Abstract
There are limited data describing acute kidney injury (AKI) in HIV-infected adult patients in resource-limited settings where tenofovir disoproxil fumarate (TDF), which is potentially nephrotoxic, is increasingly prescribed. We describe risk factors for and prognosis of AKI in HIV-infected individuals, stratified by those receiving and those naive to TDF. A prospective case cohort study of hospitalized HIV-infected adults with AKI stratified by TDF exposure. Adults (≥18 years) were recruited: clinical and biochemical data were collected at admission; their renal recovery, discharge, or mortality was ascertained as an in-patient and, subsequently, to a scheduled 3-month follow-up. Among this predominantly female (61%), almost exclusively black African cohort of 175 patients with AKI, 93 (53%) were TDF exposed; median age was 41 years (interquartile range 35–50). Median CD4 count and viral load and creatinine at baseline were 116 cells/mm3 and 110,159 copies/ml, respectively. A greater proportion of the TDF group had severe AKI on admission (61% vs. 43%, p = .014); however, both groups had similar rates of newly diagnosed tuberculosis (TB; 52%) and nonsteroidal anti-inflammatory drug (NSAID; 32%) use. Intravenous fluid was the therapeutic mainstay; only seven were dialyzed. Discharge median serum creatinine (SCr) was higher in the TDF group (p = .032) and fewer in the TDF group recovered renal function after 3 months (p = .043). Three-month mortality was 27% in both groups, but 55% of deaths occurred in hospital. Those that died had a higher SCr and more severe AKI than survivors; TB was diagnosed in 33 (70%) of those who died. AKI was more severe and renal recovery slower in the TDF group; comorbidities, risk factors, and prognosis were similar regardless of TDF exposure. Because TB is linked to higher mortality, TB coinfection in HIV-infected patients with AKI warrants more intensive monitoring. In all those with poor renal recovery, our data suggest that a lower threshold for dialysis is needed.
Keywords: : acute kidney injury, renal insufficiency, HIV, tenofovir, South Africa, mortality
Background
Acute kidney injury (AKI) is a major cause of morbidity and mortality in HIV-infected individuals.1,2 Mortality in hospitalized HIV-infected adults is higher in those with AKI, while in adults living with HIV the risk of kidney disease is almost fourfold that of HIV-uninfected adults.3,4 Antiretroviral therapy (ART) mitigates this risk.1
Numerous studies from developed settings have reported AKI in HIV-infected individuals, suggesting that the etiology of AKI is multifactorial. Moreover, both HIV and ART are directly nephrotoxic.1,3–6 Prerenal AKI from renal hypoperfusion (commonly from gastroenteritis), sepsis-induced acute tubular necrosis (ATN), and concomitant nephrotoxic drug use are common causes of AKI in HIV-infected adults.2,5,7,8 Well-described risk factors for AKI apply to both HIV-seronegative and seropositive individuals.1,3–7,9,10 However, specific risk factors in HIV-seropositive individuals include: male sex; advanced immunosuppression; long-term ART use; and preexisting renal disease.3–7,9–11
In October 2001, the Food and Drug Administration approved tenofovir disoproxil fumarate (TDF) as the first nucleotide reverse-transcriptase inhibitor (NRTI) for the treatment of HIV.12 Since April 2010, TDF has been the mainstay of first line ART in South Africa: massively increasing the number of South Africans receiving TDF.13 Although not initially reported in the early clinical trials, subsequent case reports, case series, and observational studies reported TDF-associated nephrotoxicity and, in 2010, a systematic review and meta-analysis reported a significant decline in renal function associated with TDF use.14–19
The mechanism of action appears to be the accumulation of TDF in proximal tubular cells (due primarily to genetic polymorphisms encoding defective transmembrane drug transporters), with resultant mitochondrial toxicity and subsequent renal injury, clinically presenting as the Fanconi syndrome or ATN with AKI.16,20–22 The reported prevalence of TDF-related nephrotoxicity is 2.4% occurring at 6–9 months following TDF initiation, although South African studies suggest an earlier onset.11,17–19,22–25
We report the presentation, severity, and risk factors for AKI in HIV-positive South African patients and assess the response to treatment and short-term outcomes. Furthermore, to assess the role of TDF in HIV-positive patients with AKI, analysis was stratified by TDF exposure.
Materials and Methods
This prospective case cohort study recruited patients from the Klerksdorp/Tshepong Hospital Complex, a secondary level public hospital in South Africa's North West province, from October 2014 to June 2015. We enrolled HIV-infected patients admitted to the adult medicine wards with AKI and compared those exposed to TDF to those who had not received TDF and followed them up after discharge. Eligible patients were: hospitalized, HIV-infected adults, and ≥18 years of age with a diagnosis of AKI. We excluded patients with prior or preexisting kidney disease (either self-reported or as indicated by medical records or laboratory results). Those who appeared unwilling or incapable of attending follow-up and those whose clinical records were inadequate to assess renal function or HIV treatments before the study were also excluded. The University of Witwatersrand Human Research Ethics Committee approved this study.
