Abstract
Methamphetamine (meth) addiction is a costly burden to both the individual user and society as a whole. Establishing effective pharmacotherapies to treat meth dependence is needed to help solve this health problem. The study reported herein examined the effects of varenicline, a partial α4β2 and full α7 nicotinic acetylcholine receptor agonist, on meth self-administration and reinstatement in male Sprague-Dawley rats. Following indwelling jugular catheter surgery, rats were either trained to self-administer meth or saline on a variable ratio (VR) 3 schedule of reinforcement. Self-administration sessions (2 hour duration; 19 total sessions) were conducted daily. The effect of varenicline pretreatment on meth and saline self-administration was then determined using a within-study design. All rats received varenicline (0.0, 0.3, 1.0, and 3.0 mg/kg) prior to 4 different test sessions. Dose order was randomly assigned and each test was separated by 2 standard self-administration sessions to assess stability of responding. Fifteen extinction sessions (no meth available) followed the last test. Extinction was followed by meth-primed (0.3 mg/kg IP) reinstatement tests to examine the effect of varenicline on meth-seeking behavior. All rats again received all doses of varenicline over 4 separate reinstatement tests performed on 4 consecutive days. Varenicline did not alter self-administration of meth or saline. Additionally, the 0.3 and 1.0 doses of varenicline non-specifically increased active lever responding during the reinstatement test sessions. This latter finding suggests that varenicline may increase relapse liability and should not be utilized as pharmacotherapy to treat meth dependence.
Keywords: Relapse, Chantix, Drug Dependence, Addiction, Reward Enhancement
1. Introduction
Establishing more efficacious methamphetamine (meth) cessation treatments is vital to decreasing the deleterious health effects to the individual and lowering the exorbitant economic and societal cost of meth. Pharmacotherapies that correct dopaminergic hypofunction during meth abstinence have been identified as possible meth-cessation medications (De La Garza et al, 2012; Longo et al, 2010; Reichel et al, 2009; Verrico et al, 2013). Nicotinic acetylcholine receptors (nAChRs) can modulate dopamine release (Dobbs & Mark, 2012). In fact, medications that effectively increase acetylcholine by inhibiting acetylcholinesterase reduce the positive subjective effects of meth (De La Garza et al, 2008a; De La Garza 2008b; De La Garza et al, 2012). Additionally, varenicline (Chantix®), a partial α4β2 and full α7 nicotinic acetylcholine receptor agonist, (Coe et al, 2005a; Mihalak et al, 2006) can reduce the positive subjective effects of meth in humans, an effect that may reflect nAChR agonism reversing dopaminergic hypofunction (Verrico et al, 2014; Zorick et al, 2009).
A reduction in the positive subjective effects of meth by varenicline points to the possible use of varenicline to treat meth dependence (Verrico et al, 2014; Zorick et al, 2009). However, research in preclinical models has raised some concerns with this premise, at least in females. Pittenger et al. (2016) examined the effects of varenicline on meth self-administration and meth-primed reinstatement in female rats. While varenicline reduced meth self-administration in that study, the reduction was not specific to meth (i.e., responding was also reduced in rats that received saline and timeout cues). Notably, varenicline treatment increased drug-seeking in meth-primed reinstatement tests. This increase in drug-seeking suggests that varenicline may in fact serve to increase relapse in females. Whether or not this increase in reinstatement and non-specific decrease in self-administration responding generalizes to male rats is the focus of this brief report.
2. Materials and Methods
2.1.1 Subjects
Fifteen male Sprague-Dawley rats were purchased from Harlan Laboratories Inc. (Indianapolis, IN, USA) at approximately 9 weeks of age. Rats were individual housed with ad libitum access to water in polycarbonate cages (35.5 × 32 ×18; length × width × depth) lined with TEK-Fresh® cellulose. Food was restricted to maintain rats at 90% of their free feeding weight. The colony room was maintained on a daily 6:00 AM lights on to 6:00 PM lights off cycle; the experiment was conducted during the light cycle. These protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
2.1.2 Apparatus
Ten conditioning chambers (ENV-008CT; Med Associates, Georgia, VT, USA) measuring 30.5×24.1×21.0 cm and enclosed in sound-attenuating cubicles were utilized in this study. A variable-speed syringe pump (PMH-100VS; Med-Associates) was located outside each cubicle. A catheter port on the back of the rat was attached to the pump syringe via Tygon® tubing threaded through a leash and out of the cubicle. A recessed receptacle (5.2×5.2×3.8 cm) fitted with a dipper arm (0.1 ml) was centered on one sidewall of each chamber. Two retractable levers were located on each side of the receptacle. A white cue light (2.54-cm diameter; 28V, 100-mA) was mounted 7 cm above each lever and a house light (two white 28V, 100-mA lamps; 10 cm above chamber ceiling) was located in the cubicle. An infrared emitter/detector unit was located 4 cm above the rod floor and 14.5 cm from the side wall containing the receptacle. Chamber activity was measure by the number of times this beam was broken.
