Abstract
INTRODUCTION
Ovariectomy and high fat diet (HFD) worsen obesity and metabolic dysfunction associated with low aerobic fitness. Exercise training mitigates metabolic abnormalities induced by low aerobic fitness, but whether the protective effect is maintained following ovariectomy and HFD is unknown.
PURPOSE
This study determined whether, following ovariectomy and HFD, exercise training improves metabolic function in rats bred for low intrinsic aerobic capacity.
METHODS
Female rats selectively bred for low (LCR) and high (HCR) intrinsic aerobic capacity (n=30) were ovariectomized, fed HFD, and randomized to either a sedentary (SED) or voluntary wheel running (EX) group. Resting energy expenditure, glucose tolerance, and spontaneous physical activity were determined midway through the experiment, while body weight, wheel running volume, and food intake were assessed throughout the study. Body composition, circulating metabolic markers, and skeletal muscle gene and protein expression was measured at sacrifice.
RESULTS
EX reduced body weight and adiposity in LCR rats (−10% and −50%, respectively; P<0.05) and, unexpectedly, increased these variables in HCR rats (+7% and +37%, respectively; P<0.05) compared to their respective SED controls, likely due to dietary overcompensation. Wheel running volume was ~5-fold greater in HCR than LCR rats, yet EX enhanced insulin sensitivity equally in LCR and HCR rats (P<0.05). This EX-mediated improvement in metabolic function was associated with gene up-regulation of skeletal muscle IL-6&-10. EX also increased resting energy expenditure, skeletal muscle mitochondrial content (oxidative phosphorylation complexes and citrate synthase activity), and AMPK activation similarly in both lines (all P <0.05).
CONCLUSION
Despite a 5-fold difference in running volume between rat lines, EX similarly improved systemic insulin sensitivity, resting energy expenditure, and skeletal muscle mitochondrial content and AMPK activation in ovariectomized LCR and HCR rats fed HFD compared to their respective SED controls.
Keywords: exercise, ovariectomy, insulin sensitivity, AMPK, skeletal muscle metabolism
Introduction
Low aerobic fitness is a strong predictive factor for the development of metabolic diseases including type 2 diabetes (12). Individuals with low aerobic fitness, independent of other metabolic risk factors such as age and obesity, have a four-fold greater risk of the development of metabolic syndrome (7) and a five-fold greater rate of all-cause mortality compared to individuals with high aerobic fitness (3). Impaired aerobic capacity and skeletal muscle metabolism (e.g., mitochondrial function) are strongly linked to insulin resistance (31). Furthermore, rodent ovariectomy, a model of ovarian hormone depletion, is a good model of human menopause, as both ovariectomized rodents and postmenopausal women develop metabolic dysfunction including insulin resistance and weight gain with increased visceral fat (32, 42). Moreover, low aerobic capacity exacerbates metabolic dysfunction driven by loss of ovarian hormones (40).
The interaction between intrinsic and environmental factors is complex, and the aerobic capacity phenotype is multifactorial. To study the contribution of intrinsic aerobic fitness to the etiology of metabolic abnormality, Britton and Koch (11) developed an animal model using two-way artificial selection for aerobic capacity generating rats selectively bred for low and high aerobic capacity (i.e., LCR and HCR rats, respectively). This unique model has demonstrated critical connections between low aerobic capacity and development of metabolic dysfunction (14, 21, 34). LCR rats exhibit dyslipidemia, increased fat mass, and whole body insulin resistance (43) along with impaired glucose and lipid metabolism (14, 21). A recent study demonstrated that female LCR rats have impaired skeletal muscle mitochondrial function assessed by cytochrome c protein and fatty acid oxidation (20), which is associated with insulin resistance and greater susceptibility to metabolic syndrome (31). Our group has demonstrated that LCR rats are susceptible to ovariectomy-induced insulin resistance and the accumulation of adiposity, while HCR rats are impervious to ovariectomy with respect to those variables (25, 40). Furthermore, we recently demonstrated that, following ovariectomy, LCR rats are more susceptible to high fat diet (HFD)-induced adiposity and insulin resistance than HCR rats (24). Whether access to a voluntary running wheel can rescue metabolic dysfunction in the ovariectomized LCR model is unknown.
Exercise training and increasing physical activity are the only universally-prescribed and clinically validated approaches to enhance aerobic fitness (6). Exercise training and subsequently increased aerobic fitness reverse the prevalence of metabolic syndrome and cardiovascular disease in postmenopausal women (17). Improved aerobic fitness induced by exercise training is linked to improvements in metabolic syndrome-related variables in a dose-dependent fashion (4). Previous rodent studies (15, 36) have demonstrated that forced treadmill running improves metabolic dysfunction associated with low aerobic capacity. Compared to forced treadmill running, voluntary wheel running is less stressful, yet has also been shown to effectively reverse impaired metabolic function in rodents (13, 30). The impact of voluntary wheel running in the unique physiological condition induced by ovariectomy and low aerobic capacity has not been previously investigated.
Our previous work demonstrated that, compared to HCR, LCR rats have increased susceptibility to ovariectomy-associated weight gain and metabolic dysfunction (40), and that HFD exacerbates metabolic dysfunction in ovariectomized LCR rats (24). The ovariectomized LCR rats may therefore be an appropriate model of human menopause, since many postmenopausal women are sedentary and at increased risk of metabolic diseases compared to age-matched men and premenopausal women (10). The purpose of this present study was to determine 1) whether voluntary running could attenuate metabolic dysfunction associated with ovariectomy and HFD; and 2) whether the protective effects of voluntary running are different between HCR and LCR rats. We hypothesized that, following ovariectomy and HFD, access to a voluntary wheel running would improve metabolic function, indicated by glucose tolerance and skeletal muscle mitochondrial content, in both LCR rats and HCR rats.
Methods
Animals
Thirty female HCR and LCR rats (generation 32, 16 weeks of age) were shipped to the University of Missouri, singly housed under standard humidity and temperature on a 12h-12h light/dark cycle, and fed standard rodent chow (Harlan Teklad Rodent Diet 8604) and water ad libitum. Animal procedures were approved by Institutional Animal Care and Use Committee (IACUC) at the University of Missouri-Columbia.
Experimental design
All rats were acclimated to wheel running for 10–15 minutes daily for one week prior to surgical treatments. At approximately 30 weeks of age, all rats were ovariectomized and single-housed for a 1-week recovery period. All rats were provided with ad libitum access to a HFD (45% kcal fat, D12451, Research Diets), randomized to sedentary (SED) or voluntary wheel running exercise (EX) groups, and followed for 11 weeks. We employed a 2 (HCR vs. LCR) × 2 (SED vs. EX) design generating 4 groups: HCRSED (n=7), HCREX (n=8), LCRSED (n=7), and LCREX (n=8). Rats in the EX groups were provided access to voluntary running wheels (11cm wide with an inner diameter of 35cm; bar running surface), and running distance was monitored weekly by the Sigma sport BC 800 bicycle monitoring system (Cherry Creek Cyclery, Foster Falls, VA). Food intake (g·day−1) and body weight (g) were recorded weekly. To aid in the accurate assessment of food intake, we utilized a commercial diet that was brightly colored and therefore easy to detect in bedding, and carefully searched the bedding for any food particles that may have fallen from the food hopper. Six weeks following ovariectomy, glucose tolerance tests (GTT) were conducted. Resting energy expenditure (REE) and cage spontaneous physical activity (SPA) were measured 10 weeks following ovariectomy. On the last experimental day, rats were anesthetized with sodium pentobarbital (100mg·kg−1) following an overnight fast. Wheels were locked for ~5 hours prior to sacrifice to prevent any acute exercise effects. Blood was collected from the heart, and tissues were harvested, snap frozen immediately in liquid nitrogen, and stored at −80 °C for later analysis.
