Abstract
Preventable cardiovascular disease (CVD) risk factors are responsible for the majority of CVD-related deaths, and are increasingly recognized as a cause of morbidity and mortality for HIV-infected persons taking antiretroviral therapy (ART). Simplified tools such as the American Heart Association's ideal cardiovascular health (iCVH) construct may identify and prognosticate CVD risk in resource-limited settings. No studies have evaluated iCVH metrics in sub-Saharan Africa or among HIV-infected adults. Thus, the central aim of this study was to compare levels of iCVH metrics and their correlations with carotid atherosclerosis for HIV-infected adults versus uninfected controls in a well-phenotyped Ugandan cohort. We analyzed the prevalence of iCVH metrics in a mixed cohort of HIV-infected persons on stable ART and uninfected, population-based comparators in Mbarara, Uganda. We also assessed the validity of iCVH by correlating iCVH values with common carotid intima media thickness (CCIMT). HIV-infected persons had a mean of 4.9 (SD 1.1) iCVH metrics at ideal levels versus 4.3 (SD 1.2) for uninfected controls (p = .002). This difference was largely driven by differences in blood pressure, blood glucose, and diet. In multivariable-adjusted linear regression models, each additional iCVH metric at an ideal level was associated with a significant 0.024 mm decrease in CCIMT (p < .001).HIV-infected persons on ART in rural Uganda had more iCVH metrics at ideal levels than uninfected persons. The difference appeared driven by factors that are putatively influenced by access to routine medical care. Composite scores of iCVH metrics were associated with subclinical atherosclerosis and more predictive of atherosclerosis for uninfected persons.
Keywords: : human immunodeficiency virus, cardiovascular disease, ideal cardiovascular health, epidemiology, primary prevention, risk factors
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States and worldwide, representing more than 30% of global deaths.1,2 Established and often preventable CVD risk factors are responsible for the vast majority of these deaths; adults without traditional CVD risk factors live longer and have far lower lifetime risks for CVD than those with any CVD risk factors.3–8 To readily communicate to the population this clear and somewhat predictable contribution of risk factors to CVD morbidity and mortality, the American Heart Association created the construct of ideal cardiovascular health (iCVH) as part of its 2020 Strategic Impact Goal.9 The seven factors included in the iCVH construct are smoking, body mass index (BMI), physical activity, healthy diet score, total cholesterol, blood pressure, and fasting blood glucose. Metrics were used to categorize levels of each of these risk factors as poor, intermediate, or ideal. Subsequent studies have found that the prevalence of iCVH in the United States and worldwide is quite low. The prevalence of iCVH is ∼0.1% in the United States, 0.2% in China, and similarly low in the Middle East and eastern Europe; iCVH is somewhat more common in northern Europe, but its absolute prevalence remains well under 5%.10–13 Higher aggregate scores of iCVH metrics have been associated with less subclinical and overt CVD in North American and European cohorts.14,15
As longevity for HIV-infected persons has improved substantially in recent decades due to effective antiretroviral therapy (ART), CVD risk factors are increasingly recognized as a major preventable cause of morbidity and mortality for HIV-infected persons.16–20 This is particularly true in sub-Saharan Africa, where HIV-infected persons comprise a substantial minority of the population.21,22 Although morbidity and mortality due to CVD are thought to be increasing in sub-Saharan Africa and among HIV-infected persons, no studies have explicitly evaluated iCVH metrics or correlations of iCVH scores with subclinical CVD in sub-Saharan Africa or HIV-infected populations in any setting.23–28 Thus, understanding the patterns and prevalence of health metrics comprising iCVH among HIV-infected and uninfected persons in sub-Saharan Africa may be an essential initial step in crafting policies and ultimately interventions aimed at improving cardiovascular health in sub-Saharan Africa. Moreover, if simple, low-cost metrics like iCVH prove to be predictive of CVD morbidity, they might fill an important clinical need in low-resource settings. The objectives of this study were to (1) assess the distribution of iCVH metrics in a cohort of HIV-infected persons on stable ART in Uganda with uninfected, population-based comparators from the same clinic catchment area and (2) to validate iCVH metrics by testing their association with common carotid intima media thickness (CCIMT), a validated surrogate marker of CVD.29,30
Materials and Methods
Study site and participants
