Summary
Aims
Epidemiological evidence implicates polyphenols as potential natural therapeutics for Alzheimer's disease (AD). To investigate this prospect, five anthoxanthin polyphenols were characterized for their ability to reduce amyloid‐β (Aβ) oligomer‐induced neuronal responses by two mechanisms of action, modulation of oligomerization and antioxidant activity, as well as the synergy between these two mechanisms.
Methods
Anthoxanthin oligomerization modulation and antioxidant capabilities were evaluated and correlated with anthoxanthin attenuation of oligomer‐induced intracellular reactive oxygen species (ROS) and caspase activation using human neuroblastoma cell treatments designed to isolate these mechanisms of action and to achieve dual‐action.
Results
While modulation of oligomerization resulted in only minor reductions to neuronal responses, anthoxanthin antioxidant action significantly attenuated oligomer‐induced intracellular ROS and caspase activation. Kaempferol uniquely exhibited synergism when the two mechanisms functioned in concert, leading to a pronounced reduction in both ROS and caspase activation.
Conclusions
Together, these findings identify the dominant mechanism by which these anthoxanthins attenuate Aβ oligomer‐induced neuronal responses, elucidate their prospective synergy, and demonstrate the potential of anthoxanthin polyphenols as natural AD therapeutics.
Keywords: Amyloid‐β protein, Caspase activation, Oligomer, Polyphenol, Reactive oxygen species
Introduction
Alzheimer's disease (AD), the most common neurodegenerative disorder, affects an estimated 5.4 million Americans 1. While the incidence of other leading causes of death, including heart disease, stroke, and HIV, has decreased in recent years, fatalities due to AD reflect a 71% increase 1. The amyloid‐β protein (Aβ) is closely associated with AD pathology and hypothesized to play a key role in disease pathogenesis. While Aβ fibrils, insoluble aggregates that deposit in the brain as plaques, were originally associated with disease pathogenesis, increasing evidence indicates that smaller aggregates, or oligomers, are the primary pathogenic Aβ species 2, 3, 4, 5. This discovery has stimulated numerous studies aimed at modulating oligomer formation to render oligomers less toxic.
Epidemiological studies correlating a reduced incidence of AD with diets rich in polyphenols, compounds prevalent in fruits, vegetables, and herbs 6, 7, 8, have prompted the exploration of polyphenols as prospective natural therapeutics for AD. Numerous polyphenols have the ability to obstruct the Aβ aggregation pathway 9, 10, 11, 12, although few studies have focused on the modulation of oligomer formation by polyphenols and the associated toxicity. The fused aromatic carbon ring structure characteristic of flavonoid polyphenols has been hypothesized to bind Aβ oligomers, thereby preventing further aggregation 13, 14. Thus, polyphenols presenting this structure have the potential to modulate oligomer formation and thus reduce Aβ toxicity.
The antioxidant properties exhibited by many polyphenols present an additional mechanism by which these compounds may affect AD. Antioxidants have been explored as treatments for oxidative stress‐induced cellular apoptosis observed in neurodegenerative diseases, including AD, Parkinson's disease, and stroke 15. Polyphenol antioxidant activity may neutralize reactive oxygen species (ROS), which are stimulated by Aβ 16, 17, 18 and implicated as a contributing factor in Aβ toxicity 15, 19, 20. Polyphenols serve as the most abundant antioxidants in diet 21, and their antioxidant capabilities are suggested to contribute to their therapeutic properties 22, 23, 24. In particular, many flavonoids have demonstrated the ability to neutralize Aβ‐induced ROS 12. Together, the observed antiaggregation and antioxidant capabilities of polyphenols suggest that these natural compounds are prospective dual‐action therapeutics for AD.
This study investigated the potential of five anthoxanthin polyphenols, flavone (FLA), apigenin (API), luteolin (LUT), kaempferol (KAE), and quercetin (QUE) (Figure 1), as natural therapeutics for AD. These compounds have been studied as therapeutics for a variety of ailments, including osteoarthritis, inflammation, spasms, and cancer 25, 26. Here, experimentation examines the ability of these anthoxanthins to attenuate Aβ oligomer‐induced neuronal responses associated with AD, including increases in intracellular ROS and activation of caspases. Two mechanisms of action, modulation of oligomerization and exertion of antioxidant action, as well as the synergy between these two mechanisms, were evaluated for the ability to attenuate oligomer‐induced neuronal responses. Hydroxylated anthoxanthins modulated oligomer formation by both shifting oligomer size distribution and altering oligomer conformation. However, these changes induced only a nominal effect on Aβ physiological activity, with LUT alone significantly decreasing oligomer‐induced elevation of ROS. Alternatively, all anthoxanthins significantly attenuated oligomer‐induced intracellular ROS through their antioxidant properties, with LUT and QUE also reducing caspase activation through this mechanism. Interestingly, KAE uniquely exhibited synergism when the two mechanisms of action functioned in concert, leading to a pronounced reduction in both oligomer‐induced intracellular ROS and caspase activation. Together, these findings identify the dominant mechanism by which these anthoxanthins attenuate Aβ oligomer‐induced neuronal responses and demonstrate the potential of anthoxanthin polyphenols as natural therapeutics for AD.
Figure 1.
Anthoxanthin structures. Structures of flavone (FLA), apigenin (API), luteolin (LUT), kaempferol (KAE), and quercetin (QUE), which were evaluated for their ability to disrupt Aβ oligomerization and cellular activity.
Materials and Methods
Oligomer Preparation
Aβ 1–42 was reconstituted in cold 1,1,1,3,3,3‐hexafluoro‐2‐propanol (HFIP) to 4 mg/mL and incubated on ice (60 min). After aliquoting, HFIP was evaporated overnight (25°C). Resulting protein films were stored at −80°C. To form oligomers, protein films were reconstituted to 1.5 mM in dimethyl sulfoxide (DMSO) either alone (control) or in the presence of anthoxanthin. To initiate oligomerization, 12 mM phosphate (pH 7.4) containing 1 μM NaCl was added for final concentrations of 15 μM Aβ 1–42, 150 μM anthoxanthin, and ≤2.5% DMSO. Following 30 min incubation (25°C), reactions were either stabilized by addition of 0.1% Tween‐20 for analysis by SDS‐PAGE and Western blot or diluted for immediate cell culture treatment or analysis by 8‐anilino‐1‐naphthalenesulfonic acid (ANS) spectroscopy.
Oligomer Size Determination
To determine whether anthoxanthins alter oligomer size distribution, Tween‐20 stabilized oligomers formed in the absence (control) or presence of anthoxanthins were characterized using SDS‐PAGE and Western blot. For oligomers 25–250 kDa in size, stabilized oligomers were separated on a 4–20% Tris‐glycine gel (Bio‐rad, Hercules, CA, USA); for monomer, trimer, and tetramer, stabilized oligomers were separated on a 16.5% Tris‐tricine gel (Bio‐rad). Detection was performed using 6E10 monoclonal antibody (1:2000), and size determination was facilitated using Precision Plus WesternC and Protein Dual Xtra standards. Separation and blotting protocols are detailed in the Supporting Information. Blots were imaged using the Gel DocTM XRS+imaging system (Bio‐Rad). Image Lab software (Bio‐Rad) was used to quantify the volume intensity for larger (100–250 kDa) and smaller (25–100 kDa) oligomers and to quantify the band intensity for monomer, trimer, and tetramer. Intensity values are reported as a fraction of the control.