Ovariectomy surgeries
Ovariectomy surgeries were conducted as previously described (25). All rats were anesthetized by inhaled isofluorane at 2% during surgery. Following shaving and sanitizing the dorsal skin surface, a one-inch incision was made at mid-line of the dorsal surface. Following bilateral incisions on the muscle layer to expose the ovaries, all rats were ovariectomized and administered acetaminophen (i.p., 500 mg·kg−1). The skin incision was closed using wound clips, which were removed following the one-week recovery period.
Resting energy expenditure (REE) and cage spontaneous physical activity (SPA)
REE was assessed via indirect calorimetry (i.e. monitoring oxygen consumption and carbon dioxide production) for a 72-hour period using a metabolic monitoring system (Promethion, Sable Systems Int., Las Vegas, NV) following acclimation to the chamber environment for one day, as previously performed (40). Thirty minutes of the lowest REE were selected to calculate 24-hour REE. Individual metabolic cages were not equipped with running wheels; thus, EX animals did not have running wheel access over this 72-h observation period. SPA was measured via quantification of a three-dimensional series of infrared beams (X+Y+Z). Data were analyzed for each 12-hour light and dark circadian cycle.
Glucose tolerance tests (GTT)
GTT were conducted following an 8-hr overnight fast. Following collection of baseline blood samples from the tail vein using a 23 g × 3/4 inch blood collection set (Terumo Medical Corp., Somerset, NJ), a sterile glucose solution (2 g·kg−1) was intraperitoneally injected. A hand-held rodent glucometer (AlphaTrak, Abbott Labs, Abbot Park, IL) was used to assess blood glucose concentration at 15, 30, 45, 60, and 120 minutes. Insulin concentrations were determined from blood samples collected at 15, 30, 45, 60, and 120 minutes using rat insulin ELISA kits via manufacturer’s instructions (Alpco Corp., Salem, NH). The total area under the curve (tAUC) from baseline to 120 min was used to measure the glucose and insulin response. The Matsuda index was used to calculate insulin sensitivity using the formula: 10,000 / (fasting glucose × fasting insulin × mean postprandial glucose × mean postprandial insulin)0.5 (18). Insulin and glucose values were presented in pmol·l−1 and mmol·l−1, respectively.
Circulating metabolic markers
Fasting plasma glucose, insulin, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and non-esterified fatty acids (NEFA) were assessed via a clinical diagnostic service at the University of Missouri (Clinical Pathology Services, LLC) with an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea CA). Fasting plasma interleukin (IL) -6 and IL-10 were measured using commercially available kits (Rat Quantikine ELISA kits, R&D systems, Minneapolis, MN). The homeostatic model assessment of insulin resistance (HOMA-IR) and adipocyte- IR were used to determine a surrogate measure of insulin resistance (19). The formula of HOMA-IR index was fasting insulin (pg·ml−1) × fasting glucose (mg·dl−1); the formula of adipocyte-IR index was fasting insulin (pg·ml−1) × fasting NEFA (mmol·l−1).
Body composition assessment
On the day of sacrifice, total, fat and lean mass were determined using dual energy x-ray absorptiometry (DXA; Hologic QDR-1000, calibrated for rodents). Following anesthetization, regional fat depots (liver, perigonadal, perirenal, omental white adipose tissue and interscapular brown adipose tissue) were harvested and weighed for the assessment of regional fat distribution. Brown adipose tissue mass was expressed relative to white adipose tissue mass.
RNA extraction and real-time PCR
The gastrocnemius muscle tissues were pulverized and homogenized in TRIzol solution using a tissue homogenizer (TissueLyser LT, Qiagen, Valencia, CA). Following RNA isolation using the Qiagen’s RNeasy Muscle Tissue Kit, RNA purity and concentration were measured using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). A subset of RNA samples were assessed for integrity via RNA fragment analysis; RQN values were all >8.0. cDNA was made from total RNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed using the ABI StepOne Plus sequence detection system (Applied Biosystems) and the iTaq Universal SYBR Green Supermix (BioRad, Hercules, CA), as previously referenced by our group (40). The primers for IL-6 and -10 were purchased from IDT (Coralville, IA), following designing primer sequences using the NCBI Primer Design tool: IL-6 forward: AGA GAC TTC CAG CCA GTT GC; IL-6 Reverse: AGC CTC CGA CTT GTG AAG TG; IL-10 Forward: CTG GCT CAG CAC TGC TAT GT; IL-10 Reverse: GCA GTT ATT GTC ACC CCG GA. A 20-µl reaction mixture containing the appropriate concentrations of gene-specific primers plus 4 µl of cDNA template, and 10 µl iTaq Universal SYBR Green Supermix was loaded in each well of a 96-well plate in duplicate. The specificity of the PCR products was calculated from a dissociation melt curve analysis. 18S primer was used to amplify the endogenous control product, and mRNA expression values are expressed in 2ΔCT (ΔCT = HK CT - gene of interest CT). Each mRNA data was normalized to the HCR SED group.
Western blots
Western blotting was conducted as previously referenced (5, 29, 38). The gastrocnemius muscle tissues were pulverized and homogenized by a tissue homogenizer (TissueLyser LT, Qiagen, Valencia, CA) in Triton X-100 buffer containing protease and phosphatase inhibitors. The Thermo Scientific Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL) was used to determine total protein value. Samples mixed with lammli buffer were separated by SDS-PAGE, and in turn transferred to polyvinylidene difluoride (PVDF) membranes. Membranes containing proteins were probed with primary and secondary antibodies accordingly. Protein bands were quantified via densitometry and individual protein bands were normalized to β-Actin antibody (#4967, Abcam, Cambridge, MA). Oxidative phosphorylation (OX PHOS) complexes were assessed by an antibody containing complex I subunit NDUFB8, complex II-30kDa, complex IV subunit I, and complex V alpha subunit (Abcam, Cambridge, MA) as described previously (5). Total adenosine monophosphate-activated protein kinase (AMPK)-α, pAMPK Thr172 and pAMPK Ser485/491 antibodies were purchased from the Cell Signaling (#2532, #2531, and #4185, respectively; Beverly, MA). Each pAMPK was normalized to total AMPK as performed previously (37).
Citrate synthase activity
Citrate synthase enzyme activity was assessed by the method of Srere et al. (35). Briefly, frozen gastrocnemius muscle tissues were pulverized and homogenized in Triton X-100 solution. Homogenates were incubated for 2 min at 37°C in 170µl of reaction media containing 10.0 mM oxaloacetate and 1.0 mM 5,5’-dithiobis-2-nitrobenzoic (DTNB). Then, a 30-µl sample of 3.0 mM acetyl-CoA was added and incubated for 7 min at room temperature. A wavelength of 412 nm was used to determine reduced DTNB, an estimated citrate synthase enzyme activity, using absorbance spectrophotometry. The unit of estimated citrate synthase enzyme activity is nmol·min−1·µg−1.