Study definitions and measures
Patients were categorized as TDF exposed if they were on TDF-based ART at the time of admission or had defaulted TDF for <1 week before admission. The TDF unexposed group included individuals who: were ART naive, were taking non-TDF-based ART, had defaulted non-TDF based ART for any duration, and those who had defaulted TDF based ART for at least 8 weeks before the onset of AKI.
In all study patients, AKI was diagnosed and staged according to the Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice AKI Guideline.26 Baseline serum creatinine (SCr) before AKI was determined either by a SCr taken before, but within the preceding year of admission, or the lowest normal SCr at discharge or on follow-up.27,28 If no baseline SCr was available, an estimated baseline SCr was calculated based on a Modification of Diet in Renal Disease (MDRD) equation estimated glomerular filtration rate (eGFR) of 75 ml/min per 1.73 m2.26,29,30
Renal recovery was defined as a decreasing SCr over time and no need for continuing renal replacement therapy (RRT) and was categorized as complete (SCr recovery below 1.5 times the baseline) or partial (SCr recovery above 1.5 times the baseline).31,32 New admissions to the acute care adult medical ward were screened for eligibility on weekdays. If eligible, written informed consent was obtained and a structured interview administered assessing: ART use, previous tuberculosis (TB) history, comorbidities, concomitant gastrointestinal symptoms, drug history, and other important exposures; necessary blood and urine samples were taken, results of radiological investigations recorded, and relevant information from clinical records noted.
On discharge, a summary of the clinical course of the patient was abstracted from the medical records. Patients were then actively followed up at a visit scheduled 3 months after discharge.
Statistical analyses
Patient demographic and clinical characteristics are reported as medians and interquartile ranges (IQRs) for continuous variables and proportions with 95% confidence intervals (95% CIs) for categorical variables. These were compared between groups using the Mann–Whitney, Kruskal–Wallis, and Chi-square tests as appropriate. Univariable logistical regression was used to control for TDF use and the presence of TB coinfection. Multivariable regression was not performed due to the small sample size and some missing data. All statistical analyses were performed using STATISTICA version 12 (StatSoft).
Results
From 1 October 2014 to 30 June 2015, 199 HIV-infected patients with presumed AKI were enrolled in this study; 24 were subsequently excluded as they either had prior kidney disease (16) or had defaulted TDF based ART for <8 weeks (8). Of the remaining 175, their median age was 41 years (IQR 35–50); 61% were women; virtually all (99%) were black Africans; and their median body mass index was 21 kg/m2 (IQR 19–24) on admission (Table 1). Ninety-three (53%) were TDF exposed for a median treatment duration of 30 weeks (IQR 8–61) of whom 27 (29%) had prior ART exposure before switching to a TDF containing regimen. The majority of the TDF group (94%) received a concomitant non-NRTI (NNRTI) and 6% a protease inhibitor. Thirty-one patients in the non-TDF group were receiving ART for a median duration of 306 weeks (IQR 88–729); 62% of the non-TDF group was ART-naive on admission.
Table 1.
Baseline Demographic Characteristics, Comorbidities, Risk Factors, and Laboratory Characteristics in Acute Kidney Injury Patients at Admission Stratified by Tenofovir Disoproxil Fumarate Exposure (n = 175)
