Abstract
Background
The impact of temporal changes in cancer therapy on health status among childhood cancer survivors has not been evaluated.
Objective
Compare proportions of self-report of adverse health status outcomes across three decades among childhood cancer survivors.
Design
Cross-sectional
Setting
27 North American institutions
Participants
14,566 adults, ≥5 year survivors (median age 27, range 18-48 years) treated 1970-1999
Measurements
Patient-report of poor general or mental health, functional impairment, activity limitation, cancer-related anxiety or pain were evaluated as a function of treatment decade, cancer treatment exposures, chronic health conditions, demographics, and health habits.
Results
Despite reductions in late-mortality and proportions with severe, disabling or life threatening chronic health conditions (33.4% among survivors treated 1970-79, 21.0% among those treated 1990-99), proportions reporting adverse health status did not decrease by treatment decade. When compared to those diagnosed 1970-79, survivors diagnosed 1990-99 were more likely to report poor general health (11.2% vs. 13.7%, p < 0.001) and cancer-related anxiety (13.3% vs. 15.0%, p < 0.001). From 1970-79 to 1990-99, the proportions reporting adverse outcomes were higher (p < 0.001) among leukemia (9.5% vs. 13.9%, poor general health) and osteosarcoma (23.9% vs. 36.6%, pain) survivors. Temporal changes in treatment exposures were not associated with changes in proportions reporting adverse health status. However, smoking, not meeting physical activity guidelines, and being either underweight or obese were associated with poor health status.
Limitations
The considerable improvement in survival among children diagnosed with cancer in the 1990s compared to those diagnosed in the 1970s makes it difficult to definitively determine the impact of risk factors on later self-reported health status without considering their impact on mortality itself.
Conclusions
Because survival rates following a diagnosis of childhood cancer have improved substantially over the past thirty years, this population now includes persons who would have died in earlier eras. Unfortunately, self-reported health status among those that do survive has not improved. This is despite evolution of treatment designed to reduce toxicities, and is an important reminder that even in the modern era, cancer cure is not without consequences.”
Introduction
Progress in treatment and supportive care for children with cancer has improved survival resulting in increasing numbers of childhood cancer survivors (1, 2). Unfortunately, a substantial number of survivors have chronic medical conditions resulting from cancer and/or therapy that alter health status and interfere with daily life (3-6). Ten to 25% of survivors of childhood cancer in their twenties and thirties report adverse health status including poor general health, poor mental health, functional impairment, activity limitation, cancer-related pain and cancer-related anxiety (4, 7-9). Of concern, these estimates are not static; longitudinal data indicate increased risk for adverse health status outcomes as survivors age (9, 10).
Since adverse health status outcomes are associated with specific treatment exposures, data describing risk of late effects have been used to modify clinical trials for newly diagnosed patients with the goals of maximizing survival, decreasing late effects of cancer therapy and improving overall health status (11-14). The Childhood Cancer Survivor Study (CCSS), a multi-institutional cohort study of childhood cancer survivors, has provided a wealth of information on prevalence and predictors of adverse health status that directly apply to survivors treated in North America between 1970 and 1986 (15, 16). Analysis of the recently expanded CCSS cohort to include individuals diagnosed between 1987 and 1999 demonstrates that survivors treated in more recent decades have reduced risk for late mortality (17), due in part to reduction in therapeutic exposures. However, the proportions reporting adverse health status among survivors treated with more contemporary therapy is not known.
Given this extension in lifespan for more contemporary survivors, the aims of this analysis were to: 1) determine the proportions of adult survivors of childhood cancer reporting adverse health status outcomes by treatment decade (1970-79, 1980-89, 1990-99) compared to rates in siblings; and 2) evaluate associations between diagnosis, temporal changes in treatment, chronic health conditions, demographic characteristics and health habits on adverse health status as a function of treatment decade. We hypothesized that survivors from the 1990s, treated with contemporary therapy, would report better health status compared to survivors from the 1970s.
Methods
These analyses included childhood cancer survivors participating in the CCSS treated from 1970-1999 at 27 institutions in North America. Survivors eligible for these analyses were diagnosed when < 21 years and had survived ≥ 5 years from original diagnosis. Analyses were restricted to survivors who completed a baseline questionnaire when ≥ 18 years and consented to medical record abstraction, and siblings ≥ 18 years when they completed the baseline questionnaire (see Supplemental Figure 1) (15, 16). Study documents were approved by institutional review boards at each site; informed consent was obtained from participants. Data for these analyses were restricted to participants’ responses on the first (“baseline”) questionnaire.
Health status
Adverse health status was determined using established definitions in four domains for survivors and siblings and two additional domains for survivors (7). Participants were considered to have poor general health if they answered poor or fair when asked “Would you say that your health is excellent, very good, good, fair or poor?”, and poor mental health if responses on the Brief Symptom Inventory-18 (BSI-18) (18) resulted in a sex-specific T-score of 63 or higher on the Global Severity Index or depression, anxiety, or somatization subscales. Functional impairments were considered present among participants reporting needing help with personal care, routine needs, or difficulty attending school or work. Activity limitations were considered present among participants reporting limitations in moderate activities (e.g. walking one block) in ≥3 months of the past two years. Survivors also rated pain related to cancer or treatment (adverse status defined as medium, a lot or very bad, excruciating pain) and fears/anxiety related to cancer or treatment (adverse status defined as medium, a lot or very many, extreme fears/anxiety).
Chronic conditions
Chronic health conditions were categorized using the Common Terminology for Adverse Events version 4.03 as none (Grade 0), mild/asymptomatic (Grade 1), or moderate/minimal local or non-invasive intervention indicated (Grade 2), versus severe, medically significant, disabling (Grade 3), and life-threatening (Grade 4) (19).