Statistical analysis
All data were analyzed using SPSS 22.0 (Armonk, NY). Two-way analysis of variance (ANOVA) was used to determine statistical differences for the line main effect (HCR vs. LCR), treatment main effect (SED vs. EX), and line × treatment interaction for body composition, REE, SPA, food intake, circulating metabolic markers, HOMA-IR, adipocyte-IR, glucose/insulin responses during GTT, and skeletal muscle AMPK activity, OX PHOS complexes, citrate synthase activity, and mitochondrial biogenesis mRNA expression. When a significant line by treatment interaction existed, LSD post-hoc tests were used for pair-wise comparisons. Two-way ANOVA with repeated measures was used to determine body weight gain over time. Bivariate Pearson’s correlations were performed to determine the association between the mitochondrial content (i.e. citrate synthase activity) and insulin sensitivity (i.e. Matsuda index) in HCR and LCR rats. In all cases, P<0.05 was considered statistically different and data are reported as mean ± SEM.
Results
Body composition
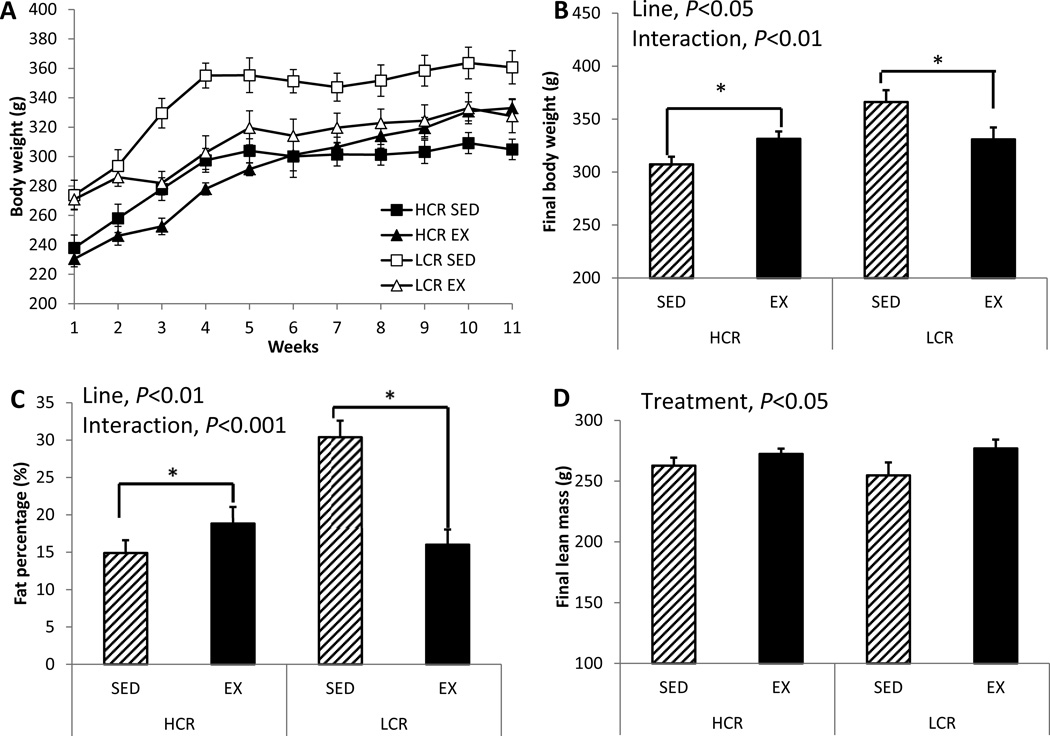
LCR rats were ~16% heavier than HCR rats at the start of this study’s observation period (line main effect for body weight, P<0.001; Figure 1A). LCR rats reduced body weight following the 11-week EX intervention; while HCR rats increased body weight following EX, (HCR +7% vs. LCR −10%; line main effect, P=0.014; line × treatment interaction, P=0.004; Figure 1B). These body weight differences were congruent with differences in fat percentage (i.e. total body fat percentage at sacrifice) such that, by the end of the intervention, LCR had greater fat percentage than HCR rats (line main effect, P=0.007; Figure 1C). EX significantly reduced fat percentage in LCR but not in the HCR (line × treatment interaction, P<0.001; Figure 1C). Similar to the change in body weight, EX caused a modest increase in adiposity in HCR rats. These changes in total adiposity with EX paralleled changes in visceral fat depot mass (perigonadal, HCR +44% vs. LCR −39%; perirenal, HCR +73% vs. LCR −57%; omental; HCR +150% vs. LCR −36%; compared to the SED groups; line × treatment interaction, P<0.01 for all variables; Table 1). Perirenal fat mass was greater in LCR than HCR (line main effect, P=0.041). EX increased lean mass in both lines (treatment main effect, P=0.048; Figure 1D).
Figure 1. EX decreases fat mass in LCR but increases in HCR.
(A) Body weight gain curves for 11 weeks, (B) final body weight, (C) % body fat, and (D) final lean mass. Values are means ± SE (n=7–8 per group). Line = line main effect, HCR vs. LCR; Treatment = treatment main effect, SED vs. EX; Interaction = line × treatment interaction. * Denotes difference (P<0.05) between SED and EX within line.
Table 1.
Body composition and metabolic characteristics
| HCR | LCR | 2-way ANOVA statistics | |||
|---|---|---|---|---|---|
| SED | EX | SED | EX | ||
| Perigonadal fat depot mass (g) |
6.71 ± 1.22 |
9.72 ± 1.75 |
14.11 ± 1.33 |
8.61 ± 1.38 |
Line, P=0.042 Line × Treatment, P=0.008 |
| Perirenal fat depot mass (g) |
3.38 ± 0.33 |
5.95 ± 1.19 |
9.70 ± 0.80 |
4.18 ± 0.81 |
Line, P=0.015 Line × Treatment, P<0.001 |
| Omental fat depot mass (g) |
0.38 ± 0.04 |
0.79 ± 0.12 |
0.75 ± 0.12 |
0.48 ± 0.09 |
Line, P=0.002 Line × Treatment, P=0.003 |
| BAT/WAT (g·g−1) |
0.030 ± 0.003 |
0.025 ± 0.004 |
0.015 ± 0.001 |
0.027 ± 0.006 |
NS |
| Liver (g) | 6.67 ± 0.54 |
8.42 ± 0.53 |
6.71 ± 0.23 |
6.42 ± 0.14 |
Line, P=0.03 Line × Treatment, P=0.023 |
| TG (mg·dl−1) |
32.71 ± 5.29 |
36.00 ± 6.33 |
52.00 ± 4.78 |
34.57 ± 1.90 |
Line × Treatment, P=0.037 |
| NEFA (mmol·l−1) |
0.31 ± 0.05 |
0.42 ± 0.07 |
0.45 ± 0.05 |
0.27 ± 0.05 |
Line × Treatment, P=0.022 |
| HDL (mg·dl−1) |
24.43 ± 1.34 |
29.00 ± 1.08 |
30.35 ± 1.31 |
29.85 ± 1.24 |
Line, P=0.026 Line × Treatment, P=0.046 |
| LDL (mg·dl−1) |
2.28 ± 0.18 |
1.50 ± 0.18 |
3.21 ± 0.26 |
2.85 ± 0.24 |
Line, P<0.001 Treatment, P=0.029 |
| Insulin (pg·ml−1) |
2.02 ± 0.49 |
2.95 ± 0.60 |
4.05 ± 0.76 |
2.87 ± 0.41 |
Line, P=0.067 (NS) |
| Glucose (mg·dl−1) |
153.8 ± 4.9 |
166.12 ± 5.6 |
160.1 ± 7.7 |
149.0 ± 2.8 |
Line × Treatment, P=0.041 |
| HOMA-IR index |
14.31 ± 4.07 |
22.40 ± 4.80 |
30.11 ± 6.13 |
19.18 ± 2.76 |
Line × Treatment, P=0.041 |
| Adipocyte-IR index |
0.714 ± 0.27 |
1.185 ± 0.41 |
1.820 ± 0.46 |
0.727 ± 0.16 |
Line × Treatment, P=0.037 |
Body composition and metabolic markers were analyzed. Two-way ANOVA for line (i.e., HCR vs. LCR), treatment (i.e., SED vs. EX) and line × treatment interaction effects were determined; values are means ± SE (n=7–8 per group). BAT= brown adipose tissue; WAT = white adipose tissue; TG= triglycerides; NEFA = non-esterified fatty acids; HDL= high-density lipoprotein; LDL=low-density lipoprotein; HOMA-IR = homeostasis model assessment of insulin resistance.