Oligomer Conformation
ANS, which binds to exposed hydrophobic molecular surfaces resulting in both a blue shift and increase in fluorescence, has been used extensively in protein folding and misfolding, including evaluation of surface‐exposed hydrophobic residues as an indication of Aβ aggregate conformation 27, 28, 29. To probe whether anthoxanthins alter oligomer conformation, ANS was combined with oligomers formed in the absence (control) or presence of anthoxanthins for final concentrations of 1 μM Aβ 1–42, 10 μM anthoxanthin, and 100 μM ANS. Fluorescence was measured as described in Supporting Information. The effect KAE has on oligomer conformation could not be evaluated via ANS due to the self‐fluorescence associated with this compound. Results are normalized to the control.
Anthoxanthin Antioxidant Capacity
Freshly dissolved anthoxanthins (10 mM in DMSO) were diluted in 75 mM potassium phosphate (pH 7.0) and assessed against Trolox standards using the OxiSelect™ Oxygen Radical Antioxidant Capacity (ORAC) activity assay (Cell Biolabs, San Diego, CA, USA), as described in Supporting Information. Results are reported as the ORAC value, or the equivalent Trolox concentration per unit concentration of anthoxanthin.
Cell Preparation and Treatment
Human neuroblastoma SH‐SY5Y cells (American Type Culture Collection, Manassas, VA, USA) were seeded at a density of 5 × 104 cells/well onto black‐sided 96‐well tissue culture plates (VWR) for intracellular ROS assays or at a density of 1 × 106 cells/well onto 22 × 22 mm glass coverslips (Fisher Scientific, Hampton, NH, USA) for caspase activation assays. Seeded cells were maintained for 24 h, as described in Supporting Information. Subsequent cellular treatments were performed by diluting Aβ 1–42 oligomers and anthoxanthin in media containing 1% FBS.
To assess the extent of oligomer‐induced cellular responses, Aβ 1–42 oligomers formed in the absence of anthoxanthins were added to cells for a final concentration of 0.01 μM Aβ 1–42. To determine the effect that anthoxanthin‐induced changes in oligomer size and conformation had on cellular responses, Aβ 1–42 oligomers formed in the presence of anthoxanthins were added to cells for final concentrations of 0.01 μM Aβ 1–42 and 0.1 μM anthoxanthin. To assess the effectiveness of anthoxanthin antioxidant capabilities at decreasing oligomer‐induced cellular responses, Aβ 1–42 oligomers formed in the absence of anthoxanthins were added to cells simultaneously with anthoxanthins for final concentrations of 0.01 μM Aβ 1–42 and 40 μM anthoxanthin. Finally, to examine the potential of anthoxanthins to act synergistically via both mechanisms, Aβ 1–42 oligomers formed in the presence of anthoxanthins were added to cells simultaneously with additional anthoxanthin for final concentrations of 0.01 μM Aβ 1–42 and 40 μM anthoxanthin. Cells treated with anthoxanthins alone confirmed negligible change from basal levels (data not shown).
Oligomer‐Induced Intracellular ROS
An OxiSelect™ Intracellular ROS assay Kit (Cell Biolabs) employing the 2′,7′‐dichlorodihydrofluorescin diacetate (DCFH‐DA) probe was implemented, as described further in Supporting Information, to assess the ability of oligomers to increase intracellular ROS as well as the effectiveness of anthoxanthins to attenuate this increase. Cells were treated as described above; cells treated with equivalent buffer dilution or 25 μM H2O2 served as a vehicle and positive control, respectively. Results are reported as the fold increase in intracellular ROS relative to the vehicle for initial evaluation of Aβ 1–42 oligomers and as the percent decrease in intracellular ROS relative to Aβ 1–42 oligomers (positive control) for samples containing anthoxanthin.
Oligomer‐Induced Caspase Activation
The Image‐iT LIVE Green Poly Caspases Detection Kit (Life Technologies, Carlsbad, CA, USA) employs a fluorescent inhibitor of caspases (FLICA) reagent for detection of caspase‐1, caspase‐3, caspase‐4, caspase‐5, caspase‐6, caspase‐7, caspase‐8, and caspase‐9 as well as Hoechst 33342 for labeling of nuclei. This assay, described further in Supporting Information, was implemented to determine the ability of Aβ 1–42 oligomers to induce caspase activation as well as the ability of anthoxanthins to attenuate this response. Cells were treated as detailed above; cells treated with equivalent buffer dilution or 1.5 U/μL TNF‐α served as a vehicle and positive control, respectively. A custom Matlab (Mathworks, Natrick, MA, USA) subroutine was used to quantify the total number of cells using Hoechst images and to determine the individual caspase activity of each cell using FLICA images. Caspase activation is reported as the fraction of caspase activated cells.
Statistical Analysis
Using Graphpad Prism 5 software (La Jolla, CA, USA), a one‐way analysis of variance (ANOVA) was performed to compare all samples to the respective control, and an unpaired t‐test was performed for comparison between samples. P < 0.05 was considered significant.
Results
Hydroxylated Anthoxanthins Alter Oligomer Size Distribution
To evaluate the ability of anthoxanthins to modulate oligomerization, Aβ 1–42 oligomers were formed in the absence (control) or presence of each selected compound. The size distribution of the resulting oligomers was assessed using SDS‐PAGE and Western blot. When separation was performed on a 4–20% Tris‐glycine gel (Figure 2A), only FLA was unable to reduce the formation of oligomers in both the 100–250 kDa (Figure 2B) and 25–100 kDa (Figure 2C) size ranges. API, LUT, KAE, and QUE all significantly reduced the quantity of 100‐ to 250‐kDa oligomers (Figure 2B), with KAE exhibiting the most pronounced effect, a > 95% reduction. API, KAE, and QUE also significantly reduced the formation of 25‐ to 100‐kDa oligomers (Figure 2C). Again, KAE exhibited the most pronounced inhibition, reducing formation of these oligomers by nearly 65%.
Figure 2.
