Abstract
Cortical function emerges from the intrinsic properties of neocortical neurons and their synaptic connections within and across lamina. Neurodevelopmental disorders affecting migration and lamination of the neocortex result in cognitive delay/disability and epilepsy. Molecular layer heterotopia (MLH), a dysplasia characterized by over-migration of neurons into layer I, are associated with cognitive deficits and neuronal hyperexcitability in humans and mice. The breadth of different inbred mouse strains that exhibit MLH and inheritance patterns of heterotopia remain unknown. A neuroanatomical survey of numerous different inbred mouse strains, 2 first filial generation (F1) hybrids, and one consomic strain (C57BL/6J-Chr 1A/J/NaJ) revealed MLH only in C57BL/6 mice and the consomic strain. Heterotopia were observed in numerous genetically-engineered mouse lines on a congenic C57BL/6 background. These data indicate that heterotopia formation is a weakly penetrant trait requiring homozygosity of one or more C57BL/6 alleles outside of chromosome 1. These data are relevant toward understanding neocortical development and disorders affecting neocortical lamination.
Keywords: neocortex, malformation, heterotopia, C57BL/6
Introduction
The neocortex is highly vulnerable to neurodevelopmental malformations caused by a neuronal migration defect. Malformations characterized by incomplete migration, such as periventricular nodular heterotopia (PVH) [1–3] and subcortical band heterotopia (SBH) [4–7], have been extensively studied. In contrast, much less is known about neocortical molecular layer heterotopia (MLH), which are characterized by over-migration of neurons into the molecular layer (layer I) and present in disorders such as dyslexia [8–10] and epilepsy [11,12]. Greater understanding of MLH has implications for various disorders with diverse clinical presentations.
MLH are present in several knockout (KO) lines [13–22] and inbred mouse strains [23,24], including C57BL/6 and C57BL/10 mice [25–27]. The cytoarchitecture of MLH in mice is indistinguishable from human heterotopia, suggesting similar causal mechanisms. Moreover, mice with heterotopia exhibit impaired learning of spatial and non-spatial memory tasks [28–32] and deficits in sensory discrimination tasks [33–36], consistent with cognitive deficits associated with MLH. Furthermore, mice with MLH have lower seizure thresholds and shorter latency to seizures following chemi-convulsant treatment [37,38], which mimic brain excitability changes observed in epileptics with MLH. Thus, behavioral changes in mice with MLH closely resemble those observed in humans with MLH, demonstrating the utility of mice as a model of human MLH.
The prevalence of MLH and underlying mechanisms of heterotopia formation remain unclear. In this study, we performed a neuroanatomical survey for the presence of MLH using 6 widely-used inbred mouse strains, including strains commonly used to produce genetically-engineered (GE) mice. We investigated the basic inheritance patterns of MLH by examining 2 first filial generation (F1) hybrids, one consomic strain, and GE mice on a congenic C57BL/6 background. Our results indicate MLH formation is a weakly penetrant trait requiring homozygosity of one or more C57BL/6 alleles outside of chromosome 1. These data are relevant for understanding neocortical development and the mechanisms of cortical lamination. Since C57BL/6 is the most widely used inbred strain in neuroscience research, our results have broad implications for their use in diverse studies and the creation of GE mice.
Materials and methods
This study was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committee of New York Institute of Technology and Wadsworth Center. Description of the housing and care of mice has been described previously [25,26,39,40]. Five week-old, A/J, DBA/2J, and B6D2F1/J male mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Five week-old, 129S6/SvEvTac and B6129SF1/Tac male mice were obtained from Taconic Farms (Germantown, NY). Breeding pairs of 129S1/SvImJ, FVB/NJ, BALB/cJ inbred mice, and C57BL/6J-Chr 1A/J/NaJ (which carry chromosome 1 from strain A/J on a C57BL/6J background) were obtained from The Jackson Laboratory to generate mice used in this study. Brains from male and female retired breeders (≥ 6 months-old) or male and female offspring from these strains (≥ 3 weeks-old) were used. Since MLH are visible as early as P2 and the presence/absence of heterotopia does not change with age [25], we compared heterotopia prevalence between groups of mice because all mice were ≥ 3 weeks-old at time of sacrifice. Data from male and female mice were combined since MLH prevalence does not differ between sexes [25,26]. Finally, no differences in heterotopia prevalence were previously observed between mice obtained directly from commercial vendors and mice bred in an academic vivarium from commercially-obtained breeders [25,26].
