Abstract
Metastatic breast cancer is a heterogeneous disease that presents in varying forms, and a growing number of therapeutic options makes it difficult to determine the best choice in each particular situation. When selecting a systemic treatment, it is important to consider the medication administered in the previous stages, such as acquired resistance, type of progression, time to relapse, tumor aggressiveness, age, comorbidities, pre- and post-menopausal status, and patient preferences. Moreover, tumor genomic signatures can identify different subtypes, which can be used to create patient profiles and design specific therapies. However, there is no consensus regarding the best treatment sequence for each subgroup of patients. During the SABCC Congress of 2014, specialized breast cancer oncologists from referral hospitals in Europe met to define patient profiles and to determine specific treatment sequences for each one. Conclusions were then debated in a final meeting in which a relative degree of consensus for each treatment sequence was established. Four patient profiles were defined according to established breast cancer phenotypes: pre-menopausal patients with luminal subtype, post-menopausal patients with luminal subtype, patients with triple-negative subtype, and patients with HER2-positive subtype. A treatment sequence was then defined, consisting of hormonal therapy with tamoxifen, aromatase inhibitors, fulvestrant, and mTOR inhibitors for pre- and post-menopausal patien ts; a chemotherapy sequence for the first, second, and further lines for luminal and triple-negative patients; and an optimal sequence for treatment with new antiHER2 therapies. Finally, a document detailing all treatment sequences, that had the agreement of all the oncologists, was drawn up as a guideline and advocacy tool for professionals treating patients with this disease.
Keywords: Metastatic breast cancer, Hormone therapy, Chemotherapy, Targeted therapies, HER2 receptor, Triple-negative tumor
Introduction
The annual incidence of breast cancer in Spain is around 27,000 cases [1], and it causes more than 6200 deaths per year [1, 2]. Metastatic breast cancer (MBC), in particular, is a disease that varies widely, depending on the site of metastasis and its aggressiveness. It may present de novo (6–10 % of breast cancers) or it may appear as recurrent disease (20–50 % of patients) [3]. It occurs in many forms, each associated with a better or poorer disease prognosis [4]. While the aim of breast cancer treatment in other stages is curative, the objectives in the metastatic stage are mainly palliative [3]. The goal, then, is to increase survival and symptom control, while minimizing toxicity. However, treatment guidelines for MBC are not yet clearly defined.
Selecting a systemic treatment for MBC is a complex process, in which different factors must be considered. Some parameters are associated with the disease itself, including hormone receptor and HER2 receptor status, tumor proliferation index, previous disease-free survival (DFS), response to previous treatments, tumor molecular signatures [5], and tumor load. Other aspects to consider include the personal characteristics of the patient, such as age, menopausal status, personal preferences, comorbidities, adverse effects of previous treatments, psychological, and socioeconomic factors, etc.
Proper knowledge of the different therapeutic options is necessary to establish optimal and homogeneous treatment sequences. Multiple clinical guidelines for breast cancer are available, such as ESMO-ABC2, SEOM, GEICAM, and ASCO, etc. [6–9]. However, there is some ambivalence about best treatment options for each line. For example, a patient with a HER2-negative, hormone receptor-positive tumor could be treated in the first line with several hormone treatments and with different chemotherapy drugs, such as taxanes, anthracyclines, vinorelbine, or capecitabine in combination with bevacizumab [5, 7, 8]. This wide spectrum, ideal for individualized treatment, can be counterproductive in terms of the uncertainty it generates. Indeed, two patients with similar biologic and clinical characteristics can be treated in opposite ways.
While considering the obvious constraints of a consensual document, it may be interesting to set down a series of general treatment recommendations. This document is a reflection of the working criteria of several oncology specialists active in this field in Spain.
Methodology
On the occasion of the San Antonio Breast Cancer Congress (SABCC) in 2014, an international group of breast cancer specialists held a parallel meeting, the aim of which was to define, on the basis of several clinical cases, the different profiles of MBC patients who may be candidates for similar treatment regimens during the natural history of their disease. Some of the Spanish oncologists who had attended the first meeting met later to review the different patient profiles, and some subgroups were established.
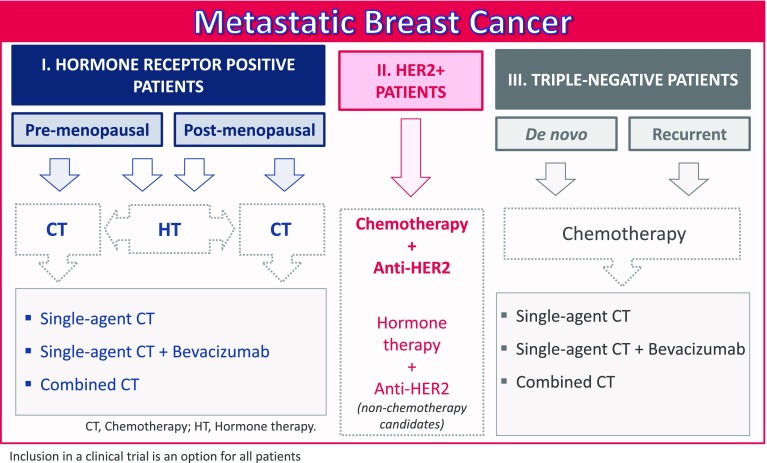
In this second meeting, the major patient profiles identified on the basis of a literature review were the following: breast cancer patients with positive hormone receptors (split into two subgroups, pre-menopausal and post-menopausal, with distinct hormone therapy approaches); patients with HER2-positive disease; and patients with triple-negative tumors (Fig. 1). The possibility of distinguishing between luminal A- and B-positive hormone receptors was discussed, but any differences were not determinant for the purpose of treatment sequencing, even if this information is useful for selecting the type of treatment (hormone therapy vs chemotherapy).
Fig. 1.
General treatment regimens for metastatic breast cancer
These proposals were discussed in a final meeting attended by a large group of Spanish breast cancer specialists, including the oncologists who had participated in the first two meetings. In this final meeting, the patient profiles were validated by all participant oncologists, who are the authors of this paper. Four working groups defined the most appropriate treatment sequences for each profile, which were subsequently validated by all participants, and the level of agreement was recorded.
Finally, the panel of breast cancer specialists drew up a document which was validated by all authors. Below is a summary of the agreed treatment sequences.
Treatment option recommendations
There was no clear agreement on the preferential treatment sequence, although there is high agreement (95 %; 19/20) on favoring the sequential single-agent chemotherapy over combined chemotherapy in MBC. Combined chemotherapy is more toxic, and while the combination of several drugs may have more impact on the tumor, it remains unclear whether the lower doses and treatment time compared to monotherapy compensate for this increased toxicity [10].
The criteria for treatment according to the different profiles identified are detailed below. The group of hormone receptor-positive patients was divided into two subgroups, depending on the menopausal status,. However, we would like to point out that any patient with any tumor profile or at any disease stage may be considered for inclusion in a clinical trial, if this possibility exists.