2.1.3 Drugs
(+)-Methamphetamine hydrochloride (Sigma-Aldrich; St. Louis, MO, USA) was dissolved in 0.9% sterile saline and infused intravenously (IV) at 35.74 μl over 1 sec at 0.043 mg/kg/infusion. Varenicline tartrate was generously provided by NIDA (RTI, Research Triangle Park, NC, USA), dissolved in 0.9% saline, and injected intraperitoneal (IP) at 1 ml/kg. Meth during drug-primed reinstatement was also administered IP at 1 ml/kg. All doses are reported as salt weight.
2.2.1 Preliminaries and Surgery
Preliminary training with the levers, indwelling jugular catheters surgery and subsequent variable ratio 3 (VR3) training with sucrose reinforcement was conducted using our labs standard protocol detailed in Pittenger et al. (2016). Two rats were excluded from the study for non-patent catheters.
2.2.2 Self-Administration
Rats were assigned into 2 groups: meth self-administration (n=7) or saline self-administration (n=6). Self-administration sessions (2 h) were conducted daily for 19 consecutive days. Active lever pressing (VR3; active vs inactive lever randomly assigned) initiated an infusion of meth or saline depending on group, retraction of both levers, and illumination of the house light for a 20 s timeout. Both levers were extended and the house light was turned off following the timeout. Inactive lever responding was recorded but had no programed outcome.
2.2.3 Effects of Varenicline on Self-Administration
Varenicline [0.0 (saline), 0.3, 1, and 3 mg/kg] was administered IP 20 min before the start of a self-administration session. Testing order of doses was pseudo-randomly assigned via a Latin-square design (i.e., all rats received all doses). Varenicline tests days were separated by 2 standard self-administration sessions to monitor deviations from baseline responding calculated as the average of the last 2 self-administration sessions before starting this test phase. No rat reached criteria for exclusion from testing; >20% variance in active lever presses or <80% of baseline responding. After varenicline testing, 2 additional self-administration sessions were given to verify stable responding before starting the extinction phase.
2.2.4 Extinction
This phase began 24 h after the last self-administration session. The 15 daily extinction sessions were identical to the self-administration sessions except meth and saline were no longer available for active lever pressing.
2.2.5 Effects of Varenicline on Meth-Primed Reinstatement
The effect of varenicline pretreatment [0.0 (saline), 0.3, 1, and 3 mg/kg] on meth-primed reinstatement was tested over 4 consecutive days. Assigned dose of varenicline, pseudo-randomly assigned via a Latin-square design, was administered 20 min before the start of the session. The meth-trigger (0.3 mg/kg) was administered 15 min before the session began. Reinstatement sessions were identical to extinction session except they were shortened to 70 min. Session length was truncated as unpublished data in our lab revealed most responding evoked by a meth-prime occurred within the first 70 min of a reinstatement session.
2.2.6 Data Analyses
Analyses of active lever pressing and locomotor activity during the self-administration and the extinction phase were performed using two-factor ANOVAs with Group (meth vs saline) as a between-subjects factor and Sessions as a within-subjects factor. Data from both varenicline test phases were analyzed using two-factor ANOVAs with Group as a between-subjects factor and Dose as a within-subjects factor. For all analyses, significant main effects or interactions were followed by post-hoc pairwise comparisons. All pairwise comparisons employed the Holm-Bonferroni method for controlling the family-wise error rate with significance set at p<0.05 (Holm, 1979).