| TDF | Non-TDF | p | |
|---|---|---|---|
| 93 (53) | 82 (47) | ||
| Demographics | |||
| Age (years), median (IQR) | 42 (35–53) | 40 (35–49) | .260 |
| Female, n (%) | 62 (67) | 45 (55) | .110 |
| Black, n (%) | 91 (98) | 83 (100) | .182 |
| Serum electrolytes | |||
| Potassium (mmol/l), median (IQR) | 4.1 (3.3–5.1) | 4.3 (3.5–5.2) | .348 |
| Bicarbonate (μmol/l), median (IQR) | 14 (10–18) | 16 (13–19) | .047 |
| Phosphate (mmol/l), median (IQR) | 1.2 (0.8–1.7) | 1.3 (0.9–1.6) | .496 |
| Renal parameters | |||
| Admission creatinine (μmol/l), median (IQR) | 282 (172–542) | 189 (146–343) | .007 |
| Admission eGFR-CKD-EPI (ml/min per 1.73 m2), median (IQR) | 14.2 (4.3–28) | 25.6 (10.4–39.5) | .002 |
| Baseline creatinine (μmol/l), median (IQR) | 77 (65–88) | 77 (62–92) | .920 |
| Urine protein:creatinine ratio (g/mmol creat), median (IQR) | 0.212 (0.111–0.316) | 0.128 (0.087–0.212) | .003 |
| Normoglycemic glycosuria, n (%) | 7 (11) | 6 (10) | .863 |
| Hyperechoic, n (%) | 71 (91) | 48 (84) | .226 |
| Right kidney size (cm), median (IQR) | 11.25 (10.3–12.2) | 11 (10.2–11.8) | .347 |
| Left kidney size (cm), median (IQR) | 11.4 (10.7–12.3) | 11 (10.4–11.8) | .135 |
| Comorbidities | |||
| Hypertension, n (%) | 22 (24) | 16 (20) | .507 |
| Diabetes, n (%) | 8 (9) | 3 (4) | .179 |
| Hepatitis B positive, n (%) | 11 (12) | 9 (11) | .86 |
| Risk factors | |||
| TB, n (%) | |||
| Completed TB treatment before admission | 35 (38) | 34 (41) | .605 |
| Newly diagnosed TB | 46 (50) | 45 (55) | .474 |
| Sputum or culture proven TB | 22 (48) | 22 (49) | .919 |
| Pneumonia, n (%) | 9 (10) | 12 (17) | .148 |
| Sepsis, n (%) | 4 (4) | 7 (9) | .249 |
| Vomiting, n (%) | 32 (34) | 14 (17) | .009 |
| Diarrhea, n (%) | 36 (39) | 17 (21) | .008 |
| NSAID use, n (%) | 26 (28) | 30 (37) | .784 |
| HIV-related and infective parameters | |||
| CD4 count (cells/mm3), median (IQR) | 147 (57–304) | 87 (41–210) | .067 |
| HIV viral load (copies/ml) | |||
| Virally suppressed (LDL or <1,000), n (%) | 56 (60) | 14 (17) | <.001 |
| Median HIV viral load in those >1,000, median (IQR) | 39,456 (4,537–326,300) | 414,365 (110,159–1,521,015) | <.001 |
| Hemoglobin (g/dl), median (IQR) | 9.6 (7.8–11.3) | 9.8 (7.4–12) | .622 |
| C-reactive protein (mg/l), median (IQR) | 127 (39–189) | 89 (37–180) | .386 |
| Albumin (g/l), median (IQR) | 20 (16–24) | 18 (15–23) | .122 |
| Ferritin (μg/l), median (IQR) | 490 (205–1,171) | 750 (366–1,415) | .047 |
eGFR, estimated glomerular filtration rate; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug; TB, tuberculosis; TDF, tenofovir disoproxil fumarate.
AKI severity and measures of kidney disease
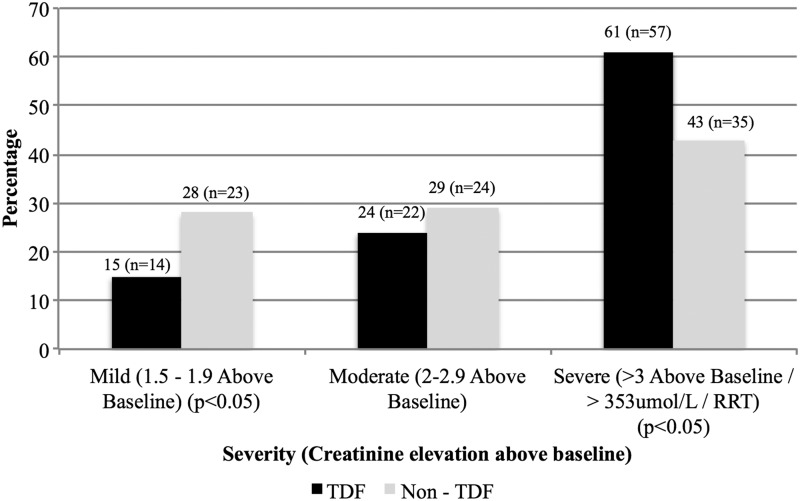
Half (53%) of all participants had severe AKI, most were in the TDF group (62%; n = 57), whereas mild AKI was predominant in the non-TDF group (62%; n = 23) (Fig. 1). The TDF group had: a higher median admission SCr (282 μmol/l; IQR 172–542 vs. 189 μmol/l; IQR 146–343; p = .007); higher median urine protein creatinine ratio (0.212 g/mmol creat; IQR 0.111–0.316 vs. 0.128 g/mmol creat; IQR 0.087–0.212; p = .003); and a lower median eGFR (CKD-EPI) (14.2 ml/min; IQR 4.3–28 vs. 25.6 ml/min; IQR 10.4–39.5; p = .002) and was more acidotic (serum bicarbonate 14 μmol/l; IQR 10–18 vs. 16 μmol/l; IQR 13–19; p = .047). The odds ratio (OR) for severe AKI in the TDF group was 1.2 (1.02–1.40; 95% CI; p = .013) compared to those who were not exposed to TDF (Table 2).
FIG. 1.
Severity of acute kidney injury on admission stratified by TDF exposure. TDF, tenofovir disoproxil fumarate.
Table 2.
