Abstract
Significance: Soluble guanylyl cyclase (sGC), which produces the second messenger cyclic guanosine 3′, 5′-monophosphate (cGMP), is at the crossroads of nitric oxide (NO) signaling: sGC catalytic activity is both stimulated by NO binding to the heme and inhibited by NO modification of its cysteine (Cys) thiols (S-nitrosation). Modulation of sGC activity by thiol oxidation makes sGC a therapeutic target for pathologies originating from oxidative or nitrosative stress. sGC has an unusually high percentage of Cys for a cytosolic protein, the majority solvent exposed and therefore accessible modulatory targets for biological and pathophysiological signaling.
Recent Advances: Thiol oxidation of sGC contributes to the development of cardiovascular diseases by decreasing NO-dependent cGMP production and thereby vascular reactivity. This thiol-based resistance to NO (e.g., increase in peripheral resistance) is observed in hypertension and hyperaldosteronism.
Critical Issues: Some roles of specific Cys thiols have been identified in vitro. So far, it has not been possible to pinpoint the roles of specific Cys of sGC in vivo and to investigate the molecular mechanisms in an animal model.
Future Directions: The role of Cys as redox sensors, intermediates of activation, and mediators of change in sGC conformation, activity, and dimerization remains largely unexplored. To understand modulation of sGC activity, it is critical to investigate the roles of specific oxidative thiol modifications that are formed during these processes. Where the redox state of sGC thiols contribute to pathologies (vascular resistance and sGC desensitization by NO donors), it becomes crucial to design therapeutic strategies to restore sGC to its normal, physiological thiol redox state. Antioxid. Redox Signal. 26, 137–149.
Keywords: : nitric oxide, guanylyl cyclase, reactive cysteine, S-nitrosation, S-nitrosylation, oxidative stress
Introduction
Soluble guanylyl cyclase (sGC) is the main receptor of nitric oxide (NO). Upon binding of NO to the heme of sGC, the catalytic activity is stimulated several hundred fold to produce cyclic guanosine 3′, 5′-monophosphate (cGMP) [see (28, 41) for general reviews on sGC]. sGC is a heterodimer with an α and a β subunit whose association is required for formation of the catalytic domain at the C-terminal part of the molecule. Each subunit has a heme-nitric oxide/oxygen binding (HNOX) N-terminal domain, but only the HNOX of the β subunit contains the heme where NO binds. In each subunit, this HNOX domain is followed by a Per/Arnt/Sim (PAS)-fold domain, a coiled-coil domain, and half of the catalytic domain (Fig. 1). While crystal structures or homology models for each separate domain of sGC exist, the three-dimensional (3D) structure of the whole heterodimeric molecule has not been solved. Therefore, a major challenge is to understand how the different domains interact and the mechanisms by which the NO activating signal propagates from the heme receptor domain to the catalytic effector domain, and its modulation.
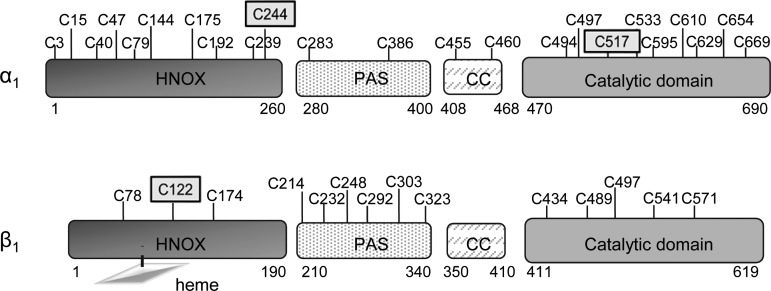
FIG. 1.
Cys location in the various domains of the human α1 and β1 sGC. The three known S-nitrosated Cys are framed. Heme is depicted with a lozenge shape. Numbering of Cys is from the human sGC sequence. Cys position is not at scale. Predicted size for each domain is indicated below. CC, coiled-coil domain; Cys, cysteine; HNOX, heme-nitric oxide/oxygen binding; PAS, Per/Arnt/Sim; sGC, soluble guanylyl cyclase.
The term “soluble” came from the fact that guanylyl cyclase (GC) activity was originally characterized in both the particulate and the soluble fractions of rat lung (17). The GC family was further characterized and comprised particulate GCs with a single transmembrane domain and a cytosolic soluble GC, which is the NO receptor. Despite the initial soluble characterization, there are many instances in which sGC has been found associated with membranes in various tissues, including cardiovascular and neuronal systems (94, 112).
Since cytosol is a reductive environment, it was experimentally tested whether oxidative conditions would alter sGC properties. Several reports from the late 70s and early 80s, before sGC was cloned, described how thiol oxidants and reductants affect basal or NO-stimulated sGC activity, and the potential involvement of disulfide bond formation in the effects (7–9, 45, 52). More recently, it was documented that posttranslational modification (PTM) of cysteine (Cys) thiols, in particular S-nitrosation (SNO), modulates sGC activity, and candidate regulatory Cys were identified in sGC (18, 34, 71, 73, 91, 95, 96).
In this review, we provide a brief history of the relationship between sGC and its sulfhydryl groups and discuss how modulation of sGC activity, and therefore of the NO-cGMP signaling pathway, is shaped by the thiol-redox cellular state. We will provide examples of the correlation between several oxidative cardiovascular diseases, NO resistance, and thiol oxidation of sGC. We will also discuss some unexpected properties of Cys in sGC and whether Cys thiols, besides making sGC vulnerable to oxidative changes under pathophysiological conditions, have a separate physiological function in NO signaling.
Early Observations of sGC Activity Dependence on Sulfhydryl Groups
The observation that sGC activity depends on the redox of the cell was first reported in the late 70s and early 80s. Thiol oxidation was later proposed to be the mechanism by which basal and NO-stimulated activity of sGC was regulated. Nonetheless, there was major discrepancy among the various reports, most likely due to the use of different cell systems, tissues, and setup. Initially, thiol blocking agents were used to show inhibition of basal and stimulated sGC in hepatic tissues; in particular, sGC inhibition by arsenite was reversed with dimercaprol (a thiol reducing agent), suggesting that vicinal dithiols were involved in the modulation of sGC activity (23). In the same study, it was noted that the effect of sGC inhibitors was different under basal and stimulated conditions and as a function of concentration, suggesting multiple mechanisms and interaction with free thiols (23).
Another early study reported that the GC activity of the soluble fraction of guinea pig splenic cells was stimulated by addition of the oxidant dehydroascorbic acid and that this effect was reversible by addition of the thiol reductant dithiothreitol (DTT) and was blocked by addition of N-Ethylmaleimide (NEM), which alkylates free thiols. Hence, in this system, sGC was modulated by an oxidative–reductive process that required sulfhydryls showing the opposite effect from the previous one (45). In contrast, another early report showed that NO responsiveness of sGC was decreased during its purification from rat liver but the NO response was rescued by addition of DTT, which was proposed to stabilize sGC (9). Likewise, desensitization of sGC to NO (e.g., sGC failure to respond to a second NO stimulation) could be prevented by the addition of thiol reductants, while reagents that induce disulfide bond between vicinal thiols (e.g., diamide) irreversibly inhibited NO-stimulated sGC activity (8).
Altogether, these initial reports in cells and tissues led to the consensus that Cys thiols were involved in modulating sGC activity but were controversial in concluding whether thiol oxidation activated or inhibited sGC activity, and whether basal or NO-stimulated or both activities were modulated by the changes in thiol redox.
Subsequently, using semi- or highly purified preparations of sGC, a consensus evolved in favor of an inhibition of sGC activity by thiol oxidizing agents and restoration with thiol reducing agents but with the caveat that the concentration of thiol oxidizing or reducing agents required was high (usually between 0.5 and 1 mM and up to 5 mM). Inactivation of purified sGC by formation of mixed disulfides, using cysteamine and Cys, was reported. This finding was supported by the observation that radioactivity was incorporated in sGC incubated with [35S]Cys (7). The authors noted that this mixed disulfide inhibition was faster at pH 9–9.5, which is expected as these elevated pH values induce ionization (reactivity) of sGC sulfhydryl groups. In the same study, it was observed that the Km for the substrate Mg2+-GTP of sGC treated with Cys or cysteamine was unchanged but the Vmax was drastically reduced, suggesting that the disulfide exchange was a regulatory mechanism. In contrast, another group observed that sGC could be protected from inhibition by dimercaprol (a vicinal dithiol compound) with excess substrate (Mg2+-GTP), indicating that Cys thiols are in the catalytic site where they might form disulfide(s) (52).
Overall, these early experiments showing a link between redox, thiols, and sGC activity led to two main hypotheses: (a) disulfide formation inhibited basal and NO-stimulated sGC activity or (b) free thiols that form disulfide bonds in sGC were necessary for NO-stimulated activity. It should be kept in mind that in these earlier studies, the presence of Cys, the role of the heme, and the heterodimer organization were unknown, as sGC (Fig. 1) was not cloned until 1988 for the β subunit and 1990 for the α subunit (60, 61, 81, 82). Moreover, the identification of NO as the endothelium-derived relaxing factor and direct activator of sGC was only settled in the late 80s (1, 40, 51, 89).