3. RESULTS
3.1 Self-Administration
For active lever-pressing (Figure 1A), there were main effects of Drug [F(1,11)=14.39; p=0.003] and Session [F(18,198)=4.60; p<0.001], as well as a significant Drug * Session interaction [F(18,198)=1.80; p=0.028]. Post-hoc analyses on the interaction revealed significantly higher active lever-pressing in meth self-administration rats than saline self-administration rats during all but the first session (ps≤0.039). Session 1 responding was higher than all but sessions 15 and 17 in the saline self-administration group (ps≤0.032); no other effects of Session were observed within the meth or saline group (ps≥0.078). For inactive lever-pressing (data not shown), there was a main effect of Session [F(18,198)=7.73; p<0.001]. Inactive lever-pressing was significantly higher on session 1 than all other sessions (ps<0.001), with no other effects of Session were detected.
Figure 1.
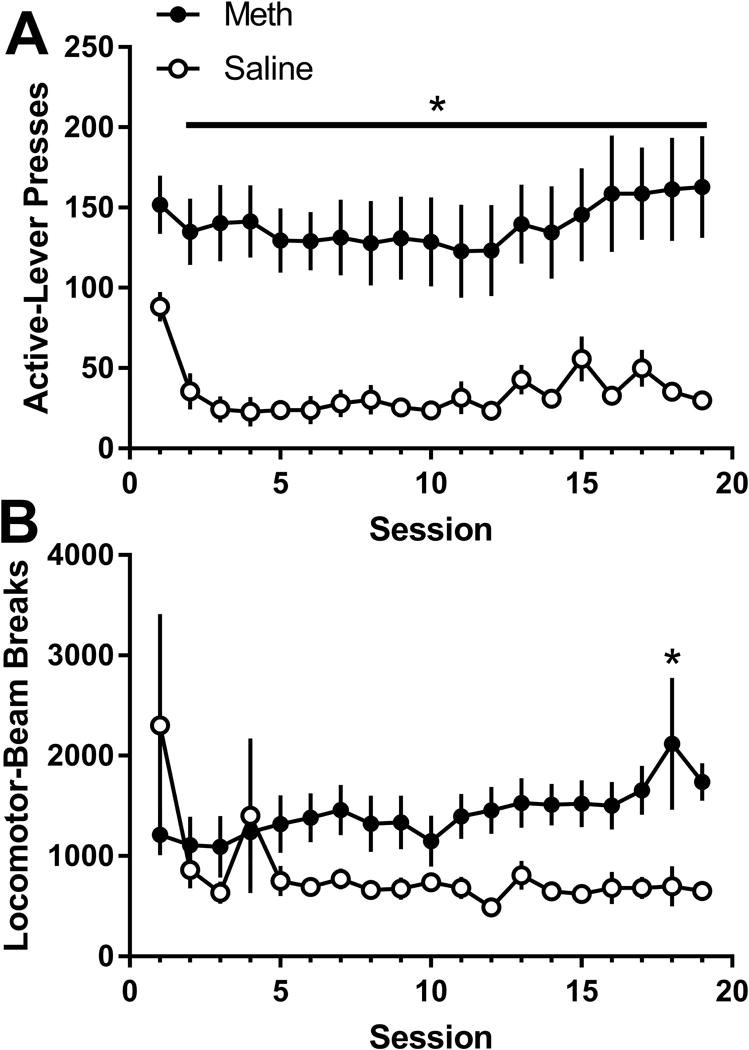
A. Active lever presses (mean ± SEM) during self-administration sesssions for the meth and saline groups. B. General locomotor activtiy during self-administration sessions for the meth and saline groups. * denotes significant differences between the meth and saline groups.
For locomotor activity during self-administration (Figure 1B), there was a main effect of Drug [F(1,11)=6.61; p=0.026] and a Drug * Session interaction [F(1,198)=2.00; p=0.012]. Locomotor activity in the meth self-administration group was higher than the saline self-administration group on session 18 (p=0.029), but not on any other session (ps≥0.249).
3.2 Effects of Varenicline on Self-Administration
For active lever-pressing (Figure 2A), there was only a significant main effect of Drug [F(1,11)=9.61; p=0.010]. This data pattern indicates that varenicline did not alter self-administration of meth or saline. No significant main effects or interactions were detected for inactive lever-pressing or locomotor activity.
Figure 2.