Data for this study were derived from the Ugandan Noncommunicable Diseases and Aging Cohort (UGANDAC) Study (NCT02445079), an ongoing cohort study of HIV-infected persons in southwestern Uganda and an age and sex-matched, population-based control group of uninfected persons from the surrounding clinic catchment area.31,32 HIV-infected study participants were selectively sampled from the Uganda AIDS Rural Treatment Outcomes cohort study, which began in 2005 and consists of HIV-infected persons initiating ART, and has been described in detail previously.33 Eligibility criteria for HIV-infected participants were age >40 years and at least 3 years of ART use. The control sample was drawn from a whole-population cohort study of adults currently being conducted in Nyakabare Parish, a network of eight villages located ∼25 km from the Immune Suppression Syndrome Clinic. This parish was chosen for the study because it has a sociodemographic composition similar to other parishes in the region and because village leaders described relatively little presence by outside nongovernmental organizations. Of 1,851 persons identified in the population census as eligible, 1,814 consented to participate in the baseline survey, for an overall response rate of 98%. Thus, the control sample was drawn from a population that is representative of rural, southwestern Uganda. The local economy is largely characterized by subsistence agriculture, and food insecurity is pervasive.34 Nearly all (95%) households in the parish report relying on public, rather than private, sources of water for household use; the median household is located 0.25 km from its primary water source.35
Ideal cardiovascular health measurements
All study participants completed questionnaires to evaluate dietary, physical activity, and smoking history. Participants also underwent anthropomorphic measurement, blood pressure measurement, and blood collection for lipid profile, hemoglobin A1c testing, CD4+ T-cell count, and HIV viral load. Serum chemistry tests were performed using the Abbott Architect Clinical Chemistry Analyzer (Abbott Diagnostics, Abbott Park, IL). Hemoglobin A1c levels were measured using the Bayer A1c Now+ point of care assay (Bayer Pharmaceuticals, Pittsburgh, PA).
Each of the seven iCVH metrics was evaluated and categorized as being at ideal or nonideal levels for each participant using previously published cutoff values.8 Ideal smoking status consisted of never smoking or having quit >12 months ago; ideal BMI was <25 kg/m2; ideal physical activity consisted of ≥150 min per week of moderate intensity activity, ≥75 min per week of vigorous intensity physical activity, or a combination thereof; ideal total cholesterol was <200 mg/dl; and ideal blood pressure was <120/<80 mmHg. Ideal diet was adapted from the standard definition of dietary score because full data on the volume of dietary intake were not available. We used fruit and vegetable intake as a proxy for diet score (with ≥20 total servings of fruits and vegetables weekly as the cutoff for ideal intake) because previous studies have demonstrated associations between high fruit and vegetable intake and decreased CVD risk.36,37 We used hemoglobin A1c rather than fasting plasma glucose as the proxy for ideal glucose status because 40 participants were either not fasting when blood glucose was checked or had missing blood glucose data, whereas all participants had complete hemoglobin A1c data. We used a hemoglobin A1c threshold of <5.7% as a proxy of ideal glucose status in light of current conventions that consider this level of HbA1c to be normal.38 The only iCVH metrics for which any participants had incomplete data were diet (three HIV-infected and six uninfected persons had missing data) and cholesterol (two uninfected persons had missing data). Given the paucity of missing data and resulting limited impact on overall iCVH scores, we designated these 11 missing values as “not ideal” diet or cholesterol.
Common carotid intima media thickness measurement
CCIMT was measured using a standardized protocol39 by a single trained operator using a Sonosite M-Turbo (Sonosite, Bothell, WA). A total of six images for each participant were obtained from anterior, lateral, and posterior positions in the left and right carotid arteries. These images were then evaluated and scored by a trained, board-certified cardiologist. Far-wall CCIMT was measured in 1 cm segments proximal to the carotid bulb using clinically available, semiautomated border-detection software (SonoCalc, version 5.0; Sonosite). Mean values of all adequate images were summarized as mean CCIMT for each participant. Study personnel were blinded to participant information and HIV serostatus during image quality assurance and CCIMT measurement. For quality assurance, the ultrasound technologist completed paired, prestudy ultrasonography on two separate days for 10 volunteers and found mean CCIMTs of 0.538 mm (t = 0.0012 and p = .999) on both days; the coefficient of variation was 4.9%.
Statistical analyses
We first summarized levels of each of the seven iCVH metrics for HIV-infected versus uninfected participants and compared the distributions between study groups using student t tests. Next we fit linear regression models to compare mean CCIMT for HIV-infected versus uninfected participants. Finally, multivariable-adjusted linear regression was used to estimate the association between iCVH metrics at ideal levels and CCIMT, stratifying by HIV serostatus. To evaluate the potential utility of a simplified iCVH score without laboratory-based metrics (i.e., that could potentially be used in resource-limited settings), we then repeated these analyses excluding cholesterol and blood glucose (and thus the iCVH score includes only smoking, BMI, blood pressure, physical activity, and dietary intake).
