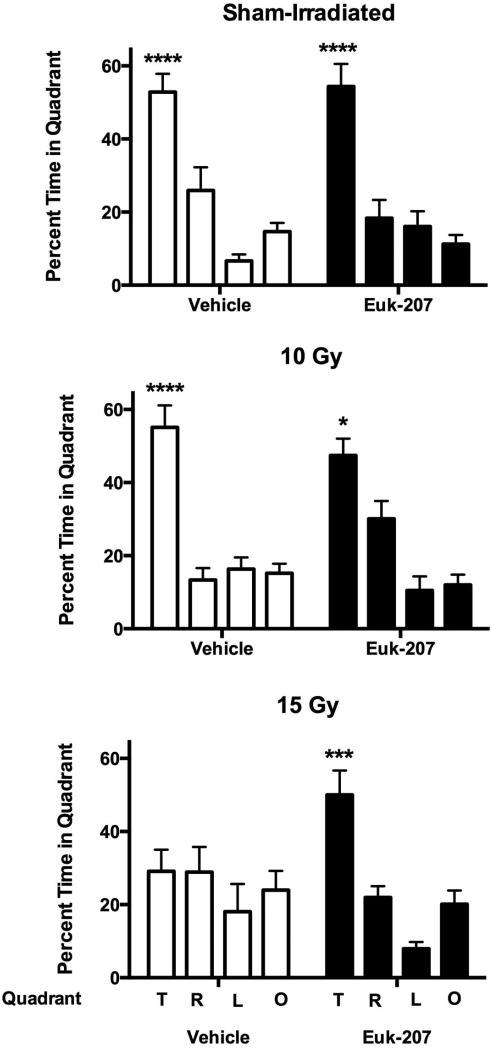
All groups improved their performance during the visible (effect of session: F (3, 40) = 67.394, p < 0.001) and hidden (effect of session: F (5, 40) = 14.654, p < 0.001) platform sessions and there were no effects of irradiation, treatment, or irradiation × treatment interactions (Fig. 1). Similar results were seen when distance moved was used as performance measure (effect of session; visible platform: (F (3, 40) = 11.425, p < 0.001); hidden platform: (F (5, 40) = 4.667, p = 0.002). However, detrimental effects of irradiation and mitigating effects of EUK-207 were seen in cognitive performance in the probe trial (no platform) following last day of hidden platform training. Sham-irradiated mice and mice irradiated with 10 Gy showed good cognitive performance and spent more time in the target quadrant than in any other quadrant (Fig. 2; sham-irradiated mice treated with vehicle: F (3, 28) = 23.59, p < 0.0001; sham-irradiated mice treated with EUK-207: F (3, 28) = 18.05, p < 0.0001; mice irradiated with a dose of 10 Gy and treated with vehicle: F (3, 28) = 25.22, p < 0.0001; mice irradiated with a dose of 10 Gy and treated with EUK-207: F (3, 28) = 17.79, p < 0.0001). However, vehicle-treated mice irradiated with 15 Gy failed to search significantly more time in the quadrant where the platform was hidden before than any other quadrant (Fig. 2). In contrast, EUK-207-treated mice irradiated with 15 Gy showed good cognitive performance and spent more time searching in the target quadrant than in any other quadrant (F (3, 28) = 17.62, p < 0.0001, Fig. 2).
Fig. 1.
Water maze learning curves of sham-irradiated mice (top panel), mice irradiated at a dose of 10 Gy (middle panel), or mice irradiated at a dose of 15 Gy (lower panel). Open circles: vehicle-treated mice; solid circles: EUK-207 treated mice. All groups improved their performance during the visible and hidden platform sessions. N = 8 mice/radiation dose/treatment.
Fig. 2.
Cognitive performance of sham-irradiated mice (top panel), mice irradiated at a dose of 10 Gy (middle panel), or mice irradiated at a dose of 15 Gy (lower panel) in the water maze probe trial. Sham-irradiated mice and mice irradiated with 10 Gy treated with vehicle or EUK-207 showed good cognitive performance. Vehicle-treated mice irradiated with 15 Gy did not show good cognitive performance but EUK-207-treated mice irradiated with 15 Gy did. T: target quadrant; R: right quadrant; L: left quadrant; and O: opposite quadrant. N = 8 mice/radiation dose/treatment. ****p < 0.001 versus any other quadrant; ***p < 0.005 versus right quadrant, p < 0.001 versus left and opposite quadrants; *p < 0.05 versus right quadrant, p < 0.001 versus left and opposite quadrants.
