Abstract
Background
In Germany, enhanced primary care (‘GP-centered health care’) is being promoted in order to strengthen the role of GPs and improve the quality of primary care. The aim of this study was to evaluate the impact of a GP-centered healthcare program, established in 2011 in the German federal state of Thuringia, on healthcare costs, care coordination, and pharmacotherapy.
Methods
We conducted a retrospective case–control study based on insurance claims data. Participants were followed from 18 months before the start of the program to 18 months after its introduction. The intervention and control groups were matched via propensity scores.
Results
40 298 participants enrolled in the program for a minimum of 18 months (between July 2011 and December 2012) were included in the intervention arm of the study. The mean age was 64.8 years. There was no significant difference in total direct costs (primary outcome) between cases and controls. Turning to secondary outcomes, the number of GP consultations rose sharply (+47%; p<0.001), there were less patients who consulted more than one GP (–41.4%; p<0.001), and less specialist consultations without referral (–5.8%; p<0.001) among patients in the intervention group. The number of patients who participated in Disease Management Programs (DMPs) increased (+17.7%; p<0.001), as did the number of GP home visits (+5.0%; p<0.001), specialist consultations (+4.1%; p<0.01), and the number of hospitalizations (+4.3%; p=0.006). The costs for pharmaceuticals were lowered by 3.9% (p<0.001).
Conclusion
The study indicates that the GP-centered healthcare program does not lead to lower direct health care costs. However, it may lead to more intense and better coordinated healthcare in older, chronically ill patients with multiple conditions. Further studies are needed on long-term effects and clinical endpoints.
About 10 years ago, a comprehensive review of the literature showed that primary care–orientated health care systems provide better quality of care and higher cost-effectiveness (1, 2). Since then, many policies and programs have been applied to strengthen primary care across the world. Examples include new payment schemes for general practitioners (GPs), medical homes, and collaborative care (3– 5). In Germany, enhanced primary care programs started in 2004 with the creation of a legal framework to support ‘GP-centered health care’ (6). These programs viewed primary care as an important element of community-based care that takes particular account of the growing number of the chronically sick (7– 9). The success factors of enhanced primary care programs have recently been well described by Bodenheimer et al. (10), and include committed leadership, data-driven improvement, team-based care, continuity of care, prompt access to care, comprehensiveness, and care coordination.
While respecting the general principle of allowing patients to choose their physicians, statutory health insurance (SHI) funds were encouraged (since 2004) and since 2007 obliged to offer contracts to GPs aimed at offering financial incentives to enhance primary care. Patients benefit from the program by choosing one specific GP whom they were obliged to consult before seeing a specialist. More than 75 of such programs now exist in Germany, and more than 16 000 GPs and 3.7 million patients participate in them (11). A major SHI—AOK PLUS, which covers 41% of the population in central Germany—established a GP-centered healthcare program in 2011 in the German federal state of Thuringia. This study aims to evaluate the impact of this program on healthcare costs, care coordination, and pharmacotherapy.
Methods
Ethics approval was granted by the Local Ethics Committee of Jena University Hospital, approval No. 4058–04/14. The trial was prospectively registered with Current Controlled Trials: ISRCTN29418540. The design, performance, and report of the claims data analysis were based on the recommendations of the GPS (Good Practice Secondary Data Analysis) (12) and the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) recommendations (13).
Data and study population
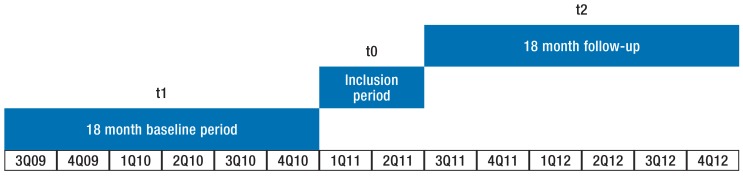
In our retrospective case–control study based on insurance claims data, we followed 80 596 patients aged 18 years and older for 36 months (1 July 2009 to 31 December 2010 [t1], 1 July 2011 to 31 December 2012 [t2]) (figure).
Figure.
Timeline for the evaluation study
Q, quarter
Control group and matching
The control group (CG) of patients receiving usual care consisted of the same number of patients as the intervention group (IG) (40 298 patients in each group) and had been matched via propensity scores in order to correct for possible differences between the groups (cf. eBox 2 for matching variables).
eBOX 2. Matching variables of cost and care utilization as well as morbidity, estimated for the 18–month baseline period (t1).