In the early 90s, the idea that the sGC activity was modulated by thiol-based redox was in a state of desuetude because sGC resides in the reducing environment of the cytosol, which is believed to be unfavorable for disulfide bond formation, and because the earlier studies did not offer a clear physiological significance of redox variations on sGC activity or cGMP production.
Cys Frequency in sGC
Computational analysis of large protein data sets (67) indicates that Cys is one of the least abundant residues in proteins but one of the most conserved. Originally, the main function of Cys was described as structural through formation of disulfide bonds, in particular in the extracellular domains of membrane proteins, with the oxidative environment allowing maintenance of disulfide bond. These disulfides are mostly engineered in the endoplasmic reticulum (ER) by protein disulfide isomerase (PDI) (50, 98). However, Cys are more than structural and have additional biological functions. Cys are catalytic, a function that can be redox-dependent as in oxidoreductases, or redox-independent as in phosphatases, they bind metals (Zn2+) and regulate protein function through reversible PTMs (43). These biological functions are tightly linked to the reactivity of their thiols (70), which is expected to be higher in oxidative environments. This correlates with the higher abundance of Cys in extracellular domains and secreted proteins compared to intracellular proteins, with a Cys frequency of 3.50% versus 1.59% in extracellular and intracellular proteins, respectively (84). In single transmembrane proteins, the Cys frequency in cytosolic domains is much lower (1.03%) than in the extracellular domains (3.24%) (83). As such, it is remarkable that human sGC, a cytosolic protein, has a high percentage of Cys: 3.3% in the α subunit and 2.3% in the β subunit (Table 1; Fig. 1). While the elevated Cys frequency is intriguing, whether these thiols have a function in sGC and/or the NO-cGMP signaling or other pathways associated with sGC would be entirely dependent on their reactivity, as described below.
Table 1.
Frequency and Solvent-Exposed Cysteine in Soluble Guanylyl Cyclase
| sGC | HNOX domain | PAS-fold | Coiled-coil | Catalytic domain | |
|---|---|---|---|---|---|
| Cys frequency% | |||||
| α | 3.3 | 3.8 | 1.7 | 3.3 | 3.6 |
| β | 2.3 | 1.6 | 4.6 | NDa | 2.4 |
| Cys exposedb/Cys total | |||||
| α | 15/27c | NDd | 5/8 | 1/2 | 8/13 |
| β | 1/3 | ||||
There is no Cys in the coiled-coil domain of β.
Solvability was estimated using the program AREAMOL. Solvent accessibility was determined for both the Cys and the sulfur atom (Focco van den Akker, pers. comm.). Sulfur group of Cys with solvent accessible surface as percentage >20: βC174, βC248, βC303, αC610, αC629, and βC571; with percentage >2: αC386, βC214, βC292, αC497, αC533, αC595, βC489, βC541.
27 is the total of Cys for which structural prediction could be made.
There is no 3D structure or homology modeling for the mammalian α HNOX domain.
Cys, cysteine; HNOX, heme-nitric oxide/oxygen binding; ND, not determined; PAS, Per/Arnt/Sim; sGC, soluble guanylyl cyclase.
Solvent-Exposed Cys in sGC
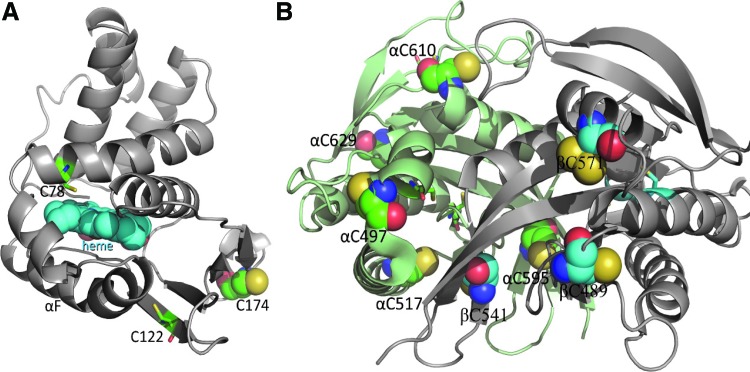
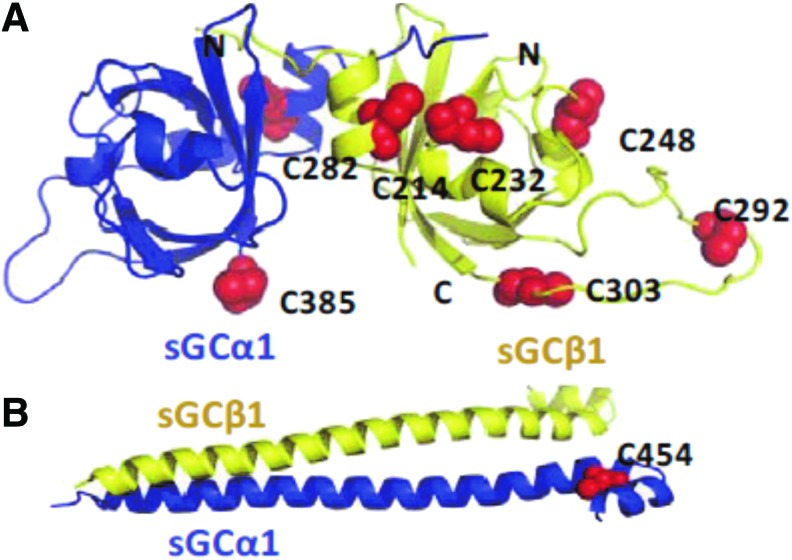
It is still a prevalent view that Cys in soluble proteins, including sGC, have limited function because of the reducing environment of the cytosol. Nonetheless, many lines of evidence point toward the existence of highly reactive cytosolic Cys susceptible to thiol oxidation due to the intrinsic properties of specific thiols and their highly local (e.g., oxidative) environment [for reviews see (6, 69)]. In addition, Marino and Gladyshev proposed, using high-throughput computational analysis of more than 1000 protein structures, that Cys with a pKa close to cytosolic physiological pH should be more sensitive to small redox changes and more readily reactive (70). Consequently, these authors predicted that Cys exposed at the surface of proteins (unlike buried ones) have increased reactivity and polarity and thereby could act as reactive thiolate (67). As shown in Figures 2 and 3, Cys that are solvent exposed in the known 3D and homology domains of sGC were modeled following analysis of their solvent accessibility (Table 1; Dr. Focco van den Akker, pers. comm.). The heme HNOX domain of β subunit (Fig. 2A) of sGC had one predicted exposed Cys (out of three). In the catalytic domain of sGC, the combined α and β subunits displayed 8 exposed Cys out of 13 (Fig. 2B). In the PAS-fold domains, the percentage of solvent exposed Cys was high, with five out of eight Cys solvent exposed (Fig. 3A), while the coiled-coil domain has only two Cys, one of them surface exposed (Fig. 3B). We could not predict the Cys exposed in the α-HNOX domain for the lack of a 3D model. Table 1 indicates that not only is the Cys frequency high but also more than 50% of Cys is predicted to be solvent exposed in sGC. The number of surface Cys could be an overestimation as some of these exposed Cys could be buried because of unknown domain interactions in the full-length sGC molecule (the whole molecule 3D structure is not solved).
FIG. 2.
Prediction of solvent-exposed Cys in the β HNOX domain (A) and catalytic domain (B) models of sGC. The α subunit is shown in green ribbons and the β subunit in gray ribbons. Cys are depicted as balls-and-sticks if the Cys is buried and as a sphere if solvent exposed. Figures are drawn using PYMOL program. Illustrations are from Dr. Focco van den Akker (pers. comm.). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 3.
Prediction of solvent-exposed Cys in the PAS-fold domain (A) and coiled-coil (B) models of sGC. The α subunit is shown in blue ribbons and the β subunit in yellow ribbons. The solvent-exposed Cys are depicted as red spheres. Figures are drawn using PYMOL program. Illustrations are from Dr. Focco van den Akker (pers. comm.). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
This number of exposed Cys is still highly unusual, as Cys is the most buried residue in the proteome as observed by Marino and Gladyshev (67). In this latter report, using a bioinformatics approach, the authors proposed that the normally low frequency of exposed Cys is explained by their increased sensitivity and reactivity to small redox changes, which would interfere with the normal protein function, regulation, or interactions. Conversely, because of the pressure of selection, they predicted that only few Cys are exposed except if they have a specific function. If this is so, the remarkably high number of exposed Cys in sGC (Figs. 2 and 3) suggests that they may have conserved functions related to their reactivity. Whether these Cys have a function and whether they are regulatory, catalytic, or involved in protein–protein interaction and/or act as redox sensor is, in part, addressed below. For example, the PAS fold domain with five out of eight Cys located at the surface of sGC is an ancient sensory signaling domain that can bind small ligands such as oxygen, is involved in protein–protein interactions and, interestingly, can sense redox potential (106). In sGC, we found that the PAS-fold domain is involved in dimerization and transduction of the NO signal (65) and it also binds heat shock protein (90 kDa) (Hsp90) (69), but it remains unknown whether these exposed reactive Cys in the PAS-fold domain are involved in those interactions, and/or redox sensing. Likewise, whether surface Cys of the catalytic domain are involved in modulating sGC activity and by what mechanism (e.g., via PTM that induces conformational changes) have not been elucidated. In addition, the HNOX domain of α has the highest Cys frequency (3.8%) and even though we could not predict whether these Cys were solvent exposed (because of the lack of a homology model for this domain), this opens intriguing possibilities for the role of this mostly uncharacterized domain. Of note, Stasch et al. proposed that Cys239 and Cys244 of the α HNOX domain were involved in the regulatory site binding to the activator Bay-41-2272 (102), however, it was later shown by site-directed mutagenesis that these two Cys were probably reactive to the photoaffinity label used (62).