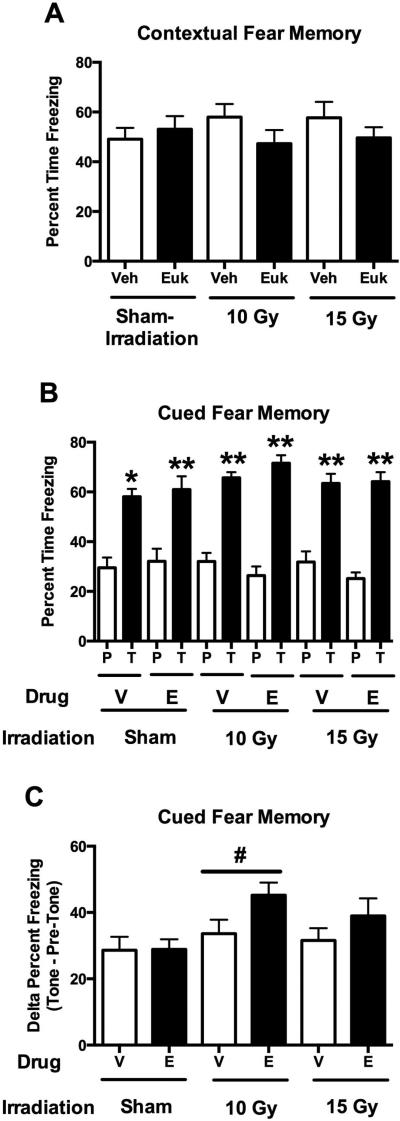
No effects of irradiation, treatment or radiation × treatment interaction were seen for baseline freezing prior to the first tone on the training day or for hippocampus-dependent contextual fear memory (Fig. 3A). All groups showed profound contextual fear memory. For hippocampus-independent cued fear memory, freezing levels during the tone were significantly higher than those prior to the tone ((F (1, 42) = 431.2, p < 0.001, Fig. 3B). When pre- and post-tone freezing levels during the cued memory tests were analyzed using a repeated-measures design, there was a radiation × tone interaction (F (2, 42) = 3.513, p = 0.039) and a trend towards a treatment × tone (F (1, 42) = 2.977, p = 0.060) interaction. Based on the radiation × tone interaction, we also analyzed the post-tone freezing alone and the difference in freezing pre- and post-tone. There was a trend towards an effect of irradiation for freezing during the tone with increased freezing levels in EUK-207 treated mice (F (2, 47) = 2.977, p = 0.062, Fig. 3B). When the difference between freezing levels prior and following the tone in the cued fear memory test was analyzed, there was an effect of irradiation (F (2, 47) = 3.513, p = 0.039, Fig. 3C) with a larger increase in freezing during the tone as compared to prior to the tone in mice irradiated with 10 Gy than in sham-irradiated mice (p = 0.014). In addition, there was a trend towards an effect of EUK-207 with a larger increase in freezing in mice irradiated with 10 or 15 Gy (F (1, 47) = 3.731, p = 0.060, Fig. 3C).
Fig. 3.
A. Contextual fear memory of sham-irradiated mice and mice in irradiated with 10 or 15 Gy treated with vehicle (V) or EUK-207 (E). All groups showed profound contextual fear memory. There were no effects of irradiation, treatment, or radiation × treatment interaction. B. Cued fear memory of sham-irradiated mice and mice in irradiated with 10 or 15 Gy treated with vehicle (V) or EUK-207 (E). Freezing levels prior to the tone (P) and during the tone (T) that was associated with the shock during the training are shown. There was a trend towards an effect of EUK-207 but that did not reach significance. All groups showed significantly higher freezing levels during the tone than prior to the tone. **p < 0.0001 versus pre-tone; *p = 0.002 versus pre-tone. C. Analysis of the difference between freezing levels at baseline and following the tone in the cued fear memory test. There was a larger increase in freezing during the tone as compared to prior to the tone in mice irradiated with 10 Gy than in sham-irradiated mice. There was a trend towards an effect of EUK-207 with a larger increase in freezing in mice irradiated with 10 or 15 Gy but that did not reach significance. #p = 0.014. N = 8 mice/radiation dose/treatment.