Univariable Analysis to Determine Risk of Severe Acute Kidney Injury and Mortality at 3-Month Follow-Up
| Severe AKI | Mortality after 3-month follow-up | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| TDF exposure | 1.20 (1.02–1.40) | .013 | 1.00 (0.86–1.14) | .993 |
| HIV viral load suppressed | 1.10 (0.95–1.27) | .196 | 1.07 (0.90–1.26) | .763 |
| TB coinfection | 1.05 (0.90–1.22) | .516 | 1.21 (1.06–1.38) | .003 |
| Vomiting | 1.14 (1.00–1.30) | .046 | 1.14 (0.99–1.30) | .073 |
| Diarrhea | 1.20 (1.05–1.30) | .007 | 1.12 (0.96–1.30) | .164 |
| NSAID use | 1.06 (0.92–1.22) | .409 | 0.97 (0.83–1.14) | .706 |
| Severe AKI | N/A | N/A | 1.10 (0.97–1.26) | .144 |
95% CI, 95% confidence interval; AKI, acute kidney injury; OR, odds ratio.
Markers of proximal tubular dysfunction (urine phosphaturia and glycosuria, serum hypophosphatemia and hypokalemia) were not markedly deranged in either group. Laboratory results suggestive of interstitial nephritis were present in many, with high rates of leukocyturia (n = 47; 32%) and hematuria (n = 81; 55%) in sterile urine specimens, but there was no significant difference in the two groups—only 29 (17%) of all patients had a culture positive urinary tract infection.
Comorbidities and risk factors for AKI
Half of both groups (50% TDF vs. 55% non-TDF; p = .474) were newly diagnosed with TB, and overall a prior history of TB treatment was reported by 39%. Gastrointestinal symptoms, vomiting, and diarrhea were reported more frequently in the TDF group (39% vs. 21%; p = .008) and twice as many patients in the TDF group reported chronic diarrhea (p = .522). Furthermore, in patients with severe AKI, the OR of vomiting [OR–1.14 (1.00–1.30; 95% CI; p = .046)] and diarrhea [OR–1.20 (1.05–1.30; 95% CI; p = .007)] was significantly increased (Table 2).
Noninfectious comorbidities: hypertension (22%) and diabetes (6%) were reported at similar rates in both groups. Receipt of traditional medication was infrequently self-reported, but high rates of nonsteroidal anti-inflammatory drug (NSAID) use, within the month before admission, were reported by both groups. A greater number of TDF-exposed patients were virally suppressed than ART-treated patients from the non-TDF group (p = .017); and in those not suppressed, the median viral load was lower in the TDF group (p = .002).
Treatment and outcomes
The overwhelming majority of patients received intravenous fluid therapy (96%) and only seven received RRT.
In those who received RRT, the admission SCr was significantly higher than those treated with intravenous fluid therapy: median SCr 553 μmol/l; IQR 471–623 vs. 216 μmol/l; IQR 156–438, p = .021; furthermore, all seven patients presented with severe AKI, p = .037. However, there were no statistical differences in the two groups in: TDF exposure and risk factors for AKI.
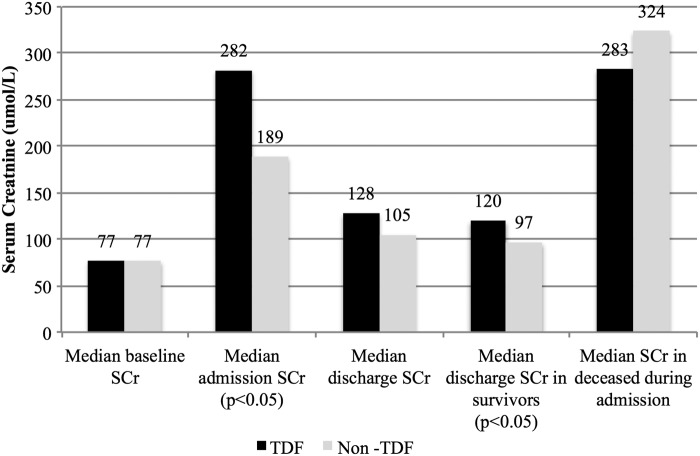
Eighty-five percent of all patients recovered sufficiently to be discharged from hospital. Even though the median length of hospital stay was the same in both groups (9 days), the TDF group had delayed renal recovery with higher SCr on discharge: median SCr 120 μmol/l; IQR 87–240 vs. 97 μmol/l; IQR 75–137; p = .032 (Fig. 2). Moreover, a smaller proportion of TDF exposed patients had complete renal recovery at the 3-month postdischarge follow-up visit (61% vs. 78%; p = .043) (Table 3).
FIG. 2.
Comparison of median SCr values stratified by TDF exposure at various time intervals: baseline before admission; on admission; on discharge; in survivors to discharge; and in deaths during hospital stay. SCr, serum creatinine.
Table 3.
