Abstract
HIV-infected individuals are at high risk of developing atherosclerosis and cardiovascular disease, in part, due to HIV-induced impairment of cholesterol metabolism. In vitro studies demonstrated that HIV-1 protein Nef inhibits activity of ABCA1, the main cellular cholesterol transporter, leading to cholesterol accumulation in macrophages and conversion of these cells into foam cells, characteristic for atherosclerosis. However, the mechanisms of Nef-mediated effects on cholesterol metabolism in vivo are not well characterized. In this study, we generated Nef-transgenic mice and evaluated the accumulation of neutral lipids in liver and aorta of these animals. Nef expression was low in all transgenic mice, with some mice carrying the Nef transgene, but not expressing the Nef RNA. Using Oil Red O staining, we demonstrated increased levels of neutral lipids in liver and aorta of mice expressing Nef relative to transgenic animals, with no detectable Nef expression or control wild-type mice. These results provide direct evidence that Nef promotes cholesterol deposition in tissues.
Keywords: : cholesterol efflux, Nef, HIV-1, lipid droplets
Combined antiretroviral therapy (cART) has dramatically changed the clinical course of HIV infection, converting it from an acute frequently fatal disease to a chronic condition. Consequently, primary clinical concerns in HIV-infected subjects have shifted from AIDS and AIDS-related conditions to HIV-associated comorbidities. Atherosclerosis and cardiovascular disease (CVD) are particularly prominent, with HIV-infected subjects being at increased1,2 and earlier3 risk for developing atherosclerosis and dying from CVD.
Various factors may contribute to HIV-associated atherosclerosis, including persistent inflammation and immune activation due to bacterial translocation through damaged mucosa4 and side effects of anti-HIV medications.5 In addition, HIV can directly affect cholesterol metabolism of infected cells by inhibiting activity of the cellular cholesterol transporter, ABCA1.6 As a result of such inhibition, cholesterol efflux from macrophages is blocked and cells accumulate cholesterol, converting into foam cells, which are characteristic cells for atherosclerotic plaques.
Our previous in vitro studies demonstrated that Nef is the HIV-1 protein responsible for downregulation of ABCA1.6,7 However, the only in vivo evidence for the effects of Nef on cholesterol metabolism are studies where recombinant Nef was injected into mice.8 These studies demonstrated an enhanced development of atherosclerosis in a mouse model of this disease (apoe−/− mice on high fat diet), and in wild-type C57BL/6 mice fed high-fat/high-cholesterol diet, Nef induced accumulation of lipid-laden macrophages in adventitia of aorta. These findings are consistent with the role of Nef in impairment of cholesterol efflux.6
In this study, we aimed to establish whether endogenously produced Nef causes accumulation of cholesterol in tissues in vivo, a key event in the proposed function of Nef in pathogenesis of HIV-induced atherosclerosis.8 To characterize the in vivo effects of Nef on lipid metabolism, we used Nef-transgenic mice. The model where Nef is expressed in all cells was selected because previous studies demonstrated that Nef released from infected cells induces ABCA1 downregulation in uninfected cells of SIV-infected macaques, and recombinant Nef inhibits cholesterol efflux in treated cells similar to the inhibition observed in HIV-infected cells.9 It has also been shown that Nef mRNA can be packaged in exosomes and induce Nef expression in uninfected cells.10
Transgenic mice were generated on C57BL/6 background using genetic construct with chicken actin gene promoter and cytomegalovirus enhancer, and removable STOP-cassette11,12 containing LoxP-flanked chloramphenicol acetyltransferase gene in front of Nef-IRES-EGFP bicistronic cassette expressing consensus Nef sequence.13 Transgenic construct was tested in vitro in HEK293 cells cotransfected with plasmids containing Nef cassette and PGK-Cre. Several transgenic founders were bred with PGK-cre deleter mice, and all progenies showed low levels of Nef expression. Specifically, Nef protein was undetectable in plasma and tissues (spleen, aorta, liver) from these animals by IHC, Western blotting, or ELISA [the threshold of the ELISA assay (Immunodiagnostics #108) was 10 pg/mL], but Nef-specific RNA could be detected by reverse transcription-polymerase chain reaction (RT-PCR) in some mice carrying the Nef transgene.
All transgenic animals were genetically identical and differed from the wild-type animals only by the presence of the Nef transgene. Therefore, variability of Nef expression levels in individual transgenic animals was likely due to varying levels of epigenetic silencing of the transgene, although special study would be required to test this assumption. The fact that the Nef expression was so low in these transgenic mice was actually advantageous for our purposes, as it approximated the situation in cART-treated subjects where HIV replication is suppressed to undetectable levels. Mice carrying the Nef transgene, but not expressing Nef RNA, served as an internal control, as they are genetically identical to Nef-expressing siblings.