Histological and analytical methods were previously described by our lab [25,26,39]. We also used digital histological data from the Allen Brain Atlas (ABA; www.brain-map.org) and Mouse Brain Architecture Project (MBAP; http://mouse.brainarchitecture.org) to document heterotopia as previously described [25,27,39,41,42]. Data from the ABA Mouse Strains dataset contains high-resolution sagittal and coronal sections from the following strains (The Jackson Laboratory): 129S1/SvlmJ, C57BL/6J, Cast/EiJ, DBA/2J, PWD/PhJ, SPRET/EiJ, WSB/EiJ. Methods used in the creation of the Mouse Strains dataset were previously reported [43]. Data from the ABA Transgenic Characterization dataset (http://connectivity.brain-map.org/transgenic) contains histology from 221 Cre-driver lines that have been crossed with one of 38 “reporter” mouse lines (loxP, etc.). Methods and mice used in the creation of the Transgenic Characterization dataset were previously described [44,45]. Data from the MBAP Transgenic Cell Counts dataset (http://mouse.brainarchitecture.org/cellcounts/) contains histology from 79 F1 hybrid mice generated by crosses between Cre-driver lines and reporter lines (loxP, etc.). Methods and mice used in the creation of the Transgenic Cell Counts dataset were found on the MBAP website.
Results
Prevalence of MLH in common inbred strains
The prevalence of MLH in C57BL/6J and C57BL/10J strains is well established [25–27], but only a small number of brains from a few other inbred mouse strains have been examined [25]. Consequently, we used well-established histological methods [25–27] to examine brains from numerous inbred mice including: 129S6/SvEvTac (n=20), 129S1/SvImJ (n=17), A/J (n=17), BALB/cJ (n=16), DBA/2J (n=20), FVB/NJ (n=18) strains. We used methods previously described [25,27,39,41,42] to examine high-resolution digital histological material from the Mouse Strains dataset from the ABA, including 129S1/SvImJ (n=117 cases), C57BL/6J (n=314), Cast/EiJ (n=117 cases), DBA/2J (n=117 cases), PWD/PhJ (n=93 cases), SPRET/EiJ (n=117 cases), WSB/EiJ (n=93 cases) strains. Remarkably, we did not observe MLH in any inbred strain except C57BL/6J brains in the ABA Mouse Strains dataset. Additionally, heterotopia were not observed in DBA/2J or 129S1/SvImJ mice in the ABA or in the cohort examined using primary histological data. This suggests that causal alleles for heterotopia formation are present in C57BL/6 linage.
Inheritance of MLH in the C57BL/6 linage
Since first filial generation (F1) hybrid mice are heterozygous at all loci where the parental strains differ in allelic composition, we examined F1 hybrid mice to determine if MLH formation is a recessive trait. After establishing that DBA/2J mice and 129S6/SvEvTac mice do not exhibit MLH, we tested whether F1 hybrid crosses of these inbred strains with C57BL/6 mice would exhibit heterotopia. We did not observe MLH in 20 male B6D2F1/J or 20 male B6129SF1/Tac mice, which were generated by crossing a female C57BL/6 mouse with a male DBA/2J mouse or male 129S6/SvEvTac mouse, respectively. This indicates that homozygosity, at one or more loci, is required for heterotopia formation.
Since consomic strains are characterized by the substitution of a homologous chromosome of a donor inbred strain backcrossed into a host inbred strain, they can be used to determine the chromosome associated with a given trait [46,47]. We examined brains from C57BL/6J-Chr 1A/J/NaJ mice, which have introgressed chromosome 1 from A/J on an inbred C57BL/6J background. We observed MLH in 6 out of 22 (27.27%) consomic mice. Fisher’s Exact Test of the prevalence of MLH in C57BL/6J-Chr 1A/J/NaJ mice compared to measures previously determined for C57BL/6J mice (35.71%) [26] revealed no significant difference. The absence of MLH in A/J mice indicates that the locus responsible for heterotopia formation is outside chromosome 1 of the C57BL/6 background.
Presence of MLH in genetically-engineered C57BL/6 congenic mice
These results suggest that GE mice produced from C57BL/6 ES cells and maintained on this background would exhibit MLH. Furthermore, GE mice produced from ES cells of another inbred strain but backcrossed to a C57BL/6 background would also exhibit MLH. We tested this prediction by examining over 1500 histological cases of F1 hybrid mice generated by crossing one of 221 Cre-driver lines with one of 38 reporter lines in the ABA Transgenic Characterization dataset. A total of 57 cases from this database exhibited MLH. Quantitative analyses of MLH prevalence were not performed because not every histological section is available for examination for each case. Thus, errors of omission (i.e. cases erroneously reporting absence of MLH) may explain the small number of cases of MLH in this dataset.