Patients with hormone receptor-positive and HER2-negative tumors
Approximately 67–70 % of all metastatic breast tumors contain estrogen and progesterone hormone receptor-positive cells [11, 12]. The initial endocrine treatment administered will vary according to the patient’s menopausal status.
Hormone therapy drugs administered in standard clinical practice include tamoxifen (selective estrogen receptor modulator) [13, 14], fulvestrant (selective estrogen receptor antagonist) [15, 16], and aromatase inhibitors (estrogen synthesis blockers). The latter, including letrozole and anastrozole [13], are non-steroidal and reversible, while other compounds, such as exemestane, are steroidal and bind irreversibly [17]. They are used alone or in association with luteinizing hormone-releasing hormone (LHRH) analogs [18–20] or anti-target therapies, such as everolimus [17, 21]. Everolimus is an mTOR complex inhibitor which modulates cell growth and proliferation and can reverse resistance to hormone therapy.
For this patient profile, the hormone treatment for pre-menopausal and post-menopausal subgroups will be defined first, and then, the chemotherapy indications will be described.
Hormone therapy in pre-menopausal patients
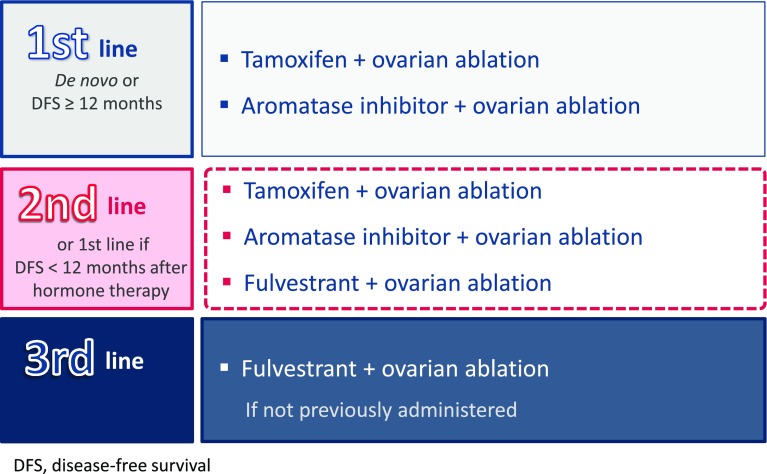
In this subgroup, the aim of hormone therapy is to achieve post-menopausal hormone levels in the pre-menopausal patient. If the patient is diagnosed with metastasis de novo, or she has had a DFS of 12 months or more and few symptoms, recommended the first-line treatment is ovarian ablation along with tamoxifen (SEOM level of certainty: high; strength of recommendation: A), or an aromatase inhibitor, which increases the clinical benefit with an acceptable safety profile (ESMO Categories of Evidence and Consensus: IA). A meta-analysis of the combination of tamoxifen and ovarian ablation showed a significant increase in overall survival (OS) (HR 0.78; p = 0.002) [18] and ovarian ablation combined with aromatase inhibitor showed a median time to progression (TTP) of 12 months [19].
In the second-line hormone treatment, or for patients with DFS of less than 12 months after treatment, ovarian ablation is recommended (SEOM level of certainty: low; strength of recommendation: B). This may be combined with an aromatase inhibitor [19], fulvestrant [20] or tamoxifen [18], depending on the patient’s previous treatment (ESMO Categories of Evidence and Consensus: IB) (SEOM level of Certainty: low; strength of recommendation: B). Fulvestrant and ovarian ablation has shown a median TTP of 6 months and a median OS of 32 months [20].
Finally, for the third-line treatment, we recommend ovarian ablation and fulvestrant [20], if this drug has not been previously used (Fig. 2).
Fig. 2.
Proposed hormone therapy for pre-menopausal patients with hormone receptor-positive disease
Hormone therapy in post-menopausal patients
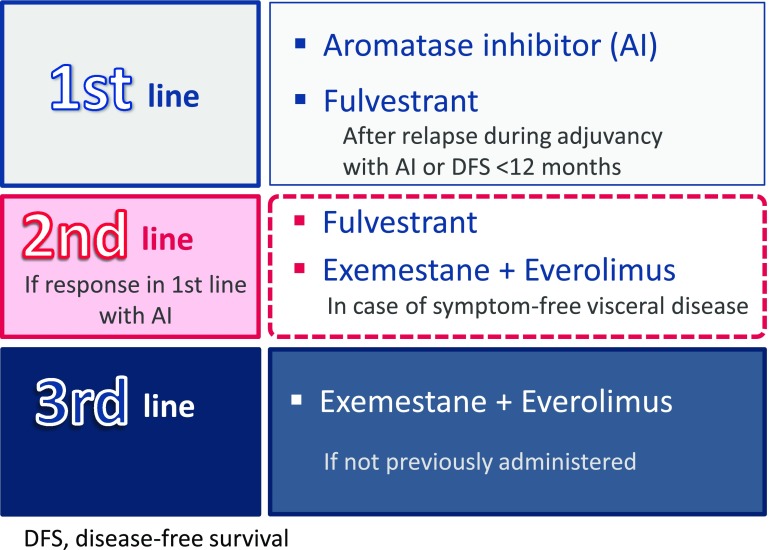
In the first-line hormone therapy in post-menopausal patients (including cases of visceral metastasis, in the absence of visceral crisis), aromatase inhibitors are recommended, providing a median progression-free survival (PFS) of between 8 and 10 months, an objective response rate (ORR) of 33–46 %, and clinical benefit around 55 % [13, 15, 24] (ESMO Categories of Evidence and Consensus: IA) (SEOM level of certainty: high; strength of recommendation: A). In patients diagnosed with recurrence during adjuvant treatment with an aromatase inhibitor or who have a DFS of less than 12 months, treatment with fulvestrant is recommended [15, 24], (ESMO Categories of Evidence and Consensus: IB) (SEOM level of certainty: moderate; strength of recommendation: B), or the combination of exemestane and everolimus [17, 21], depending on the patient’s clinical status (ESMO Categories of Evidence and Consensus: IB). Efficacy data for fulvestrant show a TTP of 23.4 months and an OS of 54 months [15, 24], while for the combination of exemestane and everolimus, median PFS during the centralized evaluation was 10.6 months [17, 21]. In the sub-analysis by subgroups, median PFS for patients that had progressed to adjuvant treatment before 12 months was 15.2 months.
If the patient responded to the first-line treatment with an aromatase inhibitor, fulvestrant is recommended as the second line (median PFS 4.8 months [16]), or in the absence of symptoms of visceral disease, another option is the combination of exemestane and everolimus, which improves PFS [17, 21] (ESMO Categories of Evidence and Consensus: IA) (SEOM level of certainty: high; strength of recommendation: A).