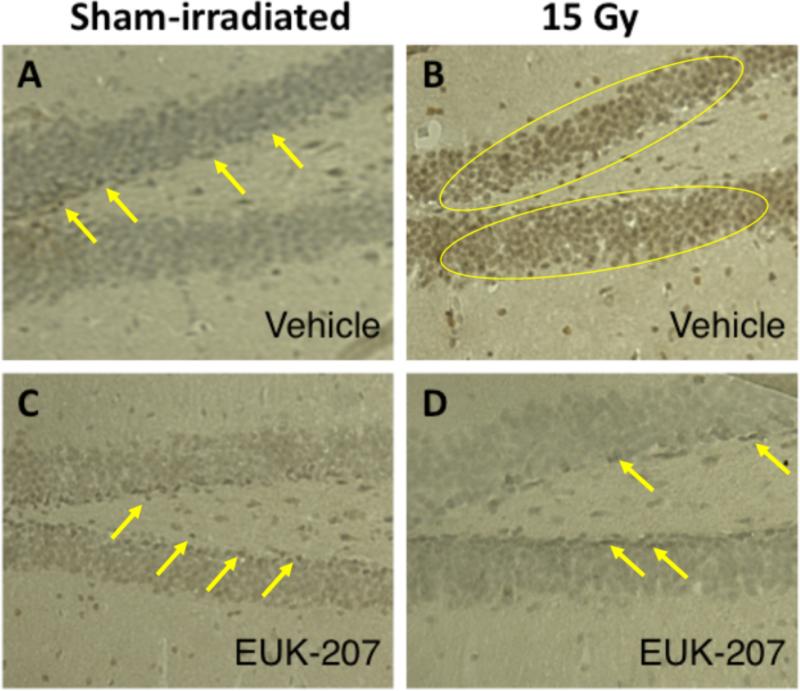
Finally, we assessed whether EUK-207 affected increases in 3-NT staining, a marker of oxidative damage indicating peroxynitrite-mediated protein modification in the tissue (35, 36). Consistent with the water maze probe trial data, 3-NT staining was higher in the brains of mice irradiated with 15 Gy (Fig. 4B) than in sham-irradiated mice (Fig. 4A). This increase was not seen in EUK-207 treated mice irradiated with 15 Gy (Fig. 4D), as compared with sham-irradiated mice treated with EUK-207 (Fig. 4C).
Fig. 4.
Effects of EUK-207 on 15 Gy radiation-induced immunoreactive staining for 3-NT. A. Sham-irradiated mice treated with vehicle. B. Mice irradiated with 15 Gy treated with vehicle. C. Sham-irradiated mice treated with vehicle. D. Mice irradiated with 15 Gy treated with EUK-207. EUK-207 mitigated the radiation-induced increase in 3-NT staining. 3-NT staining was higher in mice irradiated with 15 Gy compared to sham-irradiated mice. This increase was not seen in EUK-207-treated mice irradiated with 15 Gy, as compared to sham-irradiated mice treated with EUK-207. Yellow arrows and yellow circles identify representative positively stained cells.
4. Discussion
This study showed that EUK-207, given starting 24 hours following radiation exposure, mitigated 15 Gy-induced cognitive impairments in the water maze probe trial. In addition, EUK-207 did not affect cognitive performance of sham-irradiated mice in the water maze or of mice irradiated with 10 Gy. Irradiation and EUK-207 did not affect contextual fear memory. Both contextual fear memory and cognitive performance in the water maze probe trial are hippocampus-dependent. However, only cognitive performance in the water maze probe trial was affected three months following low linear energy transfer (LET) irradiation. A dissociation between cognitive performance in the water maze and contextual learning in fear conditioning was previously reported [38, 39]. Consistent with the current study, whole-body high-LET irradiation affected water maze performance, but not contextual fear conditioning within one month following radiation exposure in human apoE mice [40] or 13 months following high LET irradiation [41]. These data emphasized the importance of including different hippocampus-dependent cognitive tests in assessing effects of irradiation on the brain.