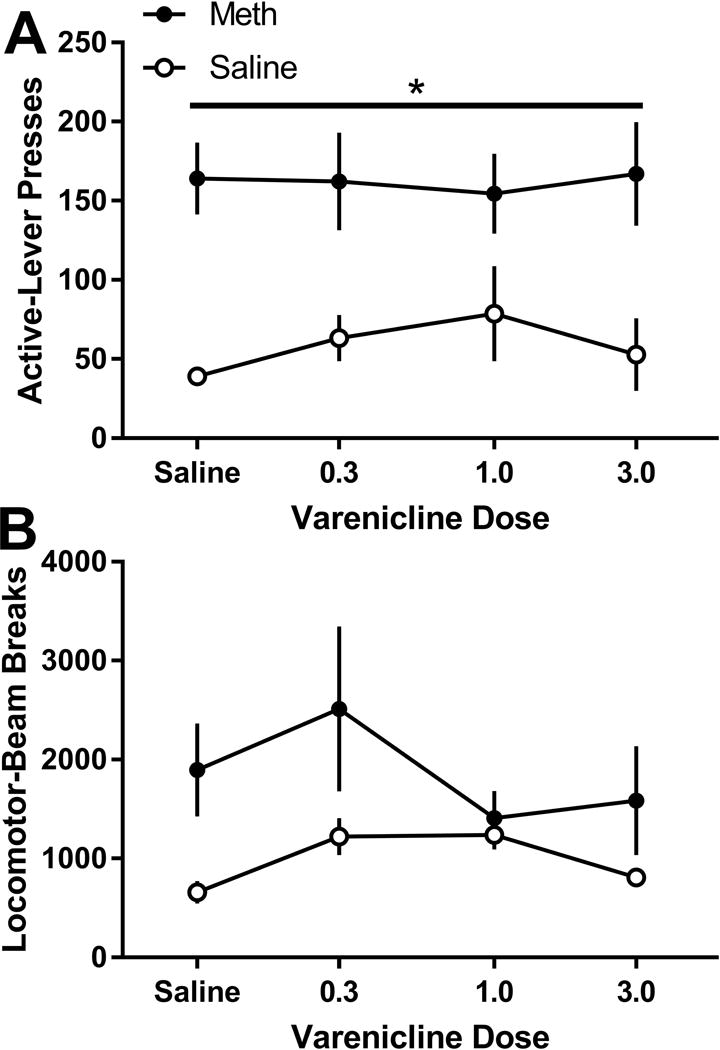
A. Active lever presses (mean ± SEM) during self-administration following treatment with varenicline for the meth and saline groups. B. General locomotor activtiy during self-administration following treatment with vareniclie for the meth and saline groups. * denotes significant differences between the meth and saline groups.
3.3 Extinction
For active lever-pressing (Figure 3A), there was a significant main effect of Session [F(14,154)=2.85; p<0.001] and a Drug * Session interaction [F(12,154)=3.68; p<0.001]. Active lever-pressing in the meth self-administration group was higher than the saline self-administration group only for the first extinction session (p<0.001). For inactive lever-pressing, only the main effect of Session [F(14,154)=4.19; p<0.001] was significant. Overall, inactive lever-pressing on session 1 was higher than all but session 3 (ps≤0.002). Analysis of locomotor activity (Figure 3B) during extinction found no significant main effects but there was a significant Drug * Session interaction [F(14,154)=1.77; p=0.048]. Although the interaction was significant, there were no significant differences in activity between the meth and the saline group (ps≥0.214).
Figure 3.