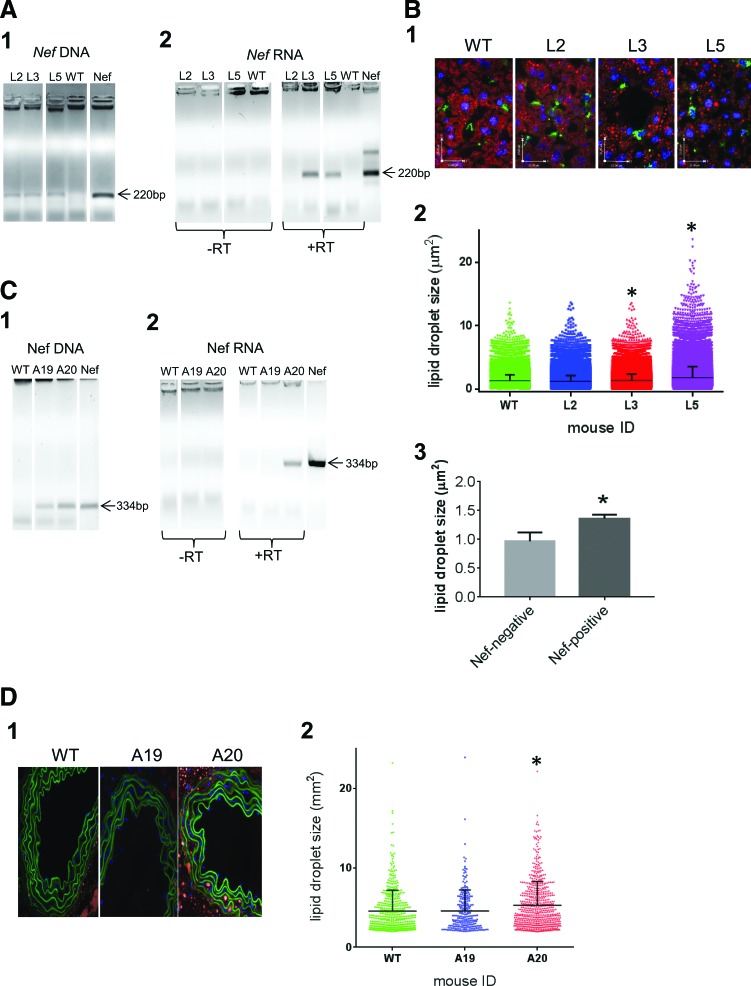
The first set of animals was used for analysis of liver sections. This set included three transgenic (L2, L3, L5) mice (descendants of the same founder) and two wild-type mice, as tested by polymerase chain reaction (PCR) of genomic DNA (Fig. 1A1) using primers amplifying Nef fragment that spans base pairs 341-560 (forward primer 5′-CTACCACACACAAGGCTACTTC-3′, reverse primer 5′-GCTGTCAAACCTCCACTCTAAC-3′). Nef RNA was detected in two out of three transgenic mice (animals L3 and L5), as revealed by RNA PCR (Fig. 1A2). Staining liver sections with Oil Red O (ORO, stains neutral lipids in red), anti-mouse CD68 (Bio-Rad) mAb followed by goat anti-rat IgG conjugated with Alexa488 (Life Technologies, stains macrophages in green), and DAPI revealed increased amount of ORO-stained lipid droplets in liver sections from Nef-expressing mice L3 and L5 (Fig. 1B1). Quantitative analysis of ORO-stained droplets using Volocity software (PerkinElmer Life Sciences) demonstrated a significant increase (p < .0001 by one-way ANOVA with post-hoc Tukey HSD test) in the size of lipid droplets in Nef-expressing mice L3 and L5 relative to the WT mouse or nonexpressing transgenic mouse L2 (Fig. 1B2).
FIG. 1.