Results
Participant characteristics and prevalence of ideal cardiovascular health metrics
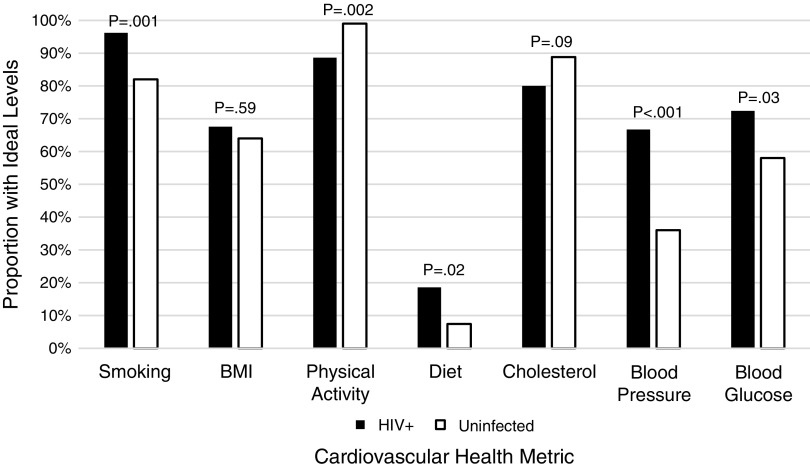
Mean age of HIV-infected and uninfected study participants was 49.3 and 51.8, respectively, and both groups had approximately half female participants (Table 1 and Fig. 1). Only 2.9% of HIV-infected persons and 2.0% of uninfected controls had iCVH, defined as all seven iCVH metrics being at ideal levels. HIV-infected persons had a mean of 4.9 (±1.1) iCVH metrics at ideal levels versus 4.3 (±1.2) for uninfected controls (p = .002). This difference appeared to be driven primarily by substantial differences in ideal blood pressure (66.7% for HIV-infected participants versus 36.0% for uninfected controls), blood sugar (72.4% versus 58.0%), and diet (18.6% versus 7.4%).
Table 1.
Cohort Characteristics
| Variable | HIV-infected (N = 105) | Uninfected (N = 100) | p |
|---|---|---|---|
| Age (mean, SD, IQR) | 49.3 ± 6.2 (45–51) | 51.8 ± 8.9 (46–54) | .02 |
| Female (%) | 51.4 | 50.0 | .84 |
| Smoking status, n (%) | |||
| Current smoker | 4 (3.8) | 18 (18.0) | |
| Prior smoker | 35 (33.3) | 32 (32.0) | |
| Never smoker | 66 (62.9) | 50 (50.0) | |
| Ideal smoking status (never smoker or quit >12 months ago) | 101 (96.2) | 82 (82.0) | .001 |
| BMI | |||
| Mean BMI (kg/m2) (SD) | 22.8 ± 3.8 | 22.9 ± 5.0 | |
| Ideal BMI (<25 kg/m2), n (%) | 71 (67.6) | 64 (64.0) | .59 |
| Physical activity | |||
| Minutes of vigorous activity per week (mean, SD) | 1,591 ± 847 | 1,561 ± 803 | |
| Minutes of moderate activity per week (mean, SD) | 849 ± 986 | 938 ± 977 | |
| Ideal physical activity (≥150 min/week moderate intensity; or ≥75 min/week vigorous intensity), n (%) | 93 (88.6) | 99 (99.0) | .002 |
| Diet | |||
| Servings of vegetables per week (mean, SD) | 7.1 ± 4.9 | 5.1 ± 3.7 | |
| Servings of fruit per week | 8.0 ± 7.2 | 5.0 ± 4.8 | |
| Ideal diet (proxy score based on available data), n (%) | 19 (18.6) | 7 (7.4) | .02 |
| Cholesterol | |||
| Total cholesterol (mg/dL; mean, SD, IQR) | 159.0 ± 40.3 | 161.3 ± 34.1 | |
| Ideal cholesterol (total cholesterol <200 mg/dL), n (%) | 84 (80) | 87 (88.8) | .09 |
| Blood pressure | |||
| Systolic blood pressure (mmHg; mean, SD) | 113.4 ± 18.1 | 122.2 ± 17.8 | |
| Diastolic blood pressure (mmHg; mean, SD) | 72.0 ± 11.1 | 78.6 ± 10.5 | |
| Ideal blood pressure (<120/<80 mmHg), n (%) | 70 (66.7) | 36 (36.0) | <.001 |
| Blood glucose/diabetes | |||
| Hemoglobin A1c (%; mean, SD) | 5.6 ± 1.3 | 5.6 ± 0.5 | |
| Ideal glucose status (hemoglobin A1c<5.7%), n (%) | 76 (72.4) | 58 (58.0) | .03 |
| Number of ideal cardiovascular health metrics (N, %) | |||