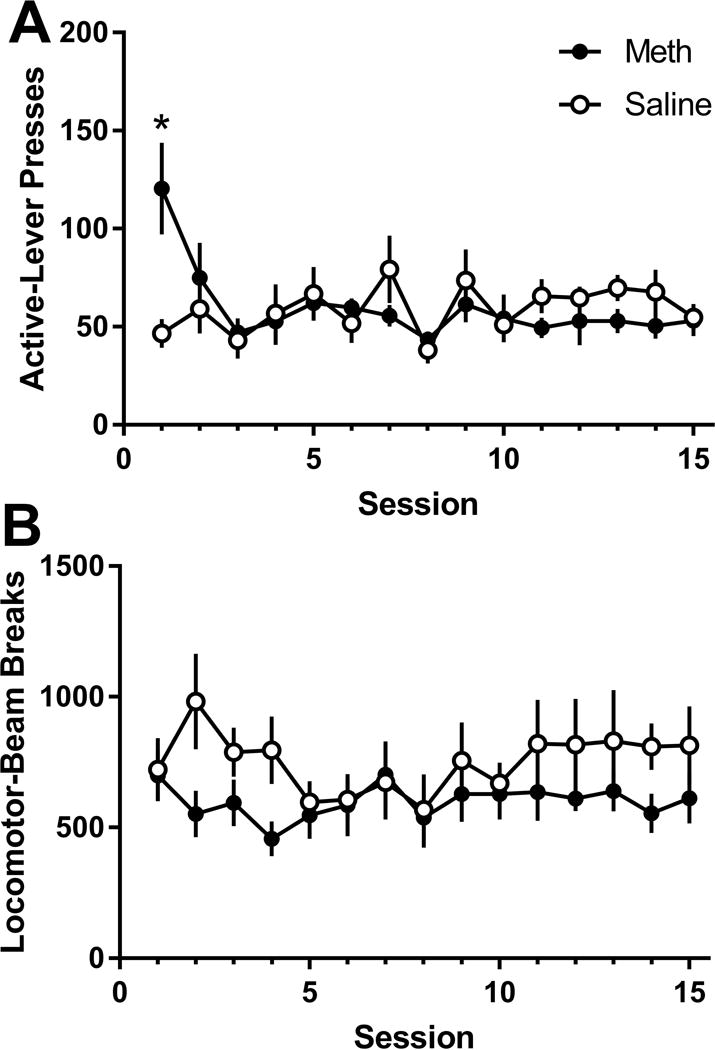
A. Active lever presses (mean ± SEM) during extinction sessions for the meth and saline groups. B. General locomotor activtiy during extinction sessions for the meth and saline groups. * denotes significant differences between the meth and saline groups.
3.4 Effects of Varenicline on Meth-Primed Reinstatement
For active lever-pressing (Figure 4A), there was only a main effect of Varenicline Dose [F(3,33)=6.71; p=0.001]. This main effect reflected higher responding after pretreatment with 0.3 and 1.0 mg/kg varenicline than either saline (vehicle) or 3.0 mg/kg (ps≤0.017). That is, following 0.3 and 1.0 mg/kg varenicline treatment, rats with or without a history of meth self-administration actually showed greater active lever pressing (i.e., reinstatement behavior). Likewise, there was only a main effect of Varenicline Dose [F(3,33)=6.33; p=0.002] for locomotor activity (Figure 4B). There was significantly higher locomotor activity after pretreatment with 0.3 and 1.0 mg/kg varenicline relative to saline (vehicle; ps≤0.004). No effects were detected on inactive lever-pressing.
Figure 4.
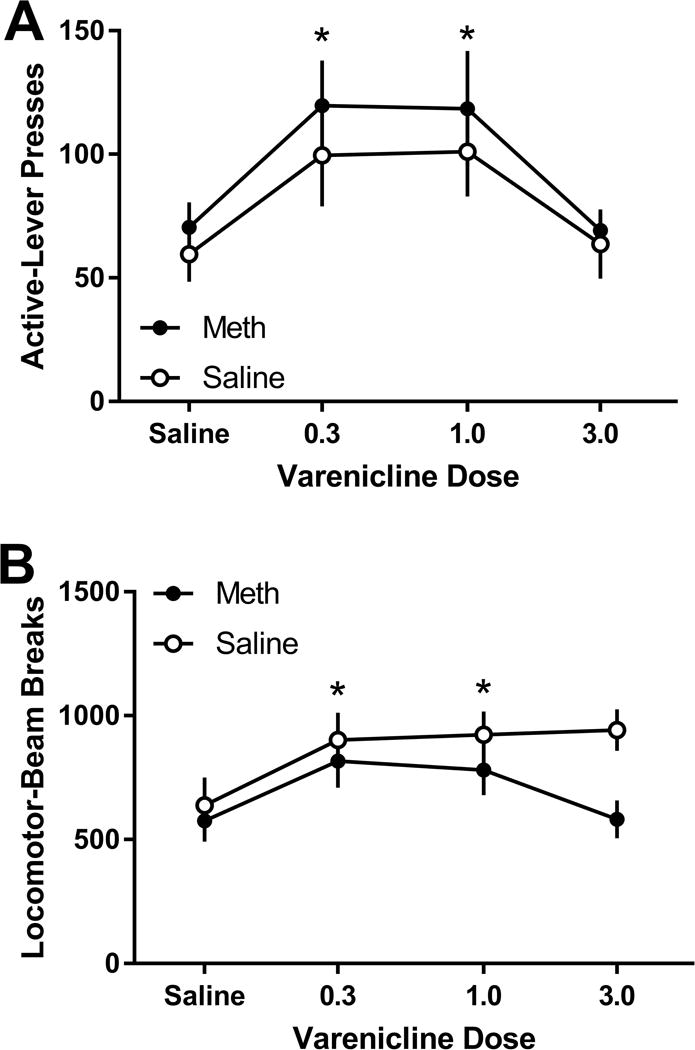
A. Active lever presses (mean ± SEM) during reinstatement testing following varenicline administration for the meth and saline groups. B. General locomotor activtiy during reinstatement following treatment with vareniclnie for the meth and saline groups. * denotes significant differences following adminstration of 0.3 and 1.0 mg/kg varenicline from saline treatment.