In the water maze probe trial, there was no detrimental effect observed of irradiation at a dose of 10 Gy. Consistent with these data, we did not see significant impairments of mice irradiated with 10 Gy of gamma-rays on performance in the water maze three months following exposure using a similar design as used in this study [2]. It is conceivable that a more challenging water maze training paradigm might reveal hippocampus-dependent cognitive injury at this dose of irradiation.
The age of an irradiated animal would appear to be critically important to the response. In mice irradiated at 3 weeks of age, 5 Gy caused cognitive injury [42]. In mice irradiated at 2 months of age, 10 Gy caused impairments in the Barnes maze, but injury was not detected using the Morris water maze (4). In the current study, mice at 2 months of age, older than the mice in the other studies, were used.
ROS is required for normal synaptic plasticity and learning and memory [16, 18, 43, 44]. Therefore, it was important to assess whether EUK-207 had any effects on the cognitive performance of sham-irradiated mice. No detrimental effects of EUK-207 on cognitive performance of sham-irradiated mice were seen. The mitigating effect of EUK-207 against 15 Gy-induced cognitive impairments might be mediated by reduced levels of ROS in EUK-207 treated mice. Although we could not quantify 3-NT levels in the different experimental groups due to loss of samples during shipment, the representative immunohistochemical images indicated that EUK-207 prevented an increase in 3-NT staining in the brains of mice irradiated with 15 Gy while having no or minimal effects on 3-NT staining in sham-irradiated mice. This is consistent with previous reports that treatment with EUK-207 or other salen Mn complexes suppressed biochemical and immunohistochemical indicators of tissue oxidative stress in various disease models [27, 32]. Interestingly, in mice lacking the extracellular form of superoxide dismutase, 3-NT staining was higher at baseline but showed a less profound increase following irradiation than that seen in wild-type mice and this, too, was associated with cognitive protection [5]. Importantly, MCAT mice, expressing catalase as a transgene in the mitochondria, were substantially protected, as compared with wild-type mice, from radiation-induced cognitive dysfunction [45]. This supports the hypothesis that the mitigation of radiation-induced cognitive impairments by EUK-207 is attributable to the catalase and mitochondria-protective activities of EUK-207 and other salen manganese complexes [45, 46]. Unfortunately, because CNS tissues from the cognitively-tested mice were lost in transit, it was not feasible to assess markers of reactive oxygen species, oxidative damage, or synaptic function, in specific brain regions and to therefore further address the mechanism of action of EUK-207 in its cognitive protection.
Low LET irradiation at a dose of 10, but not 15, Gy enhanced cued fear memory as compared to sham-irradiated mice. These data indicate that the effects of irradiation are not limited to effects on hippocampal function. Interestingly, high LET irradiation (250 MeV/amu O ions (25 keV/μm)) also enhanced cued fear memory one month after exposure at dose of 0.4 and 0.8 Gy, but not at 1.6 Gy [47]. Post-training low LET irradiation also enhanced cued fear memory two weeks after exposure [48]. Effects of low LET irradiation on arousal might contribute to these effects, as increased arousal following exposure to novel environments enhances cued fear memory in rodents [49] and verbal memory in humans [50, 51]. The combination of novelty/arousal and radiation might also pertinent to nuclear accidents and dirty bomb scenarios.
As this was a proof-of-principle study to demonstrate efficacy of EUK-207 in a relevant mouse model, we used a delivery system that would provide good drug exposure. This delivery system had been used to demonstrate efficacy of EUK-207 and related compounds in numerous other in vivo injury and degeneration models, including chronic models with even longer treatment periods [28, 32-34]. It would be premature to conclude that no beneficial effects will be seen if the treatment were given for less than three months or if another administration route were used. There are subcutaneously injected sustained slow-release formulations used clinically, including polymeric slow release microspheres, which might be explored for further development of EUK-207 [52]. If long time periods are needed to mitigate CNS radiation-induced cognitive injury, such formulations might be most convenient for treating larger exposed populations after a radiological accident or terrorism event because a single subcutaneous injection would enable drug release for a prolonged period. However, based on other animal model data with EUK-207, we recognize that periodic (daily or less frequent) subcutaneous injections are also potentially useful modes of delivery to consider [31]. Formulation and dosing optimization studies will, of course, be crucial elements of the development plan for an agent such as EUK-207 to mitigate CNS radiation injury.