Heme-Binding and Catalytic Cys in sGC
The first comprehensive study of the function of specific Cys in sGC (37) used site-directed mutagenesis of Cys conserved between the α and β subunits; for example, the many Cys that are conserved inside each subunit for various species were not considered. Also in this study, the assays were limited to the measurement of the basal, Mn2+, and NO-stimulated activities, and in the presence of millimolar DTT, hence limiting the investigation of potential regulatory function. Still, this initial study highlighted the role of HNOX βC78 and PAS βC214 in heme binding and sensitivity to NO. More specifically, the authors showed that the mutation βC78S in the HNOX domain lost the ability to bind heme and thereby the mutant was NO insensitive; C214S, in the PAS-domain of the β subunit, created a mutant with decreased NO responsiveness that could be restored with heme addition, suggesting that this mutant lost its heme. Interestingly, this study also identified two Cys in the catalytic domain βC541 and αC595 (human sequence numbering), which when mutated led to a sGC with close to none NO-stimulated activity. These two Cys are integral components of the catalytic pocket; in fact, in another study, βC541 was predicted to interact directly with the guanine ring of the substrate GTP, hence contributing to substrate specificity (104). The equivalent of βC541 in the α subunit catalytic domain, αC595, is located in the pseudo-symmetric pocket of the catalytic site, and its mutation into Serine led to an increase in basal activity and diminished response to the activator 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) (36). Together with a later study (62), the mutational analysis of αC595 suggested that YC-1 could bind to this pseudo-symmetric site; however, other studies have shown binding sites for YC-1 in other parts of the molecule (57, 89a, 102). αC595 is also predicted to be involved in the interaction with inhibitory nucleotides in this pseudo-symmetric pocket (12) and could be the key for sGC potential function as ATP sensor (92). It was subsequently observed that mutation of two residues, including αC595, changes inhibitory kinetics of ATP when NO was in excess (104a). Of note, βC541, αC595, and βC214 are predicted to be solvent exposed (Figs. 2 and 3), but it is still unknown whether this potential increased reactivity is necessary to their catalytic/conformational function.
Regulatory Cys and Their Thiol Oxidation in sGC
Cys thiol oxidation in sGC
Cys can undergo a set of thiol oxidations, including disulfide (S = S), sulfenic acid (S–OH), nitrosation (or nitrosylation), and sulfhydration to name some of the reversible ones. The fact that these PTMs are reversible suggests that they could be involved in dynamic signaling. These PTMs are now recognized as specific and mostly dependent on the ability of the thiol to ionize to a thiolate anion, which is dependent upon solvent exposure (see above) or upon basic neighboring residues(s) if buried (30a). More recently, potential regulatory Cys in sGC have been shown to be engaged in disulfide bonds, mixed disulfide exchange, or modified by SNO, as described below.
Role of disulfide bonds in sGC is still controversial
In 2006, a comprehensive study by Wolin's group reported that relaxation of pulmonary arteries and NO-stimulated sGC activity were decreased by the disulfide inducer diamide, confirming earlier studies, and by inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) generation via the pentose pathway using 6-aminonicotinamide (76). These inhibitory effects could be reversed by DTT treatment, suggesting a mechanism based on changes in sGC thiol redox status. On the contrary, a more recent study reported that activation of sGC was decreased when treated with DTT and other thiol reductants, as well as with the thiol antioxidant, dihydrolipoic acid (113). The heterodimer formation, which is required for activity as α and β C-terminal regions associate to form the catalytic domain, was decreased under these conditions. The authors suggested that the heterodimer formation is highly sensitive to redox changes because of potential inter-subunit disulfide(s). Likewise, the study reported that hypoxia increased heterodimeric sGC and increased cGMP production in response to NO, yet had minimal effect on vasodilation. These discrepancies between the impact of disulfide reductants and oxidants on sGC activity and NO physiological function are probably due to the different experimental designs. It should be emphasized that both studies infer the role of disulfides. Interestingly, the in vitro activity of purified sGC, which is dependent on heterodimerization, is not inhibited by high concentration of thiol reductants (Glutathione [GSH] and DTT are routinely used in sGC activity assays), thus suggesting that requirement of disulfide(s) in sGC could involve endogenous regulatory factors. So far, no Cys involved in disulfide bonds has been identified in sGC; this identification might require the development of new mass spectrometric (MS) methods. In contrast, MS has allowed the identification in sGC of another thiol PTM, SNO (see below).
Mixed disulfide exchange in sGC-PDI interaction
Several binding partners for sGC were identified in the past decade, including PDS95, Hsp90, Hsp70, CCTeta, AGAP1, LGN, with proposed roles in modulation of sGC activity, recruitment to a specific complex, localization, and heme insertion (2, 13, 46, 75, 94, 107). Some of the mechanisms of interaction have been described. So far, only the recently observed interaction with the ER-localized oxidoreductase PDI requires a mixed disulfide exchange and is dependent on the redox state of the cells (47). In vitro, only a mixture of oxidized PDI with reduced sGC could form a complex and in cells, a PDI-trap mutant (in which the PDI redox-active site is mutated to CxxS) could pull down sGC. This pulled-down complex was resolved by DTT to show a redox-based interaction between sGC and PDI. The interaction was also imaged by proximity ligation assay in the cytosol of smooth muscle cells. In an exogenous expression system, the NO-stimulated activity of sGC was inhibited by PDI (47). A recent study using lysine crosslinking, proteomics analysis, and computational modeling identifies the catalytic domain of sGC as the site for interaction with PDI; while the Cys of sGC engaged in the mixed disulfide have not been identified yet, the second catalytic site of PDI (C397xxC400, human numbering) was shown by Cys-directed mutagenesis to be the Cys active site involved in the mixed disulfide exchange (48). One could speculate that PDI catalyzes the formation or reduction of disulfide in sGC and this, in turn, could affect NO-stimulated activity. However, the biological relevance of the PDI-sGC interaction is yet to be established, but the fact that this interaction is through a mixed disulfide exchange could suggest a redox sensor role for sGC.
Identification and role of SNO in sGC
As an enzyme-linked receptor, the mechanism by which sGC is desensitized or/and inactivated to terminate the NO signal has been the subject of many studies. Herein, we will define desensitization as the inability of sGC to respond to a second NO stimulation, which translates into a decreased NO-stimulated production of cGMP. Some of the most common desensitization mechanisms for receptors, such as internalization, translocation, or phosphorylation, appear to play, so far, little or no role in the case of sGC. Conversely, the thiol oxidation, SNO, was shown to lower the sGC response to an NO donor following pretreatment with an S-nitrosating agent S-nitrosoglutathione (GSNO). S-nitrosated sGC (SNO-sGC) was also detected in the biologically relevant human umbilical vein endothelial cells and in the aorta treated with vascular endothelial growth factor and acetylcholine, respectively. Using purified sGC treated with GSNO, two Cys were first identified by MS to be S-nitrosated: Cys122 in the β subunit and Cys243 (C244 in Fig. 1) in the α subunit, both in the HNOX domains (95). Site-directed mutagenesis and further experiments in an exogenous expression system confirmed that thiol oxidation of βC122 and αC243 confers resistance to NO stimulation.
It is important to note that site-directed mutagenesis cannot reveal the type of thiol oxidation that occurs (e.g., sulfenation, glutathionylation, SNO, or disulfide) but does confirm functional involvement of the targeted Cys. Nitrosative sGC inactivation was later confirmed by other groups in different systems, using dinitrosyl iron complex (DNIC), a more physiologically relevant S-nitrosating agent (73), and in mice overexpressing endothelial nitric oxide synthase (eNOS) (87). In the case of DNIC-dependent SNO, inactivation by SNO of Cys took place when GSH was depleted and was reversed with addition of GSH (73). This dependence on GSH suggests that an oxidative microenvironment might be required for SNO to take place. Conversely, another study exploring inhibition of sGC activity by the nitrosating agent S-nitrosocysteine (CSNO) showed that transport of high concentration of CSNO into cells through the l-amino acid transport system induces GSH depletion. In this report, the authors proposed that deactivation of sGC could be due indirectly to increased cellular oxidative stress leading to thiol oxidation that might not be SNO (91). S-glutathionylation, for example, is another thiol oxidation that could take place (56). As a corollary, CSNO treatment was shown to induce activation of cGMP/cAMP protein kinase via the formation of disulfide with SNO serving as an intermediate (in that case the presence of a vicinal thiol is required) (11).