Sex
Age
Insurance status (voluntarily insured, retired, family insurance)
Morbidity groups (118 groups according to the German morbidity-based risk-adjustment scheme)
Level of long-term care (none, low, middle, high)
DMP breast cancer
DMP type 1 diabetes
DMP type 2 diabetes
DMP coronary heart disease
DMP asthma
DMP chronic obstructive pulmonary disease
Number of drug prescriptions
Number of GP consultations
Number of specialist consultations
Number of therapeutic aids
Number of remedies
Number of home care prescriptions
Length of hospital stay
Duration of post-acute care
Duration of outpatient rehabilitation
Duration of sick leave
Cost of drug prescriptions
Cost of physician consultations (GP and specialist)
Cost of therapeutic aids
Cost of remedies
Cost of home care
Cost of hospital care
Cost of post-acute care
Cost of outpatient rehabilitation
Cost of inpatient rehabilitation
Intervention
The GP-centered healthcare program complements regular GP care (“add-on contract”). The elements of the program for participating GPs were:
Mandatory participation in clinical GP–peer group trainings (quality circles; 3 per year, 2h each session, provided by a professional trainer)
Obligatory use of a specific IT–pharmacotherapy tool to support rational pharmacotherapy
Bonuses to support the prescription of generics and recommended substances and to limit growth in the overall number of prescriptions
Financial incentives to employ trained health-care assistants in patient-care (‘VERAH’, a qualification consisting of 3 years of vocational (on-the-job) training including half a day of school per week and about 200 units of additional training in patient-care)
A lump sum payment for each patient enrolled in the program (€2/three months)
Additional lump sum payments stratified according to the individual degree of morbidity of the enrolled patients (€6, €3, and/or €2, per three-month period).
The financial incentives were offered in addition to the regular payment system for GP care. The participating patients committed themselves to choose the contracted GP as a first-line contact to the health care service without being bound to the program by any financial or other incentives.
Outcome measures
We examined the differences between intervention and control groups using a large number of process and effect measures based on health economic and clinical reasoning, former evaluations of complex outpatient interventions, as well as recommendations on measures for the quality of outpatient care (14). Total direct costs from a health insurance perspective were defined as the primary outcome of this study. Secondary outcomes included partial costs and resource utilization, care coordination, and pharmacotherapy.
The partial cost and resource utilization parameters included outpatient GP and specialist care (including laboratory costs and ambulatory surgery), drug prescriptions (prescribed by physician and collected at pharmacy), hospital utilization, post-acute care, rehabilitation, remedies, therapeutic aids, and home care.
The care coordination parameters included the proportion of patients consulting more than one GP, specialist consultations without referral, specialist groups consulted, home visits by GPs, patients’ participation in disease management programs (DMPs), medical check-ups, emergency hospitalizations, and changes in patients’ nursing care status.
The pharmacotherapy parameters analyzed were: the number of drug prescriptions per patient, the number of prescribed medications, and the proportion of patients taking more than 5 medications.
Data analysis
We compared cases and controls with regard to any differences in the outcome parameters between the baseline period before enrollment (t1) and the follow-up period (t2) using the difference-in-differences method.
For more details on our methods see eBox 1.
eBOX 1. Methods.
Data and study population
Individual insurance claims data for members of the AOK PLUS (a social health insurance [SHI] fund) from July 2009 to December 2012 were extracted, delivered, and prepared for further analysis. 48 955 patients in 419 GP practices enrolled in the program from January 2011 to June 2011. Of these, 40 298 were included in the intervention group for the period July 2011 to December 2012. 8657 patients were not included because their GP did not meet the inclusion criteria, their GP had closed down or handed over the practice, or because their GP had died.
Control group and matching
Thuringian AOK PLUS members, who did not participate in the GP-centered healthcare program throughout the observation period (n = 322 732, group of non-participants [Non-P]) served as a pool for potential controls (pCG). To avoid effect amalgamation, non-enrolled patients who contacted GP practices with enrolled GPs were not considered as controls. The probability of enrollment (= propensity score, PS) for each patient was identified using logistic regression methods based on a total of 222 variables including age, sex, morbidity, resource utilization, and costs (ebox 2) from the baseline period (t1: Figure 1). The regression model was used to generate the predicted probabilities, the propensity score, of a patient enrolling in the program. Propensity score matching was performed as 1:1 matching without replacement. An appropriate control patient was identified for each participant (n = 40 298). A control patient was considered appropriate when the difference to the IG patient’s PS was as low as possible [nearest neighbor with a maximum caliper of 10% in the PS]. Nagelkerke’s R² was 0.12. Statistical matching quality was high: the mean difference of PS was only 0.0010 (SE.0025). The maximum difference was 0.060 (propensity score points).
Outcome measures
The rate of patients consulting more than one GP reflects the acceptance of the GP as gatekeeper and main provider of coordinated care. Regarding the rate of specialist consultations without referral, a lower rate reflects a higher level of care control and coordination. Specialist care was descriptively analyzed in terms of utilization of single disciplines in order to learn more about the possible reasons for the observed differences in specialist care between cases and controls. The number of home visits by GPs is considered, because GPs are expected to visit immobile patients at home. The number of medical check-ups may reflect care coordination that is thought to result in more intensive prevention. The number of patients in disease management programs (DMP) reflects the level of secondary prevention. The number of emergency hospitalizations may reflect close monitoring in GP-centered care that might be expected to reduce emergencies (the share of hospital procedures with potential of substitution by outpatient procedures could not be calculated since the available data only contained those procedures that had already been checked for necessity of inpatient treatment). The number of patients taking 5 or more medications may indicate the scope of multi-medication. Good care coordination might help to avoid unnecessary multi-medication.