We recognize that brain regions affected other than the hippocampus might have contributed to the cognitive impairments in the water maze probe trial and have been benefitted from the EUK-207 intervention. Future studies with additional cognitive testing, including water maze test designs involving longer delays between training to locate the hidden platform and the probe trial, and analyses of brain regions other than the hippocampus for alterations in molecular measures pertinent to cognitive performance are warranted to explore the anatomical specificity of the radiation-induced cognitive injury and the treatment effects of EUK-207.
In summary, EUK-207 seems an ideal candidate for mitigation of radiation-induced cognitive injury with little if any impact on cognitive performance under sham-irradiated conditions. Future studies should include further investigation of the molecular mechanisms underlying these neuroprotective effects. These should include detailed assessments of its impact on not only oxidative damage, but also synaptic function, in pertinent brain regions.
Highlights.
- Hippocampus-dependent cognitive injury often characterizes radiation effects.
- The EUK-207 is a member of the class of metal-containing salen manganese (Mn) complexes that suppress oxidative stress.
- Mice irradiated at a dose of 15 Gy show cognitive impairments.
- The catalytic ROS scavenger EUK-207 mitigates these impairments.
- EUK-207 does not affect cognitive performance of sham-irradiated mice.
- EUK-207 is an ideal candidate to mitigate radiation-induced cognitive injury.
Acknowledgements
The authors wish to thank investigators at Henry Ford Health System (Detroit MI) for their input including Drs. Jae Ho Kim, Stephen L. Brown and Kenneth A. Jenrow. This work was supported by NIAID/NIH Center for Medical Countermeasures against Radiological Terrorism, Medical College of Wisconsin, Milwaukee, Wisconsin (NIAID/NIH U19-AI067734; PI: Moulder, JE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. International Journal of Radiation Oncology, Biology and Physics. 1995;31:983–98. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 2.Raber J, Fan Y, Matsumori Y, Weistein PR, Fike JR, Liu J. Irradation attenuates neurogenesis in the dentate gyrus and exacerbates ischemia-induced functional deficits. Ann Neurol. 2004;55:381–90. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- 3.Madsen TM, Kristjansen PEG, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 4.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiation Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 5.Raber J, Villasana L, Rosenberg J, Zou Y, Huang T-T, Fike JR. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismuase. Hippocampus. 2011;21:72–80. doi: 10.1002/hipo.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moulder J. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int J Radiat Biol. 2004;80:3–10. doi: 10.1080/09553000310001642920. [DOI] [PubMed] [Google Scholar]
- 7.Manthous C, Jackson L. The 9-11 commission's invitation to imagine: a pathophysiology-based approach to critical care of nuclear explosion victims. Crit Care Med. 2007;35:716–23. doi: 10.1097/01.CCM.0000257328.31668.22. [DOI] [PubMed] [Google Scholar]
- 8.Pellman T, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–23. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 9.Rojas-Palma C, Liland A, Jerstad A, Etherington G, del Rosario Perez M, Rahola T, et al. TMT Handbook - Triage, monitoring and treatment - handbook for management of the public in the event of malevolent use of radiation. Norwegian Radiation Protection Agency; Osteras: 2009. [Google Scholar]
- 10.Greenberger J, Epperly M. Radioprotective antioxidant gene therapy: potential mechanisms of action. Gene Ther Mol Biol. 2004;8:31–44. [Google Scholar]
- 11.Zhao W, Robbins M. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16:130–43. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 12.Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. NeurosurgClinNAm. 2007;18:115–27. x. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Tseng B, Giedzinski E, Izadi A, Suarez T, Lan M, Tran K, et al. Consequences of radiation-induced oxidative stress in neural stem and precursor cells exposed to charged particle irradiation. AntioxidRedoxSignal. 2013;20:1410–22. doi: 10.1089/ars.2012.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rola R, Zou Y, Huang T-T, Fishman K, Baure J, Rosi S, et al. Lack of extracellular superoxide dismutase (EC-SOD) in the microenvironment impacts radiation-induced changes in neurogenesis. Free Radic Biol Med. 2007;42:1133–45. doi: 10.1016/j.freeradbiomed.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR. Radiation response of neural precursor cells: limking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiation Res. 2004;161:17–27. doi: 10.1667/rr3112. [DOI] [PubMed] [Google Scholar]