4 Discussion
Some research has reported that varenicline decreases self-administration of nicotine, ethanol, and cocaine. However, other investigators have reported increases in ethanol and cocaine intake, as well as no effect with varenicline pretreatment; for a mini-review of the effects of varenicline on nicotine, ethanol, and cocaine self-administration and reinstatement see Pittenger et al. (2016). In the study presented herein, varenicline did not alter methamphetamine self-administration. The inability of varenicline to alter meth-taking behavior in male rats is similar to findings with female rats in that there were no specific effects of varenicline on meth self-administration in either sex (Pittenger et al., 2016). In the female rats, there was a non-specific effect of varenicline slightly reducing responding in both the saline and meth groups. This reduction was not seen in the males. The variation may be a result of inherent differences between males and females. Indeed, studies have identified meth self-administration differences between males and females (Reichel et al, 2012; Roth & Carroll, 2004). Future work programmatically examining possible sex differences in varenicline treatment of meth dependence would be needed to further elucidate the mechanism of these differences.
In this study, responding during the meth-primed reinstatement tests was increased by pretreatment with 0.3 and 1.0 mg/kg varenicline regardless of whether rats had a history of meth or saline self-administration. A similar non-specific enhancement was reported in female rats (Pittenger et al., 2016). The increase in general locomotor activity following administration of both 0.3 and 1.0 mg/kg varenicline may suggest possible non-specific activation resulting in increased responding. This account, however, is largely discredited by the lack of altered responding on the inactive lever following administration of these doses of varenicline.
There are at least two other explanations for the non-specific enhancement of active lever pressing induced by varenicline in this study. First, varenicline may be substituting for meth, effectively increasing the dose of meth utilized as a drug-prime during the reinstatement tests. Indeed, varenicline has been shown to partially substitute for the discriminative-stimulus effects of meth (Desai & Bergman, 2014). Additionally, the level of responding evoked by meth in a reinstatement test is sensitive to the dose (Anggadiradja et al, 2004; Reichel et al, 2012). The finding that 3.0 mg/kg varenicline had no effect may reflect a summative meth-like dose (i.e., varenicline + meth) that is on the descending portion of the meth-prime reinstatement curve.
The second possible explanation is that varenicline may be functioning to enhance other mildly reinforcing stimuli associated with active-lever responding. Recall that timeout stimuli including illumination of the house light and lever retraction were present throughout the experiment. Previous research has clearly shown that nicotine can enhance weakly reinforcing sensory stimuli (Donny et al., 2003; Chaudhri et al., 2006). More recently, this enhancement effect has been demonstrated with other nAChR agonists including varenicline (Barrett, 2014; Schassburger et al., 2015). This account is supported by varenicline enhancing responding not only in the meth self-administration group, but also in the saline group with responding presumably maintained by the weakly reinforcing sensory stimuli of the timeout period. This increase in reinstatement susceptibility does not appear to be an irregularity of our protocol. Increased responding during meth-primed reinstatement is concordant with reinstatement findings of other drugs of abuse including nicotine (La Foll et al., 2012; Wouda et al., 2011) and cocaine (Guillem and Peoples, 2010). Enhancement of responding is not ubiquitous. Other investigators have found no effect or a reduction in drug reinstatement and the reasons for such difference are not clear [for a summary of the literature see Pittenger et al. (2016)].