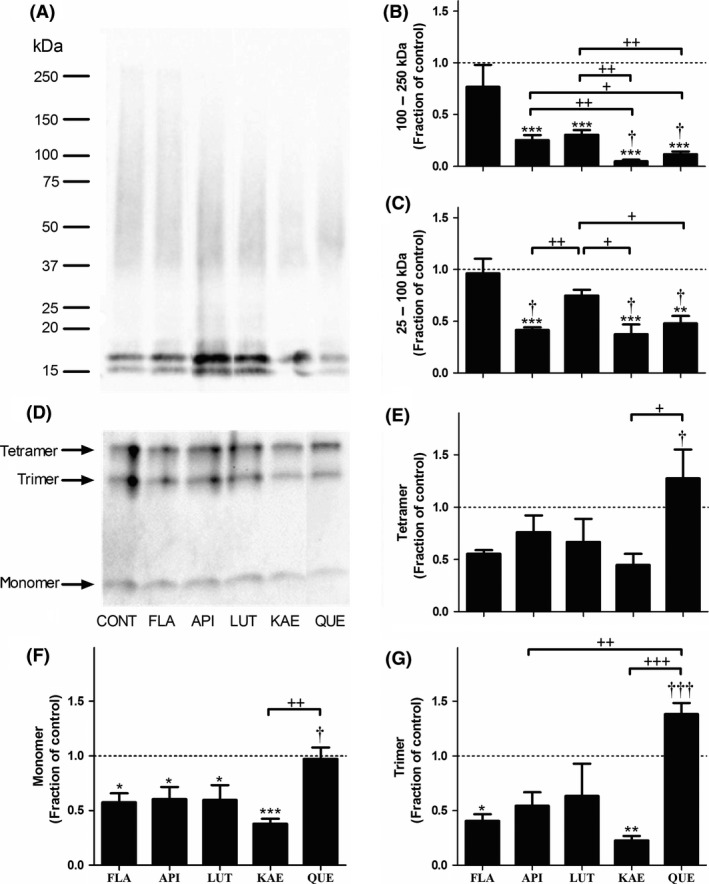
Hydroxylated anthoxanthins alter Aβ oligomer size distribution. Oligomers were prepared from Aβ 1–42 (15 μM) in the absence (CONT, control) or presence of 150 μM anthoxanthins flavone (FLA), apigenin (API), luteolin (LUT), kaempferol (KAE), or quercetin (QUE) by dilution from DMSO into 12 mM phosphate (pH 7.4) containing 1 μM NaCl. Following oligomerization (30 min, 25°C), oligomers were stabilized via addition of Tween‐20 (0.1%), resolved by SDS‐PAGE on either a 4–20% Tris‐glycine gel (panel A) or a 16.5% Tris‐tricine gel (panel D), transferred to nitrocellulose membrane, and probed with 6E10 antibody. Images are representative of 3–5 independent experiments. Using volumetric analysis in conjunction with the 4–20% Tris‐glycine gel images (panel A), oligomer species within size ranges of 100–250 kDa (panel B) and 25–100 kDa (panel C) were quantified. Using band intensity analysis in conjunction with the 16.5% Tris‐tricine gel images (panel D), tetramer (panel E), trimer (panel G), and monomer (panel F) species were quantified. Reported results are normalized to the control, shown as a dashed line with a value of 1 and representing no change. Error bars indicate SEM, n = 3–5. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control; † P < 0.05 and ††† P < 0.001 versus flavone; + P < 0.05, ++ P < 0.01, and +++ P < 0.001 between samples.
When separation was performed on a 16.5% Tris‐tricine gel (Figure 2D), none of the anthoxanthins significantly altered the amount of tetramer formed (Figure 2E), and only the presence of FLA and KAE resulted in a significant decrease in trimer formation (Figure 2G). Dimer species were not evaluated as they can be effected by SDS when cross‐linking is not employed to stabilize the dimer structure 30. The most pronounced anthoxanthin‐induced change occurred in the amount of monomeric Aβ present (Figure 2F), with all anthoxanthin samples except QUE exhibiting significantly less monomer than the control.
Hydroxylated Anthoxanthins Modify Oligomer Conformation
To determine whether the presence of anthoxanthins during oligomerization alters oligomer conformation, Aβ 1–42 oligomers formed in the absence (control) or presence of each anthoxanthin were assessed for changes in surface hydrophobicity using ANS. This fluorescent dye binds to exposed hydrophobic residues to give a shifted, enhanced fluorescence (Figure 3A–E). Although ANS binding was unchanged by FLA and API, LUT significantly reduced oligomer surface hydrophobicity, while QUE significantly increased oligomer surface hydrophobicity (Figure 3F). KAE self‐fluorescence prohibited evaluation of the effect of this anthoxanthin upon oligomer surface hydrophobicity. Thus, hydroxylated anthoxanthins not only modify oligomer conformation but do so in varying ways.
Figure 3.
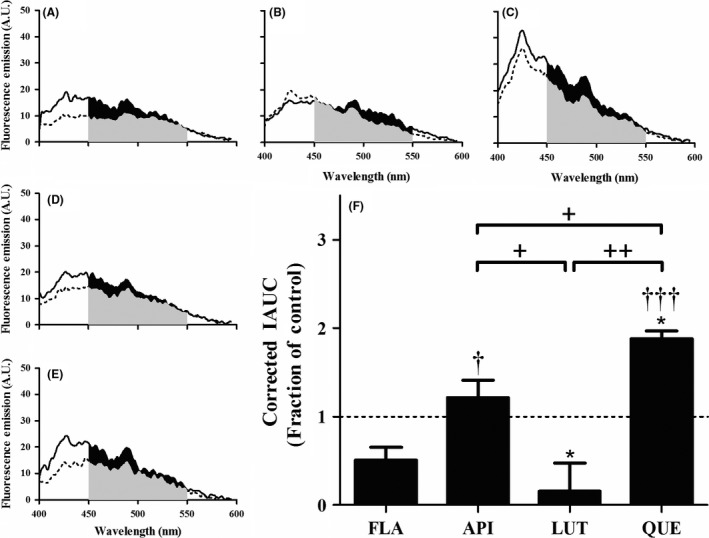
Hydroxylated anthoxanthins alter Aβ oligomer surface hydrophobicity. Oligomers were prepared from Aβ 1–42 (15 μM) in the absence (CONT, panel A) or presence of 150 μM anthoxanthins flavone (FLA, panel B), apigenin (API, panel C), luteolin (LUT, panel D), or quercetin (QUE, panel E), as described in Figure 2. Resulting oligomer products were diluted (15‐fold) into 100 μM ANS, and fluorescence (emission: 400–600 nm; excitation: 350 nm) was measured for samples (solid line) and corresponding blanks (anthoxanthin, ANS) (dashed line). KAE self‐fluorescence prohibited evaluation of ANS binding to oligomers made in the presence of this anthoxanthin. (F) Fluorescence values were determined as the IAUC from 450 to 550 nm with blank subtraction. Corrected fluorescence was normalized to the control, shown as a dashed line with a value of 1 and representing no change. Error bars indicate SEM, n = 3–4. *P < 0.05 versus control; † P < 0.05 and ††† P < 0.001 versus flavone; + P < 0.05 and ++ P < 0.01 between samples.
Oligomers Increase Intracellular ROS and Caspase Activity in Human Neuroblastoma Cells
To verify the physiological activity of Aβ 1–42 oligomers, their ability to increase intracellular ROS and stimulate caspase activity was evaluated using SH‐SY5Y human neuroblastoma cells. Oligomer preparations visualized via transmission electron microscopy (TEM), as described in Supporting Information, exhibit small spheroids that can become conjoined (Figure S1). When cells were treated for 24 h with an oligomer preparation diluted to 0.01 μM Aβ 1–42, a 50% increase in intracellular ROS was observed (Figure 4A). In fact, this increase is similar to that elicited by 25 μM H2O2, a ROS known to induce oxidative damage 31, 32. Similarly, 24‐h exposure of SH‐SY5Y cells to 0.01 μM Aβ 1–42 oligomerization products activated caspases, with approximately 50% of treated cells exhibiting caspase activity (Figure 4B,C), paralleling activity elicited by 1.5 U/μL TNF‐α, a known inducer of caspase in neuronal cell models 33, 34. Together, these results demonstrate the damaging physiological effects of oligomers at nanomolar Aβ concentrations.
Figure 4.