| 1 | 1 (1.0) | 0 | |
| 2 | 4 (3.8) | 4 (4.0) | |
| 3 | 6 (5.7) | 21 (21.0) | |
| 4 | 21 (20.0) | 29 (29.0) | |
| 5 | 38 (36.2) | 32 (32.0) | |
| 6 | 32 (30.5) | 12 (12.0) | |
| 7 | 3 (2.9) | 2 (2.0) | .002 |
| Prevalent clinical diagnoses (by self-report), n (%) | |||
| Cardiovascular disease | 14 (13.3) | 13 (13.0) | |
| Hypertension | 13 (9.5) | 7 (7.0) | |
| Hyperlipidemia | 2 (1.9) | 1 (1.0) | |
| Heart failure | 0 | 3 (3.0) | |
| Diabetes | 3 (2.9) | 2 (2.0) | |
| Renal failure | 1 (1.0) | 1 (1.0) | |
| Stroke | 1 (1.0) | 1 (1.0) | |
| Cancer | 3 (2.9) | 1 (1.0) | |
| Medication use | |||
| Antihypertensive medication | 7 (6.7%) | 1 (1.0%) | |
| Lipid-lowering medication | 0 | 0 | |
BMI, body mass index; IQR, interquartile range; SD, standard deviation.
FIG. 1.
Proportion of HIV-infected and uninfected participants with ideal levels of CVH metrics. CVH, cardiovascular health.
Ideal cardiovascular health metrics and common carotid intima media thickness
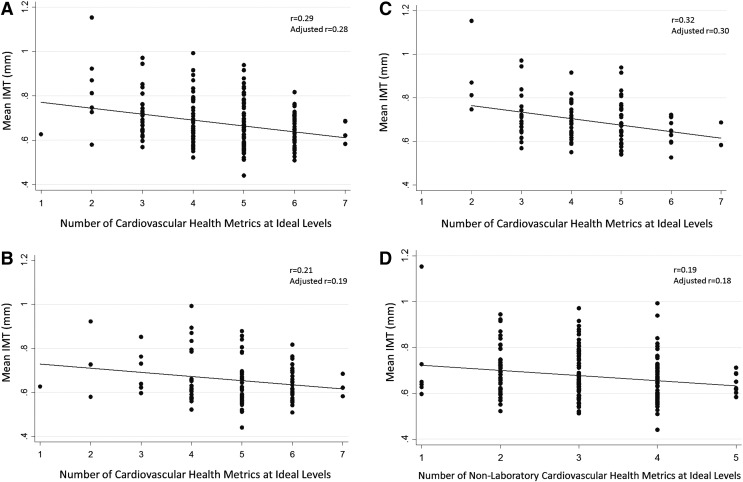
When we evaluated associations between iCVH metrics and CCIMT, a higher number of iCVH metrics at ideal levels were consistently associated with significantly lower CCIMT (Table 2). In univariate linear regression models, each additional iCVH metric at an ideal level was associated with a −0.027 mm difference in CCIMT (95% CI −0.039, −0.015; p < .001) and HIV serostatus was associated with a −0.038 mm difference in CCIMT (95% CI −0.068, −0.010; p = .009). When iCVH metrics and HIV serostatus were included in a multivariable linear regression model with CCIMT as the outcome, each additional iCVH metric at an ideal level was associated with a −0.024 mm difference in CCIMT (95% CI −0.036, −0.012; p < .001). Thus, for instance, persons with two iCVH metrics at ideal levels had predicted CCIMT of 0.751 mm, compared with a predicted CCIMT of 0.654 mm for persons with six iCVH metrics at an ideal level. Compared with the sample standard deviation of 0.106, this comparison between persons on the lower versus higher end (but not quite the extremes) of the iCVH spectrum represented an effect size of (0.751–0.654)/(0.106) = 0.915 standard deviation units, which represents a large effect size. Notably, in these multivariable models, the association between HIV serostatus and CCIMT was no longer significant (95% CI −0.054, 0.003; p = .08), nor was the interaction term for CCIMT and HIV serostatus (p = .38). The overall correlation (Pearson's r) between iCVH metrics and CCIMT was 0.29 (Fig. 2A) and the correlation was marginally weaker for HIV-infected (r = 0.19; Fig. 2B) than for uninfected (r = 0.32; Fig. 2C) participants.
Table 2.