Analysis of neutral lipids in tissue sections from Nef-transgenic mice. (A) Total DNA (A1) and RNA (A2) were extracted from liver sections of transgenic (L2, L3, L5) and one of the wild-type mice and analyzed by PCR (1 and left panels in 2) or reverse transcription-polymerase chain reaction (RT-PCR) (right panels in 2). (B1) Liver sections were stained with ORO (stains neutral lipids in red), anti-CD68 (stains macrophages in green), and DAPI (stains nuclei in blue) and observed on fluorescent microscope. (B2) Sizes of 66,274 ORO-stained vesicles in the liver sections from WT mouse, 22,400 vesicles from L2, 63,503 vesicles from L3, and 39,450 vesicles from L5 transgenic animals were analyzed using Volocity software and are presented as mean ± SEM. *p < .0001 relative to the WT and L2 mice, calculated by one-way ANOVA with post-hoc Tukey HSD test. (B3) Nef-negative group combined two wild-type mice and one transgenic mouse not expressing Nef (L2), Nef-positive group was represented by L3 and L5 mice. Bars show mean ± SEM. *p = .045, unpaired Student's two-tailed t-test. (C) Total DNA (C1) and RNA (C2) were extracted from aorta sections of transgenic (A19, A20) and wild-type mice and analyzed by PCR (C1 and left panels in C2) or RT-PCR (right panels in 2). (D1) Aorta sections were stained with ORO (stains neutral lipids in red) and DAPI (stains nuclei in blue) and observed on fluorescent microscope. Green staining of elastin was due to autofluorescence. (D2) Sizes of the ORO-stained vesicles within aorta wall were analyzed using Volocity software and are presented as mean ± SEM. *p < .0001 relative to the WT and A19 mice, calculated by one-way ANOVA with post-hoc Tukey HSD test. PCR, polymerase chain reaction; SEM, standard error of the mean. Color images available online at www.liebertpub.com/aid
Given the limited number of mice available for this study, we could not statistically compare the three groups, that is, nontransgenic mice, transgenic mice with no Nef expression, and transgenic mice with Nef expression. Therefore, we combined nontransgenic (two animals) and transgenic mice with no Nef expression (one animal) into one group (Nef negative) and compared it to the Nef-expressing group (Nef positive, two animals). Results presented in Figure 1B3 demonstrate that Nef-expressing animals accumulated significantly more lipid droplets in liver than Nef-negative mice (p = .045 by unpaired Student's t-test). Of particular interest, ORO staining did not colocalize with CD68 staining (there were few CD68-positive cells), suggesting that lipids accumulated in other than macrophages cells (e.g., hepatocytes, which also express Nef in these mice), and Nef did not induce massive macrophage migration into the liver. This conclusion is consistent with only a very low inflammation observed in Nef-injected mice.8
The second set of mice was used to stain aorta. This set included two transgenic animals (A19 and A20, descendants of the same founder, which was different from the founder of mice used for liver analysis) and three wild-type mice, as confirmed by PCR (Fig. 1C1) using primers amplifying Nef fragment spanning base pairs 26-360 (forward primer 5′-TGTGATTGGATGGCCTACTG-3′, reverse primer 5′-GTAGCCTTGTGTGTGGTAGAT-3′). One of the transgenic animals (A20) did express Nef, whereas the other (A19, used as control) did not (Fig. 1C2).
Aorta sections from these mice were stained with ORO, counterstained with DAPI, and analyzed by fluorescent microscopy. Aortic media from Nef-expressing transgenic mouse (A20) were enriched in ORO-stained lipid droplets relative to aorta sections from the wild-type mouse and the Nef-transgenic mouse that did not express Nef (Fig. 1D1). Accumulation of neutral lipids within aortic elastin fibers is characteristic to early stages in the development of atherosclerotic lesions.14
The size of ORO-stained droplets within the aorta wall was quantified for 493 vesicles from WT mouse, 635 vesicles from A20, and 238 vesicles from A19 using Volocity software. Lipid droplets from Nef-expressing transgenic mouse (A20) were significantly larger (p < .0001 by one-way ANOVA with post-hoc Tukey HSD test) than vesicles from WT mouse or transgenic mouse with silent Nef, A19 (Fig. 1D2). Given that only one Nef-expressing and one nonexpressing transgenic mice were available, statistical analysis of the groups could not be done. We therefore performed a 1-sample t-test to determine whether the size of the lipid droplets in transgenic mice was different from the size of lipid droplets in nontransgenic group. This analysis revealed that the size of droplets in Nef-expressing transgenic mouse (mean size 5.45 μm) was significantly different (p = .0422) from the droplet size in three nontransgenic mice (mean size = 4.19 μm, SD = 0.463 μm). In contrast, the droplet size in Nef-transgenic mouse not expressing Nef (mean size 4.58 μm) was not significantly different from that in nontransgenic mice (p = .2820).
Results of this study suggest that Nef expression, even at very low levels, promotes accumulation of lipid droplets in liver and aorta, which is characteristic for systemic impairment of cholesterol efflux and reverse cholesterol transport. These findings reproduce the key effect associated with injection of recombinant Nef, accumulation of lipids in aorta adventitia.6 However, in our transgenic mice, we did not observe weight gain, which was prominent in Nef-injected mice. This difference may be due to low Nef expression in transgenic animals, or/and to the fact that mice injected with Nef were fed cholesterol/fat-rich diet, whereas transgenic mice were on normal chow. Such interpretation is consistent with the model, whereby Nef and HIV infection provide an additional risk factor that potentiates the proatherogenic effect of other major risk factors, such as unhealthy diet, high cholesterol, smoking, and diabetes.