Decade, diagnosis, cancer therapy
Information related to diagnosis and treatment modalities/doses was obtained from medical records. Treatment decade (1970-79, 1980-89, 1990-99) was assigned based on diagnosis date. Time from diagnosis to survey completion was treated as a continuous variable. Diagnosis-specific (ALL, astrocytoma, medulloblastoma, HL, NHL, neuroblastoma, Wilms tumor, rhabdomyosarcoma, Ewing sarcoma and osteosarcoma) surgery, radiation and chemotherapy variables were selected for evaluation (Supplemental Table 1) based upon changes in treatment standards over time (20, 21), or because of previously established associations with adverse health status outcomes (7, 10, 22). To illustrate treatment changes over time, a variable, “treatment score”, was calculated using multivariable piecewise exponential models to estimate risk of any chronic condition ≥grade 3, with an offset of the logarithm of person years at risk, terminated at the onset of any chronic condition ≥grade 3 or censoring, and diagnosis-specific treatment exposures as risk factors (Supplemental Table 1). Specifically, this value is the standardized logarithm (those from 1970-1979 had mean 0.0, standard deviation 1) of fitted risk of having any ≥grade 3 chronic health condition (Supplemental Table 2), based on diagnosis-specific treatment exposures. Therefore, survivors with greater risk of ≥grade 3 chronic conditions have a higher treatment score (17).
Demographic characteristics, health habits
Attained age, sex, race/ethnicity, smoking history, alcohol-intake history, body mass index (BMI) and physical activity levels were determined from questionnaire responses. Age was treated as a continuous variable and race/ethnicity as a dichotomy (“non-Hispanic white”; “other”). Smoking status (>100 cigarettes) was categorized as never, past or current (23). Heavy drinking was defined as > 4 drinks/day or >14 drinks/week for males and >3 drinks/day or >7 drinks/week for females (24). BMI was categorized as underweight (<18.5 kilograms per square meter (kg/m2)), normal (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2) or obese (≥30 kg/m2) (25). Inactivity was defined as <the equivalent of 150 minutes of moderate/75 minutes of vigorous weekly physical activity (26).
Statistics
Descriptive statistics were compared between survivors and siblings and across decades with Chi-square or t-tests. Proportions reporting adverse health status were compared between siblings and survivors by treatment decade in generalized linear regression models adjusted for attained age, race and sex. Among survivors, associations between decade of treatment and adverse health status outcomes were examined within diagnosis-specific groups by contrasting relative risks (RR) from three multivariable models for each outcome, adjusted for attained age, time since diagnosis, race and sex. The first models evaluated associations between treatment decade (decade) and adverse health status. The second (decade-treatment) models were additionally adjusted for treatment variables (Supplemental Table 1), and the third (decade-chronic condition) for any grade 3-4 chronic health condition. We hypothesized that attenuation of RR from the decade-only to the decade-treatment model would suggest that treatment exposures explain, at least partially, associations between treatment decade and adverse health status. Similarly, attenuation of RR from the decade-only to the decade-chronic condition model would indicate that chronic health conditions explain, at least partially, associations between treatment decade and adverse health status. Regression was also used to evaluate associations between treatment decade, demographic characteristics and health habits with health status outcomes among survivors. Because of the availability of large numbers of ALL survivors, to reduce study costs, while still preserving statistical power, and to assure adequate numbers of less commonly diagnosed childhood cancer types, ALL survivors diagnosed 1987-1999 were under sampled. Sampling weights were applied in all modeling. SAS 9.3 (Cary, N.C.) was used for analyses.
Role of Funding Source
The funding sources had no role in the design and conduct of the study, collection, management, analysis or interpretation of the data or in preparation, review or approval of the manuscript.
Results
Characteristics of the study population are shown in Table 1. Survivors included 4,618 diagnosed 1970-79; 4,669 diagnosed 1980-89; and 5,279 1990-99. Among those eligible for these analyses, participation rates did not differ by treatment decade: 66% for 1970-79; 61% for 1980-89, and 64% for 1990-99 (p=0.22). Participation differed by diagnosis and was highest among survivors of ALL (73%) and lowest among survivors of neuroblastoma (50%). At the time they responded to the baseline survey, survivors treated 1970-79 were slightly older and further from diagnosis than those treated 1990-99, more likely to report non-Hispanic white race, less likely to be obese or report heavy drinking, and more likely to report current smoking. Siblings were more likely than survivors to be female, have health insurance and report current smoking.
Table 1.
Characteristics of the study population
| Characteristic | Siblings | Survivors | |||
|---|---|---|---|---|---|
| N=3149 | 1970-1979 N=4618 | Survivors 1980-1989 N=4669 | 1990-1999 N=5279 | ||
| Attained age at questionnaire (years) | Mean (SD) | 29.6 (7.3) | 28.5 (6.4) | 26.9 (6.0) | 25.7 (5.8) |