In the third-line endocrine therapy, exemestane combined with everolimus [17] is recommended, if not used previously (Fig. 3).
Fig. 3.
Proposed hormone therapy for hormone receptor-positive patients post-menopause or after ovarian ablation
Chemotherapy in patients with hormone receptor-positive disease
Chemotherapy for hormone receptor-positive patients includes microtubule inhibitors, such as taxanes (docetaxel [25], paclitaxel [26], and nab-paclitaxel [27], a nanoparticle albumin-bound paclitaxel), which are tubulin depolymerization inhibitors. Their mode of action consists of stabilizing GDP-bound tubulin in the microtubule. Other microtubule inhibitors are the vinca alkaloid vinorelbine [28], which is a tubulin polymerization inhibitor, and eribulin [29], a synthetic macrocyclic analog which binds to the positive growing end of the microtubules, suppressing dynamic instability.
Anthracyclines, such as doxorubicin and epirubicin, and pegylated liposomal doxorubicin [30], are cytotoxic agents that inhibit topoisomerase II, and DNA/RNA synthesis and generate oxygen free radicals. Similarly, gemcitabine is a pyrimidine antimetabolite that inhibits DNA synthesis [31], capecitabine acts as a thymidylate synthase inhibitor, and cyclophosphamide is an alkylating agent that interferes with DNA replication [32]. New agents include bevacizumab, a humanized anti-vascular endothelial growth factor (VEGF) monoclonal antibody [33].
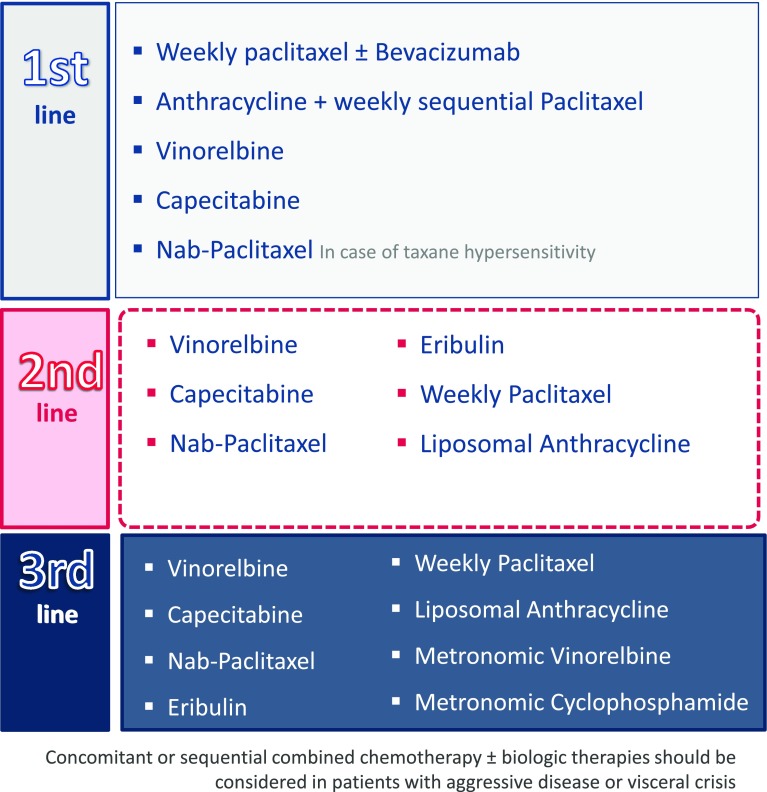
In the case of aggressive disease, combined chemotherapy, such as paclitaxel plus bevacizumab, must be considered in the first-line chemotherapy treatment [33] when a rapid tumor response is desired (SEOM level of certainty: moderate; strength of recommendation: C). Treatment with anthracyclines and taxanes, either sequential or in combination [34, 35], may also be evaluated in this situation (SEOM level of certainty: high; strength of recommendation: A), including liposomal anthracyclines (median PFS 6.9 months and median OS 21 months in the first line [30]). Nab-paclitaxel is also an option for patients with taxane hypersensitivity. It has shown a median PFS of 13 months and a median OS of 33.8 months [36]. Another possibility in this situation is to use a combination of paclitaxel and gemcitabine [31], which has shown an improvement of TTP and OS (6.1–18.6 months, respectively).
For the first-line therapy in all other cases, monotherapy can be started with weekly paclitaxel (median PFS 5.9 months and median OS 25.2 months [26, 33]) or oral drugs, such as vinorelbine [28, 37] (median PFS 4.2–4.4 months and median OS of 16.4–24 months) or capecitabine [32, 38] (median TTP 4.1 months and median OS 19.6 months), according to patient preference (ESMO Categories of Evidence and Consensus: IA). Paclitaxel can also be used in combination with bevacizumab (median PFS 11.8 months and median OS 26.7 months [33]) or sequentially with anthracyclines (SEOM level of certainty: high; strength of recommendation: A) [34, 35]. In addition, for elderly patients or those in special situations, the use of metronomic regimens should be evaluated [41] (SEOM level of certainty: moderate; strength of recommendation: B).
In the second-line therapy, the use of capecitabine [32, 38], vinorelbine [28, 37], nab-paclitaxel (median OS 14 months [27]), or eribulin (median OS 15.2 months [29, 42]) is recommended in both pre- and post-menopausal patients, depending on the regimen administered in the first line (SEOM level of certainty: high; strength of recommendation: A). Other options include weekly paclitaxel [26] or liposomal anthracyclines [30]. Again, metronomic regimens (cyclophosphamide or vinorelbine) should be considered for elderly patients or those in special situations [41]. Vinorelbine has been studied in elderly patients, obtaining an ORR of 38 %, a median PFS of 7.7 months, and an OS of 15.9 months [43], and cyclophosphamide obtained an ORR of 31 % [44].
Finally, third-line treatment in these cases consists of any of the above-listed options not previously used. At this time, metronomic regimens with vinorelbine [41, 43] or cyclophosphamide [41, 44] may be considered for all patients, not only the frail and the elderly, depending on their expectations and wishes (Fig. 4).
Fig. 4.
Proposed chemotherapy treatment for patients with hormone receptor-positive disease
Patients with HER2-positive disease
HER2 is an external membrane receptor of the breast cells that regulates proliferation. It has tyrosine kinase activity and is the product of the ERBB2 oncogene. HER2-positive disease accounts for 15–20 % of all breast cancers and is associated with a more aggressive natural history [11, 12].
A series of anti-HER2 drugs targeting this protein have been developed. These include the monoclonal antibodies, trastuzumab and pertuzumab, which bind to an extracellular component of HER2 [45]. Another compound is lapatinib, an intracellular tyrosine kinase inhibitor, which blocks the receptor signaling cascade [46]. Finally, the antibody–drug conjugate (adc) ado-trastuzumab emtansine (T-DM1) works in two ways: by disrupting HER2 signaling and by causing direct cytotoxicity [39]. Treatment of HER2-positive breast cancer generally consists of anti-HER2 inhibitors combined with the conventional chemotherapy (ESMO Categories of Evidence and Consensus: IA) (SEOM level of certainty: high; strength of recommendation: A).