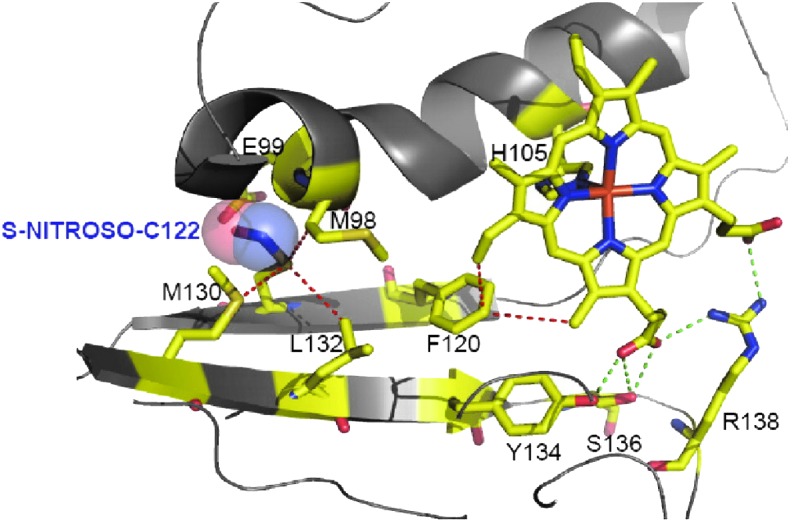
βC122 is in the sGC heme binding domain, for which a homologous structure is known (66). Thus, a modeling of the heme domain with SNO-C122 was built and predicted that a bulky SNO could induce shift of residues critical for propagation of the NO signal or/and could introduce a steric hindrance to heme incorporation (Fig. 4). This model was later refined and showed that the SNO group extended the C122 side chain by 2.5 Å, which shifted two key residues E99 in the αF helix and M130 of the β strand involved in heme stabilization (61a). βC122 is predicted to be partially buried (Fig. 2A, 1% of solvent accessible percentage for the sulfur group). This was unexpected as βC122 seems to be easily modified under oxidative stress (see below). In fact, the association between solvent exposed Cys and thiol modification is not absolute according to a study using a bioinformatics structural analysis of known SNO sites showing that ∼35% of the sulfur group in SNO-Cys was predicted to be buried (68). This was confirmed by a recent proteomics mapping of Cys modifications in the liver showing only a slightly higher solvent access for the modified Cys versus the unmodified Cys (44). The same study also showed that basic amino acids were in low proportions within a 10 Å of the modified Cys suggesting that the reactivity of buried thiol might not be a primary determinant of modification.
FIG. 4.
Model of S-nitrosated βC122 in the HNOX domain. The homology model containing SNO-C122 was generated from HNOX solved structure of Nostoc (66). The heme domain is shown with helices and strands. The NO is in ball mode and the heme, ligated to His 105, is indicated in sticks. Other critical residues for HNOX structure and propagation of NO signal are indicated. The image was obtained using PYMOL. This figure is reproduced from our publication by Mulsch et al. (95); copyright© by the National Academy of Sciences for Volumes 90–105 (1993–2008). NO, nitric oxide; SNO, S-nitrosation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Other mechanisms of desensitization by SNO of sGC have been postulated. A second mechanism is based on the finding that there was a correlation between sGC SNO levels and association with Hsp90, which led to decreased sGC heterodimerization (42). Also recently, a third mechanism of desensitization of sGC by SNO was proposed involving βC122 and an additional Cys of the β HNOX domain, C78 (34). Using electronic absorption spectroscopy, combined with sGC activity assays and blocking of free thiols with NEM, the authors proposed that ferric (oxidized) heme of sGC undergoes reductive nitrosylation, which is coupled to SNO of the two HNOX Cys, βC78 and βC122; a similar mechanism has been described for other hemoproteins, nitrophorin and hemoglobin (64, 110a). In addition, a study of the kinetics of activation of sGC by nitroxyl (HNO) showed that HNO activates ferrous sGC by binding directly to the heme (and not via release of NO) and has the ability to inhibit sGC activity via SNO of thiols (76a).
It is proposed that SNO of proteins modifies their properties, protein–protein interactions, localization, or enzymatic activity (35, 49). The fact that NO both activates sGC and, when oxidized, inhibits its activity suggests an exquisite feedback mechanism. While it is recognized that thiol oxidation of sGC could desensitize the enzyme, the challenge remains to explain the fast kinetics of inactivation that is critical for the NO-cGMP signaling pathway (3, 41). As such, experimental evidence is needed to show whether SNO of sGC contributes to the physiological fast deactivation of sGC in cells. In fact, evidence for a physiological role of SNO-sGC is not clear. One major reason is that methodological identification of SNO is challenging and the specificity of the reaction and its mechanism(s) are poorly understood (10). The difficulty of experimentally discerning SNO from further thiol oxidation was recently illustrated by a proteomic isotope-coded approach (d-SSwitch) that could quantify concurrently both SNO and S-oxidation to disulfide for specific Cys(s) and showed that oxidation dominates over SNO upon CSNO treatment (110). On the contrary, endogenous S-nitrosated proteins have been identified in vivo using a methodology based on mercury resin enrichment or chromatography fractionation with high-resolution MS. The reliability and selectivity of the method were verified using in parallel eNOS knockout mice, in which a drastic reduction of SNO was observed (29). In a subsequent study, Gould et al. identified four different Cys modifications (S–NO, S–OH, SS–G, and S–Ac) in the proteome with minimal overlap for specific Cys (11% between SNO and SSG, for instance) (44). The different outcomes between these studies could be explained by the methods used, the system (cells vs. tissues) and the treatment with NO-generating agents (nitrosative stress) or lack thereof.
sGC memory
Keeping in mind the limitations cited earlier, a speculative physiological role of SNO-sGC could be that SNO of sGC is a means by which the cells keep the memory of a previous stimulation by NO and as such control NO signaling via desensitization of its target, sGC. Of note, the SNO of proteins and of sGC is more stable than previously thought considering the cellular reductive environment [in our hands, 30 min to 1 h after washout, the SNO of sGC was still detected (96)]. Stability of SNO in proteins is difficult to reconcile with the elevated intracellular concentration of GSH (74), yet studies have shown that cellular SNO can be resistant to GSH or reducing conditions (55, 77, 88). Potential explanations might be conformational changes induced by SNO that limit the accessibility of the S-nitrosated Cys, and variations in NO concentration, oxidants, and reductants in discrete domains of cells (31). However, consensus has not been reached, in particular, because the biological chemistry of the SNO modification in vivo is still uncertain (10, 101). Several groups have proposed that the proximity of the NO source with the S-nitrosated target will favor its SNO. This is the case for arginase-1 and cyclooxygenase-2, which are S-nitrosated via direct interaction with inducible nitric oxide synthase (iNOS) (30, 59) and could be the case for sGC [as a complex with neuronal NOS via PSD95 (94) or with endothelial NOS via Hsp70/Hsp90 complex (107)]. The mechanisms proposed are increased local concentration of NO species or transnitrosation by S-nitrosated NOS (100). In summary, if SNO is a stable in vivo thiol modification, then sGC could be a form of storage for NO.
Considering the properties of NO as a gas that would diffuse in every direction, one way to control NO signaling would be to have sGC serve as a sink (41). As pointed out by others, the elevated cellular concentration of sGC in many cell types (41) or its potential location close to the source of NO production could make it an efficient trapping system. In summary, potential physiological roles of SNO-sGC could include storage or modulation of NO signaling. In support of this, we have identified by MS analysis in neonatal cardiomyocytes overexpressing sGC via adenoviral activation, nine novel SNO-Cys in sGC, with a majority predicted to be solvent exposed (unpublished results).
The particular case of Cys-mediated NO activation of sGC
The premise of a Cys-NO interaction for sGC activation is based on the hypothesis that activation of sGC by NO takes place in two steps. Initial in vitro studies using purified sGC showed that a NO-heme-sGC complex with low activity exists at low NO concentration, for example, an intermediate between basal and fully NO-stimulated sGC activity (93). Other studies, using the heme ligand butyl isocyanide, suggested that the excess NO needed to fully stimulate sGC was due to NO binding to a non-heme target rather than proximal site of heme (27). A follow-up study by the same group (33) confirmed the three functional states of sGC (basal, low-NO activity, and high-NO activity). By blocking sGC Cys with methyl methanethiosulfonate (MMTS), the authors proposed that the high NO activated state is mediated by an interaction between a Cys and NO. MS and biotin switch assay identified six potential candidates (αC175, αC610, αC629, βC174, βC292, and βC303), but the specific NO interactive Cys remains to be identified and be confirmed by site-directed mutagenesis. Of note, all these Cys are solvent exposed (Table 1; the solvability of αC175 is unknown) and as such it is not surprising that they reacted with MMTS. This effect of interaction between Cys and NO (activation) is opposite to the other desensitization effect described earlier. We have no explanation for this opposite effect and could only speculate that it is depends on which Cys are modified.