We identified 39 unique combinations of Cre-driver and reporter line hybrids with MLH as summarized in Figure 1 and Table 1. MLH were observed in F1 hybrids produced by crossing Pvalb-IRES-Cre mice with either Ai14 or Ai39 reporter mice and in F1 hybrids produced by crossing Emx1-IRES-Cre with either Ai27 or Ai32 reporter mice. All driver and reporter mice used to generate the F1 hybrids in Table 1 were identified as being on a congenic C57BL/6 background per the ABA, The Jackson Laboratory (jax.org), or the Mutant Mouse Resource and Research Center (mmrrc.org) websites. These data indicate a causal allele for heterotopia formation can be present in the background of diverse GE mice and inherited in F1 crosses of GE lines.
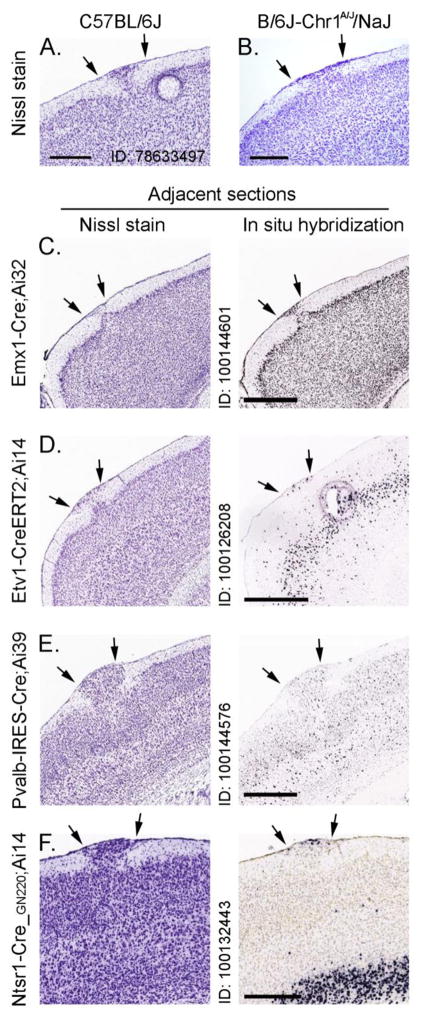
Figure 1.
A–B, Representative photomicrographs of Nissl-stained sections with heterotopia (arrows) from C57BL/6J (from the ABA Mouse Strains dataset) and C57BL/6J-Chr 1A/J/NaJ mice (abbreviated B/6J-Chr11A/J/NaJ), respectively. C–F, Representative photomicrographs of adjacent sections of F1 hybrid lines taken from the Transgenic Characterization dataset of the ABA containing heterotopia (arrows). Nissl-stained and hybridized sections in left and right panels, respectively. Hybridized gene listed next to each photomicrograph taken from the ABA. Scalebars (in microns): A = 420, B = 320, C = 798, D = 960, E = 632, F = 444.
Table 1.
List of all unique driver-reporter F1 hybrid lines with MLH found in the ABA Transgenic Characterization dataset including experiment identification number of a representative example of each mouse line.
| Experiment | F1 Hybrid Line Name | Driver line | Reporter line |
|---|---|---|---|
| 293795159 | Avp-IRES2-Cre;Ai14 | Avp-IRES2-Cre | Ai14 |
| 175707182 | Camk2a-tTA;Ai14 | Camk2a-tTA | Ai14 |
| 268328793 | Chrnb4-Cre_OL57;Ai14 | Chrnb4-Cre_OL57 | Ai14 |
| 100132436 | Crh-IRES-Cre (BL);Ai14 | Crh-IRES-Cre (BL) | Ai14 |
| 146455849 | Crh-IRES-Cre (ZJH);Ai14 | Crh-IRES-Cre (ZJH) | Ai14 |
| 80203010 | Cyp39a1-Tg1-Cre;R26-stop-EYFP | Cyp39a1-Tg1-Cre | R26-stop-EYFP |
| 100141756 | Dbh-Cre_KH212;Ai14 | Dbh-Cre_KH212 | Ai14 |
| 100141754 | Drd1a-Cre;Ai14 | Drd1a-Cre | Ai14 |
| 175194677 | EE313-lacZ-CreERT2-Tg2;Ai14 | EE313-lacZ-CreERT2-Tg2 | Ai14 |
| 167245573 | EE342-lacZ-CreERT2-Tg3;Ai14 | EE342-lacZ-CreERT2-Tg3 | Ai14 |