| Median (Range) | 29.0 (18.0, 56.0) | 28.0 (18.0, 47.0) | 26.0 (18.0, 48.2) | 24.9 (18.0, 42.4) | |
| Age at diagnosis (years) | Mean (SD) | 8.7 (5.8) | 10.1 (5.8) | 9.0 (6.1) | |
| Median (Range) | 7.7 (0.0, 21.0) | 10.3 (0.0, 21.0) | 8.5 (0.0, 21.0) | ||
| Survival time (years) | Mean (SD) | 20.3 (3.0) | 17.0 (5.6) | 16.8 (3.5) | |
| Median (Range) | 20.1 (12.9, 30.2) | 15.6 (6.4, 27.3) | 16.9 (8.4, 23.9) | ||
| % | % | %§ | %§ | ||
| Sex | Male | 47.2 | 51.1 | 52.3 | 50.6 |
| Female | 52.8 | 48.9 | 47.7 | 49.4 | |
| Race/ethnicity | Non-Hispanic White | 88.3 | 90.5 | 83.4 | 74.0 |
| Non-Hispanic Black | 2.5 | 2.8 | 4.8 | 8.2 | |
| Hispanic | 3.4 | 4.3 | 7.0 | 11.9 | |
| Asian | 0.9 | 0.6 | 1.7 | 1.9 | |
| Other/Unknown | 4.9 | 1.8 | 3.2 | 4.0 | |
| High school graduate | Yes | 94.1 | 91.7 | 92.7 | 91.5 |
| No | 5.9 | 8.3 | 7.3 | 8.5 | |
| Health insurance | Yes | 89.0 | 84.7 | 83.2 | 79.8 |
| No | 11.0 | 15.3 | 16.8 | 20.2 | |
| Body mass index* | Underweight | 3.6 | 6.3 | 5.4 | 3.8 |
| Normal | 51.4 | 52.1 | 51.0 | 47.2 | |
| Overweight | 29.1 | 27.7 | 27.4 | 27.6 | |
| Obese | 15.9 | 13.9 | 16.2 | 21.3 | |
| Smoking status | Never | 58.3 | 69.9 | 68.2 | 67.6 |
| Former | 17.1 | 11.5 | 14.8 | 16.1 | |
| Current | 24.6 | 18.6 | 16.9 | 16.3 | |
| Heavy drinking† | Yes | 13.3 | 10.1 | 16.2 | 22.5 |
| No | 86.7 | 89.9 | 83.8 | 77.5 | |
| Meets physical activity guidelines‡ | Yes | 40.4 | 34.7 | 42.4 | 46.2 |
| No | 59.6 | 65.3 | 57.6 | 53.8 | |
SD=standard deviation, %=percent, N=number, kg=kilogram, m=meter
Underweight: <18.5 kilograms/square meter (kg/m2), Normal: 18.5-24.9 kg/m2, Overweight: 25-29.9 kg/m2, Obese: ≥30kg/m2;
Consuming > 4 drinks/day or > 14 drinks/week for males and consuming > 3 drinks/day or > 7 drinks/week for females,
≥150 minutes of moderate or 75 minutes of vigorous physical activity per week;
Sampling weights applied
Temporal changes-exposures
Provided in Table 2 (Supplemental Tables 3-12) are decade-specific distributions of survivors by diagnosis and therapeutic exposure (17, 27). The percentage of survivors exposed to cranial radiotherapy (CRT) decreased across time, primarily due to the declining use of prophylactic CRT in dose ranges 1800-2400 centigray (cGy) for ALL. Therefore, those exposed to CRT in the 1990s were primarily those with CNS tumors who received higher mean doses. The percentage of survivors exposed to chest radiation also decreased from the 1970s to the 1990s (17.3% decrease HL, 13.3% decrease neuroblastoma). Use of amputation for local control of osteosarcoma decreased from 80.3% in the 1970s to 22.8% in the 1990s. Over the three decades, the percentage of survivors exposed to nearly every type of chemotherapy agent increased, but with lower cumulative doses.
Table 2.
Cancer diagnosis and treatment changes by decade
| 1970-1979 (N=4618) | 1980-1989 (N=4669) | 1990-1999 (N=5279) | |
|---|---|---|---|
| Parameter | % | %* | %* |
| Leukemia | 32.5 | 36.8 | 41.9 |
| Central Nervous System | 11.5 | 14.2 | 17.8 |
| Hodgkin Lymphoma | 17.8 | 15.6 | 11.3 |
| Non-Hodgkin Lymphoma | 7.5 | 9.8 | 8.3 |
| Wilms Tumor | 8.6 | 4.9 | 6.2 |
| Neuroblastoma | 6.3 | 3.3 | 3.6 |
| Rhabdomyosarcoma | 6.2 | 4.4 | 3.4 |
| Bone tumor | 9.5 | 10.9 | 7.5 |
| Cranial radiation | 37.5 | 29.3 | 20.4 |
| Mean (SD) dose (cGy) | 3053 (1332) | 3083 (1871) | 3310 (2164) |
| Chest radiation | 31.6 | 24.2 | 18.7 |
| Mean (SD) dose (cGy) | 3156 (1087) | 2872 (1216) | 2403 (1150) |
| Abdominal radiation | 31.7 | 19.2 | 15.6 |
| Mean (SD) dose (cGy) | 3017 (988) | 2689 (1185) | 2169 (1194) |
| Pelvic radiation | 25.2 | 15.6 | 13.2 |
| Mean (SD) dose (cGy) | 3057 (1111) | 2838 (1340) | 2297 (1364) |
| Alkylating agent† | 47.9 | 59.6 | 58.1 |
| Mean (SD) (mg/m2) | 10491 (10257) | 8301 (8265) | 8418 (9323) |
| Anthracycline‡ | 31.0 | 55.9 | 66.1 |
| Mean (SD) (mg/m2) | 335 (290) | 248 (163) | 193 (139) |
| High dose methotrexate§ | 5.1 | 13.8 | 21.3 |
| Prednisone | 47.4 | 49.4 | 48.1 |
| Dexamethasone | 3.1 | 13.0 | 25.5 |
| Vincristine | 72.3 | 71.3 | 72.0 |
| Bleomycin | 2.3 | 10.7 | 7.6 |
| Cisplatin | 0.9 | 9.0 | 8.9 |
| Amputation | 35.3 | 26.7 | 12.0 |
| Splenectomy | 74.7 | 57.9 | 9.3 |
| Hematopoietic stem cell transplant | 0.2 | 1.9 | 2.9 |
| Grade 3-4 chronic condition with onset prior to baseline | 33.4 | 25.8 | 21.0 |
Temporal changes-chronic health conditions
A decrease in proportions with severe, medically significant, disabling or life-threatening chronic health conditions is shown in Table 2, with 33.4%, 25.8%, and 21.0% of survivors (mean±SD age 26.1±6.1 years) diagnosed from 1970-79, 1980-89, and 1990-99, respectively, reporting at least one grade 3-4 chronic health condition.
Temporal changes-health status
Despite an overall decrease in radiation exposure, reduced mean chemotherapy doses, and a decline in proportions with grade 3-4 chronic health conditions, in general, patient-reported health status did not improve across treatment decades (Supplemental Table 13, Figure 1). The percentage of survivors reporting poor general health and cancer-related anxiety was highest among survivors treated 1990-99 and lowest in those treated 1970-79 (13.7% vs. 11.2% and 15% vs 13.3% respectively). Patient-reported poor general health, poor mental health, functional impairment, activity limitation or an adverse outcome in any of these four domains did not improve, with survivors more likely than siblings to report adverse health status outcomes in each (or any) domain.
Figure 1.