Regression of Ideal CVH Score and Carotid IMT
| Univariate models | Multivariable models | |||
|---|---|---|---|---|
| Coefficient (95% CI) | p | Coefficient (95% CI) | p | |
| Ideal CVH score | −0.027 (−0.039, −0.015) | <.001 | −0.024 (−0.036, −0.012) | <.001 |
| HIV serostatus | −0.038 (−0.068, −0.010) | .009 | −0.026 (−0.054, 0.003) | .08 |
| Interaction term | 0.011 | .38 | ||
CVH, cardiovascular health; IMT, intima media thickness.
FIG. 2.
(A) Mean CCIMT by number of iCVH metrics. (B) Mean CCIMT by number of iCVH metrics: HIV-infected participants. (C) Mean CCIMT by number of iCVH metrics: uninfected participants. (D) Mean CCIMT by number of nonlaboratory iCVH metrics. CCIMT, common carotid intima media thickness; iCVH, ideal cardiovascular health.
Associations between iCVH metrics and CCIMT remained significant when we excluded laboratory-based iCVH metrics (cholesterol and glucose) and included only smoking, BMI, physical activity, diet, and blood pressure (Table 3 and Fig. 2D). In univariate linear regression models, each additional iCVH metric at an ideal level was associated with a −0.023 difference in CCIMT (95% CI −0.039, −0.006; p = .007). When we added HIV serostatus to a multivariable linear regression model, this association remained statistically significant (coefficient −0.018; 95% CI −0.035, −0.001; p = .04) and the interaction term was not significant (p = .50). In this model, persons with one nonlaboratory iCVH metric at an ideal level had a predicted CCIMT of 0.728 mm compared with a predicted CCIMT of 0.657 mm for persons with five nonlaboratory iCVH metrics at ideal levels, resulting in a moderate effect size of 0.67 standard deviation units. The correlation between nonlaboratory iCVH metrics and CCIMT was marginally weaker (r = 0.19) than that between all seven iCVH metrics and CCIMT (r = 0.29).
Table 3.
Regression of Ideal CVH Score Without Laboratory Results (Cholesterol, Glucose) and Carotid IMT
| Univariate models | Multivariable models | |||
|---|---|---|---|---|
| Coefficient (95% CI) | p | Coefficient (95% CI) | p | |
| Ideal CVH score | −0.023 (−0.039, −0.006) | .007 | −0.018 (−0.035, 0.001) | .04 |
| HIV serostatus | −0.038 (−0.068, −0.010) | .009 | −0.030 (−0.060, 0.000) | .05 |
| Interaction term | 0.011 | .50 | ||
Discussion
In this analysis of a well-phenotyped cohort of HIV-infected participants on ART and uninfected controls in rural Uganda, we found that HIV-infected participants have more iCVH metrics at ideal levels. In addition, participants with higher number of iCVH metrics at ideal levels had significantly lower CCIMT. Each additional iCVH metric at an ideal level was associated with 0.024 mm lower CCIMT. Analyses from large observational cohorts have demonstrated that increments in CCIMT ranging from 0.03 to 0.20 mm are associated with 33%–300% higher risk for coronary heart disease and stroke;29,40–42 thus, in our study, an additional two to four iCVH metrics at ideal levels (corresponding to 0.048–0.096 mm lower CCIMT) may be associated with a clinically meaningful increment in risk for atherosclerotic clinical events. Longitudinal follow-up will be necessary to better understand the clinical implications of these findings in sub-Saharan Africa. Also of note, the association between iCVH metrics and CCIMT was stronger for uninfected than for HIV-infected participants.
Although the higher number of iCVH metrics at ideal levels for HIV-infected study participants compared with uninfected controls might appear counterintuitive given the association between HIV and CVD, there are several potential explanations for this finding. The iCVH metric with the highest disparity for HIV-infected versus uninfected participants was blood pressure. A total of 66.7% of HIV-infected participants had ideal blood pressure (<120/<80 mmHg) compared with only 36.0% of uninfected controls. This difference was not attributable to disparities in antihypertensive use; more HIV-infected persons (n = 7; 6.7%) than uninfected persons (n = 1; 1%) were taking antihypertensive medications, but this disparity was insufficient to explain the >30% difference between groups in prevalence of ideal blood pressure. Although some studies in sub-Saharan Africa have reported higher blood pressures among HIV-infected persons than uninfected controls, others in sub-Saharan Africa have suggested the inverse.43–45 Interestingly, one of these analyses also found that for HIV-infected persons, low blood pressure was associated with a higher mortality risk than high blood pressure.43 Relatively low blood pressure has been observed among patients with HIV infection—even among those on ART—and is thought to result from concomitant infections, bacterial translocation, autonomic dysfunction, and/or cardiometabolic derangements.1,20,43,46 Thus, it is possible that HIV-infected participants in our cohort who would otherwise have had higher blood pressures experienced downward influence on blood pressure due to chronic HIV disease. This complicates the interpretation of “ideal” blood pressure (<120/<80) for HIV-infected persons somewhat, as the higher prevalence of ideal blood pressure for HIV-infected participants in this study may simply reflect HIV disease activity.