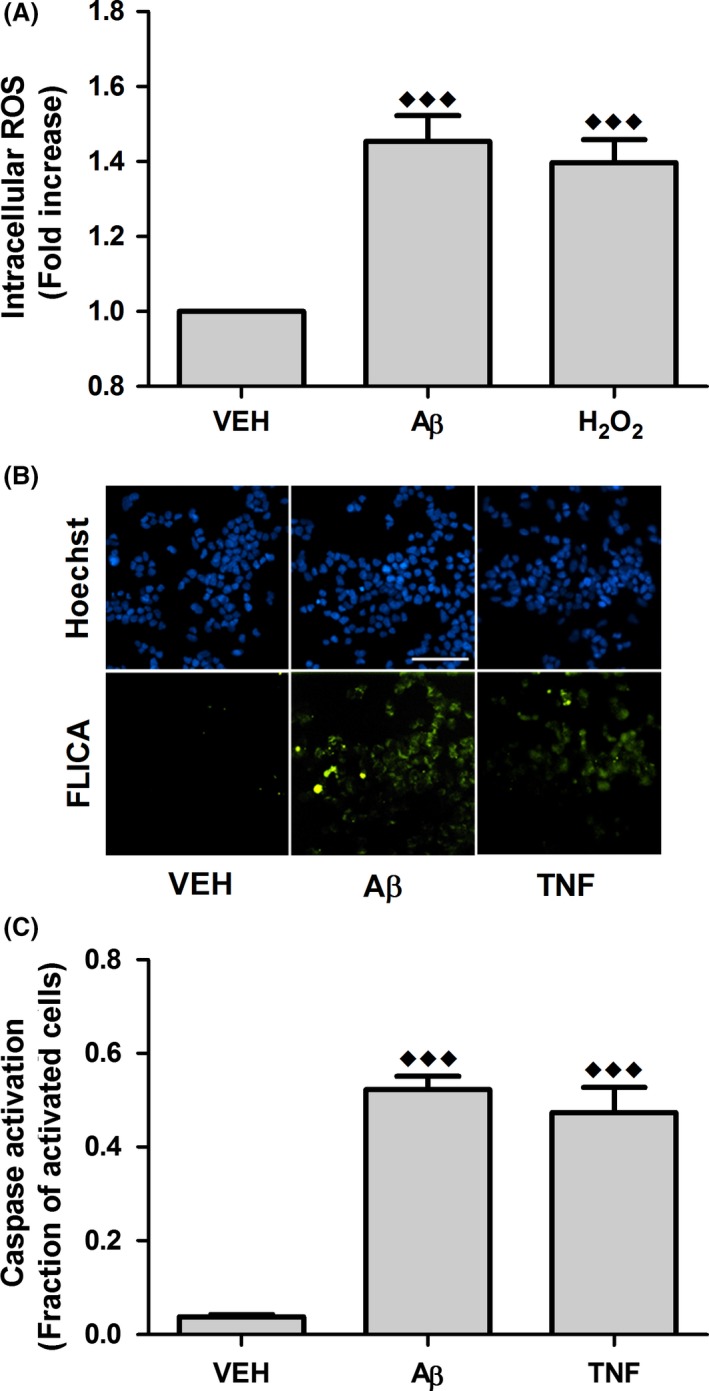
Aβ oligomers increase intracellular ROS and stimulate caspase activation in human neuroblastoma cells. SH‐SY5Y cells were incubated for 24 h with buffer equivalent (VEH, vehicle) or 0.01 μM Aβ 1–42 oligomerization products (Aβ). (A) Intracellular ROS was evaluated using the DCFH‐DA probe. Treatment with 25 μM H2O2 served as a positive control. Results are expressed as the fold increase in intracellular ROS relative to the vehicle. Error bars indicate SEM, n = 14. (B) Caspase activation was evaluated via staining with FLICA reagent for detection of caspase‐1, caspase‐3, caspase‐4, caspase‐5, caspase‐6, caspase‐7, caspase‐8, and caspase‐9 (green) in conjunction with nuclear (Hoechst) staining (blue). Treatment with 1.5 U/μL TNF‐α served as a positive control. Scale bar represents 50 μm. Images are representative of 8–13 independent experiments. (C) Cellular caspase activation was determined via analysis of Hoechst and FLICA images using custom MATLAB functions that quantify the total number of cells and the number of caspase activated cells, respectively. Results are reported as the fraction of caspase activated cells. Error bars indicate SEM, n = 8–13. ♦♦♦ P < 0.001 versus vehicle.
Anthoxanthins Exhibit Antioxidant Capacity
When antioxidant capability of anthoxanthins was evaluated using an ORAC assay, all anthoxanthins presented strong antioxidant capacity (Figure 5A), with FLA, API, LUT, and KAE exhibiting a capacity similar to that of the Trolox standard and QUE exhibiting a significantly higher antioxidant capacity than Trolox. In addition, both API and QUE displayed significantly greater antioxidant capacity than LUT and KAE, demonstrating the influence of hydroxyl placement upon anthoxanthin antioxidant capacity.
Figure 5.
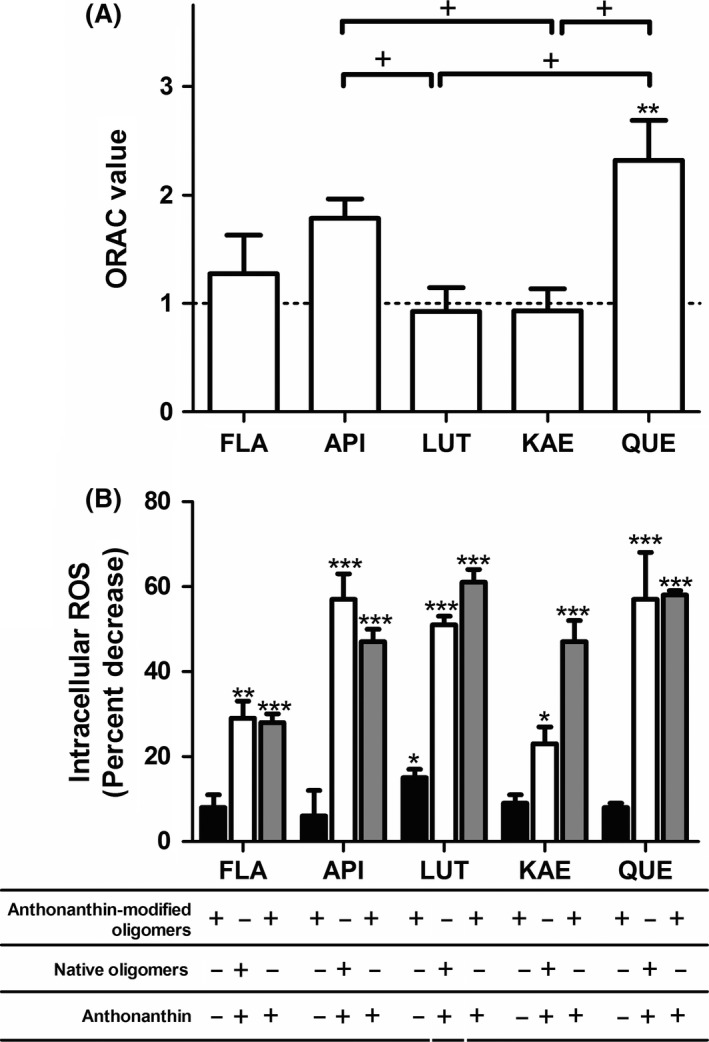
Anthoxanthins possess potent antioxidant capability and attenuate Aβ oligomer‐induced intracellular ROS in human neuroblastoma cells. (A) Anthoxanthins flavone (FLA), apigenin (API), luteolin (LUT), kaempferol (KAE), and quercetin (QUE) were diluted in 75 mM potassium phosphate (pH 7.0) for evaluation of antioxidant capacity using an ORAC assay alongside a Trolox standard. ORAC values are expressed as the equivalent Trolox concentration per molar concentration of anthoxanthin. The ORAC value of Trolox is shown as a dashed line. Error bars indicate SEM, n = 3–4. **P < 0.01 versus Trolox; + P < 0.05 between samples. (B) SH‐SY5Y cells were incubated for 24 h with indicated treatments of 0.01 μM Aβ 1–42 oligomerization products and 40 μM anthoxanthin, designed to isolate mechanisms for anthoxanthin attenuation of ROS: modulation of oligomer formation (closed bars), antioxidant action (open bars), or both mechanisms in concert (gray bars). Intracellular ROS was evaluated using the DCFH‐DA probe. Results are expressed as the percent decrease in intracellular ROS relative to treatment with Aβ 1–42 oligomers alone. Error bars indicate SEM, n = 3–4. *P < 0.05, **P < 0.01, and ***P < 0.01 versus Aβ 1–42 oligomers alone.