Energy intake, resting energy expenditure (REE), and spontaneous physical activity (SPA)
Averaged food intake increased ~30% following EX in HCR (treatment main effect, P=0.014; line × treatment interaction, P<0.001; Figure 2A and B). Moreover, this averaged food intake positively correlated with fat percentage (i.e. total body fat percentage at sacrifice) across animals (r=0.34, P<0.05), within HCR (r=0.61, P<0.05), and within LCR (r=0.53, P<0.05). HCR also had greater REE relative to body weight than LCR rats (line main effect, P<0.001; Figure 3B), and EX increased REE relative to body weight in both lines (treatment main effect, P=0.005; Figure 2C). Covarying REE for body weight did not change our results. The cage-only (i.e. no running wheel) SPA was greater in HCR than in LCR (dark cycle, line main effect, P<0.001; Figure 2D), with no effect of EX observed on SPA (i.e. non-wheel locomotor activity in home cage) in either line. Wheel running volume was ~5-fold greater in HCR EX rats than LCR EX rats (P<0.001, Figure 2E).
Figure 2. Energy intake, resting energy expenditure, and physical activity.
(A) Cumulative food intake throughout the intervention, (B) averaged food intake, (C) resting energy expenditure (REE), (D) spontaneous physical activity (SPA), and (E) wheel running volume. Values are means ± SE (n=7–8 per group). Line = line main effect, HCR vs. LCR; Treatment = treatment main effect, SED vs. EX; Interaction = line × treatment interaction. * Denotes difference (P<0.05) between SED and EX within line. # P<0.05, significantly different from HCR EX.
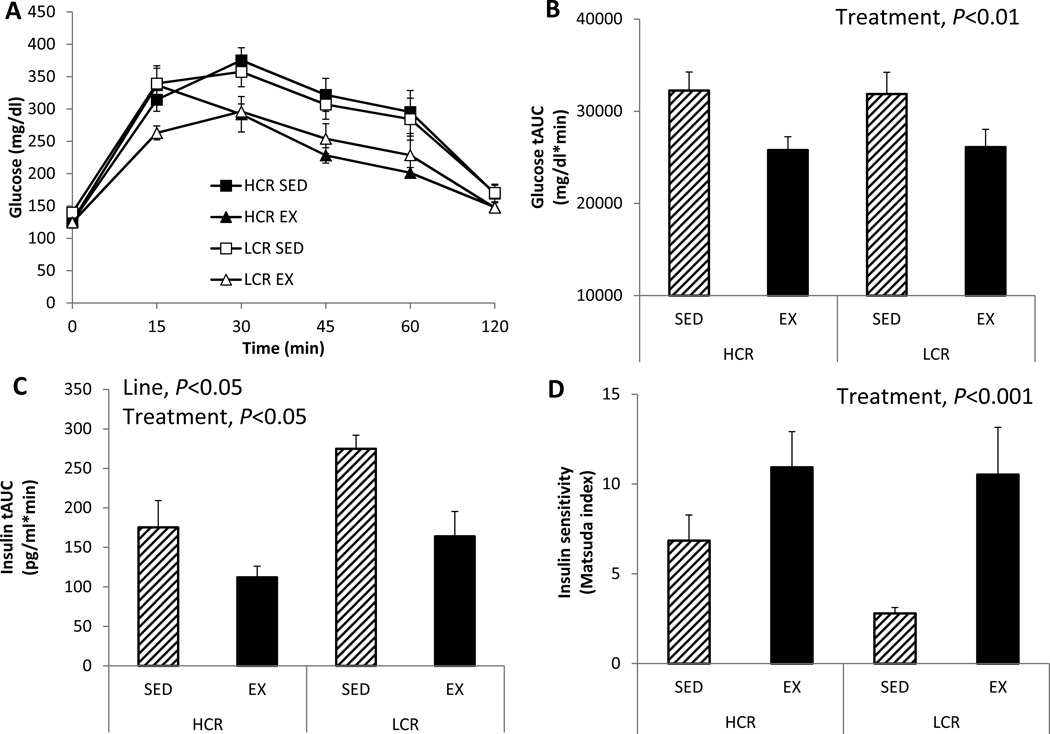
Figure 3. Despite smaller running volume than HCR, EX enhances insulin sensitivity equally in LCR and HCR.
(A) plasma glucose levels during a 2-hour intraperitoneal glucose tolerance test (GTT), (B) glucose tAUC during GTT, (C) insulin tAUC during GTT, and (D) insulin sensitivity assessed by Matsuda index. Values are means ± SE (n=7–8 per group). Line = line main effect, HCR vs. LCR; Treatment = treatment main effect, SED vs. EX.
Insulin sensitivity and systemic metabolic function
Glucose tolerance during the GTT improved following EX in both lines (treatment effect, P=0.017; Figure 3A and B) but was not statistically different between HCR and LCR rats. However, LCR rats demonstrated a hyperinsulinemic response to the glucose challenge compared to HCR (line main effect, P=0.028; Figure 3C), and EX improved the glucose-stimulated insulin response in both lines (treatment main effect, P=0.014; Figure 3C). Similarly, insulin sensitivity as assessed by the Matsuda index was significantly improved by EX in both lines (treatment main effect, P<0.001; Figure 3D) resulting in equivocal insulin sensitivity in both EX groups following the intervention, despite their stark differences in the SED condition. Circulating TG and NEFA levels unexpectedly increased following EX in HCR rats, while EX decreased those blood lipids in LCR rats (TG: HCR +10% vs. LCR −33%; NEFA: HCR +35% vs. LCR −40%; line × treatment interaction, P=0.037 and P=0.022, respectively; Table 1). HCR rats also had lower fasting HDL levels than LCR rats (line main effect, P=0.026), which increased following EX in HCR rats; on the other hand, EX decreased those in LCR rats (line × treatment interaction, P=0.046; Table 1). LCR rats did have higher LDL (line main effect, P<0.001), whereas EX attenuated LDL in both groups (HCR −34%, LCR −11%; treatment main effect, P=0.029; Table 1). Counter to our expectations, EX decreased fasting glucose in LCR rats, but increased fasting glucose in HCR rats (line × treatment interaction, P=0.041; Table 1). Similarly, HOMA- and Adipocyte-IR decreased following EX in LCR but increased following EX in HCR (line × treatment interaction, P=0.041 and P=0.037, respectively; Table 1).