An alternative, perhaps complementary, explanation for why HIV-infected participants were more likely to have ideal blood pressures is they may be more likely to have access to primary care services than uninfected controls in our sub-Saharan Africa setting. At the Mbarara Regional Referral Hospital Immune Suppression Syndrome Clinic, where participants in this cohort receive care, blood pressure is checked at each visit, and patients routinely see both a nurse and clinical officer. If HIV-infected participants in care in the region are seeing physicians more often than uninfected controls, they are likely to have higher opportunity to access both preventive and therapeutic health services. Although rates of hypertension treatment were low in both groups, 7 of 105 participants in the HIV-infected group versus only 1 of 100 in the uninfected group had an active prescription for an antihypertensive at the time of the study. This possibility is also supported by our finding that HIV-infected participants were more likely to have ideal levels of blood sugar (72.4% versus 58.0% ideal) and smoking (96.2% versus 82.0% ideal). If this is the case and HIV-infected persons have more iCVH metrics at ideal levels because they are more closely linked to care, it underscores both the value of HIV-care programs in the region and the need to translate these services into access to appropriate noncommunicable disease screening and care for uninfected populations in sub-Saharan Africa.
The association we identified between iCVH metrics and CCIMT is the first in sub-Saharan Africa to validate the iCVH scale and is consistent with previous studies in European and North American cohorts of uninfected persons. Prior studies have similarly demonstrated that persons with more iCVH metrics at ideal levels have less subclinical CVD. Although our study did not include overt CVD, others have reported associations.14,15 The stronger correlation between iCVH metrics and CCIMT for uninfected persons we noted is potentially attributable to the lack of inclusion of HIV-specific risk factors that have been associated with CVD risk, such as CD4 count and ART regimen.47 The consistent association of iCVH metrics at ideal levels with lower CCIMT in both groups lends support to the use of the iCVH scales as a relatively simple and potentially scalable approach to evaluating CVD risks in sub-Saharan Africa. Moreover, the five-item iCVH score, which excluded laboratory-based parameters (cholesterol and glucose), remained associated with CCIMT and is potentially advantageous due to easier application in resource-limited settings.
This study's findings should be interpreted in the context of its limitations. Given the relatively small size and cross-sectional nature of our study, we were unable to evaluate CVD outcomes and thus relied on CCIMT as a surrogate endpoint. However, CCIMT has been strongly associated with CVD in several populations, and the effect sizes we reported have been clinically predictive of CVD events.48–52 Moreover, the richness of phenotypic data in this cohort enabled us to capture all seven iCVH metrics and composite iCVH scores—something that, to our knowledge, has not been done in an HIV-infected nor sub-Saharan Africa cohort. The cohort we analyzed is largely representative of HIV-infected persons in rural Uganda with well-controlled disease on ART and uninfected persons (matched controls) from the same region. Our findings are therefore generalizable to this population, and data from other subpopulations on the continent will be necessary to describe these relationships more broadly.
Another limitation of this study is its cross-sectional nature, which prevented us from evaluating prospective associations of iCVH metrics with incident CVD. Our cross-sectional approach assumes that levels of iCVH metrics tend to be consistent over time and predate carotid atherosclerosis, which we believe to be a reasonable assumption given consistency of CVD risk factors seen in population-based cohorts.53 Finally, an important consideration about the iCVH metric approach, in general, is the equal weighting given to each factor and the resulting effect on risk prediction. For instance, although being a current smoker likely has a higher detrimental impact on CVD health than having a BMI ≥25 kg/m2, each of these would equally result in having one less point in an iCVH score that simply counts number of iCVH metrics at ideal levels. Although this conflation of risk factors with different effect sizes may limit the predictive utility of these iCVH metrics, our data demonstrate that it has important value as a simplified tool for health status assessment and risk prevention.
Future studies in well-phenotyped cohorts in sub-Saharan Africa are needed to confirm our findings and investigate the extent to which iCVH metrics correlate with overt CVD. If correlations between iCVH scores and CVD are consistently weaker in HIV-infected versus uninfected cohorts in sub-Saharan Africa, it would be reasonable to evaluate whether adding clinical (e.g., duration of HIV infection and ART) and/or laboratory-based (e.g., CD4+ T-cell count) HIV-specific parameters improves the validity of iCVH scores for this population. Ultimately, the use of a relatively simple, scalable approach to capturing CVD risk factors for both HIV-infected and uninfected persons in sub-Saharan Africa and similar settings would serve the important goal of improving cardiovascular health surveillance and prevention globally.