Hydroxylated Anthoxanthins Attenuate Oligomer‐Induced Intracellular ROS
As shown, anthoxanthins can modulate oligomer formation and possess strong antioxidant capability. Thus, they may attenuate Aβ oligomer‐induced intracellular ROS through either of these two mechanisms or using these mechanisms in concert. Reduction of intracellular ROS was therefore examined using cellular treatments designed to isolate these mechanisms of action.
To determine whether alterations to oligomer size (Figure 2) and conformation (Figure 3) could attenuate oligomer‐induced intracellular ROS, Aβ 1–42 oligomers were prepared in the presence of 10‐fold excess anthoxanthins and diluted to 0.01 μM for cellular treatment (24 h). When intracellular ROS was subsequently evaluated, a significant decrease was observed only with LUT (Figure 5B, closed bars). To probe whether anthoxanthin antioxidant capacity (Figure 5A) could attenuate oligomer‐induced intracellular ROS, cells were treated (24 h) simultaneously with 40 μM anthoxanthin and 0.01 μM Aβ 1–42, following oligomer formation in the absence of anthoxanthin. Here, all compounds significantly lowered intracellular ROS (Figure 5B, open bars). Treatments including API, LUT, or QUE exhibited ~50% reduction in ROS, while KAE proved inferior to these compounds (P < 0.05), eliciting ~25% reduction through antioxidant capabilities. FLA was also inferior to API and LUT at reducing intracellular ROS through antioxidant capabilities (P < 0.05 and P < 0.01, respectively).
To determine whether these two mechanisms could work in concert to decrease oligomer‐induced intracellular ROS, cells were treated simultaneously with 40 μM anthoxanthin and 0.01 μM Aβ 1–42, following oligomer formation in the presence of anthoxanthin. All treatments conferred a significant ROS reduction (Figure 5B, gray bars); however, FLA was again inferior to its hydroxylated counterparts. For dual‐mechanism treatments containing FLA, API, LUT, or QUE, the exhibited ROS reduction was similar to that observed for isolated antioxidant activity. Combined, the different treatment types demonstrate that hydroxylated anthoxanthins attenuate oligomer‐induced intracellular ROS primarily via their antioxidant properties rather than by modulating oligomerization. However, the dual‐mechanism treatment employing KAE decreased oligomer‐induced intercellular ROS significantly greater than when acting through antioxidant capability alone, demonstrating potential synergy between the two mechanisms for this compound.
Hydroxylated Anthoxanthins Reduce Oligomer Activation of Caspases
The roles of anthoxanthin modulation of oligomerization and antioxidant activity toward reducing oligomer activation of caspases were also examined. Treatment of cells (24 h) with 0.01 μM Aβ 1–42 following oligomer preparation in the presence of anthoxanthins, to isolate the role of anthoxanthin‐modulated oligomerization, failed to alter caspase activation (Figure 6A, left images; Figure 6B, closed bars). However, treatment of cells (24 h) simultaneously with 40 μM anthoxanthin and 0.01 μM Aβ 1–42 following oligomer formation in the absence of anthoxanthin, to isolate antioxidant activity, yielded a > 50% decrease in caspase activity when either LUT or QUE was included in treatments (Figure 6A, center images; Figure 6B, open bars). Furthermore, LUT and QUE were more effective (P < 0.05) at decreasing caspase activation than FLA, API, and KAE. Thus, strong anthoxanthin attenuation of oligomer‐induced intracellular ROS through antioxidant capacity (Figure 5B, open bars) is paralleled by a reduction in caspase activity.
Figure 6.
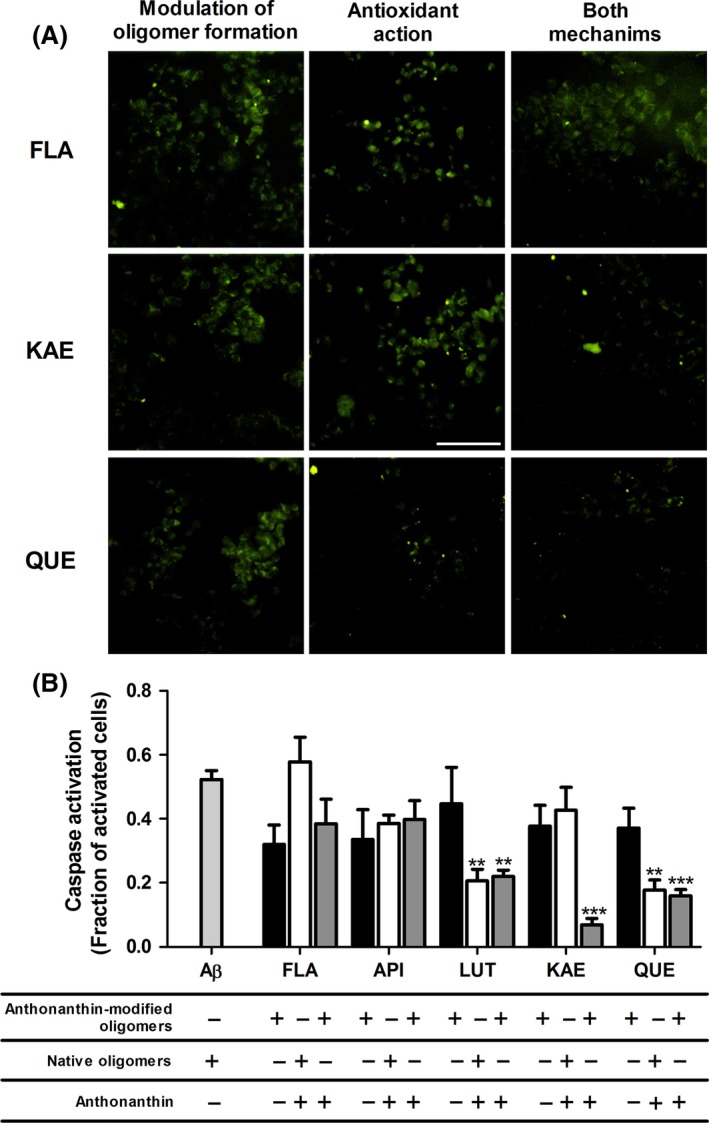
Anthoxanthins reduce Aβ oligomer activation of caspases in human neuroblastoma cells. SH‐SY5Y cells were incubated for 24 h with indicated treatments of 0.01 μM Aβ 1–42 oligomerization products and 40 μM anthoxanthin, designed to isolate mechanisms for anthoxanthin attenuation of caspase activation: modulation of oligomer formation (left images, closed bars), antioxidant action (center images, open bars), or both mechanisms in concert (right images, gray bars). Caspase activation was evaluated via staining with FLICA reagent for detection of caspase‐1, caspase‐3, caspase‐4, caspase‐5, caspase‐6, caspase‐7, caspase‐8, and caspase‐9 (green). (A) Representative images are shown for treatments including flavone (FLA), kaempferol (KAE), and quercetin (QUE); the complete image set is shown in Supporting Information (Figures S2, S3, S4). Scale bar represents 50 μm. Images are representative of 3–4 independent experiments. (B) Cellular caspase activation was quantified using FLICA images in conjunction with nuclear Hoechst staining, as described in Figure 4. Results are reported as the fraction of caspase activated cells and shown alongside treatment with Aβ 1–42 oligomers alone (light gray bar). Error bars indicate SEM, n = 3–5. **P < 0.01 and ***P < 0.001 versus Aβ 1–42 oligomers alone.