Outcomes of Mortality, Renal Recovery, and Comorbidities Measured at Discharge and 3-Month Follow-Up Stratified by Both Tenofovir Disoproxil Fumarate Exposure and Duration of Tenofovir Disoproxil Fumarate Use (n = 175)
| Duration of TDF use | |||||||
|---|---|---|---|---|---|---|---|
| TDF | Non-TDF | p | <6 Weeks | 6–24 Weeks | >24 Weeks | p | |
| 93 (53) | 82 (47) | 20 (22) | 25 (27) | 48 (51) | |||
| Mortality at discharge | |||||||
| Deceased, n (%) | 16 (17) | 10 (12) | .353 | 4 (20) | 5 (20) | 7 (15) | .787 |
| Fluid therapy alone, n (%) | 88 (95) | 80 (98) | .322 | 19 (95) | 25 (100) | 44 (92) | .324 |
| Deceased, n (%) | 13 (15) | 9 (11) | .499 | 3 (16) | 5 (20) | 5 (11) | .617 |
| Fluid and renal replacement therapy, n (%) | 5 (5) | 2 (2) | .322 | 1 (5) | 0 (0) | 4 (8) | .324 |
| Deceased, n (%) | 3 (60) | 1 (50) | .809 | 1 (100) | 0 (0) | 2 (50) | .361 |
| Duration of stay (days), median (IQR) | |||||||
| If survived | 9 (7–15) | 9 (7–14) | .722 | 8 (7–20) | 11 (7–15) | 9 (7–15) | .949 |
| If deceased | 10 (6–16) | 13 (9–16) | .286 | 11 (10–15) | 10 (7–37) | 6 (5–16) | .666 |
| Mortality after 3-month follow-up | |||||||
| Deceased, n (%) | 25 (27) | 22 (27) | .921 | 6 (30) | 8 (32) | 11 (23) | .665 |
| Duration to death (days), median (IQR) | |||||||
| Admission to death | 30 (13–40) | 64 (57–129) | .049 | 35 (30–40) | 28 (13–125) | 25 (13–88) | .783 |
| Discharge to death | 24 (18–30) | 52 (47–122) | .016 | 22 (18–25) | 23 (5–97) | 28 (6–30) | .895 |
| Renal parameters on discharge | |||||||
| Renal recovery among deceased, n (%) | |||||||
| Complete creatinine recovery | 3 (20) | 1 (10) | .504 | 0 (0) | 1 (25) | 2 (29) | .501 |
| Partial creatinine recovery | 7 (47) | 5 (50) | .870 | 2 (50) | 3 (75) | 2 (29) | .328 |
| Worsening creatinine | 5 (33) | 4 (40) | .734 | 2 (50) | 0 (0) | 3 (42) | .248 |
| Renal recovery among survivors, n (%) | |||||||
| Complete creatinine recovery | 37 (48) | 39 (54) | .456 | 9 (69) | 9 (47) | 19 (51) | .438 |
| Partial creatinine recovery | 32 (42) | 19 (26) | .051 | 4 (31) | 10 (53) | 18 (49) | .438 |
| Creatinine and eGFR (CKD-EPI) at death by renal recovery | |||||||
| By creatinine (μmol/l), median (IQR) | |||||||
| Deceased and complete recovery | 79 (78–83) | 92 (92–92) | .5 | — | 83 (83–83) | 79 (79–79) | .667 |
| Deceased and partial recovery | 283 (131–368) | 271 (139–513) | 1 | 326 (283–368) | 131 (116–147) | 574 (311–836) | .095 |
| Deceased and worsening creatinine | 572 (527–627) | 388 (366–577) | .413 | 577 (527–627) | — | 572 (227–852) | 1 |
| By eGFR (ml/min per 1.73 m2), median (IQR) | |||||||
| Deceased and complete recovery | 77 (76–78) | 63 (63–63) | .5 | — | 76 (76–76) | 78 (78–78) | .667 |
| Deceased and partial recovery | 13 (8–41) | 13 (5–38) | 1 | 10 (8–13) | 41 (33–48) | 6 (2–9) | .095 |
| Deceased and worsening creatinine | 4 (3–5) | 7 (7–12) | .413 | 4 (4–5) | — | 3 (2–18) | 1 |
| Renal parameters after 3-month follow-up | |||||||
| Renal recovery among survivors, n (%) | |||||||
| Complete creatinine recovery | 37 (61) | 40 (78) | .043 | 8 (62) | 7 (54) | 22 (63) | .849 |
| Partial creatinine recovery | 9 (15) | 2 (4) | .055 | 0 (0) | 2 (15) | 7 (20) | .221 |
Although TDF was stopped on admission in all patients, at 3-month follow-up, 55 patients (31%) had been restarted on full dose TDF based ART without apparent adverse effects, 38 (22%) of whom had been TDF exposed on admission. Only one patient who was reinitiated on TDF was subsequently changed to non-TDF based ART. No significant difference in renal recovery (p = .114) or mortality (p = .105) at 3-month follow-up was found in those reinitiated on TDF compared to those where non-TDF based ART was used following discharge.
Furthermore, among the TDF exposed group, no significant difference was noted in mortality or renal recovery at discharge or at 3-month follow-up when stratifying for duration of TDF use (Table 3).