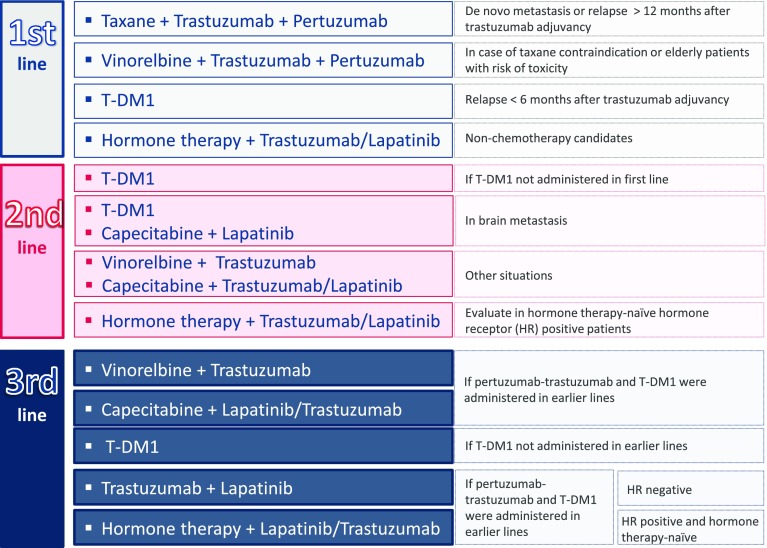
As can be seen in Fig. 5, the first-line treatment in the case of de novo metastasis (SEOM level of certainty: high; strength of recommendation: A) or relapse after the first year following the end of adjuvant treatment with trastuzumab [47] (SEOM level of certainty: moderate; strength of recommendation: B) consists of a triple combination of taxanes with trastuzumab and pertuzumab. This combination has increased OS (56.5 months) and PFS (18.7 months) [45, 48] (ESMO Categories of Evidence and Consensus: IA). If taxanes are contraindicated, they should be replaced by vinorelbine in the same combination, which has shown a median PFS of 11.4–14.3 months [49, 50] (SEOM level of certainty: low; strength of recommendation: C). This same regimen should be considered for elderly patients with a risk of taxane toxicity. Generically, combining anthracyclines with trastuzumab is not recommended, since it increases the risk of cardiotoxicity [52] If the patient relapses within the first 6 months after completing the trastuzumab adjuvant treatment, the first-line treatment with T-DM1 is recommended, since it has shown a median PFS and OS of 15.2–29.8 months, respectively, and appears to be less toxic than lapatinib combined with capecitabine [39, 53] (SEOM level of certainty: moderate; strength of recommendation: B).
Fig. 5.
Proposed treatment for patients with HER2-positive breast cancer
In hormone receptor-positive patients who are not candidates for chemotherapy, the administration of endocrine therapy combined with trastuzumab must be evaluated [54] (ESMO Categories of Evidence and Consensus: IA) (SEOM level of certainty: moderate; strength of recommendation: B). This recommendation is based on the results of the eLEcTRA study, which found that this treatment offered a median TTP of 14.1 months [55]. Hormone therapy combined with lapatinib also improves the median PFS (8.2 months) and clinical benefit rate (48 %) [56].
T-DM1 is recommended for the second-line treatment with a median PFS of 9.6 months and a median OS of 30.9 months [39] (ESMO Categories of Evidence and Consensus: IA) (SEOM level of certainty: high; strength of recommendation: A). Although the EMILIA study was not restricted to patients with brain metastases, this compound has shown activity in this subgroup of patients (median PFS 5.9 months and median OS 26.8 months [57]). Similarly, the combination of capecitabine and lapatinib improves survival outcomes in this subgroup [57, 58] (SEOM level of certainty: moderate; strength of recommendation: B). Other options in the second line could be to combine vinorelbine with trastuzumab (median TTP 15.3 months and median OS 38.8 months) [59, 60], or capecitabine with trastuzumab (median PFS 8.1 months and OS 27.3 months) [61] or capecitabine with lapatinib (median PFS 8.4 months and OS around 19 months) [62]. For the hormone therapy-naïve patients with hormone receptor-positive disease, hormone therapy combined with lapatinib [56] or trastuzumab [55] may be an option (SEOM level of certainty: moderate; strength of recommendation: B).
Third-line options depend on which regimens have already been administered. If the patient has received pertuzumab with trastuzumab and T-DM1, the recommendation is to combine trastuzumab with vinorelbine, a combination which causes fewer adverse effects than docetaxel [59, 60]. Another option is to combine capecitabine with lapatinib [46, 61] or trastuzumab (ESMO Categories of Evidence and Consensus: IB), which has shown good response rates in patients with progressive disease [61–63]. T-DM1 is recommended in patients who have not used it previously, with a median PFS of 6.2 months [64] (ESMO Categories of Evidence and Consensus: IB) (SEOM level of certainty: high; strength of recommendation: A). Finally, patients with hormone receptor-negative tumors may receive trastuzumab plus lapatinib. This combination improves clinical benefit, PFS and OS (14 months), compared to lapatinib alone [65] (SEOM level of certainty: moderate; strength of recommendation: B). If the cancer is hormone receptor-positive and the patient is hormone therapy-naïve, hormone treatment may be administered together with lapatinib [56] or trastuzumab [55] (SEOM level of certainty: moderate; strength of recommendation: B) (Fig. 5).
Patients with triple-negative breast cancer
This group of patients constitutes approximately 10–20 % of breast cancer cases [66]. Triple-negative cancer is characterized by cells that express neither hormone nor HER2 receptors. Accordingly, treatment is based on the use of chemotherapy and biological therapies. Platinum compounds, widely used in the first line, include cisplatin and carboplatin, which are alkylating-like agents that interfere with DNA replication [67].