Acknowledgments
The authors wish to thank all study participants and personnel from the UGANDAC study. This work was supported by several grants from the National Institutes of Health (P30 AI027763, R01 MH054907, and R21 HL 124712).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Compostella C, Compostella L, D'Elia R: The symptoms of autonomic dysfunction in HIV-positive Africans. Clin Auton Res 2008;18:6–12 [DOI] [PubMed] [Google Scholar]
- 2.Lim GB: Global burden of cardiovascular disease. Nat Rev Cardiol 2013;10:59. [DOI] [PubMed] [Google Scholar]
- 3.Berry JD, Dyer A, Cai X, et al. : Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daviglus ML, Stamler J, Pirzada A, et al. : Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA 2004;292:1588–1592 [DOI] [PubMed] [Google Scholar]
- 5.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL: Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: The northern Manhattan study. Circulation 2012;125:2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ES, Greenlund KJ, Hong Y: Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation 2012;125:987–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Leip EP, Larson MG, et al. : Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113:791–798 [DOI] [PubMed] [Google Scholar]
- 8.Yang Q, Cogswell ME, Flanders WD, et al. : Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 2012;307:1273–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Hong Y, Labarthe D, et al. : Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic impact goal through 2020 and beyond. Circulation 2010;121:586–613 [DOI] [PubMed] [Google Scholar]
- 10.Bi Y, Jiang Y, He J, et al. : Status of cardiovascular health in Chinese adults. J Am Coll Cardiol 2015;65:1013–1025 [DOI] [PubMed] [Google Scholar]
- 11.Jankovic S, Stojisavljevic D, Jankovic J, Eric M, Marinkovic J: Status of cardiovascular health in a transition European country: Findings from a population-based cross-sectional study. Int J Public Health 2014;59:769–778 [DOI] [PubMed] [Google Scholar]
- 12.Moghaddam MM, Mohebi R, Hosseini F, et al. : Distribution of ideal cardiovascular health in a community-based cohort of Middle East population. Ann Saudi Med 2014;34:134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen GS, Holm AS, Jorgensen T, Borglykke A: Distribution of ideal cardiovascular health by educational levels from 1978 to 2006: A time trend study from the capital region of Denmark. Eur J Prev Cardiol 2014;21:1145–1152 [DOI] [PubMed] [Google Scholar]
- 14.Laitinen TT, Pahkala K, Magnussen CG, et al. : Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: The Cardiovascular Risk in Young Finns Study. Circulation 2012;125:1971–1978 [DOI] [PubMed] [Google Scholar]
- 15.Xanthakis V, Enserro DM, Murabito JM, et al. : Ideal cardiovascular health: Associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation 2014;130:1676–1683 [DOI] [PubMed] [Google Scholar]
- 16.Freiberg MS, Chang CC, Kuller LH, et al. : HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French AL, Gawel SH, Hershow R, et al. : Trends in mortality and causes of death among women with HIV in the United States: A 10-year study. J Acquir Immune Defic Syndr 2009;51:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palella FJ, Jr., Baker RK, Moorman AC, et al. : Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34 [DOI] [PubMed] [Google Scholar]
- 19.Triant VA, Lee H, Hadigan C, Grinspoon SK: Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchetti G, Bellistri GM, Borghi E, et al. : Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS 2008;22:2035–2038 [DOI] [PubMed] [Google Scholar]
- 21.Hayes R, Weiss H: Epidemiology. Understanding HIV epidemic trends in Africa. Science 2006;311:620–621 [DOI] [PubMed] [Google Scholar]
- 22.Van de Perre P: The epidemiology of HIV infection and AIDS in Africa. Trends Microbiol 1995;3:217–222 [DOI] [PubMed] [Google Scholar]
- 23.Group ISS, Lundgren JD, Babiker AG, et al. : Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friis-Moller N, Ryom L, Smith C, et al. : An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol 2016;23:214–223 [DOI] [PubMed] [Google Scholar]
- 25.Zoghbi WA, Duncan T, Antman E, et al. : Sustainable development goals and the future of cardiovascular health: A statement from the Global Cardiovascular Disease Taskforce. Glob Heart 2014;9:273–274 [DOI] [PubMed] [Google Scholar]
- 26.Bendavid E, Holmes CB, Bhattacharya J, Miller G: HIV development assistance and adult mortality in Africa. JAMA 2012;307:2060–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bor J, Herbst AJ, Newell ML, Barnighausen T: Increases in adult life expectancy in rural South Africa: Valuing the scale-up of HIV treatment. Science 2013;339:961–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills EJ, Bakanda C, Birungi J, et al. : Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: A cohort analysis from Uganda. Ann Intern Med 2011;155:209–216 [DOI] [PubMed] [Google Scholar]
- 29.Chambless LE, Heiss G, Folsom AR, et al. : Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol 1997;146:483–494 [DOI] [PubMed] [Google Scholar]