Myokines
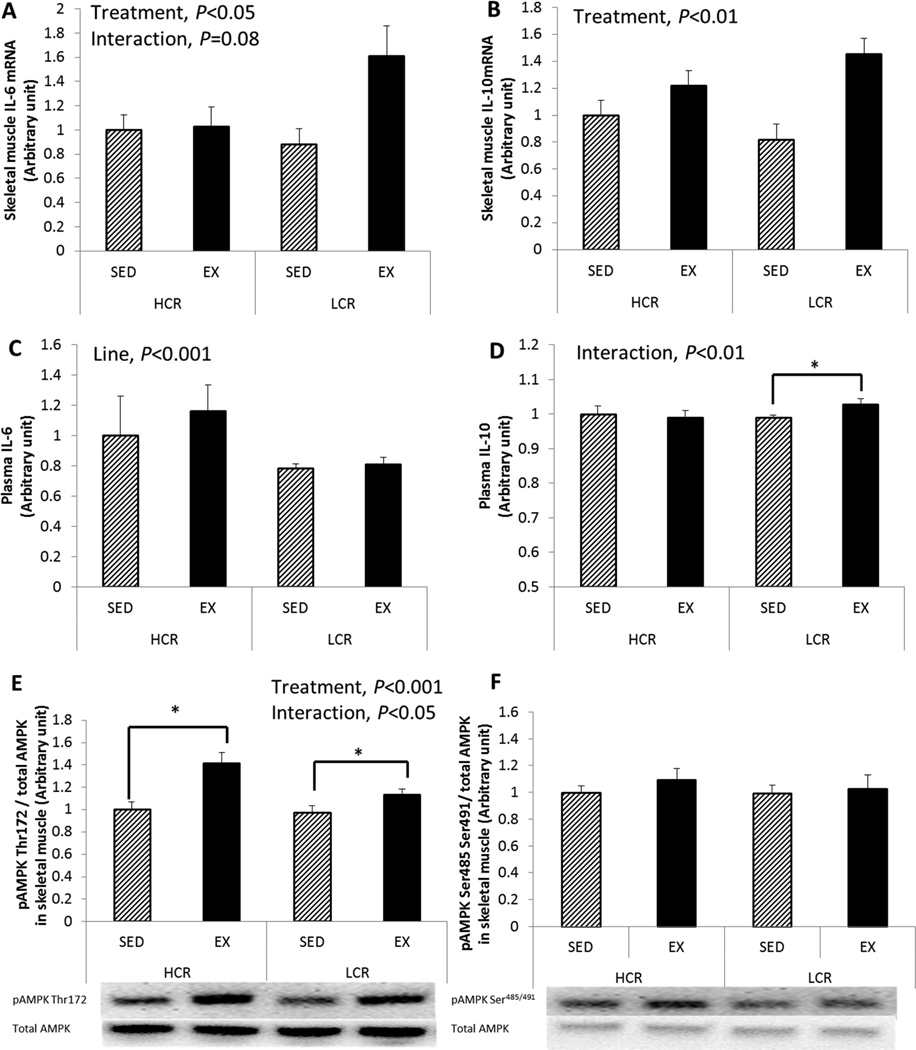
EX increased skeletal muscle IL-6 mRNA in LCR ~2-fold, but in HCR there was only a small change (treatment main effect, P=0.034; line × treatment interaction, trend P=0.080; Figure 4A). IL-10 mRNA expression increased similarly following EX in both lines (treatment main effect, P=0.003; Figure 4B); however, plasma IL-10 concentrations increased following EX only in LCR rats (line × treatment interaction, P=0.003; Figure 4D). No significant changes in plasma IL-6 concentration was found following EX in either line, while HCR had greater circulating levels of IL-6 than LCR (line main effect, P<0.001; Figure 4C). Additional gene expression data are provided in a supplementary figure (see SDC1 which includes additional genes associated with skeletal muscle metabolism).
Figure 4. EX increases IL-6 & 10 mRNA and AMPK activation in both lines.
(A) skeletal muscle IL-6 mRNA, (B) skeletal muscle IL-10 mRNA, (C) plasma IL-6, (D) plasma IL-10, (E) pAMPK Thr172 (activation site) and (F) pAMPK Ser485/491 (inhibition site). Values are normalized by total AMPK; values are means ± SE (n=6–8 per group). Line = line main effect, HCR vs. LCR; Treatment = treatment main effect, SED vs. EX; Interaction = line × treatment interaction. * Denotes difference (P<0.05) between SED and EX within line.
AMPK activity
Thr172 and Ser485/491 phosphorylation sites of the AMPKα subunit have been considered the activation and inhibition sites for AMPK activity, respectively. Both lines showed enhanced pAMPK Thr172 protein expression following EX (treatment main effect, P<0.001; Figure 4E). Specifically, HCR rats showed a greater increase in pAMPK Thr172 protein expression following EX (HCR +41% vs. LCR +17% compared to SED groups, line × treatment interaction, P=0.045). No significant change was found in pAMPK Ser485/491 protein expression between lines or treatments (Figure 4F).
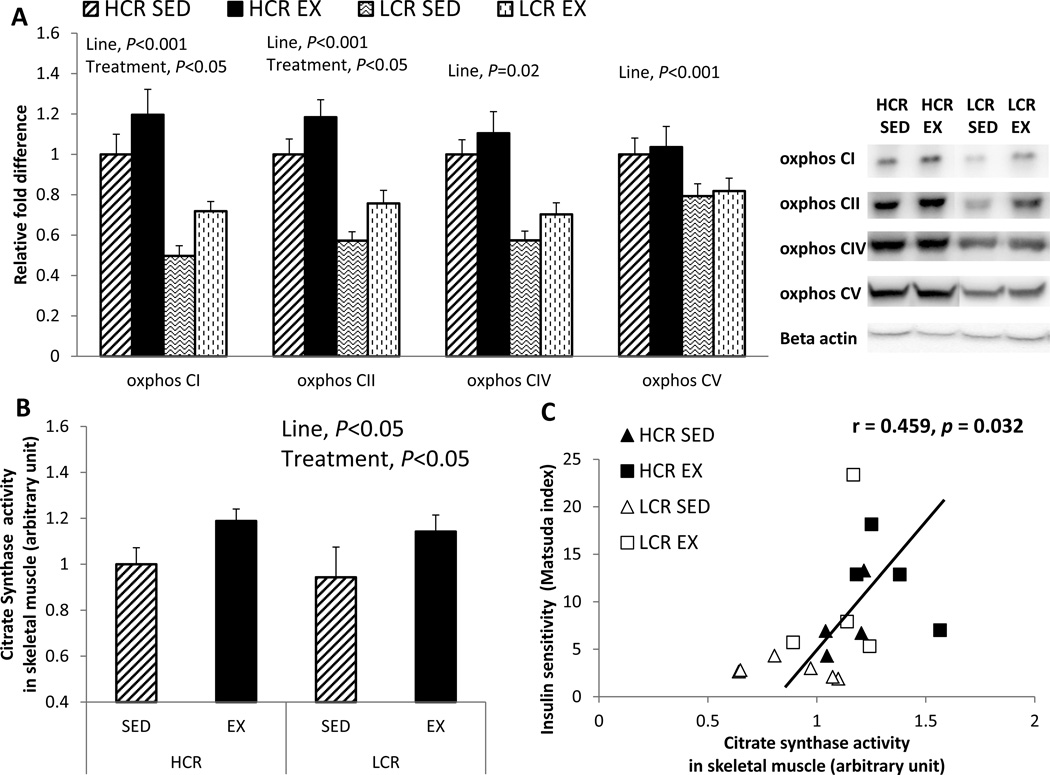
Skeletal muscle mitochondrial function
To assess inherent differences in skeletal muscle proteins associated with mitochondrial function between HCR and LCR rats, we measured protein expression of OX PHOS complex subunits via Western blot and citrate synthase enzyme activity in gastrocnemeous skeletal muscle (Figure 5A and B). HCR rats showed greater OX PHOS protein expression and citrate synthase activity than LCR rats (line main effect, P<0.001 in complex I, II, and IV; and P=0.02 and 0.05 in complex V and citrate synthase activity, respectively). OX PHOS complex I and II proteins, and citrate synthase enzyme activity increased following EX in both lines (treatment main effect, P=0.019, 0.024, and 0.013, respectively). Mitochondrial content (i.e. citrate synthase) correlated significantly with insulin sensitivity assessed by Matsuda index (r=0.459, P=0.032, Figure 5C). Furthermore, mitochondrial electron transport chain (i.e., OX PHOS) complexes I, II, and V protein content were significantly correlated with insulin sensitivity assessed by Matsuda index (complex I: r=0.445, P=0.025; complex II: r=0.625, P=0.004; and complex V: r=0.466, P=0.019), while complex IV protein and glucose transporter (i.e., Glut4) mRNA expression tended to correlate with Matsuda index (complex IV: r=0.344, P=0.069; and Glut4: r=0.349, P=0.066).