SNO and Other Thiol Oxidations of sGC in Oxidative Cardiovascular Diseases and Nitrate Tolerance
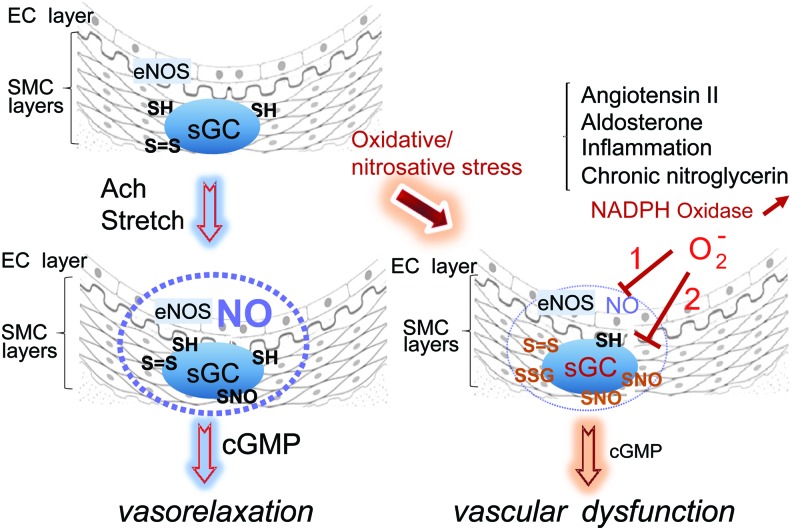
While the physiological function(s) of thiol in sGC remain ill-defined, many recent examples of the pathophysiological relevance of thiol oxidation of sGC exist, including hyperaldosteronism, inflammation, and angiotensin II (Ang II)-induced hypertension (14, 18, 71, 89b). In addition, nitrate tolerance is considered an oxidative vascular disease because of the key finding that reactive oxygen species (ROS) are generated during development of tolerance or cross-tolerance (26, 79, 80). Cross-tolerance is defined as the reduced sensitivity to NO-dependent vasodilators (other than nitroglycerin; glyceryl trinitrate [GTN]) and decreased cGMP production, following nitrate exposure (25). It is worth noting that in these diseases, the sGC activity is directly affected by the thiol oxidation (heme oxidation is not addressed in this review), which leads to NO vascular resistance, meaning decreased relaxation due to decreased response to NO stimulation. This is different from endothelial dysfunction in which the oxidative vascular diseases depress endothelium-derived NO release and hence NO bioavailability, through eNOS uncoupling or scavenging of NO by superoxide generation, among other factors (Fig. 5).
FIG. 5.
Hypothetical model of development of vascular resistance via desensitization of thiol-oxidized sGC. Left panel: Vasorelaxation inducers such as acetylcholine (Ach) or stretch lead to eNOS activation, NO production, which spreads (blue dotted sphere) through the smooth muscle cell layers and stimulates sGC activity to produce cGMP. cGMP in turn will promote vasorelaxation through protein kinase G activation. Right panel: Under conditions of oxidative or nitrosative stress created by excess Ang II, aldosterone, or chronic nitroglycerin treatment, NADPH oxidase is activated and promotes superoxide production. Inflammation will promote excess NO production via iNOS induction. Superoxide (O2−) will induce endothelial dysfunction (path 1) by scavenging NO or uncoupling eNOS, but will also induce thiol oxidation of sGC (including SNO via N2O3 formation): path2, resulting in desensitization of sGC and decreased cGMP. Both paths lead to vascular dysfunction. Free thiols, disulfide bond, SNO, and glutathionylation are depicted as SH, S = S, SNO, and SSG. Ang II, angiotensin II; cGMP, cyclic guanosine 3′, 5′-monophosphate; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Most of the reports on desensitization of sGC in nitrosative/oxidative stress described below report SNO as the PTM, yet it cannot be ruled out that other thiol oxidations could affect sGC activity. Besides methodological issues cited earlier, a kinetic model predicts that oxidation and glutathiolation might be the dominant Cys thiol oxidation induced by reactive nitrogen species (63). Likewise, issues such as stability of SNO and its specificity are not fully resolved, nor has the paradox that a Cys reactive to SNO should be reactive as well to other thiol oxidations. Potentially, GSNO or protein-driven transnitrosation reactions could be the answer for the specificity of the SNO reaction (101).
sGC nitrosation and desensitization to NO in nitrate tolerance
Many factors contribute to vascular tolerance in response to GTN therapy (25). While there is an ongoing discussion regarding the mechanism(s) of nitrate tolerance, there is a broad consensus that GTN promotes vasodilation through stimulation of sGC activity, but whether it is through NO release or NO derivative species remains controversial. The key mechanism of nitrate tolerance is impaired bioconversion of GTN by dysfunctional aldehyde dehydrogenase, whether mitochondrial (105) or/and cytosolic (5). Increased phosphodiesterase activity (58) and desensitization of sGC could also be involved. All these mechanisms are related to increased superoxide production (Fig. 5) (80). Desensitization of sGC as a contributing factor to nitrate tolerance was proposed almost 30 years ago, but the mechanism was unknown (78, 97, 108); 25 years later, SNO of sGC was proposed as a potential mechanism of nitrate tolerance in response to nitroglycerin treatment (96). As SNO in GTN-treated tissues was shown to occur via S-nitrosothiol production (32, 38, 54) and that SNO desensitizes sGC activity, we hypothesized that GTN treatment could induce SNO, hence desensitization of sGC. Desensitization/SNO of sGC was shown in cells treated with GTN, was a function of GTN concentration, and was prevented by N-acetyl cysteine pretreatment (96), supporting the earlier observation that depletion of thiols such as GSH could be involved in the development of nitrate tolerance (85, 86). In addition, mutation of sGC αC243 and βC112, previously identified as mediating NO resistance, eliminated part of the GTN-dependent desensitization of sGC. In vivo studies using a 3-day low-dosage GTN treatment to mimic clinical setting of nitrate tolerance confirmed in vitro studies and established a correlation between decreased NO-induced vasorelaxation, sGC nitrosation, and desensitization (96). In that setting, the loss of response to the NO donor 2-(N,N-diethylamino)-diazenolate-2-oxide (cross-tolerance), which was illustrated by blunted NO-dependent vasodilation, clearly pointed at the sGC dysfunction per se. In these studies, depletion of GSH could be the key to the mechanism of nitrosative inactivation of sGC by creating a favorable environment for SNO, or other thiol oxidation. Recently, nitrate tolerance was partially prevented using an sGC activator (Bay 60-2770), which activates heme-oxidized sGC, suggesting that the oxidation of heme (in addition to thiol oxidation) is also involved in nitroglycerin-induced vascular resistance (53).
Aldosterone-induced thiol oxidation and desensitization of sGC
Increased levels of aldosterone (in hyperaldosteronism, for example) are known to induce endothelial dysfunction probably through generation of superoxide via activation of NADPH oxidase (Fig. 5). The prevalent idea was that the loss in vascular reactivity was due to decreased NO bioavailability (103). This aldosterone-induced vasculopathy was recently revisited. It was shown that in vascular smooth muscle cells exposed to pathophysiologically relevant aldosterone concentrations, oxidative stress was induced (indicated by increased hydrogen peroxide levels), and sGC response to NO donors was decreased by more than 60% (71). In smooth muscle cells (SMC), aldosterone treatment induced formation of two disulfide bonds in the β subunit of sGC. Further MS analysis and site-directed mutagenesis confirmed that βC122 in the heme domain is a key residue in mediating the oxidative stress-impaired sGC activity induced by aldosterone (71). It should be noted that the experiments were conducted in cell cultures, and therefore, validation of the mechanisms of aldosterone-dependent sGC desensitization by thiol oxidation awaits an in vivo model.
Thiol oxidation and desensitization of sGC in Ang II-induced hypertension
Ang II promotes hypertension through oxidative stress by a mechanism that involves activation of NADPH oxidase (90, 111). More recently, Ang II-decreased vascular reactivity was shown to facilitate global SNO by a mechanism proposed to involve inactivation of thioredoxin reductase (14, 15). In vivo model of Ang-II-induced hypertension using intravital microscopy further showed that arterioles from cremaster muscle failed to fully vasodilate in the presence of NO donors, suggesting desensitization of sGC (18). The resistance to NO-induced vasorelaxation was associated with decreased cGMP production in response to NO stimulation and increased SNO of sGC. Infection with adenovirus expressing WT or Cys mutants in A7r5 smooth muscle cells (with no endogenous sGC) suggested that C517 in the α subunit mediates part of the NO resistance upon treatment with Ang II (18).