De novo metastasis or relapse after >12 months disease-free survival
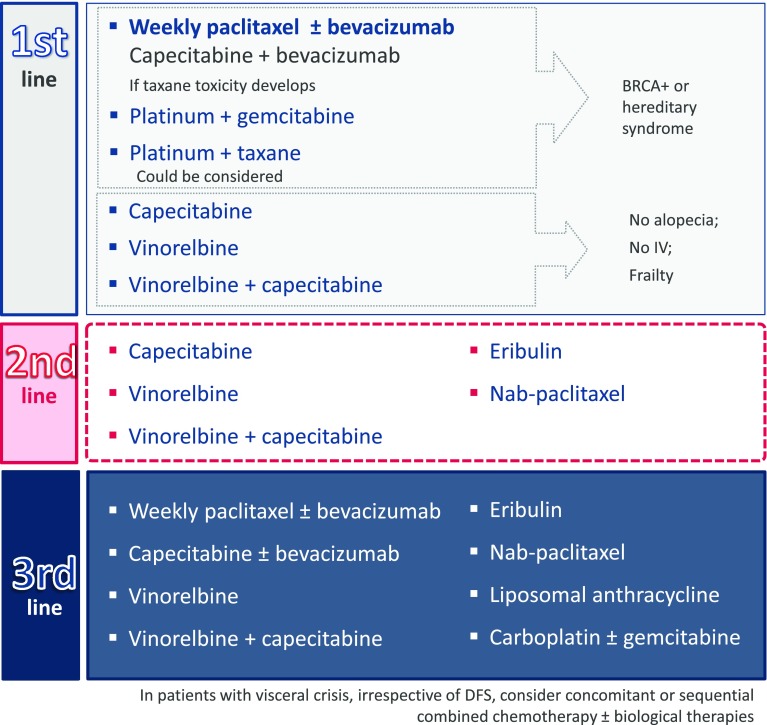
In patients with de novo metastasis or DFS >12 months, the recommendation for the first-line treatment is weekly paclitaxel, either as a single agent (ESMO Categories of Evidence and Consensus: IA) or combined with bevacizumab (SEOM level of certainty: moderate; strength of recommendation: C). The combination has been reported to increase median PFS [33]. If the patient responds to treatment, but develops early toxicity, paclitaxel can be switched to capecitabine, but not as a maintenance treatment. This recommendation is based on a phase III study which found that the PFS and safety profiles of the two combinations should, in principle, be equivalent (median PFS 6.1 months for the capecitabine arm and 6.5 for the taxane arm) [68]. Another good option for frail patients or for those who cannot or do not want to receive intravenous chemotherapy is the oral administration of capecitabine [32, 38] and vinorelbine [28, 37] separately or in combination (median OS 22.2 months with a Disease Control Rate [DCR] of 70.5 %) [69]. This is also the preferred choice for patients who want to avoid alopecia (Fig. 6) (ESMO Categories of Evidence and Consensus: IA) (SEOM level of certainty: moderate; strength of recommendation: B).
Fig. 6.
Proposed treatment for patients with triple-negative metastatic cancer de novo or with a progression-free survival greater than 12 months
BRCA-positive patients or hereditary syndrome may receive a platinum salt in monotherapy (ESMO Categories of Evidence and Consensus: IC) (SEOM level of certainty: moderate; strength of recommendation: B). A phase III study comparing carboplatin with docetaxel, both in monotherapy, achieved a median OS of 12.3–12.4 months, and a median PFS of 4.5–3.1 months, respectively, although in the mutated BRCA subgroup PFS was 6.8 months for the carboplatin arm and 4.8 months for the docetaxel arm [70]. The platinum salt can also be administered in combination with gemcitabine (SEOM level of certainty: low; strength of recommendation: B). In a global population, this combination has shown a 32 % ORR, a median PFS of 4.1 months, and a median OS of 11.1 months [71] The combination of a platinum salt with a taxane may be evaluated: high response rates have been reported (62 %) with a median TTP of 4.8 months and a median OS of 16 months [72]. Another alternative is a taxane combined with bevacizumab [33].
In the second line, the recommendation is to use capecitabine [32, 38], or vinorelbine [28, 37], or a combination of both. In this same setting, eribulin treatment has been shown to improve OS compared to other treatments selected according to the investigator’s criteria in a phase III trial and in the pooled analysis of the two available phase III studies [29, 42]. Another option would be to use nab-paclitaxel, which has shown greater efficacy and a better safety profile than tri-weekly paclitaxel [27] and tri-weekly docetaxel [36].
In the third-line treatment, any of the drugs of the previously mentioned lines that have not yet been used are recommended. Additional recommended options include liposomal anthracycline [30] and carboplatin, with or without gemcitabine [70, 71]. The results of a phase III trial suggest that liposomal anthracycline is equally effective as standard anthracycline, but associated with lower cardiotoxicity [30]. Treatment regimens are summarized in Fig. 6.
In patients with visceral crisis, irrespective of DFS, concomitant or sequential combined chemotherapy may be considered, with the possible addition of biological therapies.
Metastasis due to disease recurrence during the first 12 months
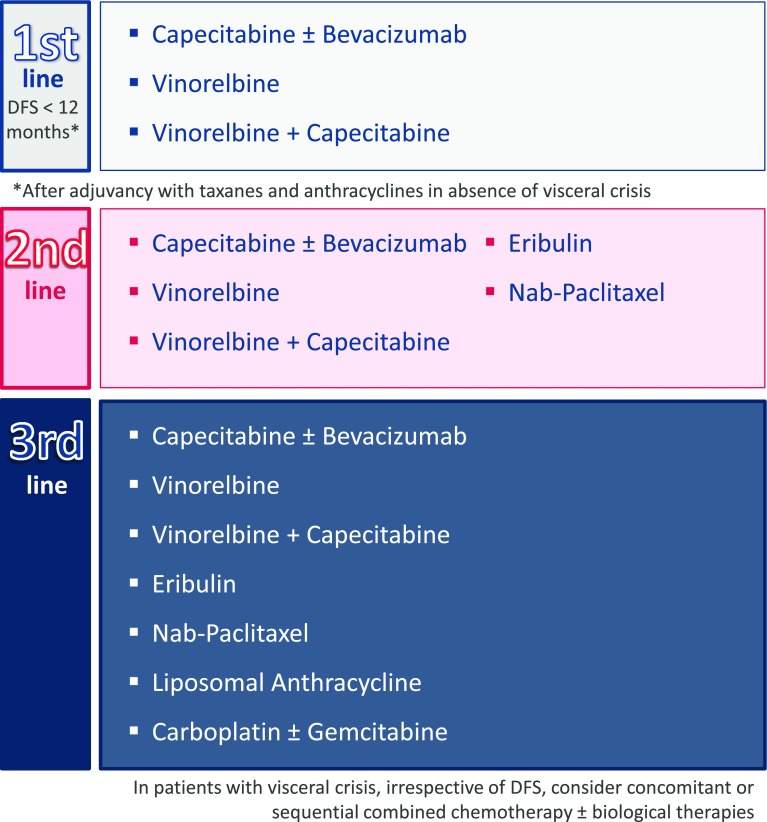
If a patient’s DFS lasts less than 12 months after adjuvant treatment with taxanes and anthracyclines, the options for the first-line treatment include vinorelbine (a good salvage therapy after failure on anthracyclines and taxanes, since it does not cross-react with taxanes [28, 37, 73]), and capecitabine (that moreover can be combined with bevacizumab [32, 38, 68] or vinorelbine [69]) (Fig. 7) (ESMO Categories of Evidence and Consensus: IB).
Fig. 7.