In those who died during their hospital stay, no difference was noted in either SCr values measured before death or in renal recovery when comparing the TDF and non-TDF groups (Table 3). Fifty-five percent of deaths occurred in hospital and they had a higher median SCr before death compared to the median SCr at discharge in survivors (p < .001). Furthermore, most patients who died in hospital had severe AKI immediately before death (p < .001).
Sixteen (9.1%) patients were lost to follow-up after discharge. In those who died following discharge, similar mortality rates were found in both groups (p = .383). When assessing overall outcome, from admission to 3-month follow-up, the mortality rate in both groups was 27%, but the duration from admission to death and discharge to death was significantly shorter in the TDF group (Table 3). Seventy percent (n = 33) of those who died were newly diagnosed with TB compared to 42% of survivors (p = .001), and the OR of mortality at 3-month follow-up in those newly diagnosed with TB was 1.21 (1.06–1.38; 95% CI, p = .003) compared to those without TB (Table 2). Similarly, elevated C-reactive protein and anemia were more frequently found in patients who died in this cohort.
Discussion
This prospective cohort suggests that HIV-infected patients with AKI who also received TDF-based ART had more severe AKI and a higher presenting median SCr compared to those on non-TDF-based ART. Moreover, the renal recovery was slower in those taking TDF. In-hospital outcomes, duration of admission, and overall mortality, however, were similar in both TDF exposed and unexposed groups. AKI in HIV-infected individuals has a poor prognosis, as more than a quarter of the patients in this cohort died.
In our study, TDF exposed HIV-infected patients who develop AKI have a similar etiology and rate and range of nephrotoxic risk factors that we measured as those not receiving TDF. However, our data suggest that TDF has an added nephrotoxic effect in patients with AKI causing: a more rapid worsening of renal function; a higher proportion with proteinuria and acidosis; and delayed renal recovery.
The data on TDF use and AKI in Southern Africa are limited; previous studies in ambulatory patients report a frequency of TDF-associated nephrotoxicity of just less than 3% and risk factors for nephrotoxicity with TDF use include: preexisting renal dysfunction, anemia, and immunosuppression.24,25 This study is the first we are aware of that reports AKI, stratified by TDF exposure, in hospitalized HIV-positive patients in South Africa, and is particularly relevant due to the massive increase in prescriptions of TDF as part of first line ART in the country.33
RRT use was limited in this cohort, despite increasing SCr in over a third of those who died during admission and despite no formal restrictions or eligibility criteria for the initiation of RRT in this setting. We postulate that RRT may have prevented some deaths. Moreover, the substantial mortality following discharge from hospital suggests that more intensive monitoring and follow-up after discharge from hospital are necessary in all patients with AKI. Further studies are required to assess the likely causes of and contributors to death and to better identify potential interventions to prevent mortality.
Consistent with our findings, international studies have reported the duration of TDF exposure before onset of decline in renal function to be 6–9 months,18,23 although studies of ambulatory patients in South Africa report a shorter median time to nephrotoxicity (3.6 months).24,25
Current recommendations suggest that TDF dose adjustment is necessary in those with renal injury while on TDF.34
Although published literature reports male gender and lower body weight as risk factors associated with AKI,18,19,23,25 the high proportion of women with AKI that we report is likely due to higher seroprevalence of HIV among South African women and national policy to initiate ART in all HIV-positive pregnant women irrespective of CD4 count.33,35
The apparent role of TB, which was frequently diagnosed in this cohort, in contributing to both morbidity and mortality following discharge is substantial. The association between AKI and TB is described in a retrospective cohort from Johannesburg, in which 26% of HIV-positive patients with AKI were diagnosed with TB, almost half of what we report.8 However, over half of the cases of TB we report were not laboratory confirmed.36
Fifty-three percent of our cohort had laboratory findings suggestive of interstitial nephritis, which could be at least partially attributed to TB and/or NSAID use, although this finding is limited by the absence of renal biopsies in this cohort.37,38 While the immune reconstitution inflammatory syndrome (IRIS) is a known cause of an interstitial nephritis, the duration of ART exposure in the majority of our patients was for a longer period than that associated with the onset of IRIS; moreover, some patients were ART naive.38,39
This study has several limitations. We restricted our sample to patients admitted to a single regional hospital in South Africa and we are unable to report the overall rate of AKI in TDF and other ART recipients. Tubular dysfunction using urine beta-2 microglobulin and fractional excretion of phosphate was not measured, and in some patients, not every study parameter was measured, but all had sufficient measured data to be eligible for inclusion.
Baseline SCr values were not available in all patients and missing baseline values were inferred using the MDRD formula (calculated using an eGFR of 75 ml/min/1.73 m2); this may have biased the classification of AKI severity in both the TDF, but particularly in the non-TDF group where baseline SCr levels were less readily available as ART-naive patients infrequently had a measured SCr before admission or on follow-up.28,40
Furthermore, no renal biopsies were performed in this cohort, particularly in those with partial renal recovery; however, in most instances, the etiology of AKI was clinically evident. Finally, the small sample size of the study and missing data values limited the regression analysis of this cohort.