NADPH oxidase activation appears to play a key role in the etiology of the oxidative vascular diseases mentioned earlier, including that induced by aldosterone, Ang II, and potentially chronic nitroglycerin. These studies suggest that increased ROS, by creating an oxidative environment in smooth muscle cells, favor thiol oxidation of sGC, which in turn desensitizes it to NO stimulation. It is unclear what type of thiol oxidation is predominant in these examples, but SNO was one of the PTM measured, and disulfide bonds were also observed. The fact that sGC was directly affected by the oxidative stress (in addition to upstream events such as eNOS uncoupling) was demonstrated in vivo by showing blunted vasodilation of arterioles in response to exogenous NO donors (18, 96).
Inhibition of smooth muscle relaxation and sGC nitrosation in cytokine-dependent inflammation
In a recent study, increased iNOS expression was induced in colonic SMC from an in vivo inflammation mouse model treated with 2,4,6-trinitrobenzene sulfonic acid, and in longitudinal muscle strips treated with proinflammatory cytokines. In this model, the authors observed a decrease in sGC activity and proposed that SNO was the underlying mechanism. In this experimental design, the NO-induced muscle relaxation was partially restored by treatment with 1400 W, an iNOS inhibitor (89b). This study suggests that excess NO production via iNOS induction, especially under oxidative stress, could lead to thiol oxidation, including SNO of sGC, hence its desensitization.
Concluding Remarks
In the past, decreased vascular reactivity in oxidative cardiovascular diseases, such as hypertension, atherosclerosis, and diabetes, was overwhelmingly attributed to endothelial dysfunction, in which NO availability is limited because of scavenging of NO by ROS or because of eNOS uncoupling, which also produces superoxide. However, it is now clear that dysfunction of the NO receptor itself is also involved in impaired vascular reactivity. The fact that sGC contains an unusually high number of Cys, of which a high proportion is predicted to be solvent exposed, could make it sensitive to redox changes, hence vulnerable to oxidative or nitrosative stress, as summarized in Figure 5. The physiological role of the solvent exposed, potentially reactive Cys in sGC, still needs to be addressed. Interestingly, a recent study described an increased risk for Moyamoya disease (vasculopathy), achalasia, and hypertension linked to mutation of αC517 to tyrosine (109). Many questions regarding the potential roles of Cys remain unanswered, including the role of the PDI-sGC association. If this complex promotes reduction or formation of disulfide linkages in sGC, one could speculate that disulfide switch (two or more disulfides were observed in sGC) could be a mechanism of propagation of the NO signal of activation between different domains of sGC. We also speculate that in vivo, sGC, because of its localization in proximity of NOS, could be S-nitrosated and if SNO is stable (a point highly debated), could be a form of storage of NO. More research is needed to determine whether sGC SNO or other thiol oxidations are modulated by PDI, which was shown to have “denitrosating” activity (99). As SNO of sGC seems to be increased by thioredoxin reductase inhibition (15), one can speculate that thioredoxin, an oxidoreductase like PDI, but cytosolic rather than ER restricted, could modulate denitrosation/nitrosation of sGC (4).
Abbreviations Used
- 3D
three-dimensional
- Ang II
angiotensin II
- CC
coiled-coil domain
- cGMP
cyclic guanosine 3′, 5′-monophosphate
- CSNO
S-nitrosocysteine
- Cys
cysteine
- DEA-NO
2-(N,N-diethylamino)-diazenolate-2-oxide
- DNIC
dinitrosyl iron complex
- DTT
dithiothreitol
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- GC
guanylyl cyclase
- GSH
glutathione
- GSNO
S-nitrosoglutathione
- GTN
glyceryl trinitrate (also known as nitroglycerin)
- HNOX
heme-nitric oxide/oxygen binding
- Hsp90
heat shock protein (90 kDa)
- iNOS
inducible nitric oxide synthase
- MMTS
methyl methanethiosulfonate
- MS
mass spectrometry
- NADPH
nicotinamide adenine dinucleotide phosphate
- ND
not determined
- NEM
N-Ethylmaleimide
- NO
nitric oxide
- PAS
Per/Arnt/Sim
- PDI
protein disulfide isomerase
- PTM
posttranslational modification
- ROS
reactive oxygen species
- sGC
soluble guanylyl cyclase
- SMC
smooth muscle cells
- SNO
S-nitrosation
- YC-1:
3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole
Acknowledgment
This work was supported, in part, by grants from the National Institute of General Medical Sciences GM112415 and GM067640.
References
- 1.Arnold WP, Mittal CK, Katsuki S, and Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A 74: 3203–3207, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balashova N, Chang F-J, Lamothe M, Sun Q, and Beuve A. Characterization of a novel type of endogenous activator of soluble guanylyl cyclase. J Biol Chem 280: 2186–2196, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bellamy TC. and Garthwaite J. Sub-second kinetics of the nitric oxide receptor, soluble guanylyl cyclase, in intact cerebellar cells. J Biol Chem 276: 4287–4292, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Benhar M, Forrester MT, Hess DT, and Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 320: 1050–1054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beretta M, Wolkart G, Schernthaner M, Griesberger M, Neubauer R, Schmidt K, Sacherer M, Heinzel FR, Kohlwein SD, and Mayer B. Vascular bioactivation of nitroglycerin is catalyzed by cytosolic aldehyde dehydrogenase-2. Circ Res 110: 385–393, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Brandes N, Schmitt S, and Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal 11: 997–1014, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandwein HJ, Lewicki JA, and Murad F. Reversible inactivation of guanylate cyclase by mixed disulfide formation. J Biol Chem 256: 2958–2962, 1981 [PubMed] [Google Scholar]
- 8.Braughler JM. Soluble guanylate cyclase activation by nitric oxide and its reversal. Involvement of sulfhydryl group oxidation and reduction. Biochem Pharmacol 32: 811–818, 1983 [DOI] [PubMed] [Google Scholar]
- 9.Braughler JM, Mittal CK, and Murad F. Effects of thiols, sugars, and proteins on nitric oxide activation of guanylate cyclase. J Biol Chem 254: 12450–12454, 1979 [PubMed] [Google Scholar]
- 10.Broniowska KA. and Hogg N. The chemical biology of S-nitrosothiols. Antioxid Redox Signal 17: 969–980, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgoyne JR. and Eaton P. Detecting disulfide-bound complexes and the oxidative regulation of cyclic nucleotide-dependent protein kinases by H2O2. Methods Enzymol 528: 111–128, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Chang FJ, Lemme S, Sun Q, Sunahara RK, and Beuve A. Nitric oxide-dependent allosteric inhibitory role of a second nucleotide binding site in soluble guanylyl cyclase. J Biol Chem 280: 11513–11519, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Chauhan S, Jelen F, Sharina I, and Martin E. The G-protein regulator LGN modulates the activity of the NO receptor soluble guanylate cyclase. Biochem J 446: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi H, Allahdadi KJ, Tostes RC, and Webb RC. Augmented S-nitrosylation contributes to impaired relaxation in angiotensin II hypertensive mouse aorta: role of thioredoxin reductase. J Hypertens 29: 2359–2368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi H, Tostes RC, and Webb RC. Thioredoxin reductase inhibition reduces relaxation by increasing oxidative stress and s-nitrosylation in mouse aorta. J Cardiovasc Pharmacol 58: 522–527, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.This reference has been deleted.
- 17.Chrisman TD, Garbers DL, Parks MA, and Hardman JG. Characterization of particulate and soluble guanylate cyclases from rat lung. J Biol Chem 250: 374–381, 1975 [PubMed] [Google Scholar]
- 18.Crassous PA, Couloubaly S, Huang C, Zhou Z, Baskaran P, Kim DD, Papapetropoulos A, Fioramonti X, Duran WN, and Beuve A. Soluble guanylyl cyclase is a target of angiotensin II-induced nitrosative stress in a hypertensive rat model. Am J Physiol Heart Circ Physiol 303: H597–H604, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.This reference has been deleted.
- 20.This reference has been deleted.
- 21.This reference has been deleted.
- 22.This reference has been deleted.