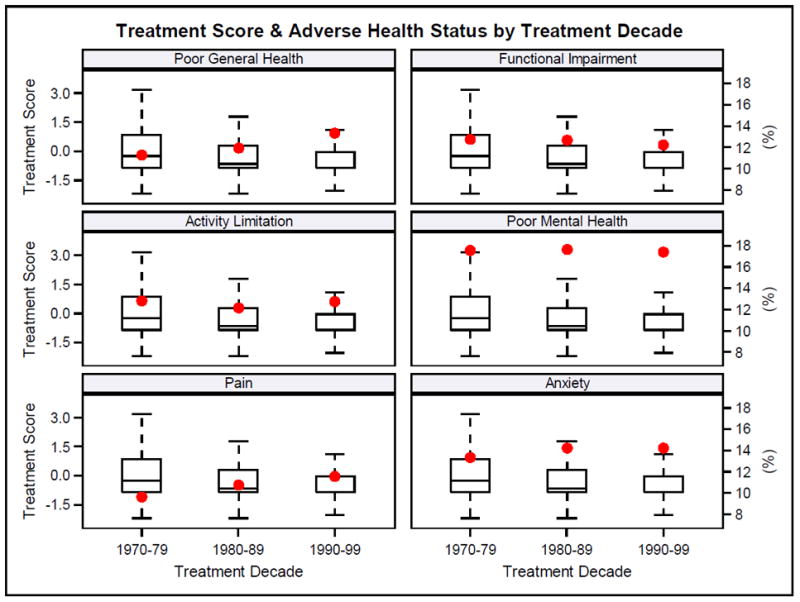
Treatment score (box and whisker plot – left y axis) and percent with adverse health status outcome (large dots – right y axis) by treatment decade.
Treatment decade (1970s, 1980s, 1990s) was associated with changes in proportions reporting adverse health status primarily among ALL, astrocytoma, medulloblastoma, and osteosarcoma survivors (Figure 2, Supplemental Table 14). The percentage of ALL survivors reporting poor general health (9.5%, 9.9%, 13.9%) and cancer-related pain (7.5%, 9.5%, 13.7%) or anxiety (11.3%, 12.2%, 16.1%) increased across treatment decades. After adjusting for treatment exposures, associations between treatment decade and both poor general health and cancer-related pain became non-significant. However, adjusting for presence of any grade 3-4 chronic health condition did not influence these associations. The association between treatment decade and cancer-related anxiety was not attenuated by including treatment exposures or chronic conditions in multivariable models. The percentage of astrocytoma survivors reporting functional impairment (33.4%, 28.9%, 18.1%) and activity limitation (21.1%, 15.4%, 12.5%) decreased across time. Adjusting for treatment exposure or chronic health conditions did not impact associations between treatment decade and functional impairment. However, the association between treatment decade and activity limitation became non-significant when treatment exposures or grade 3-4 chronic health conditions were included in models. The percentage of medulloblastoma survivors reporting functional impairment also decreased over time (45.8%, 28.6%, 26.5%), although treatment decade was not associated with this change. The proportion of osteosarcoma survivors reporting poor general health (12.8%, 9.4%, 16.3%), poor mental health (16.8%, 17.3%, 24.8%) and cancer-related pain (23.9%, 23.4%, 26.6%) increased across time. The effects of treatment decade remained significant for pain, but were attenuated for poor general and mental health when treatment exposures were included in models. Including chronic health conditions in models did not influence associations between treatment decade and poor general health, poor mental health or cancer-related pain.
Figure 2.
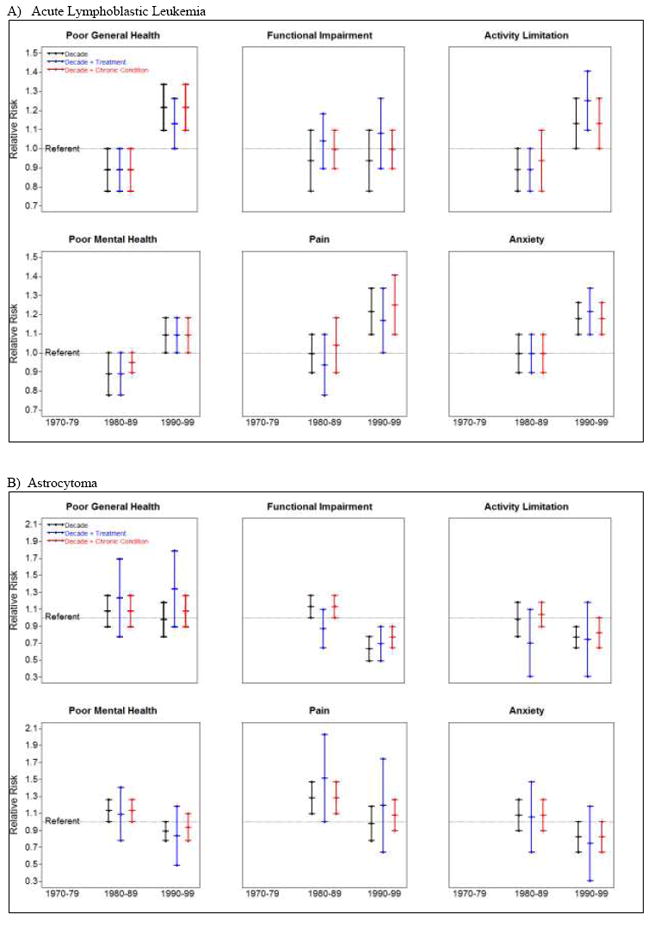
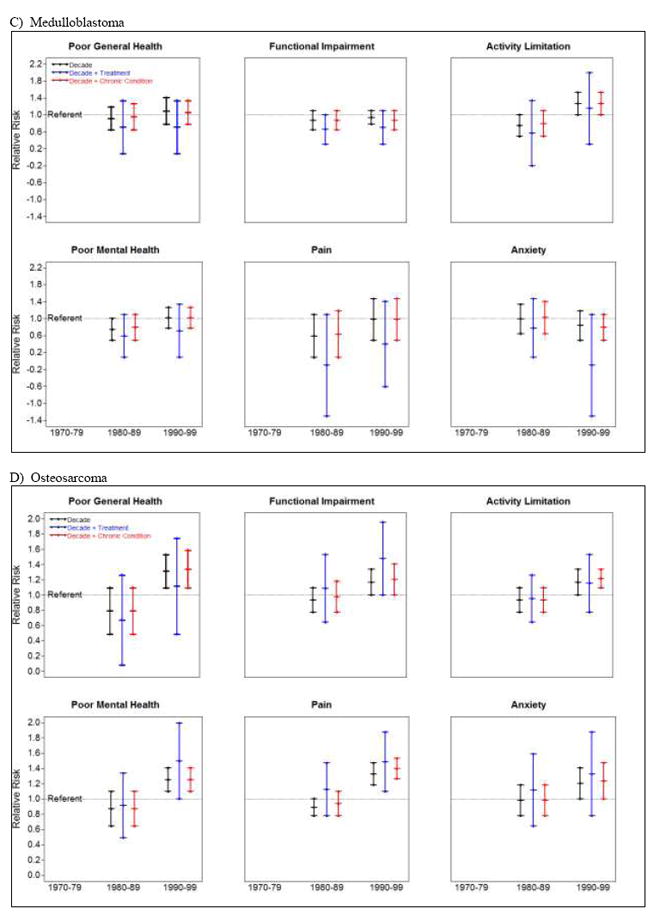
Relative risk for adverse health status by treatment decade, adjusted for treatment and chronic conditions among survivors of A) acute lymphoblastic leukemia, B) astrocytoma, C) medulloblastoma, and D) osteosarcoma.