Proposed treatment for patients with triple-negative metastatic cancer with a progression-free survival less than 12 months
For the second-line therapy, eribulin [29, 42] or nab-paclitaxel [27, 36], are recommended, as well as capecitabine, in combination with bevacizumab; another option is vinorelbine separately or in combination with capecitabine, if not previously used. In the third line, we recommend any of the drugs of the previously mentioned lines that has not yet been used (Fig. 7), including liposomal anthracycline [30] or carboplatin as a single agent or in combination with gemcitabine [70, 71].
In patients with visceral crisis, irrespective of DFS, concomitant or sequential combined chemotherapy may be considered, with the possible addition of biological therapies.
Future perspectives
The outlook for these patient profiles is evolving rapidly, and some new drugs are currently in development. Palbociclib, an oral small-molecule selective inhibitor of cyclin-dependent kinase 4/6, has been developed for patients with HER2-negative and hormone receptor-positive tumors. It was tested in PALOMA-1, a phase II trial comparing letrozole alone or in combination with palbociclib in previously untreated post-menopausal patients. Primary endpoint was PFS. The experimental arm obtained a median PFS of 20.2 vs 10.2 months in the placebo arm (HR 0.488; p = 0.004) [75]. PALOMA-3, the phase III trial, included pre- and post-menopausal patients who progressed on one prior endocrine therapy, with an aromatase inhibitor in the case of post-menopausal patients. These patients were randomized to receive fulvestrant combined with palbociclib or placebo. Efficacy results were recently reported. The study met its primary endpoint with a median PFS of 9.3 months for the experimental arm vs 4.6 months in the placebo arm (HR 0.46; p < 0.001) [76]. The FDA approved the indication of palbociclib in combination with fulvestrant after progression on a previous endocrine therapy in February, 2016 [77]. Currently, 666 patients are enrolled in PALOMA-2, a phase III trial comparing letrozole combined with palbociclib vs placebo in post-menopausal patients who are candidates for the first-line treatment with endocrine therapy. Study results have not yet been reported (NCT01740427).
Neratinib, an oral irreversible pan-ErbB receptor tyrosine kinase inhibitor, has been developed for patients with HER2-positive tumors. NEfERT-T is a randomized controlled phase II study which tested the combination of paclitaxel with neratinib or trastuzumab in the first line. Median PFS, the primary endpoint, was 12.9 months for both combinations (HR 1.02; p = 0.89). However, combination with neratinib reduces symptomatic CNS recurrences (HR 0.48; p = 0.002) and 2-year incidence of CNS metastasis (HR 0.45; p = 0.004) [78]. Another randomized phase II trial was designed to demonstrate the non-inferiority of single-agent neratinib in PFS vs capecitabine combined with lapatinib. Median PFS of neratinib was 4.5 vs 6.8 months for the combination arm. Median OS was also superior in the combination arm (19.7 vs 23.6 months) [79]. A phase I/II trial which tested the combination of capecitabine with neratinib showed a promising ORR of 64 % in patients not previously exposed to lapatinib, and 57 % in previously exposed patients. Median PFS was 40.3–35.9 weeks, respectively [80]. Currently, a phase III trial comparing capecitabine plus neratinib or lapatinib is recruiting patients who have previously received with at least two anti-HER2 therapies. This study is exploring two co-primary endpoints, PFS and OS (NCT01808573).
In patients with triple-negative tumors, olaparib, a novel orally active poly(ADP-ribose) polymerase (PARP) inhibitor which induces synthetic lethality in BRCA-deficient cells is being developed in breast cancer with BRCA 1/2 mutations. It is currently indicated in patients with platinum-sensitive relapse of a BRCA-mutated ovarian cancer. In BRCA-mutated breast cancer, a phase II trial was developed with the inclusion of 54 patients. The first cohort of 27 patients received olaparib 400 mg twice daily and the second cohort of 27 patients received 100 mg twice daily. ORR, the primary endpoint, was 41 % in the first cohort and 22 % in the second one [81]. A phase III trial, OlympiAD, is now being developed. Patients with pre-treated BRCA 1/2 mutated breast cancer were randomized to receive olaparib in monotherapy or their physicians’ choice of chemotherapy. Results of this study have not yet been reported (NCT02000622).
Conclusion
Three MBC patient profiles have been outlined in this document. They have been classified according to genomic characteristics, and subgroups have been formed according to DFS and pre- and post-menopausal status, which can be treated differently.
In their clinical practice, the specialists we consulted reserve chemotherapy for the treatment of patients with triple-negative breast cancer, aggressive hormone receptor-positive disease, visceral crisis, or hormone therapy resistance. It is also used to complement biological therapy in patients with HER2-positive disease.
Patients with hormone receptor-positive tumors who do not have aggressive disease or visceral crisis receive endocrine therapy as a first option and up to three lines of treatment may be administered.
In patients who are already hormone-resistant or who have more aggressive disease than candidates for endocrine therapy, but not as aggressive as candidates for combined chemotherapy, the treatment of choice may be single-agent therapy with vinorelbine or capecitabine, but anthracycline or paclitaxel must also be taken into consideration. In addition, in the case of aggressive disease or visceral crisis, paclitaxel can be used in combination with bevacizumab.
In patients with HER2-positive disease, the recommendation is to combine a taxane with dual anti-HER2 blockade with trastuzumab and pertuzumab. If taxanes are contraindicated, vinorelbine can be used instead. All patients with HER2-positive disease can be treated with T-DM1 in any of the lines (in the case of the first-line treatment, those with relapse diagnosed during the first 6 months after completing adjuvancy).
For patients with triple-negative cancer, a common first-line option is weekly paclitaxel, with or without bevacizumab, unless resistance has developed (DFS < 12 months), in which case capecitabine or vinorelbine may be administered. In the second line, eribulin or nab-paclitaxel are generally given, and in the third line, liposomal anthracycline and carboplatin combined with gemcitabine are added to the list of choices.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Research involving human participants and/or animals
Not applicable to this type of consensus document.
Informed consent
Not applicable to this type of consensus document.