- 16.Kishida K, Klann E. Sources and targets of reacitive oxygen species in synaptic plasticity and memory. AntioxidRedoxSignal. 2007;9:233–44. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu D, Cao P, Thiels E, Chu C, Wu G, Oury T, et al. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxde dismutase. Neurobiol Learn Mem. 2007;87:372–84. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiels E, Urbann C, Gonzalez-Burgos G, Kanterewicz B, Barrionuevo G, Chu C, et al. Impairment of long-term potentiation and associative memory in mice that overexpress superoxide dismutase. J Neurosci. 2000;20:7631–9. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen R, Johnson L, Zuloaga D, Limoli CL, Raber J. Enhanced hippocampus-dependent memory and reduced anxiety in mice overexpressing human catalase in mitochondria. J Neurochem. 2013;125:303–13. doi: 10.1111/jnc.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villasana L, Rosenthal R, Doctrow S, Pfankuch T, Zuloaga D, MacColl Garfinkel A, et al. Effects of alpha-lipoic acid on associative and spatial memory of shan-irradiaed and 56Fe-irradiated C57BL/6J mice. Pharmacol Biochem Behav. 2013;103:487–93. doi: 10.1016/j.pbb.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourgier C, Levy A, Vozemin M, Deutch E. Pharmacological strategies to spare normal tissues fromradiation damage: useless or overlooked therapeutics? Cancer Metastasis Rev. 2012;31:699–712. doi: 10.1007/s10555-012-9381-9. [DOI] [PubMed] [Google Scholar]
- 22.Calveley V, Jelveh S, Langan A, Mahmood J, Yeung I, Van Dyk J, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173:602–11. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batinic-Haberle I, Tovmasyan A, Roberts E, Vujaskovic Z, Leong K, Spasojevic I. SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid Redox Signal. 2014;20:2372–415. doi: 10.1089/ars.2012.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabbani Z, Batinic-Haberle I, Anscher M, Huang J, Day B, Alexander E, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–80. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, et al. Salen manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J Med Chem. 2002;45:4549–58. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 26.Doctrow S, Baudry M, Huffman K, Malfroy B, Melov S. Salen-manganese complexes: multifunctional catalytic antioxidants protective in models for neurodegenerative diseases of aging. In: Sessler J, Doctrow SR, McMurry T, Lippard S, editors. Medicinal Inorganic Chemistry. American Chemical Society Symposium Series 903, American Chemical Society; Washington DC: 2005. pp. 319–47. 2005 (distributed by Oxford University Press) [Google Scholar]
- 27.Liesa M, Luptak I, Qin F, Hyde B, Sahin E, Siwik D, et al. Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion. Circulation. 2011;124:806–13. doi: 10.1161/CIRCULATIONAHA.110.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doctrow S, Lopez A, Shock A, Duncan N, Jourdan M, Olasz E, et al. A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. J Invest Dermatol. 2013;133:1088–96. doi: 10.1038/jid.2012.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao F, Fish B, Szabo A, Doctrow S, Kma L, Molthen R, et al. Short-term treatment with a SOD/Catalase mimetic, EUK-207, mitigates pneumonitis and fibrosis after single-dose total-body or whole-thoracic irradiation. Radiat Res. 2012;178:468–80. doi: 10.1667/RR2953.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doctrow SR, Fish BL, Huffman K, Lazarova Z, Medhora M, Williams J, et al. Redox Active Therapeutics. In: Batinic-Haber I, Reboucas JS, editors. Salen manganese complexes mitigate radiation injury in normal tissues through modulation of tissue environment, including through redox mechanisms. Springer International Publishing; Switzerland: 2017. [Google Scholar]