Figure 5. EX increases skeletal muscle mitochondrial content in both lines.
(A) oxidative phosphorylation (OX PHOS) complex I, II, IV, and V proteins in skeletal muscle; (B) skeletal muscle citrate synthase (CS) enzyme activity; and (C) association between citrate synthase activity and insulin sensity(i.e. Matsuda index). Values are means ± SE (n=6–8 per group). Line = line main effect, HCR vs. LCR; Treatment = treatment main effect, SED vs. EX.
Discussion
Our group previously demonstrated that a western high fat diet (HFD) aggravates metabolic dysfunction (i.e. further increases adiposity and insulin resistance) in ovariectomized rats bred for low aerobic capacity (i.e., LCR) while ovariectomized rats bred for high aerobic capacity (i.e., HCR) are protected from this HFD-induced metabolic dysfunction (24). The present study determined whether voluntary wheel running exercise (EX) is sufficient to mitigate those HFD-associated metabolic consequences in ovariectomized LCR rats. We demonstrate an unexpected divergent response to EX between ovariectomized LCR and HCR rats fed HFD such that LCR display an expected reduction in adiposity, while HCR actually gain fat mass despite running ~5-times the distance covered by LCR. Moreover, these vastly different running volumes were equally effective in enhancing insulin sensitivity, resting energy expenditure, and skeletal muscle mitochondrial content and AMPK activation in LCR and HCR rats following ovariectomy and HFD consumption.
Ovariectomized rodents increase adiposity and develop insulin resistance (32); and increased dietary fat consumption further increases susceptibility to this metabolic dysfunction (44). We recently demonstrated that LCR rats are more susceptible to ovariectomy-associated metabolic dysfunction (25, 40) and the combination of ovariectomy plus HFD (24) compared to HCR. EX is a highly effective treatment to reverse metabolic dysfunction including insulin resistance via enhancing aerobic capacity (6, 17). Some studies (15, 36) have demonstrated that forced treadmill running improves metabolic dysfunction associated with low aerobic capacity, but that modality may not be translatable to postmenopausal women, who may be physically incapable of performing the equivalent duration and/or intensity of exercise. As such, no previous rodent studies have investigated how voluntary wheel running exercise (i.e., presumably, a lower dose and/or intensity) may offset metabolic dysfunction associated with ovariectomy in the setting of low aerobic capacity. In the current study, this voluntary wheel running modality (i.e., EX) significantly reduced adiposity by a remarkable 50% in LCR rats. Unexpectedly, EX actually increased adiposity by 37% in the setting of high aerobic capacity (i.e., HCR rats). However, despite this counterintuitive body composition response in HCR rats, they maintained metabolic health as indicated by insulin sensitivity. In fact, despite their starkly different training volumes and divergent body composition responses, both HCR and LCR rats exhibited a similar EX-mediated improvement in insulin sensitivity. The EX-induced increase in adiposity in HCR was likely attributed to an ‘overcompensation’ in energy intake (Figure 2A and B). Our results were somewhat similar to those generated by Lessard et al. (15) who, also using the HCR/LCR model, reported that exercise training reversed low aerobic capacity-associated impairments in whole body metabolic markers and glucose/lipid metabolism, while no improvements were found following exercise training in HCR. Unfortunately, that study did not report food intake data and how food intake was changed following EX in HCR. Although the present study cannot explain the underlying mechanism behind the overcompensation in energy intake in response to EX in the ovariectomized HCR rats, we recently found that HCR and LCR female rats differ in gene expression patterns in the nucleus accumbens brain region (23), which is the region associated with behaviors regulating energy intake and expenditure. Interestingly, HCR rats had significantly greater excitatory dopamine receptor (i.e., Drd1) expression (23), which was recently shown to drive increases in both locomotor and food intake (45). This interesting behavioral interaction observed in the present study certainly warrants future investigation.
Previous work demonstrated that even low-intensity and low-dose exercise enhances insulin sensitivity and decreases circulating lipids in sedentary, insulin resistant individuals (8, 39). Similarly, the present study demonstrates, despite running 5-fold less than HCR, LCR rats showed improvements in glucose tolerance and insulin sensitivity (measured by the Matsuda index) similar to the HCR rats. Specifically, insulin sensitivity was improved to a greater extent in LCR rats (HCR +60% vs. LCR +275%). An explanation may be that the metabolically dysfunctional LCR rats have greater potential for EX-mediated improvements compared to HCR. A human study (1) supports this hypothesis such that individuals genetically predisposed to metabolic disorders may possess greater responsiveness to exercise-associated improvements in metabolic function including insulin sensitivity. Another animal study using the HCR/LCR model also demonstrated that 6 weeks of low-intensity forced treadmill exercise training reversed low aerobic fitness-associated impairments in insulin sensitivity and skeletal muscle lipid oxidation while exercise had no such positive effect in HCR (15). In other words, since HCR rats are already lean and healthy, LCR rats exhibit greater responsiveness to exercise-related improvements in metabolic function. On the other hand, the fact that the HCR showed a similar EX-induced improvement in insulin sensitivity to the LCR rats despite 5-fold greater voluntary running activity is likely attributed to their energy overcompensation. In the absence of this energy overcompensation and increased adiposity, HCR rats probably would have exhibited greater beneficial effects on those metabolic markers in response to the high dose of exercise training relative to LCR rats.
Increased physical activity or exercise training increases skeletal muscle AMPK activation (33), which has been believed a major mediator for many cellular pathways including insulin signaling and mitochondrial biogenesis. As expected, skeletal muscle AMPK activation increased following EX in both HCR and LCR rats. HCR rats responded to EX with a greater increase in activation site of pAMPK, likely due to the fact that wheel running volume was 5-fold greater in HCR than LCR rats. Another study (22) demonstrated that treadmill-trained rats at 1.2 km/h displayed greater AMPK phosphorylation compared to rats trained at 0.8 km/h suggesting that the difference between the HCR and LCR rats studied here may have been attributed to a higher exercise intensity in HCR. Unfortunately, exercise intensity was not monitored in the present study; future studies should address this question of whether different exercise intensities determine degree of AMPK activation. One study proposed that increased calorie intake (e.g., HFD-induced hyperphagia) may attenuate AMPK activation in skeletal muscle, thus contributing to systemic insulin resistance (16). Our evidence in HCR rats, of significant hyperphagia combined with high exercise volume, may suggest that exercise is a more powerful stimulus of AMPK activation than hyperphagia.