Data analysis
For continuous parameters, the independent samples t-test was used to compare the differences between both groups (mean differences, MD). For binary outcomes, the logistic regression model with group and baseline (t1) values as independent factors was fitted (odds ratios, OR). For univariate analyses, the chi square test was used to examine group differences in binary variables. For computing, we used SAS9.3 [SAS-Institute/Cary/NC] and IBM-SPSS-Statistics V20.0 [IBM Corp./Armonk/NY].
Results
Population
The 40 298 participants in the intervention group were substantially older than the 322 732 non-participants (64.8 vs. 56.9 years) in the group from which the control group was matched, and many more were elderly than young (left-skewed distribution: 25% over age 77; skew of –0.763). They were also less often male (40.9% vs. 45.4%), more morbid in terms of multiple conditions (measured using the morbidity weight applied in the morbidity-based risk adjustment scheme in Germany (1.903 vs. 1.348), and more often participated in DMPs, meaning they were also chronically ill. After matching, there was no significant difference in propensity scores between IG and CG. Table 1 shows the characteristics of participants in the GP-centered health care program in comparison to non-participants.
Table 1. Study population: Characteristics of participants in the GP-centered health care program (intervention group. IG) in comparison to non-participants (Non-P) and control group (CG).
| Parameter | IG n = 40 298 |
Non-P n = 322 732 |
Relative difference IG vs Non-P before matching (p-value) |
CG n = 40 298 |
Relative difference IG vs CG (p-value) |
| Male sex | 40.9% | 45.4% | −11.2% (<0.001) | 40.0% | 2.2% (0.013) |
| Age: mean±SD; Median; 25% quartile; 75% quartile; skew |
64.8 ± 16.4; 69; 54; 77; −0.763 |
56.9 ± 18.8; 58; 44; 77; −0.251 |
12.2% (<0.001) |
65.1 ± 16.0; 69; 55; 77; −0.767 |
−0.5% (0.001) |
| Retired | 66.5% | 46.6% | 29.9% (<0.001) | 67.3% | −1.2% (0.023) |
| Morbidity index | 1.903 | 1.348 | 29.2% (<0.001) | 1.907 | −0.2% (0.816) |
| Number of HMGs | 2.6 | 1.6 | 38.9% (<0.001) | 2.7 | −1.2% (0.056) |
| Long-term care level 1 | 5.6% | 3.6% | 35.7% (<0.001) | 5.6% | −1.3% (0.668) |
| Long-term care level 2 | 2.7% | 1.8% | 31.8% (<0.001) | 2.8% | −4.2% (0.322) |
| Long-term care level 3 | 0.7% | 0.5% | 23.7% (<0.001) | 0.6% | 5.8% (0.487) |
| DMP type 2 diabetes | 21.4% | 6.7% | 68.8% (<0.001) | 20.5% | 3.8% (0.003) |
| DMP CHD | 11.6 % | 2.9% | 74.7% (<0.001) | 10.4% | 9.6% (<0.001) |
| DMP type 1 diabetes | 0.1% | 0.2% | −13.7% (0.357) | 0.1% | 5.3% (0.776) |
| DMP COPD | 3.3% | 0.6% | 80.5% (<0.001) | 2.7% | 19.0% (<0.001) |
| DMP asthma | 1.9% | 0.7% | 63.4% (<0.001) | 1.7% | 10.6% (0.033) |
| DMP breast cancer | 0.1% | 0.1% | 5.4% (0.707) | 0.1% | −13.5% (0.506) |
| Propensity score: mean±SD |
0.176 ± 0.125 |
0.103 ±0.077 |
0.073 (<0.001) |
0.177 ± 0.128 |
0.001 (0.281) |
p<0.05. statistically significant difference (t-test. chi square test); HMG. hierarchical morbidity group; DMP. disease management program; CHD. coronary heart disease. COPD. chronic obstructive pulmonary disease. SD. standard deviation. SE. standard error
Costs
No significant difference was found between groups for the primary outcome, total direct costs (table 2). While in the IG the average total direct costs rose from €4785 in t1 to €5439 in t2, the increase in average costs in the CG was from €4886 to €5457. The intervention effect of +€83 (95% confidence interval [CI] [–48; 215]; p = 0.215) was not significant. Relevant differences were observed for the following cost categories: GP consultations +€27 ([25; 30]; p<0.001), specialist consultations +€22 ([1.3; 43]; p = 0.0385), and drug prescriptions –€44 ([–74; –13]; p = 0.001).