| 167242744 | EE609-lacZ-CreERT2-Tg2;Ai14 | EE609-lacZ-CreERT2-Tg2 | Ai14 |
| 167246779 | EE921-lacZ-CreERT2-Tg2;Ai14 | EE921-lacZ-CreERT2-Tg2 | Ai14 |
| 100144601 | Emx1-IRES-Cre;Ai32 | Emx1-IRES-Cre | Ai32 |
| 100095125 | Emx1-IRES-Cre;Ai27 | Emx1-IRES-Cre | Ai27 |
| 100132637 | Et(cre/ERT2)7089Rdav;Ai14 | Et(cre/ERT2)7089Rdav | Ai14 |
| 196126585 | Et(EGFP/cre)16059Rdav;Ai14 | Et(EGFP/cre)16059Rdav | Ai14 |
| 100144041 | Et(EGFP/cre)16102Rdav;Ai14 | Et(EGFP/cre)16102Rdav | Ai14 |
| 100141712 | Et(EGFP/cre)16261Rdav;Ai14 | Et(EGFP/cre)16261Rdav | Ai14 |
| 156350546 | Et(icre)21468Rdav;Ai14 | Et(icre)21468Rdav | Ai14 |
| 114374599 | Et(icre/ERT2)14163Rdav;Ai14 | Et(icre/ERT2)14163Rdav | Ai14 |
| 100126208 | Etv1-CreERT2;Ai14 | Etv1-CreERT2 | Ai14 |
| 157078989 | Gabrr3-Cre_KC112;Ai14 | Gabrr3-Cre_KC112 | Ai14 |
| 100130925 | Lepr-IRES-Cre;Ai14 | Lepr-IRES-Cre | Ai14 |
| 100132443 | Ntsr1-Cre_GN220;Ai14 | Ntsr1-Cre_GN220 | Ai14 |
| 112608505 | Otof-Cre;Ai14 | Otof-Cre | Ai14 |
| 159119080 | Otof-CreERT2;Ai14 | Otof-CreERT2 | Ai14 |
| 100130512 | Oxt-IRES-Cre;Ai14 | Oxt-IRES-Cre | Ai14 |
| 100144576 | Pvalb-IRES-Cre;Ai39 | Pvalb-IRES-Cre | Ai39 |
| 100117968 | Pvalb-IRES-Cre;Ai14 | Pvalb-IRES-Cre | Ai14 |
| 146454705 | Rbp4-Cre_KL100;Ai14 | Rbp4-Cre_KL100 | Ai14 |
| 281575505 | Slc17a7-IRES2-Cre;Ai14 | Slc17a7-IRES2-Cre | Ai14 |
| 100141516 | Slc17a8-iCre;Ai14 | Slc17a8-iCre | Ai14 |
| 100104685 | Slc6a3-Cre;Ai3 | Slc6a3-Cre | Ai3 |
| 112200098 | Slc6a4-CreERT2_EZ13;Ai14 | Slc6a4-CreERT2_EZ13 | Ai14 |
| 181446571 | Syt17-Cre_NO14;Ai14 | Syt17-Cre_NO14 | Ai14 |
| 146452634 | Tac1-IRES2-Cre;Ai14 | Tac1-IRES2-Cre | Ai14 |
| 156348437 | Trib2-2A-CreERT2;Ai14 | Trib2-2A-CreERT2 | Ai14 |
| 100141745 | Ucn3-Cre_KF43;Ai14 | Ucn3-Cre_KF43 | Ai14 |
| 148054441 | Vipr2-Cre_KE2;Ai14 | Vipr2-Cre_KE2 | Ai14 |
MLH were also observed in the MBAP Transgenic Cell Counts database (Figure 2). Only 4 of 77 cases exhibited MLH which may be due to the mixed background in these congenic mice or fewer histological sections from which to evaluate. Nonetheless, heterotopia were identified in F1 hybrids crossed with the same Cre-driver mice shown to have MLH in the ABA database (e.g. Emx1-Cre and Pvalb-Cre mice). These data, from two different databases, indicate that driver-reporter F1 crosses can exhibit heterotopia.
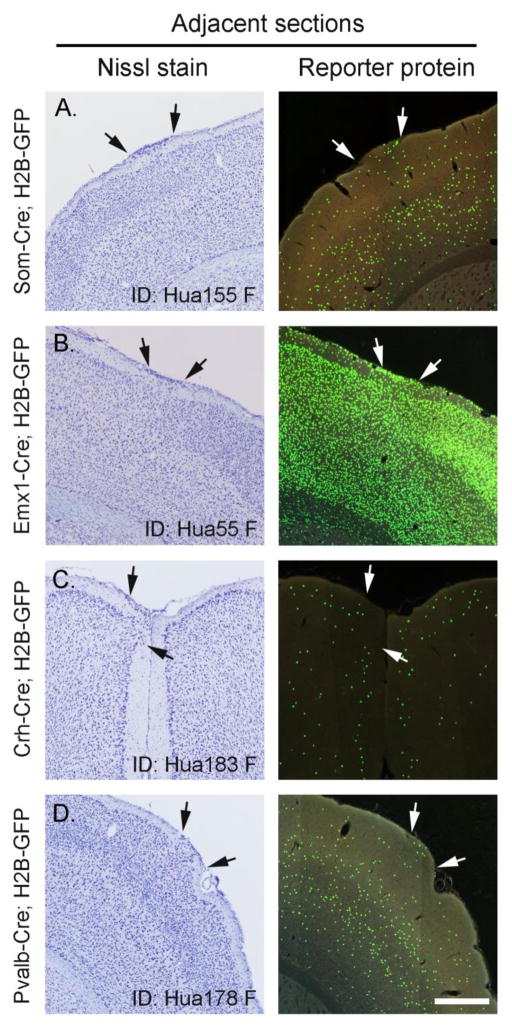
Figure 2.