Table 3 shows relative risks for adverse health status outcomes among survivors by treatment decade, demographic characteristics and health habits. In multivariable models, when compared to those treated 1970-79, those treated 1990-99 had an increased risk for poor general health and anxiety. Female sex, other race, BMI < 18.5 kg/m2 or > 24.9 kg/m2, physical inactivity and smoking were associated with increased risk for poor general health, functional impairment, poor mental health and anxiety. Heavy drinking was inversely associated with functional impairments and activity limitations, but was not associated with chronic disease status (16.6% among those with no vs. 13.1% among those with any grade 3-4 chronic condition).
Table 3.
Relative risk for adverse health status outcomes by treatment decade and by demographic characteristics and health habits among survivors.
| Poor General Health | Functional Impairment | Activity Limitation | Poor Mental Health | Cancer-related Pain | Cancer-related Anxiety | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | RR (95% CI) | % | RR (95% CI) | % | RR (95% CI) | % | RR (95% CI) | % | RR (95% CI) | % | RR (95% CI) | ||
| Treatment decade | 1970-1979 | 4618 | 11.2 | 1.0 | 12.8 | 1.0 | 12.8 | 1.0 | 17.5 | 1.0 | 9.6 | 1.0 | 13.3 | 1.0 |
| 1980-1989 | 4669 | 11.8 | 1.2 (1.0, 1.4) | 12.2 | 1.1 (0.9, 1.2) | 11.6 | 0.9 (0.8, 1.0) | 17.3 | 1.0 (0.9, 1.1) | 10.6 | 1.1 (1.0, 1.3) | 14.0 | 1.0 (0.9, 1.1) | |
| 1990-1999 | 5279 | 13.3 | 1.3 (1.1, 1.5) | 11.3 | 1.0 (0.9, 1.2) | 11.9 | 1.0 (0.9, 1.2) | 17.8 | 1.0 (0.9, 1.1) | 11.3 | 1.3 (1.1, 1.5) | 14.4 | 1.0 (0.9, 1.1) | |
| Sex | Male | 7492 | 11.5 | 1.0 | 11.0 | 1.0 | 9.6 | 1.0 | 16.6 | 1.0 | 9.7 | 1.0 | 11.0 | 1.0 |
| Female | 7074 | 13.0 | 1.3 (1.1, 1.4) | 13.0 | 1.6 (1.4, 1.7) | 14.7 | 1.9 (1.7, 2.1) | 18.6 | 1.1 (1.0, 1.2) | 11.7 | 1.3 (1.2, 1.5) | 17.2 | 1.5 (1.4, 1.7) | |
| Non-Hispanic white | Yes | 12062 | 11.2 | 1.0 | 11.4 | 1.0 | 11.7 | 1.0 | 17.1 | 1.0 | 10.3 | 1.0 | 13.9 | 1.0 |
| No | 2504 | 17.1 | 1.7 (1.5, 1.9) | 14.7 | 1.3 (1.2, 1.5) | 13.8 | 1.3 (1.1, 1.4) | 19.6 | 1.2 (1.1, 1.3) | 12.2 | 1.2 (1.1, 1.4) | 14.6 | 1.1 (1.0, 1.2) | |
| Smoking status | Never | 9936 | 9.7 | 1.0 | 10.8 | 1.0 | 10.9 | 1.0 | 14.2 | 1.0 | 8.5 | 1.0 | 11.9 | 1.0 |
| Former | 2074 | 13.1 | 1.4 (1.2, 1.6) | 11.8 | 1.2 (1.0, 1.4) | 13.8 | 1.2 (1.1, 1.4) | 19.3 | 1.4 (1.2, 1.5) | 12.7 | 1.4 (1.2, 1.6) | 17.5 | 1.4 (1.2, 1.5) | |
| Current | 2472 | 21.9 | 2.8 (2.5, 3.1) | 16.9 | 1.8 (1.6, 2.1) | 14.9 | 1.5 (1.3, 1.7) | 29.7 | 2.1 (1.9, 2.2) | 17.2 | 2.2 (2.0, 2.5) | 19.4 | 1.6 (1.4, 1.7) | |
| Heavy drinking* | No | 12252 | 12.1 | 1.0 | 12.7 | 1.0 | 12.2 | 1.0 | 16.9 | 1.0 | 10.4 | 1.0 | 12.9 | 1.0 |
| Yes | 2311 | 13.2 | 0.9 (0.8, 1.1) | 8.7 | 0.5 (0.4, 0.6) | 11.5 | 0.7 (0.6, 0.8) | 20.7 | 1.1 (1.0, 1.2) | 11.9 | 1.0 (0.8, 1.1) | 19.6 | 1.2 (1.0, 1.3) | |
| Body mass index† | Normal | 7129 | 8.5 | 1.0 | 10.0 | 1.0 | 10.2 | 1.0 | 15.9 | 1.0 | 8.9 | 1.0 | 13.6 | 1.0 |
| Underweight | 758 | 16.3 | 2.0 (1.6, 2.5) | 19.7 | 2.1 (1.7, 2.6) | 19.7 | 2.1 (1.7, 2.5) | 20.4 | 1.3 (1.1, 1.5) | 14.1 | 1.8 (1.4, 2.2) | 14.9 | 1.1 (0.9, 1.3) | |
| Overweight | 3905 | 11.2 | 1.3 (1.2, 1.5) | 10.4 | 1.0 (0.9, 1.2) | 11.3 | 1.1 (1.0, 1.3) | 17.2 | 1.1 (1.0, 1.2) | 10.7 | 1.2 (1.1, 1.4) | 12.8 | 1.0 (0.9, 1.1) | |
| Obese | 2394 | 22.3 | 2.7 (2.4, 3.1) | 17.2 | 1.7 (1.5, 1.9) | 15.9 | 1.5 (1.3, 1.7) | 22.0 | 1.3 (1.2, 1.5) | 14.3 | 1.5 (1.3, 1.8) | 16.6 | 1.2 (1.1, 1.3) | |
| Meets physical activity guidelines‡ | Yes | 5777 | 7.8 | 1.0 | 9.1 | 1.0 | 8.4 | 1.0 | 15.5 | 1.0 | 9.6 | 1.0 | 13.7 | 1.0 |
| No | 8348 | 15.3 | 1.9 (1.7, 2.2) | 13.9 | 1.4 (1.3, 1.6) | 14.7 | 1.7 (1.5, 1.9) | 19.1 | 1.2 (1.1, 1.3) | 11.3 | 1.1 (1.0, 1.2) | 14.2 | 1.0 (0.9, 1.0) | |
N=number, %=percent, RR=relative risk, CI=confidence interval
Regression models used a binomial distribution with a log link. Adjusted for sampling weights, attained age and survival time in years which were not significant.