- 23.Craven PA. and DeRubertis FR. Effects of thiol inhibitors on hepatic guanylate cylase activity. Biochim Biophys Acta 524: 231–244, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Craven PA. and DeRubertis FR. Restoration of the responsiveness of purified guanylate cyclase to nitrosoguanidine, nitric oxide, and related activators by heme and hemeproteins. Evidence for involvement of the paramagnetic nitrosyl-heme complex in enzyme activation. J Biol Chem 253: 8433–8443, 1978 [PubMed] [Google Scholar]
- 25.Daiber A. and Munzel T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: emphasis on redox biology and oxidative stress. Antioxid Redox Signal 23: 899–942, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daiber A, Oelze M, Wenzel P, Wickramanayake JM, Schuhmacher S, Jansen T, Lackner KJ, Torzewski M, and Munzel T. Nitrate tolerance as a model of vascular dysfunction: roles for mitochondrial aldehyde dehydrogenase and mitochondrial oxidative stress. Pharmacol Rep 61: 33–48, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Derbyshire ER. and Marletta MA. Butyl isocyanide as a probe of the activation mechanism of soluble guanylate cyclase. Investigating the role of non-heme nitric oxide. J Biol Chem 282: 35741–35748, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Derbyshire ER. and Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem 81: 533–559, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Doulias PT, Greene JL, Greco TM, Tenopoulou M, Seeholzer SH, Dunbrack RL, and Ischiropoulos H. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc Natl Acad Sci U S A 107: 16958–16963, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn J, Gutbrod S, Webb A, Pak A, Jandu SK, Bhunia A, Berkowitz DE, and Santhanam L. S-nitrosation of arginase 1 requires direct interaction with inducible nitric oxide synthase. Mol Cell Biochem 355: 83–89, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Eaton P. Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic Biol Med 40: 1889–1899, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Espey MG, Miranda KM, Thomas DD, and Wink DA. Distinction between nitrosating mechanisms within human cells and aqueous solution. J Biol Chem 276: 30085–30091, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Feelisch M, Noack E, and Schroder H. Explanation of the discrepancy between the degree of organic nitrate decomposition, nitrite formation and guanylate cyclase stimulation. Eur Heart J 9 Suppl A: 57–62, 1988 [DOI] [PubMed] [Google Scholar]
- 33.Fernhoff NB, Derbyshire ER, and Marletta MA. A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc Natl Acad Sci U S A 106: 21602–21607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernhoff NB, Derbyshire ER, Underbakke ES, and Marletta MA. Heme-assisted S-nitrosation desensitizes ferric soluble guanylate cyclase to nitric oxide. J Biol Chem 287: 43053–43062, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster MW, Hess DT, and Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15: 391–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friebe A, Russwurm M, Mergia E, and Koesling D. A point-mutated guanylyl cyclase with features of the YC-1-stimulated enzyme: implications for the YC-1 binding site? Biochemistry 38: 15253–15257, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Friebe A, Wedel B, Harteneck C, Foerster J, Schultz G, and Koesling D. Functions of conserved cysteines of soluble guanylyl cyclase. Biochemistry 36: 1194–1198, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Fung HL, Chong S, and Kowaluk E. Mechanisms of nitrate action and vascular tolerance. Eur Heart J 10 Suppl A: 2–6, 1989 [DOI] [PubMed] [Google Scholar]
- 39.This reference has been deleted.
- 40.Furchgott RF. and Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 41.Garthwaite J. New insight into the functioning of nitric oxide-receptive guanylyl cyclase: physiological and pharmacological implications. Mol Cell Biochem 334: 221–232, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Ghosh A, Stasch JP, Papapetropoulos A, and Stuehr DJ. Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J Biol Chem 289: 15259–15271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Go YM, Chandler JD, and Jones DP. The cysteine proteome. Free Radic Biol Med 84: 227–245, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gould NS, Evans P, Martinez-Acedo P, Marino SM, Gladyshev VN, Carroll KS, and Ischiropoulos H. Site-specific proteomic mapping identifies selectively modified regulatory cysteine residues in functionally distinct protein networks. Chem Biol 22: 965–975, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haddox MK, Stephenson JH, Moser ME, and Goldberg ND. Oxidative-reductive modulation of guinea pig splenic cell guanylate cyclase activity. J Biol Chem 253: 3143–3152, 1978 [PubMed] [Google Scholar]
- 46.Hanafy KA, Martin E, and Murad F. CCTeta, a novel soluble guanylyl cyclase-interacting protein. J Biol Chem 279: 46946–46953, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Heckler EJ, Crassous P-A, Baskaran P, and Beuve A. Protein disulfide isomerase interacts with soluble guanylyl cyclase via a redox-based mechanism and modulates its activity. Biochem J 452: 161–169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heckler EJ, Kholodovych V, Jain M, Liu T, Li H, and Beuve A. Mapping soluble guanylyl cyclase and protein disulfide isomerase regions of interaction. PLoS One 10: e0143523, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hess DT, Matsumoto A, Kim SO, Marshall HE, and Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Hogg PJ. Disulfide bonds as switches for protein function. Trends Biochem Sci 28: 210–214, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Ignarro LJ, Byrns RE, Buga GM, and Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res 61: 866–879, 1987 [DOI] [PubMed] [Google Scholar]
- 52.Ignarro LJ, Kadowitz PJ, and Baricos WH. Evidence that regulation of hepatic guanylate cyclase activity involves interactions between catalytic site -SH groups and both substrate and activator. Arch Biochem Biophys 208: 75–86, 1981 [DOI] [PubMed] [Google Scholar]
- 53.Jabs A, Oelze M, Mikhed Y, Stamm P, Kroller-Schon S, Welschof P, Jansen T, Hausding M, Kopp M, Steven S, Schulz E, Stasch JP, Munzel T, and Daiber A. Effect of soluble guanylyl cyclase activator and stimulator therapy on nitroglycerin-induced nitrate tolerance in rats. Vascul Pharmacol 71: 181–191, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Janero DR, Bryan NS, Saijo F, Dhawan V, Schwalb DJ, Warren MC, and Feelisch M. Differential nitros(yl)ation of blood and tissue constituents during glyceryl trinitrate biotransformation in vivo. Proc Natl Acad Sci U S A 101: 16958–16963, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jourd'heuil D, Jourd'heuil FL, and Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J Biol Chem 278: 15720–15726, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Keszler A, Zhang Y, and Hogg N. Reaction between nitric oxide, glutathione, and oxygen in the presence and absence of protein: how are S-nitrosothiols formed? Free Radic Biol Med 48: 55–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kharitonov VG, Sharma VS, Magde D, and Koesling D. Kinetics and equilibria of soluble guanylate cyclase ligation by CO: effect of YC-1. Biochemistry 38: 10699–10706, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, Beavo JA, Berk BC, and Yan C. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation 104: 2338–2343, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Kim SF, Huri DA, and Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310: 1966–1970, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Koesling D, Harteneck C, Humbert P, Bosserhoff A, Frank R, Schultz G, and Bohme E. The primary structure of the larger subunit of soluble guanylyl cyclase from bovine lung. Homology between the two subunits of the enzyme. FEBS Lett 266: 128–132, 1990 [DOI] [PubMed] [Google Scholar]
- 61.Koesling D, Herz J, Gausepohl H, Niroomand F, Hinsch KD, Mulsch A, Bohme E, Schultz G, and Frank R. The primary structure of the 70 kDa subunit of bovine soluble guanylate cyclase. FEBS Lett 239: 29–34, 1988 [DOI] [PubMed] [Google Scholar]
- 61a.Kumar V, Martin F, Hahn MG, Schaefer M, Stamler JS, Stasch JP, van den Akker F. Insights into BAY 60-2770 activation and S-nitrosylation-dependent desensitization of soluble guanylyl cyclase via crystal structures of homologous nostoc H-NOX domain complexes. Biochemistry 52: 3601–3608, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamothe M, Chang F-J, Balashova N, Shirokov R, and Beuve A. Functional characterization of nitric oxide and YC-1 activation of soluble guanylyl cyclase: structural implication for the YC-1 binding site?. Biochemistry 43: 3039, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Lancaster JR., Jr. Nitroxidative, nitrosative, and nitrative stress: kinetic predictions of reactive nitrogen species chemistry under biological conditions. Chem Res Toxicol 19: 1160–1174, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, Singel DJ. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci USA 100: 461–466, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma X, Sayed N, Baskaran P, Beuve A, and van den Akker F. PAS-mediated dimerization of soluble guanylyl cyclase revealed by signal transduction histidine kinase domain crystal structure. J Biol Chem 283: 1167–1178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma X, Sayed N, Beuve A, and van den Akker F. NO and CO differentially activate soluble guanylyl cyclase via a heme pivot-bend mechanism. EMBO J 26: 578–588, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marino SM. and Gladyshev VN. Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces. J Mol Biol 404: 902–916, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marino SM. and Gladyshev VN. Structural analysis of cysteine S-nitrosylation: a modified acid-based motif and the emerging role of trans-nitrosylation. J Mol Biol 395: 844–859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marino SM. and Gladyshev VN. Redox biology: computational approaches to the investigation of functional cysteine residues. Antioxid Redox Signal 15: 135–146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marino SM. and Gladyshev VN. Analysis and functional prediction of reactive cysteine residues. J Biol Chem 287: 4419–4425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J. and Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem 284: 7665–7672, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.This reference has been deleted.