References
- 1.Sanchez MJ, Payer T, De Angelis R, Larranaga N, Capocaccia R, Martinez C. Cancer incidence and mortality in Spain: estimates and projections for the period 1981–2012. Ann Oncol. 2010;21(3):iii30–iii36. doi: 10.1093/annonc/mdq090. [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24(3):792–800. doi: 10.1093/annonc/mdt010. [DOI] [PubMed] [Google Scholar]
- 3.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 4.Puig-Vives M, Sanchez MJ, Sanchez-Cantalejo J, Torrella-Ramos A, Martos C, Ardanaz E, et al. Distribution and prognosis of molecular breast cancer subtypes defined by immunohistochemical biomarkers in a Spanish population-based study. Gynecol Oncol. 2013;130(3):609–614. doi: 10.1016/j.ygyno.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 5.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)dagger. Ann Oncol. 2014;25(10):1871–1888. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Di Leo A, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(29):3307–3329. doi: 10.1200/JCO.2014.56.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavila J, Lopez-Tarruella S, Saura C, Munoz M, Oliveira M, De la Cruz-Merino L, et al. SEOM clinical guidelines in metastatic breast cancer 2015. Clin Transl Oncol. 2015;17(12):946–955. doi: 10.1007/s12094-015-1476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guía GEICAM de Práctica Clínica Para el Diagnóstico y Tratamiento del Cáncer de Mama Metastásico.2015. https://www.google.es/url?sa = t&rct = j&q = &esrc = s&source = web&cd = 2&ved = 0ahUKEwioh7_oo9TMAhVClxoKHUTJCAwQFgguMAE&url = http%3A%2F%2Fwww.guiasalud.es%2FGPC%2FGPC_538_AF%2520GUIA%2520GEICAM_resumida.pdf&usg = AFQjCNH73GDVc2bzyRoeGVJC2uHKYyTkHw&sig2 = tTLxmquVf6D_FWm0i5sfdg&bvm = bv.121926005,d.d2 s&cad = rja
- 10.Dear RF, McGeechan K, Jenkins MC, Barratt A, Tattersall MH, Wilcken N. Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2013;12:CD008792. doi: 10.1002/14651858.CD008792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandoval C, Rahal R, Forte T, Klein-Geltink J, He D, Bryant H. Indicator measures er/pr and her2 testing among women with invasive breast cancer. Curr Oncol. 2013;20(1):62–63. doi: 10.3747/co.20.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 13.Bonneterre J, Thurlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18(22):3748–3757. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 14.Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(30):4883–4890. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson JF, Lindemann JP, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503–511. doi: 10.1007/s10549-012-2192-4. [DOI] [PubMed] [Google Scholar]
- 16.Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14(10):989–998. doi: 10.1016/S1470-2045(13)70322-X. [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klijn JG, Blamey RW, Boccardo F, Tominaga T, Duchateau L, Sylvester R. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J Clin Oncol. 2001;19(2):343–353. doi: 10.1200/JCO.2001.19.2.343. [DOI] [PubMed] [Google Scholar]
- 19.Cheung KL, Agrawal A, Folkerd E, Dowsett M, Robertson JF, Winterbottom L. Suppression of ovarian function in combination with an aromatase inhibitor as treatment for advanced breast cancer in premenopausal women. Eur J Cancer. 2010;46(16):2936–2942. doi: 10.1016/j.ejca.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Bartsch R, Bago-Horvath Z, Berghoff A, DeVries C, Pluschnig U, Dubsky P, et al. Ovarian function suppression and fulvestrant as endocrine therapy in premenopausal women with metastatic breast cancer. Eur J Cancer. 2012;48(13):1932–1938. doi: 10.1016/j.ejca.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Yardley DA, Noguchi S, Pritchard KI, Burris HA, 3rd, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30(10):870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pritchard KI. Ovarian suppression/ablation in premenopausal ER-positive breast cancer patients. Issues and recommendations. Oncology (Williston Park) 2009;23(1):27–33. [PubMed] [Google Scholar]
- 23.El-Saghir NS, El H, II, Makarem JA, Otrock ZK. Combined ovarian ablation and aromatase inhibition as first-line therapy for hormone receptor-positive metastatic breast cancer in premenopausal women: report of three cases. Anticancer Drugs. 2006;17(8):999–1002. doi: 10.1097/01.cad.0000224456.28898.37. [DOI] [PubMed] [Google Scholar]
- 24.Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiowka M, Hewson N, et al. Fulvestrant 500 mg Versus Anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol. 2015;33(32):3781–3787. doi: 10.1200/JCO.2015.61.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 26.Perez EA, Vogel CL, Irwin DH, Kirshner JJ, Patel R. Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2001;19(22):4216–4223. doi: 10.1200/JCO.2001.19.22.4216. [DOI] [PubMed] [Google Scholar]
- 27.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 28.Freyer G, Delozier T, Lichinister M, Gedouin D, Bougnoux P, His P, et al. Phase II study of oral vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 2003;21(1):35–40. doi: 10.1200/jco.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 29.Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 31.Albain KS, Nag SM, Calderillo-Ruiz G, Jordaan JP, Llombart AC, Pluzanska A, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;26(24):3950–3957. doi: 10.1200/JCO.2007.11.9362. [DOI] [PubMed] [Google Scholar]
- 32.Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, Bell D, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12(9):1247–1254. doi: 10.1023/A:1012281104865. [DOI] [PubMed] [Google Scholar]
- 33.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 34.Alba E, Martin M, Ramos M, Adrover E, Balil A, Jara C, et al. Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first-line treatment of metastatic breast cancer: a Spanish Breast Cancer Research Group (GEICAM-9903) phase III study. J Clin Oncol. 2004;22(13):2587–2593. doi: 10.1200/JCO.2004.08.125. [DOI] [PubMed] [Google Scholar]
- 35.Chan S, Friedrichs K, Noel D, Pinter T, Van Belle S, Vorobiof D, et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol. 1999;17(8):2341–2354. doi: 10.1200/JCO.1999.17.8.2341. [DOI] [PubMed] [Google Scholar]
- 36.Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27(22):3611–3619. doi: 10.1200/JCO.2008.18.5397. [DOI] [PubMed] [Google Scholar]
- 37.Martin M, Ruiz A, Munoz M, Balil A, Garcia-Mata J, Calvo L, et al. Gemcitabine plus vinorelbine versus vinorelbine monotherapy in patients with metastatic breast cancer previously treated with anthracyclines and taxanes: final results of the phase III Spanish Breast Cancer Research Group (GEICAM) trial. Lancet Oncol. 2007;8(3):219–225. doi: 10.1016/S1470-2045(07)70041-4. [DOI] [PubMed] [Google Scholar]
- 38.Bajetta E, Procopio G, Celio L, Gattinoni L, Della Torre S, Mariani L, et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol. 2005;23(10):2155–2161. doi: 10.1200/JCO.2005.02.167. [DOI] [PubMed] [Google Scholar]
- 39.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biganzoli L, Lichtman S, Michel JP, Papamichael D, Quoix E, Walko C et al. Oral single-agent chemotherapy in older patients with solid tumours: a position paper from the International Society of Geriatric Oncology (SIOG). Eur J Cancer. 2015. [DOI] [PubMed]
- 41.Biganzoli L, Lichtman S, Michel JP, Papamichael D, Quoix E, Walko C, et al. Oral single-agent chemotherapy in older patients with solid tumours: a position paper from the International Society of Geriatric Oncology (SIOG) Eur J Cancer. 2015;51(17):2491–2500. doi: 10.1016/j.ejca.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014;148(3):553–561. doi: 10.1007/s10549-014-3144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Addeo R, Sgambato A, Cennamo G, Montella L, Faiola V, Abbruzzese A, et al. Low-dose metronomic oral administration of vinorelbine in the first-line treatment of elderly patients with metastatic breast cancer. Clin Breast Cancer. 2010;10(4):301–306. doi: 10.3816/CBC.2010.n.039. [DOI] [PubMed] [Google Scholar]
- 44.Gebbia V, Boussen H, Valerio MR. Oral metronomic cyclophosphamide with and without methotrexate as palliative treatment for patients with metastatic breast carcinoma. Anticancer Res. 2012;32(2):529–536. [PubMed] [Google Scholar]
- 45.Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 47.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 48.Dang C, Iyengar N, Datko F, D’Andrea G, Theodoulou M, Dickler M, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33(5):442–447. doi: 10.1200/JCO.2014.57.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson M, López-Vega J, Petit T, Zamagni C, Freudensprung U, Robb S et al. Interim safety and efficacy of pertuzumab, trastuzumab and vinorelbine for first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer [Abstract 361PD]. ESMO2014; 26–30 September; Madrid, Spain, 2014.