Consuming > 4 drinks/day or > 14 drinks/week for males and consuming > 3 drinks/day or > 7 drinks/week for females,
Underweight: <18.5 kilograms/square meter (kg/m2), Normal: 18.5-24.9 kg/m2, Overweight: 25-29.9 kg/m2, Obese: ≥30kg/m2.
≥150 minutes of moderate or 75 minutes of vigorous physical activity per week.
Bold text indicates RR estimates with p < 0.05
Conclusions
While contemporary therapy for certain childhood cancers has resulted in reduction of late mortality, extending the lifespan of survivors (17), our findings suggest that there is not concurrent improvement in patient-reported health status among survivors. This is important as perceived health and well-being have been associated with long-term mortality in other adult cohorts (28-30). Overall, we observed increases in the percentages of childhood cancer survivors treated from 1990-99 who reported poor general health and anxiety, and found that accounting for specific treatment exposures, in most instances, did not explain effects of treatment decade on adverse health status outcomes. Demographic characteristics and high-risk health behaviors were associated with an increased risk for adverse health status. Thus, while the results of our analysis do not generally support anticipated benefits to health status expected from reductions in therapy, they do provide potential targets for clinical counseling and intervention among the increasing number of long-term survivors of childhood cancer.
There are several potential explanations for lack of improvement in health status outcomes reported by survivors treated over three decades. First, there has been a substantial increase in survival among children diagnosed with cancer. Estimates indicate that five-year survival rates for children diagnosed with cancer improved from 62.8% in 1975 to 79.8% in 1999 (2). It is possible that those who would have died in the earliest era, but lived in the most recent era, are those that on average, would report worse later health outcomes. It is likely that contemporary cohorts contain a greater proportion of survivors cured of higher risk disease, who would have previously died because of their cancer or treatment complications. Risk-adapted therapy minimized treatment intensity for children with low-risk disease in the 1990s, whereas children with high risk for relapse or mortality continued to receive more intensive treatment (31). This is exemplified among ALL survivors, for whom we found a small but consistent increase in the percentage reporting adverse outcomes in three of six health status domains. Among children with ALL, gains in survival over the study period were realized with evolution of therapies designed to cure children with high-risk disease (32), whereas decreases in long-term morbidity resulted from reduction of therapies to minimize late-effects in those with low-risk disease (33). Our data suggest that a smaller proportion of ALL survivors have medically significant, detectable chronic conditions (treated for high-risk disease), whereas a more sizable proportion may have subclinical chronic conditions that interfere with daily function. Second, there has been a decrease in late mortality among childhood cancer survivors (1), potentially because surveillance and identification allows for earlier medical management of subclinical health conditions. Early screening and disease management may reduce mortality, but may not positively influence perception of health status (34), which may result in survivors rating their health as worse. Finally, it is possible that survivors of childhood cancer treated in a more recent era who were more likely to have access to organized follow-up care (35) and educational materials about medical late effects (36), and who were treated when disclosure of mental health issues and pain were more acceptable, developed different internal standards (expectations) upon which to judge health status outcomes. Thus, those treated 1990-99 may be more likely to anticipate and/or report adverse outcomes than those treated 1970-79.(37)
In previous reports from the CCSS, the highest proportions of functional limitations were among CNS and bone tumor survivors (7). Since treatment approaches for these disease groups evolved over the decades to minimize adverse events, we expected rates of functional limitation to decrease over time. We observed improvement among astrocytoma and medulloblastoma survivors, where changes in treatment included improved surgical techniques and reductions in CRT dose/volume (20, 38, 39). However, there was no improvement among osteosarcoma survivors, where the biggest change in treatment was replacement of amputation with limb-sparing surgery. Our findings are consistent with literature that indicates a dose-response association (7% (1-13%) per Gray) between CRT dose and risk for adverse events among childhood brain tumor survivors (40), and with meta-analyses that report no difference in long-term disability among childhood sarcoma survivors who require amputation compared to those who had limb-sparing surgery (41).
The increase from the 1970s to the 1990s in percentage of ALL and osteosarcoma survivors who report cancer-related pain years after diagnosis is concerning. Among ALL survivors, this may be related to increased use of intrathecal therapy to treat CNS disease in the more recent era. Data indicate that back pain is a frequent complaint of adult survivors of ALL (42), and that back pain and neuropathy are associated with the number of intrathecal chemotherapy administrations (43). In osteosarcoma survivors, this may be due to increases in numbers of younger patients eligible for limb-sparing related to availability of expandable prostheses in the 1990s, many of whom required multiple surgeries to manage orthopedic complications. Dotan et al (44) reported an average of 6.2 complications, requiring 83 additional surgeries, among 22/38 children. Likewise, Ruggieri et al (45) reported implant-related complications among 17/32 children (72 months of follow-up) with lower extremity sarcoma who had limb-sparing using expandable prostheses.