- 73.Mayer B, Kleschyov AL, Stessel H, Russwurm M, Munzel T, Koesling D, and Schmidt K. Inactivation of soluble guanylate cyclase by stoichiometric S-nitrosation. Mol Pharmacol 75: 886–891, 2009 [DOI] [PubMed] [Google Scholar]
- 74.Mayer B, Schrammel A, Klatt P, Koesling D, and Schmidt K. Peroxynitrite-induced accumulation of cyclic GMP in endothelial cells and stimulation of purified soluble guanylyl cyclase. Dependence on glutathione and possible role of S-nitrosation. J Biol Chem 270: 17355–17360, 1995 [DOI] [PubMed] [Google Scholar]
- 75.Meurer S, Pioch S, Wagner K, Muller-Esterl W, and Gross S. AGAP1, a novel binding partner of nitric oxide-sensitive guanylyl cyclase. J Biol Chem 279: 49346–49354, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Mingone CJ, Gupte SA, Ali N, Oeckler RA, and Wolin MS. Thiol oxidation inhibits nitric oxide-mediated pulmonary artery relaxation and guanylate cyclase stimulation. Am J Physiol Lung Cell Mol Physiol 290: L549–L557, 2006 [DOI] [PubMed] [Google Scholar]
- 76a.Miller TW , Cherney MM, Lee AJ, Francoleon NE, Farmer PJ, King SB, Hobbs AJ, Miranda KM, Burstyn JN, Fukuto JM. The effects of nitroxyl (HNO) on soluble guanylate cyclase activity: interactions at ferrous heme and cysteine thiols. J Biol Chem 284: 21788–21796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell DA. and Marletta MA. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol 1: 154–158, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Mulsch A, Busse R, and Bassenge E. Desensitization of guanylate cyclase in nitrate tolerance does not impair endothelium-dependent responses. Eur J Pharmacol 158: 191–198, 1988 [DOI] [PubMed] [Google Scholar]
- 79.Munzel T, Daiber A, and Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res 97: 618–628, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Munzel T, Sayegh H, Freeman BA, Tarpey MM, and Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest 95: 187–194, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakane M, Arai K, Saheki S, Kuno T, Buechler W, and Murad F. Molecular cloning and expression of cDNAs coding for soluble guanylate cyclase from rat lung. J Biol Chem 265: 16841–16845, 1990 [PubMed] [Google Scholar]
- 82.Nakane M, Saheki S, Kuno T, Ishii K, and Murad F. Molecular cloning of a cDNA coding for 70 kilodalton subunit of soluble guanylate cyclase from rat lung. Biochem Biophys Res Commun 157: 1139–1147, 1988. 2905128 [Google Scholar]
- 83.Nakashima H. and Nishikawa K. The amino acid composition is different between the cytoplasmic and extracellular sides in membrane proteins. FEBS Lett 303: 141–146, 1992 [DOI] [PubMed] [Google Scholar]
- 84.Nakashima H. and Nishikawa K. Discrimination of intracellular and extracellular proteins using amino acid composition and residue-pair frequencies. J Mol Biol 238: 54–61, 1994 [DOI] [PubMed] [Google Scholar]
- 85.Needleman P, Jakschik B, and Johnson EM., Jr. Sulfhydryl requirement for relaxation of vascular smooth muscle. J Pharmacol Exp Ther 187: 324–331, 1973 [PubMed] [Google Scholar]
- 86.Needleman P. and Johnson EM., Jr. Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther 184: 709–715, 1973 [PubMed] [Google Scholar]
- 87.Oppermann M, Suvorava T, Freudenberger T, Dao VT, Fischer JW, Weber M, and Kojda G. Regulation of vascular guanylyl cyclase by endothelial nitric oxide-dependent posttranslational modification. Basic Res Cardiol 106: 539–549, 2011 [DOI] [PubMed] [Google Scholar]
- 88.Paige JS, Xu G, Stancevic B, and Jaffrey SR. Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chem Biol 15: 1307–1316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palmer RM, Ferrige AG, and Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 89a.Purohit R, Fritz BG, The J, Issaian A, Weichsel A, David CL, Campbell E, Hausrath AC, Rassouli-Taylor L, Garcin ED, Gage MJ, Montfort WR. YC-1 binding to the β subunit of soluble guanylyl cyclase overcomes allosteric inhibition by the α subunit. Biochemistry 53: 101–114, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89b.Rajagopal S, Nalli AD, Kumar DP, Bhattacharya S, Hu W, Mahavadi S, Grider JR, Murthy KS. Cytokine-induced S-nitrosylation of soluble guanylyl cyclase and expression of phosphodiesterase 1A contribute to dysfunction of longitudinal smooth muscle relaxation. J Pharmacol Exp Ther 352: 509–518, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, and Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riego JA, Broniowska KA, Kettenhofen NJ, and Hogg N. Activation and inhibition of soluble guanylyl cyclase by S-nitrosocysteine: involvement of amino acid transport system L. Free Radic Biol Med 47: 269–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruiz-Stewart I, Tiyyagura SR, Lin JE, Kazerounian S, Pitari GM, Schulz S, Martin E, Murad F, and Waldman SA. Guanylyl cyclase is an ATP sensor coupling nitric oxide signaling to cell metabolism. Proc Natl Acad Sci U S A 101: 37–42, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Russwurm M. and Koesling D. NO activation of guanylyl cyclase. EMBO J 23: 4443–4450, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Russwurm M, Wittau N, and Koesling D. Guanylyl cyclase/PSD-95 interaction: targeting of the nitric oxide- sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J Biol Chem 276: 44647–44652, 2001 [DOI] [PubMed] [Google Scholar]
- 95.Sayed N, Baskaran P, Ma X, van den Akker F, and Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci U S A 104: 12312–12317, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Duran WN, and Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res 103: 606–614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schroder H, Leitman DC, Bennett BM, Waldman SA, and Murad F. Glyceryl trinitrate-induced desensitization of guanylate cyclase in cultured rat lung fibroblasts. J Pharmacol Exp Ther 245: 413–418, 1988 [PubMed] [Google Scholar]
- 98.Sevier CS. and Kaiser CA. Formation and transfer of disulphide bonds in living cells. Nat Rev Mol Cell Biol 3: 836–847, 2002 [DOI] [PubMed] [Google Scholar]
- 99.Sliskovic I, Raturi A, and Mutus B. Characterization of the S-denitrosation activity of protein disulfide isomerase. J Biol Chem 280: 8733–8741, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Smith BC, Fernhoff NB, and Marletta MA. Mechanism and kinetics of inducible nitric oxide synthase auto-S-nitrosation and inactivation. Biochemistry 51: 1028–1040, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith BC. and Marletta MA. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr Opin Chem Biol 16: 498–506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schroder H, Schroeder W, Stahl E, Steinke W, Straub A, and Schramm M. NO-independent regulatory site on soluble guanylate cyclase. Nature 410: 212–215, 2001 [DOI] [PubMed] [Google Scholar]
- 103.Struthers AD. Aldosterone-induced vasculopathy. Mol Cell Endocrinol 217: 239–241, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Sunahara RK, Beuve A, Tesmer JJ, Sprang SR, Garbers DL, and Gilman AG. Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclases. J Biol Chem 273: 16332–16338, 1998 [DOI] [PubMed] [Google Scholar]
- 104a.Sürmeli NB, Müskens FM, Marletta MA. The Influence of Nitric Oxide on Soluble Guanylate Cyclase Regulation by Nucleotides: ROLE OF THE PSEUDOSYMMETRIC SITE. J Biol Chem 290: 15570–15580, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, Ullrich V, Mulsch A, Schulz E, Keaney JF, Jr, Stamler JS, and Munzel T. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest 113: 482–489, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taylor BL. and Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev 63: 479–506, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Venema RC, Venema VJ, Ju H, Harris MB, Snead C, Jilling T, Dimitropoulou C, Maragoudakis ME, and Catravas JD. Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am J Physiol Heart Circ Physiol 285: H669–H678, 2003 [DOI] [PubMed] [Google Scholar]
- 108.Waldman SA, Rapoport RM, Ginsburg R, and Murad F. Desensitization to nitroglycerin in vascular smooth muscle from rat and human. Biochem Pharmacol 35: 3525–3531, 1986 [DOI] [PubMed] [Google Scholar]
- 109.Wallace S, Guo DC, Regalado E, Mellor-Crummey L, Banshad M, Nickerson DA, Dauser R, Hanchard N, Marom R, Martin E, Berka V, Sharina I, Ganesan V, Saunders D, Morris S, and Milewicz DM. Disrupted nitric oxide signaling due to GUCY1A3 mutations increases risk for moyamoya disease, achalasia and hypertension. Clin Genet [Epub ahead of print]; DOI: 10.1111/cge.12739, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang YT, Piyankarage SC, Williams DL, and Thatcher GR. Proteomic profiling of nitrosative stress: protein S-oxidation accompanies S-nitrosylation. ACS Chem Biol 9: 821–830, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110a.Weichsel A, Maes EM, Andersen JF, Valenzuela JG, Shokhireva TKh, Walker FA, Montfort WR. Heme-assisted S-nitrosation of a proximal thiolate in a nitric oxide transport protein. Proc Natl Acad Sci USA 102: 594–599, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, and Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic Biol Med 31: 1456–1464, 2001 [DOI] [PubMed] [Google Scholar]
- 112.Zabel U, Kleinschnitz C, Oh P, Nedvetsky P, Smolenski A, Muller H, Kronich P, Kugler P, Walter U, Schnitzer JE, and Schmidt HHHW. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nat Cell Biol 4: 307, 2002 [DOI] [PubMed] [Google Scholar]
- 113.Zheng X, Ying L, Liu J, Dou D, He Q, Leung SWS, Man RYK, Vanhoutte PM, and Gao Y. Role of sulfhydryl-dependent dimerization of soluble guanylyl cyclase in relaxation of porcine coronary artery to nitric oxide. Cardiovasc Res 90: 565–572, 2011 [DOI] [PubMed] [Google Scholar]