Treatment of cells with 40 μM anthoxanthin and 0.01 μM Aβ 1–42 following oligomerization in the presence of anthoxanthin, to facilitate both mechanisms in concert, again resulted in a significant decrease in caspase activity when either LUT or QUE was included in treatments, while treatments including FLA or API failed to attenuate oligomer activation of caspases (Figure 6A, right images; Figure 6B, gray bars). Similar to ROS reduction, these results parallel isolated antioxidant activity. Interestingly, KAE again showed potential for synergy by significantly decreasing caspase activity when treatments facilitated both mechanisms, despite performing ineffectively when either mechanism was isolated. In fact, within dual‐mechanism treatments, KAE was more effective than all other compounds (FLA, QUE P < 0.05; API, LUT P < 0.01).
Conclusion
Epidemiological studies demonstrating that polyphenol‐rich diets correlate with a reduced incidence of AD have rendered these dietary components promising candidates for natural AD therapeutics 6, 7, 8. Moreover, several polyphenols exhibit properties that engender the potential to attenuate AD, namely the propensity to mitigate disease through antioxidant capabilities 22, 23, 24 and modulation of Aβ aggregation 13, 14, 35, 36. This study evaluated the ability of five polyphenols, anthoxanthins FLA, API, LUT, KAE, and QUE, to act via these mechanisms, either individually or synergistically, to ameliorate Aβ oligomer‐induced neuronal responses. These investigations reveal that the studied compounds primarily attenuate oligomer‐induced cellular responses through antioxidant capacity and identify KAE as capable of synergistic action.
Caspase activity regulates cell networks responsible for apoptosis and inflammation 37, which are characteristic of AD brain. Moreover, caspase activation can further the pathology of AD by inducing tau cleavage, promoting tau tangle formulation, and cleaving APP 20, 38. In addition, upregulated ROS play a role in AD pathology as well as Aβ cytotoxicity 6, 10, 15, 20. In parallel with these pathogenic observations, Aβ 1–42 oligomers stimulated a 1.4‐fold increase in intracellular ROS (Figure 4A) and elevated caspase activation by 50% (Figure 4B) in an SH‐SY5Y neuronal culture model. These results are in agreement with other reports that Aβ aggregates, including oligomers, increase both intracellular ROS and caspase activation 39, 40, 41, 42 and support ameliorating these responses as a therapeutic strategy for AD. Thus, the potential of anthoxanthins as AD therapeutics was assessed through their ability to attenuate oligomer‐induced intracellular ROS and caspase activation.
Like many polyphenols already recognized as antioxidants 43, 44, 45, each anthoxanthin exhibited high antioxidant capacity (Figure 5A). Moreover, this antioxidant capacity translated to an attenuation of Aβ 1–42 oligomer‐induced cellular responses, with all anthoxanthins significantly decreasing intracellular ROS (Figure 5B, open bars). Antioxidant activity of LUT and QUE also attenuated caspase activity (Figure 6B, open bars), suggesting that Aβ‐mediated ROS could play a role in activation of caspases. The superiority of LUT and QUE may be attributed to their 3′,4′ catechol structure, implicated previously as a key structural element for cellular protection by polyphenols 46. Alternatively, LUT and QUE may attenuate caspase activity, in part, through other mechanisms that API and KAE fail to engage. Oxidative stress, increased cytochrome c, and mitochondria dysfunction are all factors capable of initiating caspase activation 47. LUT and QUE are reported to reduce oxidative damage by elevating glutathione 46, 48, and QUE can reduce cytochrome c 49, 50, 51. Furthermore, QUE, but not API, has been shown to reduce H2O2 production caused by mitochondria dysfunction in rat brain 49.
Each hydroxylated anthoxanthin exhibited the ability to reduce the formation of >25‐kDa oligomers (Figure 2A–C), and several anthoxanthins also altered oligomer surface hydrophobicity (Figure 3). Parallel reductions in monomer, trimer, and tetramer species (Figure 2D–G) suggest that anthoxanthins are not interacting with early oligomers to halt assembly, but instead are shifting the distribution to large aggregate structures. Other polyphenols appear to interact with Aβ aggregates only after aggregates acquire certain structures 12, 52, 53. Thus, anthoxanthins may likewise be capable of intervening only at a later stage of oligomerization. Despite these alterations in oligomer formation, only LUT modulation of oligomerization reduced intracellular ROS (Figure 5B, closed bars), and this change was not paralleled by attenuation of caspase activation (Figure 6B, closed bars). Interestingly, LUT was inferior to the other hydroxylated anthoxanthins at altering oligomer size distribution; however, it was the only compound capable of reducing oligomer surface hydrophobicity. Increased oligomer surface hydrophobicity has been implicated as an essential factor governing oligomer toxicity 54, 55, 56, and computational models have indicated that increased surface hydrophobicity promotes oligomer insertion into the cell membrane 55. Combined, these results suggest that modulating oligomer conformation may be a more effective therapeutic strategy than altering oligomer size distribution. In juxtaposition to this study, other reports have identified polyphenols that substantially influence cellular responses via modulation of Aβ aggregation 10, 14, 35, 44. These differences may result from implementation in this study of more controlled cellular assays to effectively isolate the mechanism of action (antiaggregation vs. antioxidant) or, alternatively, the ability of different polyphenols to attenuate Aβ physiological activity via different mechanisms.
While anthoxanthin modulation of oligomerization had minimal effect on cellular responses, the comparative study of anthoxanthins with varying structure did identify key structural elements that facilitate alteration of oligomer size distribution. Only FLA was unable to reduce the formation of large oligomers (Figure 2A–C) and was also unable to alter oligomer conformation (Figure 3), indicating the significance of hydroxylation. In addition, the superior modulation of oligomer size distribution by flavonols KAE and QUE over their flavone counterparts, API and LUT, (Figure 2B) demonstrates the importance of hydroxyl placement at the three position. However, a hydroxyl at the 3′ position was less crucial, with LUT and QUE displaying no increase in modulation of oligomerization compared to their analogs lacking 3′ hydroxylation, API and KAE. Other structure‐activity studies have concluded that the 4′ hydroxyl group within polyphenols fisetin and 3,3′,4′,5,5′‐pentahydroxyflavone is essential to their ability to inhibit Aβ fibril formation 57, 58. All hydroxylated anthoxanthins here exhibit 4′ hydroxylation, reinforcing that this functionalization is instrumental in inhibiting aggregation. In contrast to the current oligomerization results, however, these studies also cited the 3′ hydroxyl group as crucial for inhibition of Aβ fibril formation, suggesting that polyphenol structural elements needed to alter oligomerization and fibril formation may not always coincide.