Considerable evidence suggests that low aerobic capacity and diminished mitochondrial function result in lipid accumulation, skeletal muscle insulin resistance, and eventually, greater susceptibility to the metabolic syndrome (31). Indeed, skeletal muscle mitochondrial markers, including PGC-1α and protein expression of oxidative phosphorylation complexes, were previously found to be greater in HCR rats compared to LCR rats (40). Those markers did not, however, appear to be affected by ovariectomy, arguing against the hypothesis that protection observed in HCR against OVX-mediated metabolic dysfunction is due to greater skeletal muscle mitochondrial biogenesis and content (40). However, we have also observed a significant attenuation in those same mitochondrial markers following HFD in ovariectomized LCR and HCR rats (24) suggesting that HFD, more than ovariectomy, may impair skeletal muscle mitochondrial function. Importantly, EX rescued HFD-associated impairment in mitochondrial markers in both HCR and LCR rats; and increased mitochondrial content (i.e. citrate synthase and OX PHOS complex I, II, and V) was associated with enhanced insulin sensitivity. Some previous studies (9, 15) showed no relationship between mitochondrial markers and insulin sensitivity in terms of protection against metabolic dysfunction. In another study of LCR rats (15), enhanced insulin sensitivity following exercise was not associated with increased mitochondrial markers including citrate synthase and β-HAD, but did increase protein content of Glut4, a key regulator of carbohydrate and lipid metabolism. Helge et al. (9) also demonstrated that low-intensity exercise enhanced Glut4 expression in the absence of increased mitochondrial biogenesis markers and enzymes. The present study suggests that EX enhanced mitochondrial content (i.e. citrate synthase activity and protein expression of OX PHOS complexes) along with increased Glut4 gene expression (see Supplemental Figure 1, SDC1, which provides additional skeletal muscle gene expression data) in both LCR and HCR rats. In addition to a significant correlation between mitochondrial content (i.e. citrate synthase and OX PHOS complex I, II, and V) and calculated insulin sensitivity, we also found that this Glut4 gene expression tended to correlate with insulin sensitivity. This difference in EX-induced changes in mitochondrial markers between the present and previous studies (9, 15) may be explained by the fact that the present study investigated the ability of EX to rescue metabolic disturbances associated with both ovariectomy and HFD, while the previous studies were conducted under standard metabolic conditions. However, it should be noted that our study did not assess mitochondrial function directly by assessing respiration ex vivo; thus more sophisticated mitochondrial analyses should be performed in the future to more accurately address the question of whether exercise-mediated improvements in mitochondrial function and/or content contribute to exercise-mediated improvements in insulin sensitivity. Additionally, we only assessed an association between mitochondrial markers and insulin sensitivity; this analysis cannot imply causation. Thus, future studies should determine the extent to which HFD or ovariectomy alters the effect of EX on mitochondrial content/function, glucose transport, and insulin sensitivity.
During acute exercise, skeletal muscle-derived cytokines (i.e. myokines) including IL-6 and IL-10 are released into the circulation (28). Petersen et al. (28) proposed that these myokines act locally to facilitate insulin action, glucose uptake, and fat oxidation via activation of AMPK and PI3 kinase, and also peripherally to enhance lipolysis in adipose tissue. In fact, IL-6 deficient mice develop insulin resistance and obesity (41), effects that are recovered by IL-6 administration. During exercise, IL-6 increases in circulation; this has been reported to be followed by increased circulating IL-10 and IL-1 receptor antagonist, which are anti-inflammatory cytokines (26). We wanted to determine the role of these myokines on metabolic dysfunction in the condition of HFD and ovariectomy. Enhanced insulin sensitivity following EX paralleled increased gene expression of skeletal muscle IL-6 and IL-10 both in LCR and HCR rats. Interestingly, despite running 5-fold less than HCR, LCR experienced a significant 2-fold increase IL-6 gene expression following EX, whereas the increase in IL-6 expression following EX in HCR was only modest; a similar line difference was noted in the EX-mediated increase in IL-10. The underlying mechanism by which LCR showed greater myokine responses to EX compared to HCR is not known and should be addressed in future studies.
The relationship between skeletal muscle gene expression and circulating myokines is not clear. A limitation of the present study is that the running wheels were locked ~5 hours prior to sacrifice to prevent any acute effect of training; this may have resulted in discrepancy between skeletal muscle gene expression and circulating IL-6 and IL-10 levels. That is, it is possible that circulating cytokine levels declined while wheels were locked. Pedersen et al. (27) showed that circulating IL-6 levels peak immediately following exercise and rapidly decreases to baseline levels. It is interesting that, in the present study, skeletal muscle gene expression of IL-6 and IL-10 paralleled the noted enhancements in insulin sensitivity and AMPK activation.
Findings of this current study should be interpreted in light of its limitations. One such limitation is in the level of stringency with respect to the molecular analyses. While the gene expression data were generated from RNA harvested from tissues that were frozen immediately following animal sacrifice in order to preserve optimal integrity and quality, it is possible that RNA integrity was not optimal in some cases. That is because, although we used a subset of mRNA samples to determine quality assurance for mRNA expression via RNA quality number (RQN) assessment, not every sample was tested in this way. Importantly, each of the randomly-selected mRNA samples generated optimal RQN levels. Furthermore, the current study did not determine the linear dynamic range for each gene/protein assessed along with the respective control for genes/proteins. Thus, the above should be viewed as methodological limitations.
In summary, voluntary wheel running exercise (i.e., EX) rescued HFD-induced insulin resistance and adiposity in the ovariectomized LCR rat model, an established animal model of postmenopausal metabolic dysfunction (10, 40). Especially since hormone replacement therapy has been shown to adversely influence cardiovascular disease in some women (2) and is controversial for a variety of other reasons, alternative strategies such as exercise training have been emphasized to attenuate the metabolic dysfunction that occurs post-menopause. Currently unknown is whether high volume or intensity of exercise training is necessary to offset post-menopausal metabolic symptoms. Among the most important findings of the present study is that, despite running only a fraction of the distance covered by high-fit HCR rats, low-fit LCR rats experienced comparable improvements in glucose tolerance, insulin sensitivity, resting energy expenditure, and skeletal muscle mitochondrial content compared to HCR rats. These findings highlight the importance of exercise training in mitigating post-menopausal metabolic dysfunction and suggest that the amount of exercise required may depend upon the fitness status of the individual. That is, for low-fit (e.g., previously sedentary) postmenopausal women, modest levels of voluntary exercise may effectively mitigate metabolic dysfunction.
Supplementary Material
Acknowledgments
This research was supported by MU Research Council grant (VVP), NIH R01DK088940 (JPT), NIH P40OD021331 (LGK and SLB), and NIH K01HL125503 (JP). We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp. Contact LGK lgkoch@umich.edu or SLB brittons@umich.edu for information on the LCR and HCR rats: these rat models are maintained as an international resource with support from the Department of Anesthesiology at the University of Michigan, Ann Arbor, Michigan.