Table 2. Comparative Outcomes (difference in differences [DiD]) for healthcare costs.
| Outcome measure | t1 | t2 | t2 vs t1 | DiD | ||||
| Healthcare costs (mean values per patient) |
IG € (SD) |
CG € (SD) |
IG € (SD) |
CG € (SD) |
IG € |
CG € |
Absolute intervention effect*1 (p-value [95% CI]) |
Relative change*2 % |
| Primary outcome | ||||||||
| Mean total direct costs per patient*3 | 4785 (8048) |
4886 (8485) |
5439 (9648) |
5457 (10 029) |
654 | 571 | 83 (0.215 [−48; 215]) |
1.7 |
| Secondary outcomes | ||||||||
| Cost of GP consultations | 438 (246) |
420 (258) |
480 (278) |
435 (278) |
42 | 15 | 27 (<0.001 [25; 30]) |
6.2 |
| Cost of specialist consultations | 551 (1515) |
590 (1928) |
529 (1500) |
546 (1500) |
−22 | −44 | 22 (0.385 [1.3; 43]) |
4.1 |
| Cost of drug prescriptions | 1108 (2974) |
1117 (2321) |
1201 (3406) |
1254 (3406) |
94 | 137 | −44 (0.001 [−74; −13]) |
−3.9 |
| Cost of hospital care | 2150 (5838) |
2219 (6294) |
2447 (6897) |
2418 (6897) |
297 | 199 | 98 (0.096 [−17; 214]) |
4.6 |
| Cost of post-acute care | 81 (575) |
79 (609) |
83 (605) |
83 (609) |
−3 | −0.19 | −2.45 (0.673 [−13.84; 8.93]) |
−3.0 |
| Cost of inpatient rehabilitation | 6 (117) |
7 (162) |
6 (137) |
8 (162) |
1 | 2 | −1 (0.608 [−3; 2]) |
−11.7 |
| Cost of outpatient rehabilitation | 2 (13) |
3 (17) |
2 (14) |
3 (17) |
1 | 1 | 0.25 (0.055 [−0.01; 0.5]) |
13.5 |
| Cost of remedies prescriptions | 92 (330) |
114 (391) |
94 (337) |
114 (391) |
22 | 19 | 3 (0.079 [−0.83; 6.59]) |
3.1 |
| Cost of therapeutic aids prescriptions | 236 (811) |
277 (847) |
233 (811) |
273 (847) |
41 | 40 | 1 (0.862 [−10; 12]) |
0.4 |
| Cost of homecare prescriptions | 121 (821) |
209 (1687) |
122 (1131) |
221 (1687) |
89 | 99 | −11 (0.369 [−36; 14]) |
−8.4 |
p<0.05. statistically significant difference (t-test); *1 intervention effect: mean difference; *2 relative change: share of intervention effect in IG t1 in %
*3 total direct cost = costs of outpatient physician care (GP care + specialist care). drug prescriptions. inpatient hospital care. post-acute care. rehabilitation. remedies. therapeutic aids;
IG. intervention group; CG. control group; CI. confidence interval; SD. standard deviation
Resource utilization
The total number of GP consultations increased remarkably in the IG, by 47.4% (mean difference [MD] 3.7; [3.6; 3.7]; p<0.001), see Table 3. In absolute terms the number of specialist consultations remained almost constant in the IG and decreased in the CG. Further analyses showed that this effect (+4.1%; MD 0.5 [0.4; 0.6]; p<0.001) was mainly the result of a stronger rise in the number of GP-ordered laboratory tests and ophthalmologic consultations, and a lesser decrease in the number of diagnostic imaging tests in the IG. Hospital utilization increased by 4.3% in the IG (MD 0.03 [0.01;0.06]; p = 0.006). This was particularly the case for day care, but also for inpatient care.
Table 3. Secondary comparative outcomes (difference in differences [DiD]) for resource utilization.