As in the general population (46), smoking, body composition, inactivity, non-white race and female sex were associated with adverse health status outcomes among childhood cancer survivors. This indicates that survivors may be able to influence health status outcomes by modifying high-risk personal behaviors. Survivors who smoke, are inactive or obese should be directed to interventions designed to eliminate smoking (47), increase activity (48) and achieve a healthy weight (48). In addition, clinicians who treat female and non-white survivors and researchers designing interventions should consider these factors (49, 50) when counseling patients or developing targeted strategies to improve health status. Of note, we found an association between heavy drinking and functional impairments and activity limitations in our cohort. These data seem implausible, but are consistent with other studies among middle age adults where the highest odds for physical limitations are among never-drinkers and among individuals who quit drinking because of illness (51). Because we did not collect data on drinking history in our cohort, we were unable to directly evaluate this. However, there were no differences in the percentages of individuals who reported heavy drinking by chronic condition status.
These results should be considered in the context of potential study limitations. First of all, these analyses include many parallel comparisons, not adjusted for multiplicity. Chance alone could account for some of our findings. Second, not every survivor eligible for CCSS agreed to participate. Even though we stratified our analyses by diagnoses, participants may have been healthier or sicker than non-participants which could bias estimates either toward or away from the null. Third, the considerable improvement in survival among children diagnosed with cancer in the 1990s compared to those diagnosed in the 1970s makes it difficult to definitively determine the impact of risk factors on later self-reported health status without considering their impact on mortality itself. Finally, our evaluation of the impact of personal risk factors was concurrent with ascertainment of health status making it impossible to determine the temporality of this association.
Improved survival following a diagnosis of childhood cancer is a success story in modern medicine. Surprisingly, our data demonstrate lack of improvement in health status among childhood cancer survivors over thirty years of changing cancer therapy, an important reminder that cancer cure is not without some consequences. Current long-term follow-up guidelines are primarily focused on screening for chronic disease and do not give specific recommendations for surveillance of perceived health status. Fortunately, there has been recent recognition of the importance of patient-reported outcomes as part of curative therapy trials (48). These data may be important in providing opportunities to modify treatments with extreme toxicity and directing early intervention during survivorship for survivors who report adverse health status. Evaluations of associations between changes in specific treatment exposures and health status outcomes for individual primary cancer types, and the development and validation of risk prediction models to better determine who needs screening (52), are important next steps. In addition, because data indicate that risk for adverse health status increases with age, including measures of perceived health status should be considered in long-term follow-up settings.
Supplementary Material
Consort diagram.
Acknowledgments
Funding: This work was supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
KKN, KEJ, WL, YY, and YC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
LLR, JPN, MS designed the cohort study. KKN, PN, MMH, KO and WL conceived these data analysis. GTA, TMG, MS, JPN, KCO, KRK, KKN, MMH and LLR participated in data collection. KKN, KEJ, WL, YY and YC analyzed the data. All authors interpreted the data and participated in writing and revising the manuscript.
The corresponding author attests that everyone who made a significant contribution to this manuscript has been listed and that all authors had access to all study data.
The corresponding author takes full responsibility for the accuracy of the analysis and had authority over manuscript preparation and the decision to submit the manuscript for publication. All authors approve the manuscript and agree to adhere to all terms outlined in the Annals of Internal Medicine information for authors including terms of copyright.
The authors declare no conflicts of interest.
Trial Registration: National Institutes of Health #NCT01120353
Reference List
- 1.Armstrong GT, Pan Z, Ness KK, Srivastava D, Robison LL. Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. J Clin Oncol. 2010;28(7):1224–31. doi: 10.1200/JCO.2009.24.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review 1975-2012. National Cancer Institute; Bethesda, MD: Apr, 2015. http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–63. doi: 10.1158/1055-9965.EPI-14-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32(12):1218–27. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–92. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 8.Ozono S, Ishida Y, Honda M, Okamura J, Asami K, Maeda N, et al. General health status and late effects among adolescent and young adult survivors of childhood cancer in Japan. Jpn J Clin Oncol. 2014;44(10):932–40. doi: 10.1093/jjco/hyu102. [DOI] [PubMed] [Google Scholar]
- 9.Reulen RC, Winter DL, Lancashire ER, Zeegers MP, Jenney ME, Walters SJ, et al. Health-status of adult survivors of childhood cancer: a large-scale population-based study from the British Childhood Cancer Survivor Study. Int J Cancer. 2007;121(3):633–40. doi: 10.1002/ijc.22658. [DOI] [PubMed] [Google Scholar]
- 10.Hudson MM, Oeffinger KC, Jones K, Brinkman TM, Krull KR, Mulrooney DA, et al. Age-dependent changes in health status in the Childhood Cancer Survivor cohort. J Clin Oncol. 2015;33(5):479–91. doi: 10.1200/JCO.2014.57.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodgson DC, Hudson MM, Constine LS. Pediatric hodgkin lymphoma: maximizing efficacy and minimizing toxicity. Semin Radiat Oncol. 2007;17(3):230–42. doi: 10.1016/j.semradonc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuh GE, Loredo LN, Yonemoto LT, Bush DA, Shahnazi K, Preston W, et al. Reducing toxicity from craniospinal irradiation: using proton beams to treat medulloblastoma in young children. Cancer J. 2004;10(6):386–90. doi: 10.1097/00130404-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331–7. [PubMed] [Google Scholar]