Allowing both antioxidant and antiaggregation mechanisms to act in concert revealed the relative contributions of these two mechanisms and identified any synergistic interaction. As expected, anthoxanthins unable to significantly impact oligomer‐induced cellular responses by modulating oligomer formation acted predominately as antioxidants (Figure 5B, 6B). Although capable of reducing oligomer‐induced cellular responses via both mechanisms, LUT also acted primarily through antioxidant capabilities. These findings correlate with other studies that have suggested antioxidant capability is the dominant mechanism by which polyphenols rescue cells from Aβ cytotoxicity 12, 59. In contrast, despite its inability to affect cellular responses via modulation of oligomer formation, KAE exhibited an enhanced ability to attenuate cellular responses when both mechanisms acted in concert (Figures 5B and 6B). While KAE antioxidant activity decreased intracellular ROS induced by native oligomers, this reduction was doubled when cells were treated with KAE‐modified oligomers. In parallel, the modest, although significant, reduction in Aβ oligomer‐induced intracellular ROS resulting from KAE antioxidant activity alone was not sufficient to render any change in caspase activation. However, Aβ oligomer‐induced caspase activation was reduced by 90% when KAE antioxidant activity was paired with KAE‐modified oligomers. These combined results demonstrate synergistic activity by KAE. The effectiveness of KAE in attenuating Aβ‐induced cellular responses corroborates other investigations. KAE has been shown to protect against Aβ‐induced toxicity in SH‐SY5Y cells 60, although via unexplored mechanisms. Additionally, KAE's antioxidant and antiaggregation capabilities lowered Aβ‐induced cytotoxicity in PC12 cells 44. Thus, this study provides additional support for KAE as a potential AD therapeutic and further demonstrates that its effectiveness is achieved via synergistic action.
This study has demonstrated the ability of five anthoxanthins, FLA, API, LUT, KAE, and QUE, to exert antioxidant capabilities toward the reduction of Aβ 1–42 oligomer‐induced intracellular ROS, and for LUT and QUE to also attenuate oligomer‐induced caspase activation through antioxidant action. In contrast, modulation of oligomerization by anthoxanthins had negligible effect on oligomer‐induced cellular responses, with only LUT exhibiting the ability to attenuate intracellular ROS via this mechanism. Accordingly, with the exception of KAE, all anthoxanthins predominately reduced cellular responses through antioxidant capabilities. KAE, however, attenuated both oligomer‐induced intracellular ROS and caspase activation via synergism between antioxidant and antiaggregation mechanisms, despite exerting little effect when these mechanisms were isolated. Together, these findings identify the promise of anthoxanthins as natural therapeutics for AD and demonstrate their potential for synergistic action.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1. Morphology of native Aβ 1–42 oligomers.
Figure S2. Anthoxanthin modulation of oligomerization does not alter Aβ oligomer‐induced activation of caspases in human neuroblastoma cells.
Figure S3. Anthoxanthin antioxidant capability reduces Aβ oligomer‐induced activation of caspases in human neuroblastoma cells.
Figure S4. KAE synergistically reduces Aβ oligomer‐induced activation of caspases in human neuroblastoma cells.
Appendix S1. Extended methods.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences at the National Institutes of Health via funding through the Centers of Biomedical Research Excellence Program (COBRE, P20GM103641 to M.A.M.)
References
- 1. 2016 Alzheimer's Disease Facts and Figures [Internet]. [cited 2016 Apr 14]. Available from: http://www.alz.org/documents_custom/2016-facts-and-figures.pdf [DOI] [PubMed]
- 2. Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: An emperor in need of clothes. Nat Neurosci 2012;15:349–357. [DOI] [PubMed] [Google Scholar]
- 3. McLean CA, Cherny RA, Fraser FW, et al. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol 1999;46:860–866. [DOI] [PubMed] [Google Scholar]
- 4. Mc Donald JM, Savva GM, Brayne C, et al. The presence of sodium dodecyl sulphate‐stable Aβ dimers is strongly associated with Alzheimer‐type dementia. Brain 2010;133:1328–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murakami K, Murata N, Noda Y, et al. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid β protein oligomerization and memory loss in mouse model of Alzheimer disease. J Biol Chem 2011;286:44557–44568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butterfield DA, Castegna A, Pocernich CB, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer's disease. J Nutr Biochem 2002;13:444–461. [DOI] [PubMed] [Google Scholar]
- 7. Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer's disease: The Kame project. Am J Med 2006;119:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barberger‐Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: The Three‐City cohort study. Neurology 2007;69:1921–1930. [DOI] [PubMed] [Google Scholar]
- 9. Ngoungoure VLN, Schluesener J, Moundipa PF, Schluesener H. Natural polyphenols binding to amyloid: A broad class of compounds to treat different human amyloid diseases. Mol Nutr Food Res 2015;59:8–20. [DOI] [PubMed] [Google Scholar]
- 10. Ebrahimi A, Schluesener H. Natural polyphenols against neurodegenerative disorders: Potentials and pitfalls. Ageing Res Rev 2012;11:329–345. [DOI] [PubMed] [Google Scholar]
- 11. Lakey‐Beitia J, Berrocal R, Rao KS, Durant AA. Polyphenols as therapeutic molecules in Alzheimer's disease through modulating amyloid pathways. Mol Neurobiol 2015;51:466–479. [DOI] [PubMed] [Google Scholar]
- 12. Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des 2006;67:27–37. [DOI] [PubMed] [Google Scholar]
- 13. Lemkul JA, Bevan DR. Destabilizing Alzheimer's Aβ 42 protofibrils with morin: Mechanistic insights from molecular dynamics simulations. Biochemistry 2010;49:3935–3946. [DOI] [PubMed] [Google Scholar]
- 14. Williams RJ, Spencer JPE. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med 2012;52:35–45. [DOI] [PubMed] [Google Scholar]
- 15. Zhao B. Natural antioxidants for neurodegenerative diseases. Mol Neurobiol 2005;31:283–293. [DOI] [PubMed] [Google Scholar]
- 16. Viña J, Lloret A, Vallés SL, et al. Mitochondrial oxidant signalling in Alzheimer's disease. J Alzheimers Dis 2007;11:175–181. [DOI] [PubMed] [Google Scholar]
- 17. Smith WW, Gorospe M, Kusiak JW. Signaling mechanisms underlying Aβ toxicity: Potential therapeutic targets for Alzheimer's disease. CNS Neurol Disord Drug Targets 2006;5:355–361. [DOI] [PubMed] [Google Scholar]
- 18. Block ML. NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci 2008;9:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuo L, Motherwell MS. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson's disease. Gene 2013;532:18–23. [DOI] [PubMed] [Google Scholar]
- 20. Zuo L, Hemmelgarn BT, Chuang C‐C, Best TM. The role of oxidative stress‐induced epigenetic alterations in amyloid‐β production in Alzheimer's disease. Oxid Med Cell Longev 2015;2015:604658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer's disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem 2008;56:4855–4873. [DOI] [PubMed] [Google Scholar]