| Outcome measure (secondary) | t1 | t2 | t2 vs t1 | DiD | ||||
| Resource utilization (mean values per patient) |
IG (SD) |
CG (SD) |
IG (SD) |
CG (SD) |
IG | CG | Absolute intervention effect*1 (p-value [95% CI]) |
Relative change*2 % |
| GP consultations (billed cases) |
7.7 (3.8) |
7.8 (4.1) |
12.2 (5.2) |
8.7 (5.2) |
4.5 | 0.9 | 3.7 (<0.001 [3.6; 3.7]) |
47.4 |
| Specialist consultations (billed cases) |
12.1 (9.0) |
12.4 (9.9) |
10.4 (8.1) |
10.2 (8.1) |
−1.7 | −2.2 | 0.5 (<0.001 [0.4; 0.6]) |
4.1 |
| Hospital care (billed cases) |
0.76 (1.42) |
0.78 (1.52) |
0.80 (1.55) |
0.78 (1.55) |
0.04 | 0.00 | 0.03 (0.006 [0.01; 0.06]) |
4.3 |
| Post-acute care | 0.05 (0.25) |
0.03 (0.20) |
0.05 (0.24) |
0.03 (0.20) |
−0.02 | −0.02 | 0.00 (0.531 [−0.01;0.00]) |
−2.6 |
| Inpatient rehabilitation | 0.00 (0.06) |
0.00 (0.06) |
0.00 (0.05) |
0.00 (0.06) |
0.00 | 0.00 | 0.00 (0.159 [0.00; 0.00]) |
(–)*3 |
| Outpatient rehabilitation | 0.02 (0.16) |
0.04 (0.22) |
0.02 (0.17) |
0.04 (0.22) |
0.02 | 0.01 | 0.01 (0.091 [0; 0.01]) |
11.5 |
| Remedies | 1.32 (2.92) |
1.41 (2.95) |
1.36 (2.93) |
1.42 (2.95) |
0.09 | 0.06 | 0.03 (0.034 [−0.01; 0.07]) |
2.3 |
| Therapeutic aids | 1.7 (4.12) |
2.3 (4.6) |
1.7 (4.2) |
2.2 (4.6) |
0.5 | 0.5 | 0.02 (0.897 [−0.03; 0.08]) |
1.4 |
| Homecare prescriptions | 0.5 (2.8) |
0.9 (3.5) |
0.5 (2.8) |
0.8 (3.5) |
0.31 | 0.3 | 0.02 (0.268 [−0.01; 0.05]) |
3.5 |
p<0.05. statistically significant difference (t-test); *1 intervention effect: mean difference; CI: 95%; *2 relative change: share of intervention effect in IG t1 in %;
*3 due to the very small number of a maximum of 14 inpatient rehabilitation prescriptions per group with 40 298 patients and per observation period. the relative change is not shown;
IG. intervention group; CG. control group; CI. confidence interval; SD. standard deviation
Care coordination
The number of patients consulting more than one GP decreased in the IG and increased in the CG (table 4). The intervention effect was –41.4% (odds ratio [OR] 0.59 [0.56; 0.61]; p<0.001). The rate of specialist consultations without referral increased less in the IG compared to the CG (–5.8%; MD –0.92 [–1.27; –0.57]; p<0.001). The increase in the number of home visits by GPs was more pronounced in the IG than in the CG (+5.0%; MD 0.08 [0.04; 0.13]; p<0.001). The number of patients participating in DMPs rose in the IG (+17.7% (MD 0.07 [0.06; 0.07]; p<0.001) but declined in the CG. In t1, the number of medical check-ups was much higher in the IG than in the CG. In t2, the numbers declined more in the IG, falling by 3.5% (MD –0.016 [–0.024; –0.008]; p<0.001).
Table 4. Secondary comparative outcomes (difference in differences [DiD]) for care coordination.
| Outcome measure (secondary) | t1 | t2 | t2 vs t1 | DiD | ||||
| Care coordination | IG (SD) |
CG (SD) |
IG (SD) |
CG (SD) |
IG | CG | Absolute intervention effect*3 (p-value [95% CI]) |
Relative change*4 % |
| Share of patients consulting more than one GP*1 | 19.0% | 20.9% | 13.5% | 21.1% | −5.42*5 | 0.23*5 | 0.59 (<0.001 [0.56; 0.61]) |
−41.4 |
| Share of specialist consultations without referral*2 | 15.5% | 18.0% | 15.6% | 18.9% | 0.04*5 | 0.95*5 | −0.01 (<0.001 [−0.013; −0.006]) |
−5.8 |
| Number of different specialist groups consulted per patient (mean)*2 |
3.68 (2.15) |
3.62 (2.19) |
3.69 (2.15) |
3.57 (2.21) |
0.01 | −0.05 | 0.06 (<0.001 [0.04; 0.08]) |
1.6 |
| Number of home visits by GPs per patient (mean)*2 |
1.63 (5.19) |
1.41 (4.67) |
1.93 (5.30) |
1.64 (4.83) |
0.31 | 0.23 | 0.08 (<0.001 [0.04; 0.13]) |
5.0 |
| Number of DMP participants (mean value per patient)*2 |
0.38 (0.61) |
0.36 (0.59) |
0.44 (0.66) |
0.35 (0.59) |
0.06 | −0.01 | 0.07 (<0.001 [0.06; 0.07]) |
17.7 |
| Number of medical check-ups (mean value per patient)*2 |
0.45 (0.61) |
0.31 (0.53) |
0.42 (0.59) |
0.29 (0.51) |
−0.04 | −0.02 | −0.016 (<0.001 [−0.024; −0.008]) |
−3.5 |
| Number of emergency hospitalizations (mean value per patient)*2 |
0.24 (0.64) |
0.25 (0.68) |
0.31 (0.77) |
0.31 (0.80) |
0.07 | 0.06 | 0.001 (0.868 [−0.011; 0.013]) |
0.4 |
| Increase in nursery care level (0. 1. 2. 3) | 0: 91.1% 1: 5.6% 2: 2.7% 3: 0.7% |
0: 86.8% 1: 7.6% 2: 4.3% 3: 1.3% |
0: 90.9% 1: 5.6% 2: 2.8% 3: 0.6% |
0: 86.9% 1: 7.6% 2: 4.3% 3: 1.3% |
5.9% | 5.8% | 0.1*5 (0.578 [−0.3; 0.5]) |
0.1*6 |
p<0.05. statistically significant difference (t-test respectively chi square test); *1 binary variable; *2 metric variable; *3 intervention effect for metric variables: mean difference; intervention effect for binary variables: odds ratio; *4 relative change for metric variables: share of intervention effect in IG t1 in %; relative change for binary variables: odds ratio –1. i.e. percentage change in odds;
*5 percentage points; *6 relative change equals absolute intervention effect because of equal denominator (n = 40 298 patients of IG); DMP. disease management program; IG. intervention group;
CG. control group; CI. confidence interval; SD. standard deviation
For the IG, the following data was available on the employment of specialized healthcare assistants: 23.2% of the GPs in the IG employed at least one specialized healthcare assistant. Specialized healthcare assistants were more frequently employed in practices with more than one GP and in practices in rural areas. They conducted 7107 home visits, which represented 10.8% of all home visits in t2 in the IG.