A–D, Representative photomicrographs taken from F1 hybrid lines from the MBAP Transgenic Cell Counts database containing heterotopia (arrows). Nissl-stained and reporter protein expression shown in the left and right panels, respectively. Scalebar (in microns): A–B = 500; C–D = 600 microns.
Discussion
Heterotopia in the C57BL/6 linage require homozygosity at one or more alleles outside chromosome 1 and the sex chromosomes
We provide novel data on the prevalence and inheritance of spontaneous neurodevelopmental malformations of the neocortex using a combination of inbred and consomic strains as well as F1 hybrid GE mice. Our results indicate MLH may be unique to the C57BL linage as heterotopia were not observed in any other inbred strains examined. This suggests that other strains within the C57BL linage may also exhibit heterotopia, which is consistent with our previous observations of MLH in C57BL/10 mice. Since the natural history and genetic evolution/drift of this linage is well understood, documenting which C57BL stains exhibit MLH is instrumental toward a mechanistic understanding of heterotopia formation.
Using two different F1 hybrid mice produced from parental strains, including C57BL/6 and a strain with 0% prevalence for MLH (DBA/2J or 129S6/SvEvTac), our results indicate that homozygosity for one or more C57BL/6 alleles is required for heterotopia formation. This genetic model for heterotopia formation is consistent with observations of MLH in recombinant inbred mice, which by definition are homozygous at all alleles for some combination of each parental genotype [6,26,28–30,32]. Consequently, quantitative trait loci studies using a panel of recombinant inbred mice is a potential avenue to determine the genetic locus/loci responsible for MLH formation.
We used consomic mice to demonstrate that heterotopia formation requires one or more alleles outside of chromosome 1. Thus, future neuroanatomical analyses from an entire panel of consomic mice would reveal one or more strains that never exhibit heterotopia, thereby revealing the chromosome(s) where casual alleles are found. Since the prevalence of MLH in male and female C57BL/6 mice is the same [39] and F1 mice of both sexes do not display these malformations, these data argue that alleles on the sex chromosomes or chromosome 1 are not required for heterotopia formation.
Neocortical MLH in genetically-engineered mice
We document neocortical MLH in F1 hybrids of Cre-driver and reporter mouse lines even though most of these lines are on a mixed/congenic C57BL/6 background. The finding that MLH may be exclusive to the C57BL/6 linage suggests that heterotopia formation in these GE mice results from the inheritance of one or more causal C57BL/6 alleles during crossing. Consequently, GE mice created with C57BL/6 ES cells will exhibit some prevalence of heterotopia. Likewise, GE mice created with ES cells from another inbred strain but backcrossed to the C57BL/6 background will also exhibit heterotopia. Thus, results from studies using GE mice that exhibit heterotopia should be carefully evaluated so as not to attribute heterotopia formation to a genetic manipulation that was experimentally produced.
Implications of MLH on the use of C57BL/6J mice in neuroscience research
Our findings have broad implications on the use of C57BL/6 mice or GE mice on this background in neuroscience research. Firstly, results from neuroanatomical studies of neocortical development in C57BL/6 mice using experimental agents/perturbations to disrupt growth or neuronal migration should be carefully evaluated as some percentage of control and experimentally-treated mice are expected to exhibit heterotopia solely due to the genetic background. For example, in a study where C57BL/6 dams were treated with sodium acetazolamide, 34% of acetazolamide-exposed pups had heterotopia which was not significantly different than the 28% of water-treated control pups with heterotopia [48]. Secondly, results from behavioral studies using C57BL/6 mice or GE mice on this background should be carefully evaluated. Heterotopia in control and experimentally-treated mice may affect performance on the task(s) tested or interact with treatment regimes. This assertion is supported by previous findings that mice with MLH exhibit deficits in learning, memory [28–30,32], and sensory discrimination tasks [33,34,36] as well as neocortical hyperexcitability based on response to chemi-convulsant treatment [38,49]. Finally, the extent to which gene/protein expression changes are observed in neurons and glia in heterotopia remains unknown; these changes could affect neocortical expression studies, particularly when the presence/absence of heterotopia is not known prior to tissue harvesting. Thus, until the potential effects of neocortical MLH are elucidated, results from studies using C57BL/6 mice should be carefully evaluated. Histological confirmation of the presence/absence of heterotopia in mice used in a study would be critical to interpretation of experimental results.