- 22. Kelley BJ, Knopman DS. Alternative medicine and Alzheimer disease. Neurologist 2008;14:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun AY, Wang Q, Simonyi A, Sun GY. Botanical phenolics and brain health. NeuroMol Med 2008;10:259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Darvesh AS, Carroll RT, Bishayee A, Geldenhuys WJ, Van der Schyf CJ. Oxidative stress and Alzheimer's disease: Dietary polyphenols as potential therapeutic agents. Expert Rev Neurother 2010;10:729–745. [DOI] [PubMed] [Google Scholar]
- 25. Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci 1999;65:337–353. [DOI] [PubMed] [Google Scholar]
- 26. Ganapathy M, Bhunia S. Nutraceuticals: The new generation therapeutics. Adv Tech Biol Med 2016;04:1000179. [Google Scholar]
- 27. Lindgren M, Hammarström P. Amyloid oligomers: Spectroscopic characterization of amyloidogenic protein states. FEBS J 2010;277:1380–1388. [DOI] [PubMed] [Google Scholar]
- 28. Fändrich M. Oligomeric intermediates in amyloid formation: Structure determination and mechanisms of toxicity. J Mol Biol 2012;421:427–440. [DOI] [PubMed] [Google Scholar]
- 29. Bolognesi B, Kumita JR, Barros TP, et al. ANS binding reveals common features of cytotoxic amyloid species. ACS Chem Biol 2010;5:735–740. [DOI] [PubMed] [Google Scholar]
- 30. Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid β‐protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A 2003;100:330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gough DR, Cotter TG. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis 2011;2:e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell 2007;26:1–14. [DOI] [PubMed] [Google Scholar]
- 33. Tanabe A, Shiraishi M, Sasaki Y. Myristoylated alanine‐rich C kinase substrate accelerates TNF‐α‐induced apoptosis in SH‐SY5Y cells in a caspases‐6 and/or ‐7‐dependent manner. Adv Biosci Biotechnol 2015;06:572–582. [Google Scholar]
- 34. Pregi N, Wenker S, Vittori D, Leirós CP, Nesse A. TNF‐alpha‐induced apoptosis is prevented by erythropoietin treatment on SH‐SY5Y cells. Exp Cell Res 2009;315:419–431. [DOI] [PubMed] [Google Scholar]
- 35. Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti‐amyloidogenic and fibril‐destabilizing effects of polyphenols in vitro: Implications for the prevention and therapeutics of Alzheimer's disease. J Neurochem 2003;87:172–181. [DOI] [PubMed] [Google Scholar]
- 36. Rivière C, Richard T, Vitrac X, Mérillon JM, Valls J, Monti JP. New polyphenols active on β‐amyloid aggregation. Bioorg Med Chem Lett 2008;18:828–831. [DOI] [PubMed] [Google Scholar]
- 37. McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 2013;5:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer's disease: Amyloid‐β oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol 2009;87:181–194. [DOI] [PubMed] [Google Scholar]
- 39. Sponne I, Fifre A, Koziel V, Oster T, Olivier JL, Pillot T. Membrane cholesterol interferes with neuronal apoptosis induced by soluble oligomers but not fibrils of amyloid‐β peptide. FASEB J 2004;18:836–838. [DOI] [PubMed] [Google Scholar]
- 40. Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid β induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci 2006;26:6011–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shelat PB, Chalimoniuk M, Wang JH, et al. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem 2008;106:45–55. [DOI] [PubMed] [Google Scholar]
- 42. He Y, Cui J, Lee JC‐M, et al. Prolonged exposure of cortical neurons to oligomeric amyloid‐β impairs NMDA receptor function via NADPH oxidase‐mediated ROS production: Protective effect of green tea (‐)‐epigallocatechin‐3‐gallate. ASN Neuro 2011;3:e00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yordi EG, Pérez EM, Matos MJ, Villares EU. Antioxidant and pro‐oxidant effects of polyphenolic compounds and structure‐activity relationship evidence In: Bouayed J, Bohn T, editors. Nutr Well‐Being Health. Croatia: InTech, 2012;23–48. [Google Scholar]
- 44. Cheung AWH, Choi RCY, Chu GKY, et al. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: A comparison of different flavonoids in activating estrogenic effect and in preventing β‐amyloid‐induced cell death. J Agric Food Chem 2007;55:2438–2445. [DOI] [PubMed] [Google Scholar]
- 45. Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: Antioxidants and beyond. Am J Clin Nutr 2005;81:215–217. [DOI] [PubMed] [Google Scholar]
- 46. Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 2001;30:433–446. [DOI] [PubMed] [Google Scholar]
- 47. Eckert A, Keil U, Marques CA, et al. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer's disease. Biochem Pharmacol 2003;66:1627–1634. [DOI] [PubMed] [Google Scholar]
- 48. Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Aβ(1‐42): Relevance to Alzheimer's disease. J Nutr Biochem 2009;20:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lagoa R, Graziani I, Lopez‐Sanchez C, Garcia‐Martinez V, Gutierrez‐Merino C. Complex I and cytochrome c are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim Biophys Acta 2011;1807:1562–1572. [DOI] [PubMed] [Google Scholar]
- 50. Vladimirov YA, Proskurnina EV, Demin EM, et al. Dihydroquercetin (taxifolin) and other flavonoids as inhibitors of free radical formation at key stages of apoptosis. Biochemistry 2009;74:301–307. [DOI] [PubMed] [Google Scholar]
- 51. Chow J‐M, Shen S‐C, Huan SK, Lin H‐Y, Chen Y‐C. Quercetin, but not rutin and quercitrin, prevention of H2O2‐induced apoptosis via anti‐oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem Pharmacol 2005;69:1839–1851. [DOI] [PubMed] [Google Scholar]
- 52. DaSilva KA, Shaw JE, McLaurin J. Amyloid‐β fibrillogenesis: Structural insight and therapeutic intervention. Exp Neurol 2010;223:311–321. [DOI] [PubMed] [Google Scholar]
- 53. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 2005;280:5892–5901. [DOI] [PubMed] [Google Scholar]
- 54. Ladiwala AR, Litt J, Kane RS, et al. Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity. J Biol Chem 2012;287:24765–24773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Viola KL, Klein WL. Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis. Acta Neuropathol 2015;129:183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jang H, Connelly L, Arce FT, et al. Alzheimer's disease: Which type of amyloid‐preventing drug agents to employ? Phys Chem Chem Phys 2013;15:8868–8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ushikubo H, Watanabe S, Tanimoto Y, et al. 3,3′,4′,5,5′‐Pentahydroxyflavone is a potent inhibitor of amyloid β fibril formation. Neurosci Lett 2012;513:51–56. [DOI] [PubMed] [Google Scholar]
- 58. Akaishi T, Morimoto T, Shibao M, et al. Structural requirements for the flavonoid fisetin in inhibiting fibril formation of amyloid β protein. Neurosci Lett 2008;444:280–285. [DOI] [PubMed] [Google Scholar]
- 59. Bastianetto S, Quirion R. Natural extracts as possible protective agents of brain aging. Neurobiol Aging 2002;23:891–897. [DOI] [PubMed] [Google Scholar]
- 60. Sharoar MG, Thapa A, Shahnawaz M, et al. Keampferol‐3‐O‐rhamnoside abrogates amyloid beta toxicity by modulating monomers and remodeling oligomers and fibrils to non‐toxic aggregates. J Biomed Sci 2012;19:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Morphology of native Aβ 1–42 oligomers.
Figure S2. Anthoxanthin modulation of oligomerization does not alter Aβ oligomer‐induced activation of caspases in human neuroblastoma cells.
Figure S3. Anthoxanthin antioxidant capability reduces Aβ oligomer‐induced activation of caspases in human neuroblastoma cells.
Figure S4. KAE synergistically reduces Aβ oligomer‐induced activation of caspases in human neuroblastoma cells.
Appendix S1. Extended methods.