Footnotes
Conflict of Interest: None. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Supplemental Digital Content
Supp Fig 1 Park MSSE 9 2016.pdf—gene expression in skeletal muscle
References
- 1.Barwell ND, Malkova D, Moran CN, et al. Exercise training has greater effects on insulin sensitivity in daughters of patients with type 2 diabetes than in women with no family history of diabetes. Diabetologia. 2008;51(10):1912–1919. doi: 10.1007/s00125-008-1097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassuk SS, Manson JE. Menopausal hormone therapy and cardiovascular disease risk: utility of biomarkers and clinical factors for risk stratification. Clinical chemistry. 2014;60(1):68–77. doi: 10.1373/clinchem.2013.202556. [DOI] [PubMed] [Google Scholar]
- 3.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes care. 2004;27(1):83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Earnest CP, Johannsen NM, Swift DL, Lavie CJ, Blair SN, Church TS. Dose effect of cardiorespiratory exercise on metabolic syndrome in postmenopausal women. Am J Cardiol. 2013;111(12):1805–1811. doi: 10.1016/j.amjcard.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher JA, Meers GM, Linden MA, et al. Impact of various exercise modalities on hepatic mitochondrial function. Medicine and science in sports and exercise. 2014;46(6):1089–1097. doi: 10.1249/MSS.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodyear LJ. The exercise pill--too good to be true? The New England journal of medicine. 2008;359(17):1842–1844. doi: 10.1056/NEJMcibr0806723. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Barlow CE, Farrell SW, Vega GL, Haskell WL. Cardiorespiratory fitness and metabolic risk. The American journal of cardiology. 2012;109(7):988–993. doi: 10.1016/j.amjcard.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Hansen D, Dendale P, Jonkers RA, et al. Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA(1c) in obese type 2 diabetes patients. Diabetologia. 2009;52(9):1789–1797. doi: 10.1007/s00125-009-1354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helge JW, Overgaard K, Damsgaard R, et al. Repeated prolonged whole-body low-intensity exercise: effects on insulin sensitivity and limb muscle adaptations. Metabolism: clinical and experimental. 2006;55(2):217–223. doi: 10.1016/j.metabol.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Kim C. Does menopause increase diabetes risk? Strategies for diabetes prevention in midlife women. Womens Health (Lond Engl) 2012;8(2):155–167. doi: 10.2217/whe.11.95. [DOI] [PubMed] [Google Scholar]
- 11.Koch LG, Britton SL, Wisloff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends in cardiovascular medicine. 2012;22(2):29–34. doi: 10.1016/j.tcm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA : the journal of the American Medical Association. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 13.Laye MJ, Rector RS, Warner SO, et al. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. The Journal of physiology. 2009;587(Pt 14):3729–3739. doi: 10.1113/jphysiol.2009.172601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lessard SJ, Rivas DA, Chen ZP, et al. Impaired skeletal muscle beta-adrenergic activation and lipolysis are associated with whole-body insulin resistance in rats bred for low intrinsic exercise capacity. Endocrinology. 2009;150(11):4883–4891. doi: 10.1210/en.2009-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessard SJ, Rivas DA, Stephenson EJ, et al. Exercise training reverses impaired skeletal muscle metabolism induced by artificial selection for low aerobic capacity. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300(1):R175–R182. doi: 10.1152/ajpregu.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindholm CR, Ertel RL, Bauwens JD, Schmuck EG, Mulligan JD, Saupe KW. A high-fat diet decreases AMPK activity in multiple tissues in the absence of hyperglycemia or systemic inflammation in rats. Journal of physiology and biochemistry. 2013;69(2):165–175. doi: 10.1007/s13105-012-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. The New England journal of medicine. 2002;347(10):716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Naples SP, Borengasser SJ, Rector RS, et al. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2010;35(2):151–162. doi: 10.1139/h09-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noland RC, Thyfault JP, Henes ST, et al. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;293(1):E31–E41. doi: 10.1152/ajpendo.00500.2006. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira NR, Marques SO, Luciano TF, et al. Treadmill training increases SIRT-1 and PGC-1 alpha protein levels and AMPK phosphorylation in quadriceps of middle-aged rats in an intensity-dependent manner. Mediators of inflammation. 2014;2014:987017. doi: 10.1155/2014/987017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Park YM, Kanaley JA, Padilla J, et al. Effects of intrinsic aerobic capacity and ovariectomy on voluntary wheel running and nucleus accumbens dopamine receptor gene expression. Physiology & behavior. 2016;164(Pt A):383–389. doi: 10.1016/j.physbeh.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park YM, Kanaley JA, Zidon TM, et al. Ovariectomized High Fit Rats Are Protected against Diet-Induced Insulin Resistance. Medicine and science in sports and exercise. 2016;48(7):1259–1269. doi: 10.1249/MSS.0000000000000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park YM, Rector RS, Thyfault JP, et al. Effects of ovariectomy and intrinsic aerobic capacity on tissue-specific insulin sensitivity. American journal of physiology. Endocrinology and metabolism. 2016;310(3):E190–E199. doi: 10.1152/ajpendo.00434.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen BK. The diseasome of physical inactivity--and the role of myokines in muscle--fat cross talk. The Journal of physiology. 2009;587(Pt 23):5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological reviews. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 28.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 29.Rector RS, Thyfault JP, Morris RT, et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. American journal of physiology. Gastrointestinal and liver physiology. 2008;294(3):G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 30.Rector RS, Uptergrove GM, Borengasser SJ, et al. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. American journal of physiology. Endocrinology and metabolism. 2010;298(6):E1179–E1187. doi: 10.1152/ajpendo.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54(1):8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 32.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. The Journal of clinical investigation. 2013;123(7):2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seifert EL, Bastianelli M, Aguer C, et al. Intrinsic aerobic capacity correlates with greater inherent mitochondrial oxidative and H2O2 emission capacities without major shifts in myosin heavy chain isoform. J Appl Physiol (1985) 2012;113(10):1624–1634. doi: 10.1152/japplphysiol.01475.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srere PA. Citrate synthase. Methods in Enzymology. 1969;13:3–11. [Google Scholar]
- 36.Stephenson EJ, Lessard SJ, Rivas DA, et al. Exercise training enhances white adipose tissue metabolism in rats selectively bred for low- or high-endurance running capacity. Am J Physiol Endocrinol Metab. 2013;305(3):E429–E438. doi: 10.1152/ajpendo.00544.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thyfault JP, Cree MG, Zheng D, et al. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. American journal of physiology. Cell physiology. 2007;292(2):C729–C739. doi: 10.1152/ajpcell.00311.2006. [DOI] [PubMed] [Google Scholar]
- 38.Thyfault JP, Rector RS, Uptergrove GM, et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587(Pt 8):1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venables MC, Jeukendrup AE. Endurance training and obesity: effect on substrate metabolism and insulin sensitivity. Medicine and science in sports and exercise. 2008;40(3):495–502. doi: 10.1249/MSS.0b013e31815f256f. [DOI] [PubMed] [Google Scholar]
- 40.Vieira-Potter VJ, Padilla J, Park YM, et al. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. American journal of physiology. Regulatory, integrative and comparative physiology. 2015;308(6):R530–R542. doi: 10.1152/ajpregu.00401.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallenius V, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nature medicine. 2002;8(1):75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 42.Walton C, Godsland IF, Proudler AJ, Wynn V, Stevenson JC. The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. European journal of clinical investigation. 1993;23(8):466–473. doi: 10.1111/j.1365-2362.1993.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 43.Wisloff U, Najjar SM, Ellingsen O, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307(5708):418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 44.Yonezawa R, Wada T, Matsumoto N, et al. Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. American journal of physiology. Endocrinology and metabolism. 2012;303(4):E445–E456. doi: 10.1152/ajpendo.00638.2011. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Ottenheimer D, DiLeone RJ. Activity of D1/2 Receptor Expressing Neurons in the Nucleus Accumbens Regulates Running, Locomotion, and Food Intake. Frontiers in behavioral neuroscience. 2016;10:66. doi: 10.3389/fnbeh.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.