Highlights.
Neocortical MLH is a weakly penetrant, divergent trait among inbred mice.
Homozygosity, at one or more loci, is required for the formation of heterotopia.
MLH formation requires one or more C57BL/6 alleles outside of chromosome 1.
MLH are present in diverse genetically-engineered lines on a C57BL/6 background.
Acknowledgments
This work was supported by NYIT departmental funds to R.L.R. and NIMH grant MH10368 to V.J.B. The authors thank the Veterinarian Sciences and Animal Care staff at NYIT and Wadsworth Center for the excellent care they provided for our mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarkisian MR, Bartley CM, Chi H, Nakamura F, Hashimoto-Torii K, Torii M, Flavell RA, Rakic P. MEKK4 signaling regulates filamin expression and neuronal migration. Neuron. 2006;52:789–801. doi: 10.1016/j.neuron.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheen VL, Dixon PH, Fox JW, Hong SE, Kinton L, Sisodiya SM, Duncan JS, Dubeau F, Scheffer IE, Schachter SC, Wilner A, Henchy R, Crino P, Kamuro K, DiMario F, Berg M, Kuzniecky R, Cole AJ, Bromfield E, Biber M, Schomer D, Wheless J, Silver K, Mochida GH, Berkovic SF, Andermann F, Andermann E, Dobyns WB, Wood NW, Walsh CA. Mutations in the X-linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum Mol Genet. 2001;10:1775–83. doi: 10.1093/hmg/10.17.1775. [DOI] [PubMed] [Google Scholar]
- 3.Sheen VL, Torres AR, Du X, Barry B, Walsh CA, Kimonis VE. Mutation in PQBP1 is associated with periventricular heterotopia. Am J Med Genet A. 2010;152A:2888–90. doi: 10.1002/ajmg.a.33507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert ME, Ramos RL, McCloskey DP, Goodman JH. Subcortical Band Heterotopia in Rat Offspring Following Maternal Hypothyroxinaemia: Structural and Functional Characteristics. J Neuroendocrinol. 2014;26:528–541. doi: 10.1111/jne.12169. [DOI] [PubMed] [Google Scholar]
- 5.Bai LJJ, Ramos RL, Ackman JB, Thomas AM, Lee RV. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–83. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- 6.Ramos RLSM, Toia AR, Pasternack D, Dotzler TP, Cuoco JA, Esposito AW, Le M, Parker AK, Goodman JH. Neuroanatomical characterization of the cellular and axonal architecture of subcortical band heterotopia in the BXD29-Tlr4lps-2J/J mouse cortex. Neuroscience. 2016:S0306–4522. doi: 10.1016/j.neuroscience.2016.08.049. [DOI] [PubMed] [Google Scholar]
- 7.Ramos RL, Bai J, LoTurco JJ. Heterotopia formation in rat but not mouse neocortex after RNA interference knockdown of DCX. Cereb Cortex. 2006;16:1323–31. doi: 10.1093/cercor/bhj074. [DOI] [PubMed] [Google Scholar]
- 8.Galaburda AMKT. Cytoarchitectonic abnormalities in developmental dyslexia: a case study. Ann Neurol. 1979;6:94–100. doi: 10.1002/ana.410060203. [DOI] [PubMed] [Google Scholar]
- 9.Galaburda AM, GN, Sherman GF, Rosen GD, Aboitiz F. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol. 1985;18:222–33. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- 10.Humphreys PGA, Kaufmann WE. Developmental dyslexia in women: neuropathological findings in three patients. Ann Neurol. 1990;28:727–38. doi: 10.1002/ana.410280602. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson SH, Rydenhag B, Uvebrant P, Malmgren K, Nordborg C. Widespread microdysgenesis in therapy-resistant epilepsy--a case report on post-mortem findings. Acta Neuropathol. 2002;103:74–7. doi: 10.1007/s004010100426. [DOI] [PubMed] [Google Scholar]
- 12.Nordborg C, Eriksson S, Rydenhag B, Uvebrant P, Malmgren K. Microdysgenesis in surgical specimens from patients with epilepsy: occurrence and clinical correlations. J Neurol Neurosurg Psychiatry. 1999;67:521–4. doi: 10.1136/jnnp.67.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radner S, Banos C, Bachay G, Li YN, Hunter DD, et al. β2 and γ3 laminins are critical cortical basement membrane components: ablation of Lamb2 and Lamc3 genes disrupts cortical lamination and produces dysplasia. Dev Neurobiol. 2013;73:209–229. doi: 10.1002/dneu.22057. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Jin Z, Koirala S, Bu L, Xu L, Hynes RO, Walsh CA, Corfas G, Piao X. GPR56 Regulates Pial Basement Membrane Integrity and Cortical Lamination. J Neurosci. 2008;28:5817–5826. doi: 10.1523/JNEUROSCI.0853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iguchi T, Sakata K, Yoshizaki K, Tago K, Mizuno N, Itoh H. Orphan G Protein-coupled Receptor GPR56 Regulates Neural Progenitor Cell Migration via a G 12/13 and Rho Pathway. J Biol Chem. 2008;283:14469–14478. doi: 10.1074/jbc.M708919200. [DOI] [PubMed] [Google Scholar]