- 50.Andersson M, Lopez-Vega JM, Petit T, Zamagni C, Donica M, Kamber J et al., editors. The co-administration of pertuzumab (P) and trastuzumab (T) as a single infusion, followed by vinorelbine (V), in first-line (1L) treatment of HER2-positive locally advanced or metastatic breast cancer (MBC) patients (pts): VELVET study interim analysis. ASCO Annu Meet Proc; 2015.
- 51.Andersson M, López-Vega JM, Petit T, Zamagni C, Freudensprung U, Robb S, et al. 361PD Interim safety and efficacy of pertuzumab, trastuzumab and vinorelbine for first-line (1 l) treatment of patients (pts) with HER2-positive locally advanced or metastatic breast cancer (MBC) Ann Oncol. 2014;25(4):iv120-iv. [Google Scholar]
- 52.Du F, Yuan P, Zhu W, Wang J, Ma F, Fan Y, et al. Is it safe to give anthracyclines concurrently with trastuzumab in neo-adjuvant or metastatic settings for HER2-positive breast cancer? A meta-analysis of randomized controlled trials. Med Oncol. 2014;31(12):340. doi: 10.1007/s12032-014-0340-x. [DOI] [PubMed] [Google Scholar]
- 53.Ellis PA, Barrios CH, Eiermann W, Toi M, Im Y-H, Conte PF et al., editors. Phase III, randomized study of trastuzumab emtansine (T-DM1){±} pertuzumab (P) vs trastuzumab + taxane (HT) for first-line treatment of HER2-positive MBC: Primary results from the MARIANNE study. ASCO Ann Meet Proc; 2015.
- 54.Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27(33):5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 55.Huober J, Fasching PA, Barsoum M, Petruzelka L, Wallwiener D, Thomssen C, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer—results of the eLEcTRA trial. Breast. 2012;21(1):27–33. doi: 10.1016/j.breast.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Johnston S, Pippen J, Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27(33):5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 57.Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–119. doi: 10.1093/annonc/mdu486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 59.Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol. 2011;29(3):264–271. doi: 10.1200/JCO.2010.30.8213. [DOI] [PubMed] [Google Scholar]
- 60.Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007;110(5):965–972. doi: 10.1002/cncr.22885. [DOI] [PubMed] [Google Scholar]
- 61.Pivot X, Manikhas A, Zurawski B, Chmielowska E, Karaszewska B, Allerton R, et al. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33(14):1564–1573. doi: 10.1200/JCO.2014.57.1794. [DOI] [PubMed] [Google Scholar]
- 62.von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol. 2009;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 63.Bartsch R, Wenzel C, Altorjai G, Pluschnig U, Rudas M, Mader RM, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007;25(25):3853–3858. doi: 10.1200/JCO.2007.11.9776. [DOI] [PubMed] [Google Scholar]
- 64.Krop IE, Kim SB, Gonzalez-Martin A, LoRusso PM, Ferrero JM, Smitt M, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 65.Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 66.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23(6):vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 67.Decatris MP, Sundar S, O’Byrne KJ. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer Treat Rev. 2004;30(1):53–81. doi: 10.1016/S0305-7372(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 68.Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 69.Campone M, Dobrovolskaya N, Tjulandin S, Chen SC, Fourie S, Mefti F, et al. A three-arm randomized phase II study of oral vinorelbine plus capecitabine versus oral vinorelbine and capecitabine in sequence versus docetaxel plus capecitabine in patients with metastatic breast cancer previously treated with anthracyclines. Breast J. 2013;19(3):240–249. doi: 10.1111/tbj.12098. [DOI] [PubMed] [Google Scholar]
- 70.Tutt A, Ellis P, Kilburn L, Gilett C, Pinder S, Abraham J et al., editors. The TNT trial: A randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012). San Antonio Breast Cancer Conference; 2014.
- 71.O’Shaughnessy J, Schwartzberg L, Danso MA, Miller KD, Rugo HS, Neubauer M, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32(34):3840–3847. doi: 10.1200/JCO.2014.55.2984. [DOI] [PubMed] [Google Scholar]
- 72.Loesch D, Robert N, Asmar L, Gregurich MA, O’Rourke M, Dakhil S, et al. Phase II multicenter trial of a weekly paclitaxel and carboplatin regimen in patients with advanced breast cancer. J Clin Oncol. 2002;20(18):3857–3864. doi: 10.1200/JCO.2002.08.129. [DOI] [PubMed] [Google Scholar]
- 73.Seidman AD. Gemcitabine as single-agent therapy in the management of advanced breast cancer. Oncology (Williston Park) 2001;15(2Suppl3):11–14. [PubMed] [Google Scholar]
- 74.Zelek L, Barthier S, Riofrio M, Fizazi K, Rixe O, Delord JP, et al. Weekly vinorelbine is an effective palliative regimen after failure with anthracyclines and taxanes in metastatic breast carcinoma. Cancer. 2001;92(9):2267–2272. doi: 10.1002/1097-0142(20011101)92:9<2267::AID-CNCR1572>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 75.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 76.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016. [DOI] [PubMed]
- 77.Palbociclib (IBRANCE Capsules). US Food and Drug Administration. 2016. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm487080.htm2016
- 78.Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016. [DOI] [PubMed]
- 79.Martin M, Bonneterre J, Geyer CE, Jr, Ito Y, Ro J, Lang I, et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2 + advanced breast cancer. Eur J Cancer. 2013;49(18):3763–3772. doi: 10.1016/j.ejca.2013.07.142. [DOI] [PubMed] [Google Scholar]
- 80.Saura C, Garcia-Saenz JA, Xu B, Harb W, Moroose R, Pluard T, et al. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2014;32(32):3626–3633. doi: 10.1200/JCO.2014.56.3809. [DOI] [PubMed] [Google Scholar]
- 81.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]