- 15.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–18. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–39. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 17.Feijen EA, Leisenring WM, Stratton KL, Ness KK, van der Pal HJ, Caron HN, et al. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol. 2015;33(32):3774–80. doi: 10.1200/JCO.2015.61.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derogatis LR. BSI Brief Symptom Inventory: Administration, Scoring, and Procedure Manual. 4. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 19.U.S.Department of Health and Human Services, National Institutes of Health, National Cancer Institute. [November 19 2015];Common Terminology Criteria for Adverse Events (CTCAE) 4.03. 2010 http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 20.Green DM, Kun LE, Matthay KK, Meadows AT, Meyer WH, Meyers PA, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60(7):1083–94. doi: 10.1002/pbc.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudson MM, Neglia JP, Woods WG, Sandlund JT, Pui CH, Kun LE, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334–43. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Leisenring WM, Ness KK, Robison LL, Armstrong GT, Yasui Y, et al. Racial/Ethnic Differences in Adverse Outcomes Among Childhood Cancer Survivors: The Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(14):1634–43. doi: 10.1200/JCO.2015.66.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emmons K, Li FP, Whitton J, Mertens AC, Hutchinson R, Diller L, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the childhood cancer survivor study. J Clin Oncol. 2002;20(6):1608–16. doi: 10.1200/JCO.2002.20.6.1608. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Alcohol and Public Health. Atlanta, GA: [August 26 2015]. http://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm. [Google Scholar]
- 25.National Heart Lung and Blood Institute Expert Panel on the Identification Evaluation and Treatment of Overweight and Obesity in Adults. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults. Bethesda, MD: 1998. [Google Scholar]
- 26.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. Washington, DC: [August 26 2015]. www.health.gov/paguidelines. [Google Scholar]
- 27.Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61(1):53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gana K, Broc G, Saada Y, Amieva H, Quintard B. Subjective wellbeing and longevity: Findings from a 22-year cohort study. J Psychosom Res. 2016;85:28–34. doi: 10.1016/j.jpsychores.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Floud S, Pirie K, Green J, Peto R, Beral V, et al. Does happiness itself directly affect mortality? The prospective UK Million Women Study. Lancet. 2016;387(10021):874–81. doi: 10.1016/S0140-6736(15)01087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steptoe A, Deaton A, Stone AA. Subjective wellbeing, health, and ageing. Lancet. 2015;385(9968):640–8. doi: 10.1016/S0140-6736(13)61489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson MM, Link MP, Simone JV. Milestones in the curability of pediatric cancers. J Clin Oncol. 2014;32(23):2391–7. doi: 10.1200/JCO.2014.55.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013;50(3):185–96. doi: 10.1053/j.seminhematol.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Essig S, Li Q, Chen Y, Hitzler J, Leisenring W, Greenberg M, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2014;15(8):841–51. doi: 10.1016/S1470-2045(14)70265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naus MJ, Ishler MD, Parrott CE, Kovacs SA. Cancer survivor adaptation model: conceptualizing cancer as a chronic illness. J Clin Psychol. 2009;65(12):1350–9. doi: 10.1002/jclp.20622. [DOI] [PubMed] [Google Scholar]
- 35.Eshelman-Kent D, Kinahan KE, Hobbie W, Landier W, Teal S, Friedman D, et al. Cancer survivorship practices, services, and delivery: a report from the Children’s Oncology Group (COG) nursing discipline, adolescent/young adult, and late effects committees. J Cancer Surviv. 2011;5(4):345–57. doi: 10.1007/s11764-011-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–90. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 37.Eaves ER, Sherman KJ, Ritenbaugh C, Hsu C, Nichter M, Turner JA, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12. doi: 10.1186/s12906-015-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellison DW, Clifford SC, Gajjar A, Gilbertson RJ. What’s new in neuro-oncology? Recent advances in medulloblastoma. Eur J Paediatr Neurol. 2003;7(2):53–66. doi: 10.1016/s1090-3798(03)00014-x. [DOI] [PubMed] [Google Scholar]
- 39.Mueller S, Chang S. Pediatric brain tumors: current treatment strategies and future therapeutic approaches. Neurotherapeutics. 2009;6(3):570–86. doi: 10.1016/j.nurt.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dijk IW, Cardous-Ubbink MC, van der Pal HJ, Heinen RC, van Leeuwen FE, Oldenburger F, et al. Dose-effect relationships for adverse events after cranial radiation therapy in long-term childhood cancer survivors. Int J Radiat Oncol Biol Phys. 2013;85(3):768–75. doi: 10.1016/j.ijrobp.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Stokke J, Sung L, Gupta A, Lindberg A, Rosenberg AR. Systematic review and meta-analysis of objective and subjective quality of life among pediatric, adolescent, and young adult bone tumor survivors. Pediatr Blood Cancer. 2015;62(9):1616–29. doi: 10.1002/pbc.25514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowers DC, Griffith T, Gargan L, Cochran CJ, Kleiber B, Foxwell A, et al. Back pain among long-term survivors of childhood leukemia. J Pediatr Hematol Oncol. 2012;34(8):624–9. doi: 10.1097/MPH.0b013e31827080de. [DOI] [PubMed] [Google Scholar]
- 43.Khan RB, Hudson MM, Ledet DS, Morris EB, Pui CH, Howard SC, et al. Neurologic morbidity and quality of life in survivors of childhood acute lymphoblastic leukemia: a prospective cross-sectional study. J Cancer Surviv. 2014;8(4):688–96. doi: 10.1007/s11764-014-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dotan A, Dadia S, Bickels J, Nirkin A, Flusser G, Issakov J, et al. Expandable endoprosthesis for limb-sparing surgery in children: long-term results. J Child Orthop. 2010;4(5):391–400. doi: 10.1007/s11832-010-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruggieri P, Mavrogenis AF, Pala E, Romantini M, Manfrini M, Mercuri M. Outcome of expandable prostheses in children. J Pediatr Orthop. 2013;33(3):244–53. doi: 10.1097/BPO.0b013e318286c178. [DOI] [PubMed] [Google Scholar]
- 46.Zack MM Centers for Disease Control and Prevention (CDC) Health-related quality of life - United States, 2006 and 2010. MMWR Surveill Summ. 2013;62(Suppl 3):105–11. [PubMed] [Google Scholar]
- 47.Emmons KM, Puleo E, Sprunck-Harrild K, Ford J, Ostroff JS, Hodgson D, et al. Partnership for health-2, a web-based versus print smoking cessation intervention for childhood and young adult cancer survivors: randomized comparative effectiveness study. J Med Internet Res. 2013;15(11):e218. doi: 10.2196/jmir.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braam KI, van der Torre P, Takken T, Veening MA, van Dulmenden Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2013;4 doi: 10.1002/14651858.CD008796.pub2. CD008796. [DOI] [PubMed] [Google Scholar]
- 49.Woodruff SI, Talavera GA, Elder JP. Evaluation of a culturally appropriate smoking cessation intervention for Latinos. Tob Control. 2002;11(4):361–7. doi: 10.1136/tc.11.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosas LG, Stafford RS. Practical Research Strategies for Reducing Social and Racial/Ethnic Disparities in Obesity. Int J Obes (Lond) 2012;2012(2):s16–s22. doi: 10.1038/ijosup.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Y, Pikhart H, Malyutina S, Pajak A, Kubinova R, Nikitin Y, et al. Alcohol consumption and physical functioning among middle-aged and older adults in Central and Eastern Europe: results from the HAPIEE study. Age Ageing. 2015;44(1):84–9. doi: 10.1093/ageing/afu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consort diagram.