- 16.Niewmierzycka ARL, Mills J, St-Arnaud R, Dedhar S. Integrin-Linked Kinase Deletion from Mouse Cortex Results in Cortical Lamination Defects Resembling Cobblestone Lissencephaly. J Neurosci. 2005;25:7022–7031. doi: 10.1523/JNEUROSCI.1695-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haubst NGM, Georges-Labouesse E, De Arcangelis A, Mayer U. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–3254. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- 18.Halfter W, Dong S, Yip Y-P, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–40. doi: 10.1523/JNEUROSCI.22-14-06029.2002. 20026580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–421. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 20.Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- 21.Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave K-A, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK Deficiency in Cells Contributing to the Basal Lamina Results in Cortical Abnormalities Resembling Congenital Muscular Dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/S0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu H, Yang Y, Eade A, Xiong Y, Qi Y. Breaches of the pial basement membrane and disappearance of the glia limitans during development underlie the cortical lamination defect in the mouse model of muscle-eye-brain disease. J Comp Neurol. 2007;501:168–183. doi: 10.1002/cne.21238. [DOI] [PubMed] [Google Scholar]
- 23.Sherman GF, Galaburda AM, Geschwind N. Cortical anomalies in brains of New Zealand mice: a neuropathologic model of dyslexia? Proc Natl Acad Sci U S A. 1985;82:8072–4. doi: 10.1073/pnas.82.23.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman GF, Galaburda AM, Behan PO, Rosen GD. Neuroanatomical anomalies in autoimmune mice. Acta Neuropathol. 1987;74:239–42. doi: 10.1007/BF00688187. [DOI] [PubMed] [Google Scholar]
- 25.Ramos RL, Smith PT, DeCola C, Tam D, Corzo O, Brumberg JC. Cytoarchitecture and transcriptional profiles of neocortical malformations in inbred mice. Cereb Cortex. 2008;18:2614–28. doi: 10.1093/cercor/bhn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipoff DM, RR, Bhambri A, Fokas GJ, Sharma S, Gabel LA, Brumberg JC, Richfield EK. Neocortical molecular layer heterotopia in substrains of C57BL/6 and C57BL/10 mice. Brain Res. 2011;1391:35–43. doi: 10.1016/j.brainres.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Ramos RL, HB, Siu NY, Brunken WJ, Yee KT, Gabel LA, Van Dine SE. Cellular and axonal constituents of neocortical molecular layer heterotopia. Dev Neurosci. 2014;36:477–89. doi: 10.1159/000365100. [DOI] [PubMed] [Google Scholar]
- 28.Boehm GW, Sherman GF, Hoplight BJ, Hyde LA, Waters NS, Bradway DM, Galaburda AM, Denenberg VH. Learning and memory in the autoimmune BXSB mouse: effects of neocortical ectopias and environmental enrichment. Brain Res. 1996;726:11–22. [PubMed] [Google Scholar]
- 29.Hoplight BJ, Sherman GF, Hyde LA, Denenberg VH. Effects of Neocortical Ectopias and Environmental Enrichment on Hebb-Williams Maze Learning in BXSB Mice. Neurobiol Learn Mem. 2001;76:33–45. doi: 10.1006/nlme.2000.3980. [DOI] [PubMed] [Google Scholar]
- 30.Hyde LA, Sherman GF, Hoplight BJ, Denenberg VH. Working memory deficits in BXSB mice with neocortical ectopias. Physiol Behav. 2000;70:1–5. doi: 10.1016/S0031-9384(00)00239-0. [DOI] [PubMed] [Google Scholar]
- 31.Hyde LA, Hoplight BJ, Harding S, Sherman GF, Mobraaten LE, Denenberg VH. Effects of ectopias and their cortical location on several measures of learning in BXSB mice. Dev Psychobiol. 2001;39:286–300. doi: 10.1002/dev.1006. [DOI] [PubMed] [Google Scholar]