The small number of transgenic mice available for this study is a clear limitation, which needs to be addressed in the future. Future studies would also be required to determine the chain of molecular events behind the Nef-induced accumulation of lipid droplets. Nef is known to impair the function of the ABCA1 cholesterol transporter,6 but may also, through its association with calnexin,7 affect other proteins involved in lipid synthesis or transport. Nef interaction with Acyl-CoA thioesterase 8 (ACOT8)15,16 may also influence fatty acid metabolism.17 Findings described in this report provide the rationale for mechanistic studies into the role of Nef in pathogenesis of atherosclerosis and metabolic diseases in HIV-infected subjects.
Acknowledgments
We thank Dr. G. Willimsky for providing pCAG-CAT-Z, F. Rozov and M. Nosenko for technical assistance, and K. Korneev and D. Kuprash for helpful discussions. The following reagent was obtained through the AIDS Reagent Program, Division of AIDS, NIAID, NIH: pT7consnefhis6 from Dr. Ron Swanstrom. This study was supported by NIH grants R01HL101274, R21AI108533, P30AI087714, and RFBR grant #13-04-91458 and Leading Schools grant #10014.2016.4.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Escaut L, Monsuez JJ, Chironi G, et al. : Coronary artery disease in HIV infected patients. Intensive Care Med 2003;29:969–973 [DOI] [PubMed] [Google Scholar]
- 2.Neumann T, Woiwoid T, Neumann A, et al. : Cardiovascular risk factors and probability for cardiovascular events in HIV-infected patients: Part I. Differences due to the acquisition of HIV-infection. Eur J Med Res 2003;8:229–235 [PubMed] [Google Scholar]
- 3.Patel AA, Budoff MJ: Coronary artery disease in patients with HIV infection. Am J Cardiovasc Drugs 2015;15:81–87 [DOI] [PubMed] [Google Scholar]
- 4.Troseid M, Manner IW, Pedersen KK, Haissman JM, Kvale D, Nielsen SD: Microbial translocation and cardiometabolic risk factors in HIV infection. AIDS Res Hum Retroviruses 2014;30:514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boccara F, Lang S, Meuleman C, et al. : HIV and coronary heart disease: Time for a better understanding. J Am Coll Cardiol 2013;61:511–523 [DOI] [PubMed] [Google Scholar]
- 6.Mujawar Z, Rose H, Morrow MP, et al. : Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol 2006;4:e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jennelle L, Hunegnaw R, Dubrovsky L, et al. : HIV-1 protein Nef inhibits activity of ATP-binding cassette transporter A1 by targeting endoplasmic reticulum chaperone calnexin. J Biol Chem 2014;289:28870–28884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui HL, Ditiatkovski M, Kesani R, et al. : HIV protein Nef causes dyslipidemia and formation of foam cells in mouse models of atherosclerosis. FASEB J 2014;28:2828–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asztalos BF, Mujawar Z, Morrow MP, et al. : Circulating Nef induces dyslipidemia in simian immunodeficiency virus-infected macaques by suppressing cholesterol efflux. J Infect Dis 2010;202:614–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan MB, Lang MJ, Huang MB, et al. : Nef exosomes isolated from the plasma of individuals with HIV-associated dementia (HAD) can induce Abeta1-42 secretion in SH-SY5Y neural cells. J Neurovirol 2016;22:179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki K, Araki M, Miyazaki J, Vassalli P: Site-specific recombination of a transgene in fertilized eggs by transient expression of Cre recombinase. Proc Natl Acad Sci U S A 1995;92:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willimsky G, Blankenstein T: Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature 2005;437:141–146 [DOI] [PubMed] [Google Scholar]
- 13.Shugars DC, Smith MS, Glueck DH, Nantermet PV, Seillier-Moiseiwitsch F, Swanstrom R: Analysis of human immunodeficiency virus type 1 nef gene sequences present in vivo. J Virol 1993;67:4639–4650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bobryshev YV, Lord RS: Accumulation of co-localised unesterified cholesterol and neutral lipids within vacuolised elastin fibres in athero-prone areas of the human aorta. Atherosclerosis 1999;142:121–131 [DOI] [PubMed] [Google Scholar]
- 15.Watanabe H, Shiratori T, Shoji H, et al. : A novel acyl-CoA thioesterase enhances its enzymatic activity by direct binding with HIV Nef. Biochem Biophys Res Commun 1997;238:234–239 [DOI] [PubMed] [Google Scholar]
- 16.Liu LX, Heveker N, Fackler OT, et al. : Mutation of a conserved residue (D123) required for oligomerization of human immunodeficiency virus type 1 Nef protein abolishes interaction with human thioesterase and results in impairment of Nef biological functions. J Virol 2000;74:5310–5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt MC, Solaas K, Kase BF, Alexson SE: Characterization of an acyl-coA thioesterase that functions as a major regulator of peroxisomal lipid metabolism. J Biol Chem 2002;277:1128–1138 [DOI] [PubMed] [Google Scholar]