Comparison of the two groups suggests that risks factors, etiology, and mortality of AKI in HIV-infected in-patients, irrespective of TDF use, are similar, but that coexisting TDF use appears to result in more severe and longer lasting AKI. Our findings suggest that a lower threshold for RRT may be needed in those with limited or no SCr recovery and intensive follow-up after discharge from hospital may reduce mortality in all HIV-infected AKI patients, and especially in those with concomitant TB.
Acknowledgments
The authors express gratitude to the clinicians and staff of the Klerksdorp/Tshepong Hospital Complex for their assistance and care provided to the patients and thank the staff of the Perinatal HIV Research Unit (PHRU) for their support. The project was cofunded by PHRU and Council for Scientific and Industrial Research (CSIR).
Authors' Contributions
F.S., N.M., E.V., and D.M. conceived the idea and designed the study. K.M. and P.A. contributed to data collection and data interpretation. F.S. drafted the article, with assistance from N.M., E.V., and S.N. All authors approved the final article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Wyatt CM, Arons RR, Klotman PE, Klotman ME: Acute renal failure in hospitalized patients with HIV: Risk factors and impact on in-hospital mortality. AIDS 2006;20:561–565 [DOI] [PubMed] [Google Scholar]
- 2.Kalim S, Szczech LA, Wyatt CM: Acute kidney injury in HIV-infected patients. Semin Nephrol 2008;28:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG: Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int 2010;78:478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islam FM, Wu J, Jansson J, Wilson DP: Relative risk of renal disease among people living with HIV: A systematic review and meta-analysis. BMC Public Health 2012;12:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franceschini N, Napravnik S, Eron JJ, Jr, Szczech LA, Finn WF: Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int 2005;67:1526–1531 [DOI] [PubMed] [Google Scholar]
- 6.Roe J, Campbell LJ, Ibrahim F, Hendry BM, Post FA: HIV care and the incidence of acute renal failure. Clin Infect Dis 2008;47:242–249 [DOI] [PubMed] [Google Scholar]
- 7.Lopes JA, Melo MJ, Viegas A, Raimundo M, Camara I, Antunes F, et al. : Acute kidney injury in hospitalized HIV-infected patients: A cohort analysis. Nephrol Dial Transplant 2011;26:3888–3894 [DOI] [PubMed] [Google Scholar]
- 8.Vachiat AI, Musenge E, Wadee S, Naicker S: Renal failure in HIV-positive patients—A South African experience. Clin Kidney J 2013;6:584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes JA, Melo MJ, Raimundo M, Fragoso A, Antunes F: Long-term risk of mortality for acute kidney injury in HIV-infected patients: A cohort analysis. BMC Nephrol 2013;14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miro JM, Cofan F, Trullas JC, Manzardo C, Cervera C, Tuset M, et al. : Renal dysfunction in the setting of HIV/AIDS. Curr HIV/AIDS Rep 2012;9:187–199 [DOI] [PubMed] [Google Scholar]
- 11.Wikman P, Safont P, Del Palacio M, Moreno A, Moreno S, Casado JL: The significance of antiretroviral-associated acute kidney injury in a cohort of ambulatory human immunodeficiency virus-infected patients. Nephrol Dial Transplant 2013;28:2073–2081 [DOI] [PubMed] [Google Scholar]
- 12.DeChristoforo R, Penzak SR: Tenofovir: A nucleotide analogue reverse-transcriptase inhibitor for treatment of HIV infection. Am J Health Syst Pharm 2004;61:86–98;quiz 99–100. [DOI] [PubMed] [Google Scholar]
- 13.South African National Department of Health. Clinical guidelines for the management of HIV & AIDS in adults and adolescents. Pretoria: Department of Health, 2010. Available at www.sahivsoc.org, accessed April14, 2015 [Google Scholar]
- 14.Schooley RT, Ruane P, Myers RA, Beall G, Lampiris H, Berger D, et al. : Tenofovir DF in antiretroviral-experienced patients: Results from a 48-week, randomized, double-blind study. AIDS 2002;16:1257–1263 [DOI] [PubMed] [Google Scholar]
- 15.Schaaf B, Aries SP, Kramme E, Steinhoff J, Dalhoff K: Acute renal failure associated with tenofovir treatment in a patient with acquired immunodeficiency syndrome. Clin Infect Dis 2003;37:e41–e43 [DOI] [PubMed] [Google Scholar]
- 16.Woodward CL, Hall AM, Williams IG, Madge S, Copas A, Nair D, et al. : Tenofovir-associated renal and bone toxicity. HIV Med 2009;10:482–487 [DOI] [PubMed] [Google Scholar]
- 17.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M: Systematic review and meta-analysis: Renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 2010;51:496–505 [DOI] [PubMed] [Google Scholar]
- 18.Nelson MR, Katlama C, Montaner JS, Cooper DA, Gazzard B, Clotet B, et al. : The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: The first 4 years. AIDS 2007;21:1273–1281 [DOI] [PubMed] [Google Scholar]