- 32.Waters NS, Sherman GF, Galaburda AM, Denenberg VH. Effects of cortical ectopias on spatial delayed-matching-to-sample performance in BXSB mice. Behav Brain Res. 1997;84:23–29. doi: 10.1016/S0166-4328(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 33.Clark MG, Sherman GF, Bimonte HA, Fitch RH. Perceptual auditory gap detection deficits in male BXSB mice with cerebrocortical ectopias. Neuroreport. 2000;11:693–696. doi: 10.1097/00001756-200003200-00008. [DOI] [PubMed] [Google Scholar]
- 34.Peiffer AM, Dunleavy CK, Frenkel M, Gabel LA, LoTurco JJ, Rosen GD, Fitch RH. Impaired detection of variable duration embedded tones in ectopic NZB/BINJ mice. Neuroreport. 2001;12:2875–2879. doi: 10.1097/00001756-200109170-00024. [DOI] [PubMed] [Google Scholar]
- 35.Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in ectopic BXSB/MpJ mice. Neuroreport. 2002;13:2277–80. doi: 10.1097/01.wnr.0000044223.79663.3b. [DOI] [PubMed] [Google Scholar]
- 36.Frenkel M, Sherman GF, Bashan KA, Galaburda AM, LoTurco JJ. Neocortical ectopias are associated with attenuated neurophysiological responses to rapidly changing auditory stimuli. Neuroreport. 2000;11:575–9. doi: 10.1097/00001756-200002280-00029. [DOI] [PubMed] [Google Scholar]
- 37.Gabel LA, LoTurco JJ. Electrophysiological and morphological characterization of neurons within neocortical ectopias. J Neurophysiol. 2001;85:495–505. doi: 10.1152/jn.2001.85.2.495. [DOI] [PubMed] [Google Scholar]
- 38.Gabel LA, Manglani M, Ibanez N, Roberts J, Ramos RL, Rosen GD. Differential seizure response in two models of cortical heterotopia. Brain Res. 2013;1494:84–90. doi: 10.1016/j.brainres.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangaru Z, Salem E, Sherman M, Van Dine SE, Bhambri A, Brumberg JC, Richfield EK, Gabel LA, Ramos RL. Neuronal Migration Defect of the Developing Cerebellar Vermis in Substrains of C57BL/6 Mice: Cytoarchitecture and Prevalence of Molecular Layer Heterotopia. Dev Neurosci. 2013;35:28–39. doi: 10.1159/000346368. [DOI] [PubMed] [Google Scholar]
- 40.Eisener-Dorman AF, Lawrence DA, Bolivar VJ. Behavioral and genetic investigations of low exploratory behavior in Il18r1/mice: We can’t always blame it on the targeted gene. Brain Behav Immun. 2010;24:1116–1125. doi: 10.1016/j.bbi.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Dine SE, Siu NY, Toia A, Cuoco JA, Betz AJ, Bolivar VJ, Torres G, Ramos RL. Spontaneous malformations of the cerebellar vermis: Prevalence, inheritance, and relationship to lobule/fissure organization in the C57BL/6 lineage. Neuroscience. 2015;310:242–251. doi: 10.1016/j.neuroscience.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Ramos RL, Van Dine SE, George E, Patel D, Hoplight BJ, Leheste JR, Richfield EK, Torres G. Molecular layer heterotopia of the cerebellar vermis in mutant and transgenic mouse models on a C57BL/6 background. Brain Res Bull. 2013;97:63–68. doi: 10.1016/j.brainresbull.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Morris JA, Royall JJ, Bertagnolli D, Boe AF, Burnell JJ, Byrnes EJ, Copeland C, Desta T, Fischer SR, Goldy J, Glattfelder KJ, Kidney JM, Lemon T, Orta GJ, Parry SE, Pathak SD, Pearson OC, Reding M, Shapouri S, Smith KA, Soden C, Solan BM, Weller J, Takahashi JS, Overly CC, Lein ES, Hawrylycz MJ, Hohmann JG, Jones AR. Divergent and nonuniform gene expression patterns in mouse brain. Proc Natl Acad Sci. 2010;107:19049–19054. doi: 10.1073/pnas.1003732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, Bohn P, Mortrud M, Ouellette B, Kidney J, Smith KA, Dang C, Sunkin S, Bernard A, Oh SW, Madisen L, Zeng H. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits. 2014;8 doi: 10.3389/fncir.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24:221–5. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- 47.Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O’Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–8. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- 48.Sherman GF, Holmes LB. Cerebrocortical microdysgenesis is enhanced in c57BL/6J mice exposed in utero to acetazolamide. Teratology. 1999;60:137–42. doi: 10.1002/(SICI)1096-9926(199909)60:3<137::AID-TERA8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Gabel LA, LoTurco JJ. Layer I ectopias and increased excitability in murine neocortex. J Neurophysiol. 2002;87:2471–9. doi: 10.1152/jn.2002.87.5.2471. [DOI] [PubMed] [Google Scholar]