The results reported herein do not suggest varenicline would be a suitable pharmacotherapy in males, synonymous with our previous finding in females (Pittenger et al., 2016). Varenicline up to 3.0 mg/kg did not decrease self-administration of meth. Further, active lever responding was increased during the meth-primed reinstatement with 0.3 and 1.0 mg/kg varenicline. An increase in reinstatement behavior is particularly notable, given the importance relapse plays in meth-use disorders (Brackins et al, 2011). That is, varenicline may actually heighten vulnerability to meth relapse in males (reported herein) and females (Pittenger et al., 2016).
Acknowledgments
This work was supported by the National Institutes of Health research grant DA034389.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anggadiradja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watenabe S, Yamamoto T. Endacannabinoid system modulates relapse to methamphetamine seeking: Possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29:1470–1478. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- Barrett ST. Doctoral dissertation. University of Nebraska – Lincoln; Lincoln: 2014. A quantitative analysis of the value-enhancing effects of nicotine, bupropion, and varenicline in male and females rats. [Google Scholar]
- Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541–550. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006a;189(1):27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005a;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- De La Garza R, Mahoney JJ, Culbertson C, Shoptaw S, Newton TF. The acetylcholinesterase inhibitor rivastigmine does not alter total choices for methamphetamine, but may reduce positive subjective effects, in a laboratory model of intravenous self-administration in human volunteers. Pharmacol Biochem Behav. 2008a;89(2):200–208. doi: 10.1016/j.pbb.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, Newton TF, Haile CN, Yoon JH, Nerumalla CS, Mahoney JJ, et al. Rivastigmine reduces “Likely to use methamphetamine” in methamphetamine-dependent volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):141–146. doi: 10.1016/j.pnpbp.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, Shoptaw S, Newton TF. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Int J Neuropsychopharmacol. 2008b;11(6):729–741. doi: 10.1017/S1461145708008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Bergman J. Methamphetamine-like discriminative-stimulus effects of nicotinic agonists. J Pharmacol Exp Ther. 2014;348(3):478–88. doi: 10.1124/jpet.113.211235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LK, Mark GP. Acetylcholine from the mesopontine tegmental nuclei differentially affects methamphetamine induced locomotor activity and neurotransmitter levels in the mesolimbic pathway. Behav Brain Res. 2012;226(1):224–234. doi: 10.1016/j.bbr.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Guillem K, Peoples LL. Varenicline effects on cocaine self administration and reinstatement behavior. Behav Pharmacol. 2010;21(2):96–103. doi: 10.1097/FBP.0b013e328336e9c5. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist. 1979;6:65–70. [Google Scholar]
- Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, et al. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol. 2012;15(9):1265–1274. doi: 10.1017/S1461145711001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M, Wickes W, Smout M, Harrison S, Cahill S, White JM. Randomized controlled trial of dexamphetamine maintenance for the treatment of methamphetamine dependence. Addiction. 2010;105(1):146–154. doi: 10.1111/j.1360-0443.2009.02717.x. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel sleective nicotinic acetylcholine receptor partial agoinst, varencline, for smoking cessation. Arch Intern Med. 2015;166(15):1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Pittenger ST, Barrett S, Chou S, Bevins RA. The effects of varenicline on methamphetamine self-administration and drug-primed reinstatement in female rats. Behav Brain Res. 2016;300:150–9. doi: 10.1016/j.bbr.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 2012;223(4):371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Grant KM, Bevins RA. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug Alcohol Depend. 2009;100(1–2):54–62. doi: 10.1016/j.drugalcdep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172(4):443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Schassburger RL, Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Donny EC, et al. Differentiating the primary reinforcing and reinforcement-enhancing effects of varenicline. Psychopharmacology (Berl) 2015;232(5):975–983. doi: 10.1007/s00213-014-3732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Mahoney JJ, Thompson-Lake DG, Bennett RS, Newton TF, De La Garza R. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223–233. doi: 10.1017/S146114571300134X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 2011;216(2):267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick T, Sevak RJ, Miotto K, Shoptaw S, Swanson AN, Clement C, et al. Pilot Safety Evaluation of Varenicline for the Treatment of Methamphetamine Dependence. J Exp Pharmacol. 2009;2010(2):13–18. [PMC free article] [PubMed] [Google Scholar]