Pharmacotherapy
The number of prescriptions and medications increased in both groups, but increased slightly stronger in the IG (+0.7%; MD 0.057 [0.004; 0.110]; p = 0.035; Table 5). A stronger effect was observed in the rate of patients with 5 and more drugs prescribed by GPs over a period of 18 months: +15% (OR 1.15 [1.11; 1.19]; p<0.001).
Table 5. Secondary comparative outcomes (difference in differences [DiD]) for pharmacotherapy.
| Outcome measure (secondary) | t1 | t2 | t2 vs t1 | DiD | ||||
| Pharmacotherapy | IG (SD) |
CG (SD) |
IG (SD) |
CG (SD) |
IG | CG | Absolute intervention effect*3 (p-value [95% CI]) |
Relative change*4 % |
| Number of drug prescriptions per patient*1 | 26.4 (23.2) |
26.8 (23.4) |
28.8 (24.0) |
29.1 (24.0) |
2.4 | 2.3 | 0.1 (0.156 [−0.04; 0.28]) |
0.4 |
| Number of different medications (ATC-7) per patient*1 |
7.67 (5.48) |
7.67 (5.62) |
8.14 (5.66) |
8.09 (5.90) |
0.48 | 0.42 | 0.06 (0.035 [0.004; 0.11]) |
0.7 |
| Share of patients with 5 or more different medications (GP prescriptions. ATC-7)*2 |
57.2% | 55.9% | 61.3% | 58.4% | 4.1*5 | 2.6*5 | 1.15 (<0.001 [1.11; 1.19]) |
15 |
p<0.05. statistically significant difference (t-test respectively chi square test); *1 binary variable; *2 metric variable; *3 intervention effect for metric variables: mean difference; intervention effect for binary variables: odds ratio; *4 relative change for metric variables: share of intervention effect in IG t1 in %; relative change for binary variables: odds ratio –1. i.e. percentage change in odds;
*5 percentage points; ATC. anatomical therapeutic chemical classification system [full 7-digit form of ATC codes];
IG. intervention group; CG. control group; CI. confidence interval; SD. standard deviation
Discussion
Contrary to the study hypothesis, the results do not suggest a reduction in total direct costs by the GP-centered healthcare program. Cost increases for GP and specialist care were observable, while savings were identifiable in drug costs. We noticed that more care was provided to the 40 298 patients participating in the program, who had a mean age of 65 years as well as multiple and probably chronic conditions. This is reflected in a greater number of GP consultations, more specialist consultations (probably caused by more frequently ordered laboratory diagnostics, ophthalmologic consultations, and a lesser decrease in the number of diagnostic imaging tests), more home visits, and more patients participating in DMPs.
We would have expected a lower number of emergency hospitalizations and at least no rise in the utilization of hospitals, but this was not shown in the results. This cannot be explained by the data available. Additional enrollments of patients in the intervention group in other structured care programs such as DMPs may explain some of the intensified activities in care provided in the intervention group. The higher number of medical check-ups in participants before (and after) program implementation presumably reflects a selection effect.
The stronger rise in the number of patients taking 5 or more different drugs in the IG was accompanied by equally rising numbers of prescriptions. As these observations do not point to an increase in multi-medication, they would rather appear to reflect medication switches.
The observed effects may mainly be attributed to financial incentives, which were provided to the GP practices from the start. This is particularly true for the morbidity-oriented lump-sums that were specifically directed to chronically ill patients with multiple conditions (the majority of patiens participating in the program) who stand to benefit in particular from continuous and coordinated healthcare. The bonuses for cost-efficient pharmacotherapy and the IT-tool for pharmacotherapy (where implemented) might have produced the savings in drug costs as well as the presumed medication switches. Besides, greater participation in clinical peer meetings (quality circles) may have had some impact. In these circles led by a trained moderator, participating GPs discuss therapies on the basis of real data and patient examples in order to capture concrete optimization potential.