- 30.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M: Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007;115:459–467 [DOI] [PubMed] [Google Scholar]
- 31.Siedner MJ, Kim JH, Nakku RS, et al. : Persistent immune activation and carotid atherosclerosis in HIV-infected Ugandans receiving antiretroviral therapy. J Infect Dis 2016;213:370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siedner MJ, Kim JH, Nakku RS, et al. : HIV infection and arterial stiffness among older-adults taking antiretroviral therapy in rural Uganda. AIDS 2016;30:667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt PW, Cao HL, Muzoora C, et al. : Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS 2011;25:2123–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai AC, Bangsberg DR, Frongillo EA, et al. : Food insecurity, depression and the modifying role of social support among people living with HIV/AIDS in rural Uganda. Soc Sci Med 2012;74:2012–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai AC, Kakuhikire B, Mushavi R, et al. : Population-based study of intra-household gender differences in water insecurity: Reliability and validity of a survey instrument for use in rural Uganda. J Water Health 2016;14:280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He FJ, Nowson CA, Lucas M, MacGregor GA: Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J Hum Hypertens 2007;21:717–728 [DOI] [PubMed] [Google Scholar]
- 37.Joshipura KJ, Hu FB, Manson JE, et al. : The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med 2001;134:1106–1114 [DOI] [PubMed] [Google Scholar]
- 38.D'Agostino RB, Sr: Cardiovascular risk estimation in 2012: Lessons learned and applicability to the HIV population. J Infect Dis 2012;205(Suppl 3):S362–S367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein JH, Korcarz CE, Hurst RT, et al. : Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93–111; quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 40.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE: Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation 1997;96:1432–1437 [DOI] [PubMed] [Google Scholar]
- 41.Hodis HN, Mack WJ, LaBree L, et al. : The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 1998;128:262–269 [DOI] [PubMed] [Google Scholar]
- 42.Salonen JT, Salonen R: Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation 1993;87(Suppl 3):II56–II65 [PubMed] [Google Scholar]
- 43.Bloomfield GS, Hogan JW, Keter A, et al. : Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: A retrospective analysis of electronic health records. BMC Infect Dis 2014;14:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chillo P, Bakari M, Lwakatare J: Echocardiographic diagnoses in HIV-infected patients presenting with cardiac symptoms at Muhimbili National Hospital in Dar es Salaam, Tanzania. Cardiovasc J Afr 2012;23:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillon DG, Gurdasani D, Riha J, et al. : Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: A systematic review and meta-analysis. Int J Epidemiol 2013;42:1754–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meya DB, Katabira E, Otim M, et al. : Functional adrenal insufficiency among critically ill patients with human immunodeficiency virus in a resource-limited setting. Afr Health Sci 2007;7:101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friis-Moller N, Thiebaut R, Reiss P, et al. : Predicting the risk of cardiovascular disease in HIV-infected patients: The data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil 2010;17:491–501 [DOI] [PubMed] [Google Scholar]
- 48.Ssinabulya I, Kayima J, Longenecker C, et al. : Subclinical atherosclerosis among HIV-infected adults attending HIV/AIDS care at two large ambulatory HIV clinics in Uganda. PLoS One 2014;9:e89537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortblad KF, Lozano R, Murray CJ: The burden of HIV: Insights from the Global Burden of Disease Study 2010. AIDS 2013;27:2003–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bloomfield GS, Hogan JW, Keter A, et al. : Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One 2011;6:e22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edward AO, Oladayo AA, Omolola AS, Adetiloye AA, Adedayo PA: Prevalence of traditional cardiovascular risk factors and evaluation of cardiovascular risk using three risk equations in nigerians living with human immunodeficiency virus. N Am J Med Sci 2013;5:680–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nery MW, Martelli CM, Silveira EA, et al. : Cardiovascular risk assessment: A comparison of the Framingham, PROCAM, and DAD equations in HIV-infected persons. ScientificWorldJournal 2013;2013:969281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulmer H, Kelleher C, Diem G, Concin H: Long-term tracking of cardiovascular risk factors among men and women in a large population-based health system: The Vorarlberg Health Monitoring & Promotion Programme. Eur Heart J 2003;24:1004–1013 [DOI] [PubMed] [Google Scholar]