- 19.Young B, Buchacz K, Baker RK, Moorman AC, Wood KC, Chmiel J, et al. : Renal function in tenofovir-exposed and tenofovir-unexposed patients receiving highly active antiretroviral therapy in the HIV Outpatient Study. J Int Assoc Physicians AIDS Care (Chic) 2007;6:178–187 [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Nino MD, Izquierdo MC, Poveda J, et al. : Tenofovir nephrotoxicity: 2011 Update. AIDS Res Treat 2011;2011:354908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall AM, Hendry BM, Nitsch D, Connolly JO: Tenofovir-associated kidney toxicity in HIV-infected patients: A review of the evidence. Am J Kidney Dis 2011;57:773–780 [DOI] [PubMed] [Google Scholar]
- 22.Horberg M, Tang B, Towner W, Silverberg M, Bersoff-Matcha S, Hurley L, et al. : Impact of tenofovir on renal function in HIV-infected, antiretroviral-naive patients. J Acquir Immune Defic Syndr 2010;53:62–69 [DOI] [PubMed] [Google Scholar]
- 23.Madeddu G, Bonfanti P, De Socio GV, Carradori S, Grosso C, Marconi P, et al. : Tenofovir renal safety in HIV-infected patients: Results from the SCOLTA Project. Biomed Pharmacother 2008;62:6–11 [DOI] [PubMed] [Google Scholar]
- 24.Brennan A, Evans D, Maskew M, Naicker S, Ive P, Sanne I, et al. : Relationship between renal dysfunction, nephrotoxicity and death among HIV adults on tenofovir. AIDS 2011;25:1603–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamkuemah M, Kaplan R, Bekker LG, Little F, Myer L: Renal impairment in HIV-infected patients initiating tenofovir-containing antiretroviral therapy regimens in a primary healthcare setting in South Africa. Trop Med Int Health 2015;20:518–526 [DOI] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl 2012;2:1–138 [Google Scholar]
- 27.Siew ED, Ikizler TA, Matheny ME, Shi Y, Schildcrout JS, Danciu I, et al. : Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 2012;7:712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wald R: Predicting baseline creatinine in hospitalized patients. Clin J Am Soc Nephrol 2012;7:697–699 [DOI] [PubMed] [Google Scholar]
- 29.Okusa MD, Davenport A: Reading between the (guide)lines—The KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int 2014;85:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaiao S, Cruz DN: Baseline creatinine to define acute kidney injury: Is there any consensus? Nephrol Dial Transplant 2010;25:3812–3814 [DOI] [PubMed] [Google Scholar]
- 31.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute renal failure − Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–R212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashani K, Kellum JA: Novel biomarkers indicating repair or progression after acute kidney injury. Curr Opin Nephrol Hypertens 2015;24:21–27 [DOI] [PubMed] [Google Scholar]
- 33.South African National Department of Health. The South African antiretroviral treatment guidelines. Pretoria: Department of Health, 2013. Available at www.sahivsoc.org, accessed April14, 2015 [Google Scholar]
- 34.Jafari A, Khalili H, Dashti-Khavidaki S: Tenofovir-induced nephrotoxicity: Incidence, mechanism, risk factors, prognosis and proposed agents for prevention. Eur J Clin Pharmacol 2014;70:1029–1040 [DOI] [PubMed] [Google Scholar]
- 35.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. : South African National HIV Prevalence, Incidence and Behaviour Survery, 2012. HSRC Press, Cape Town, 2014, pp. 1–198 [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann CJ, Hoffmann JD, Kensler C, van der Watt M, Omar T, Chaisson RE, et al. : Tuberculosis and hepatic steatosis are prevalent liver pathology findings among HIV-infected patients in South Africa. PLoS One 2015;10:e0117813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkhie SM, Fine DM, Lucas GM, Atta MG: Characteristics of patients with HIV and biopsy-proven acute interstitial nephritis. Clin J Am Soc Nephrol 2010;5:798–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaidan M, Lescure FX, Brocheriou I, Dettwiler S, Guiard-Schmid JB, Pacanowski J, et al. : Tubulointerstitial nephropathies in HIV-infected patients over the past 15 years: A clinico-pathological study. Clin J Am Soc Nephrol 2013;8:930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leone S, Nicastri E, Giglio S, Narciso P, Ippolito G, Acone N: Immune reconstitution inflammatory syndrome associated with Mycobacterium tuberculosis infection: A systematic review. Int J Infect Dis 2010;14:e283–e291 [DOI] [PubMed] [Google Scholar]
- 40.Siew ED, Peterson JF, Eden SK, Moons KG, Ikizler TA, Matheny ME: Use of multiple imputation method to improve estimation of missing baseline serum creatinine in acute kidney injury research. Clin J Am Soc Nephrol 2013;8:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]