- 31.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill R. Mitigation of radiation induced lung injury by genestein and EUK-207. Int J Radiat Biol. 2011;87:889–901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clausen A, Doctrow SR, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2010;31:425–33. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A. 2003;100:8526–31. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng J, Stevenson F, Doctrow SR, Andersen J. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J Biol Chem. 2005;280:29194–8. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- 35.Melov S, Doctrow S, Schneider J, Haberson J, Patel M, Coskun P, et al. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci. 2001;21:8348–53. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruijn LI, Beal MF, Becher MW, Schulz JB, Wong PC, Price DL, et al. Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc Natl Acad Sci USA. 1997;94:7606–11. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uttenthal LO, Alonso D, Fernández AP, Campbell RO, Moro MA, Leza JC, et al. Neuronal and inducible nitric oxide synthase and nitrotyrosine immunoreactivities in the cerebral cortex of the aging rat. Microsc Res Tech. 1998;43:75–88. doi: 10.1002/(SICI)1097-0029(19981001)43:1<75::AID-JEMT11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Logue S, Paylor R, Wehner J. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned fear task. Behav Neurosci. 1997;111:104–13. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- 39.Silva AJ, Giese K, Fedorov N, Frankland PW, Kogan J. Molecular, cellular, and neuroanatomical substrates of place learning. Neurobiol Learn Mem. 1998;70:44–61. doi: 10.1006/nlme.1998.3837. [DOI] [PubMed] [Google Scholar]
- 40.Haley G, L V, C D, M.J. D, J R. ApoE Genotype-Dependent Paradoxical Short-Term Effects of 56Fe Irradiation on the Brain. Int J Radiat Oncol Biol Phys. 2012;84:793–9. doi: 10.1016/j.ijrobp.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villasana L, Benice T, Raber J. Long-term effects of 56Fe irradiation on spatial memory of mice: role of sex and apolipoprotein E isoform. Int J Radiat Oncol Biol Phys. 2011;80:567–73. doi: 10.1016/j.ijrobp.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 42.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg S, Morhardt D, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Kishida K, Pao M, Holland S, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA! Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hidalgo C, Carasso M, Munoz P, Nunez M. A role for reactive oxygen/nitrogen species and iron on neuronal plasticity. AntioxidRedoxSignal. 2007;97:245–55. doi: 10.1089/ars.2007.9.245. [DOI] [PubMed] [Google Scholar]
- 45.Parihar V, Allen B, Tran K, Chmielewski N, Craver B, Martirosian V, et al. Targeted overexpression of mitochondrial catalase prevents radiation-induced cognitive dysfunction. Antioxid Redox Signal. 2015;22:78–91. doi: 10.1089/ars.2014.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doctrow S, Liesa M, Melov S, Shirihai O, Tofilon P. Salen Mn complexes are superoxide dismutase and catalase mimetics that protect the mitochondria. Curr Inorgan Chemq. 2012;2:325–34. [Google Scholar]
- 47.Raber J, Marzulla T, Kronenberg A, Turker MS. 16Oxygen irradiation enhances cued fear memory in B6D2F1 mice. Life Sci Space Res. 2015;7:61–5. doi: 10.1016/j.lssr.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Kugelman T, Zuloaga D, Weber S, Raber J. Post-training gamma irradiation-enhanced contextual fear memory associated with reduced neuronal activation of the infralimbic cortex. Behav Brain Res. 2015;298:1–11. doi: 10.1016/j.bbr.2015.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King S, Williams C. Novelty-induced arousal enhances memory for cued classical conditoning: Interactions between peripheral adrenergic and brainstem glutamatergic systems. Learn Mem. 2009;16:625–34. doi: 10.1101/lm.1513109. [DOI] [PubMed] [Google Scholar]
- 50.Cahill L, Alkire M. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79:194–8. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 51.Fenker D, Frey J, Schuetze H, Heipertz D, Heinze H, Duzel E. Novel scenes improve recollection and recall of words. J Cogn Neurosci. 2008;20:1–16. doi: 10.1162/jocn.2008.20086. [DOI] [PubMed] [Google Scholar]
- 52.Ramazani F, Chen W, van Nostrum C, Storm G, Kiessling F, Lammers T, et al. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: State-of-the-art and challenges. Int J Pharm. 2016;499:358–67. doi: 10.1016/j.ijpharm.2016.01.020. [DOI] [PubMed] [Google Scholar]