In addition to the explicit elements of the program, however, other mechanisms may have supported the observed changes in care, ranging from improved patient adherence to closer monitoring of symptoms and improved medication planning resulting in recognition of a need for further diagnosis and therapy by the GP, as well as efforts to improve the cost-effectiveness of prescribing.
We also regard the low number of patients who consulted more than one GP among participants in the IG as an indication that acceptance of the GP as the principal care coordinator was high. The lower increase in the number of specialist consultations without referral in the IG suggests that the gate-keeping role of GPs for participating patients is more distinct than in usual care. The small effect may be attributed to the penalty of €10 that patients had to pay for each outpatient consultation without a referral; this policy was in force throughout the entire evaluation period and independent of the program. Altogether, care coordination was assessed using 8 outcome measures of which 4 improved, 3 showed no effect and 1 suggested a selection effect.
Limitations
Despite appropriate propensity score matching, there may have been systematic differences that the chosen matching parameters did not address, such as physicians’ and patients’ motivation. We did not consider program costs (financial incentives paid to the GPs; payments the SHI received from the national risk adjustment scheme). In a simulation of the minimum and maximum lump sums paid to the participating GPs, we found a statistically non-significant rise in total direct costs of between €50 and €81 (mean difference). The study was based on claims data, which had been collected for non-scientific purposes; clinical as well as patient-reported data were lacking (15). Not all measures built on claims data (i.e. billed cases of treatment) are appropriate to exactly describe health care delivery. We assume, however, that this does not affect the comparison of both arms of the study.
Generalizability
International enhanced primary care approaches stress the importance of continuous and individualized care by family physicians in cooperation with non-medical health professionals (8). The continuity of the doctor–patient relationship, which is also fundamental to the concept of the medical home (16, 17) is thought to encourage positive outcomes. This aspect has also been strengthened by the GP-centered healthcare programs in Germany, of which the one in Thuringia is an example.
Comparisons with programs of similar aims but much more complexity, in which health plans adopt a blended payment methodology for physicians that includes a pay-for-performance program (18), are not sensible. Instead, a comparison with the medical home approach in Belgium that incorporates capitation payments as well as collaborative care elements, is more suitable. The authors discovered similar outcomes such as intensified care but no increase in costs (19, 20). They point out that the quality of care was particularly improved in specific populations, such as patients with diabetes, who were not explicitly in the focus of the program (20). It may therefore be interesting to shed light on the effects of the Thuringian program in patient groups with special needs in terms of intensified monitoring and better care coordination.
Our analysis is the first one in Germany to estimate the total direct cost effects of a GP-centered healthcare program. Another evaluation study from the SHI AOK Baden-Wuerttemberg (21, 22) that started in 2008 and for which data are available for 2010 and 2012, has produced the following main results: more GP-consultations, fewer specialist consultations without a referral, lower medication costs, less multi-medication (only 2010), lower hospitalization rates (only 2012). These results are different to ours with respect to hospitalization and multi-medication. However, comparability is restricted because of the longer duration and greater intervention intensity in Baden-Wuerttemberg, regional differences in sector-specific care capacities, as well as methodological differences in limiting selection bias (22).
Key Messages.
The program did not lead to lower total direct healthcare costs.
Fewer patients in the intervention group than in the control group consulted more than one GP or consulted a specialist without referral. The total number of specialist consultations, home visits and patients participating in DMPs increased, suggesting more intensive and better coordinated care in a population of older, chronically ill patients with multiple conditions.
A similar rise in prescription numbers was observed in both groups while there was a higher increase in the proportion of patients with 5 and more different medications in the intervention group along with reduced prescription costs, suggesting that GPs prescribed medication more actively and cost-effectively.
We attribute the observed effects to improved patient adherence to close monitoring of symptoms and medication, and improved medication planning.
Further evaluations should examine long-term effects as well as changes in clinical outcomes.
Acknowledgments
Acknowledgements
The authors would like to thank the German Statutory Health Insurance Funds “AOK PLUS – Die Gesundheitskasse für Sachsen und Thüringen” for funding, the reviewers for their detailed assessment of the manuscript, and Dr. med. Florian Wolf for valuable notes.
Footnotes
Conflict of interest statement
Prof. Gensichen and Prof. Wasem have received study support (third party funding) from AOK PLUS.
Dr. Schulz and Prof. Gensichen are both general practitioners (GPs).
The remaining authors declare that no conflict of interest exists.
References
- 1.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis K, Schoenbaum SC, Audet AM. A 2020 vision of patient-centered primary care. J Gen Intern Med. 2005;20:953–957. doi: 10.1111/j.1525-1497.2005.0178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McShane M, Mitchell E. Person centred coordinated care: where does the QOF point us? BMJ. 2015;350 doi: 10.1136/bmj.h2540. h2540. [DOI] [PubMed] [Google Scholar]
- 4.Flieger SP. Implementing the patient-centered medical home in complex adaptive systems: Becoming a relationship-centered patient-centered medical home. Health Care Manage Rev Epub ahead of print. 2016 doi: 10.1097/HMR.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katon W, Von Korff M, Lin E, Simon G. Rethinking practitioner roles in chronic illness: the specialist, primary care physician, and the practice nurse. Gen Hosp Psychiatry. 2001;23:138–744. doi: 10.1016/s0163-8343(01)00136-0. [DOI] [PubMed] [Google Scholar]
- 6.Deutscher Bundestag. Gesetzentwurf der Fraktionen SPD, CDU/CSU und BÜNDNIS 90/DIE GRÜNEN - Entwurf eines Gesetzes zur Modernisierung der gesetzlichen Krankenversicherung (GKV-Modernisierungsgesetz - GMG) Drucksache. 2003 15/1525. [Google Scholar]
- 7.Sachverständigenrat. Sachverständigenrat zur Begutachtung der Entwicklung im Gesundheitswesen. Bonn: 2009. Koordination und Integration - Gesundheitsversorgung in einer Gesellschaft des längeren Lebens - Sondergutachten 2009, Langfassung. [Google Scholar]
- 8.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 9.Gensichen J, Muth C, Butzlaff M, et al. [The future is chronic: German primary care and the Chronic Care Model—the comprehensive principles in the proactive treatment of the chronically ill] Z Arztl Fortbild Qualitatssich. 2006;100:365–374. [PubMed] [Google Scholar]
- 10.Bodenheimer T, Ghorob A, Willard-Grace R, Grumbach K. The 10 building blocks of high-performing primary care. Ann Fam Med. 2014;12:166–171. doi: 10.1370/afm.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deutscher Hausärzteverband. Hausarztverträge. www.hausaerzteverband.de/cms/Hausarztvertraege.988.0.html (last accessed on 17 November 2015) [Google Scholar]
- 12.AGENS, DGSMP, DGEpi. 3. Hannover: 2012. Gute Praxis Sekundärdatenanalyse (GPS) Leitlinien und Empfehlungen 2012 Arbeitsgruppe Erhebung und Nutzung von Sekundärdaten, Deutsche Gesellschaft für Sozialmedizin und Prävention, Deutsche Gesellschaft für Epidemiologie. [Google Scholar]
- 13.The EQUATOR Network. STROBE guidelines for scientific reporting on observational studies. www.equator-network.org (last accessed on 28 August 2015) [Google Scholar]
- 14.Szecsenyi J, Broge B, Stock J. Band B: Allgemeine Indikatoren. Messgrößen für die Qualität regionaler Versorgungsmodelle. In: QISA - Das Qualitätsindikatorensystem für die ambulante Versorgung, editor. KomPart Verlagsgesellschaft. Berlin: 2009. [Google Scholar]
- 15.Swart E, Ihle P, Gothe H, Matusiewicz D, editors. Verlag Hans Huber. Bern: 2014. Routinedaten im Gesundheitswesen - Handbuch Sekundärdatenanalyse Grundlagen, Methoden und Perspektiven. 2nd, completely revised edition. [Google Scholar]
- 16.Beyer M, Erler A, Gerlach FM. Ein Zukunftskonzept für die hausärztliche Versorgung in Deutschland 1. Grundlagen und internationale Modelle - Eine Darstellung anhand der Vorschläge des Sachverständigenrats Gesundheit 2009. Z Allg Med. 2010;86:93–105. [Google Scholar]
- 17.Reid RJ, Coleman K, Johnson EA, et al. The group health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood) 2010;29:835–843. doi: 10.1377/hlthaff.2010.0158. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal MB, Sinaiko AD, Eastman D, Chapman B, Partridge G. Impact of the Rochester Medical Home Initiative on primary care practices, quality, utilization, and costs. Med Care. 2015;53:967–973. doi: 10.1097/MLR.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 19.Perelman J, Roch I, Heymans I, et al. Medical homes versus individual practice in primary care: impact on health care expenditures. Med Care. 2013;51:682–688. doi: 10.1097/MLR.0b013e318293c2df. [DOI] [PubMed] [Google Scholar]
- 20.Moureaux C, Perelman J, Mendes da Costa E, et al. Impact of the medical home model on the quality of primary care: the Belgian experience. Med Care. 2015;53:396–400. doi: 10.1097/MLR.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 21.Laux G, Szecsenyi J, Mergenthal K, et al. Hausarztzentrierte Versorgung in Baden-Wurttemberg: Ergebnisse einer qualitativen und querschnittlich quantitativen Evaluation. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2015;58:398–407. doi: 10.1007/s00103-015-2122-9. [DOI] [PubMed] [Google Scholar]
- 22.Laux G, Kaufmann-Kolle P, Bauer E, Goetz K, Stock C, Szecsenyi J. Evaluation der Hausarztzentrierten Versorgung in Baden-Württemberg auf der Basis von Routinedaten der AOK. Z Evid Fortbild Qual Gesundhwes. 2013;107:372–378. doi: 10.1016/j.zefq.2013.07.001. [DOI] [PubMed] [Google Scholar]