Abstract
Excessive levels of reactive oxygen species (ROS) and increased expression of NADPH oxidases (Nox) have been proposed to contribute to pulmonary artery hypertension (PAH) and other cardiovascular diseases (CVD). Nox enzymes are major sources of ROS but the mechanisms regulating changes in Nox expression in disease states remain poorly understood. Epigenetics encompasses a number of mechanisms that cells employ to regulate the ability to read and transcribe DNA. Histone acetylation is a prominent example of an epigenetic mechanism regulating the expression of numerous genes by altering chromatin accessibility. The goal of this study was to determine whether inhibition of histone deacetylases (HDAC) affects the expression of Nox isoforms and reduces pulmonary hypertension. In immune cells, we found that multiple HDAC inhibitors robustly decreased Nox2 mRNA and protein expression in a dose-dependent manner concomitant with reduced superoxide production. This effect was not restricted to Nox2 as expression of Nox1, Nox4 and Nox5 was also reduced by HDAC inhibition. Surprisingly, Nox promoter-luciferase activity was unchanged in the presence of HDAC inhibitors. In macrophages and lung fibroblasts, ChIP experiments revealed that HDAC inhibitors block the binding of RNA polymerase II and the histone acetyltransferase p300 to the Nox2, Nox4 and Nox5 promoter regions and decrease histones activation marks (H3K4me3 and H3K9ac) at these promoter sites. We further show that the ability of CRISPR-ON to drive transcription of Nox1, Nox2, Nox4 and Nox5 genes is blocked by HDAC inhibitors. In a monocrotaline (MCT) rat model of PAH, multiple HDAC isoforms are upregulated in isolated pulmonary arteries, and HDAC inhibitors attenuate Nox expression in isolated pulmonary arteries and reduce indices of PAH. In conclusion, HDAC inhibitors potently suppress Nox gene expression both in vitro and in vivo via epigenetically regulating chromatin accessibility.
Keywords: Histone deacetylases, Epigenetics, NADPH oxidase, Pulmonary artery hypertension
1. Introduction
Oxidative stress, triggered by the overproduction of reactive oxygen species (ROS), is considered an etiological contributor to the pathophysiology of cardiovascular diseases [1–3], cancers [4], inflammation [5] and lung injury [6]. The NADPH oxidase (Nox) enzymes are a major source of ROS, and constitute a family of 5 isoforms, that have been designated Nox1–5 [7–9]. Vascular cells express Nox1–2, Nox4 and Nox5 (except in rodents which do not possess the gene for Nox5), and Nox3 expression is primarily restricted to the inner ear [7]. The post-translational regulation of Nox1 and Nox2 depends primarily on protein-protein interaction between various subunits that include NOXA1, NOXO1, p22phox, p47phox (NCF1), and p67phox(NCF2) [7]. In contrast, Nox4 is a unique isoform that is constitutively active [10], while Nox5 is regulated by calcium and phosphorylation[11]. Nox2 and Nox4 are upregulated in pulmonary artery hypertension (PAH) [2,10,12–15] and are thought to play a vital role in the development of elevated pulmonary artery resistance and pressure [16]. This is supported by studies showing that inhibition of Nox2 by genetic or pharmacological approaches in mice ameliorates PAH [17,18]. Several inhibitors of Nox4, such as GKT137831, VCC588646 and VCC202273 have also been shown to reverse PAH in rats and mice [2,15]. Given the critical role of Nox isoforms in the development of cardiovascular disease, the mechanisms underlying their transcriptional regulation is of great interest, but remains poorly defined.
Epigenetics refers to mechanisms that encode heritable traits not related to genetics or changes in the DNA sequence. This includes reversible covalent modifications such as DNA methylation and histone acetylation and methylation as well as small and non-coding RNAs [19]. The power of epigenetics is demonstrated by the existence of multicellular organisms which are comprised of multiple cell types with distinct functions and plasticity that are all derived from a single genome. Epigenetic mechanisms that regulate gene expression are currently of great interest as drug targets to more effectively influence the multiple pathways that are altered in complex diseases. Histone modifications (acetylation and methylation), and the specific nature and organization of these modifications, is referred to as the histone code. Prominent examples include the trimethylation of lysine 4 and acetylation of lysine 9 and 14 on histone 3 which have been shown to mark regions of active transcription. The number of histone modifications and the impact on transcription is quite complex and is reviewed in detail elsewhere [20].
Acetylation of histone residues results from the balance of the actions of acetyltransferases and deacetylases which dynamically regulate chromatin state. In general, increased acetylation of histone residues is thought to weaken the interaction with DNA and thus provide greater accessibility to regulatory elements of DNA. Multiple enzymes contribute to histone acetylation (HATs) and deacetylation (HDACs). HDACs comprise a family of enzymes with 18 members that are referred to as HDAC 1–11 and SIRT 1–7. They are classified into 4 groups based on function and sequence homology: Class I (HDACs1–3 and 8), Class II (HDACs 4–7, 9–10), Class III (SIRT1–7), and Class IV (HDAC11). Chemicals that inhibit HDAC activity were first described almost 40 years ago [21] and paved the way for the development of improved inhibitors with enhanced specificity towards HDACs as well as selectivity for different classes of HDACs. The ability of HDAC inhibitors to regulate gene expression is complex, with up to 30% of the transcriptome changing in expression; the upregulation and downregulation of genes occurs in equal proportions [22], and the pattern and direction of changes in gene expression is cell type dependent [23,24]. This contrasts with the simple view that HDAC inhibition should, in principal, increase histone acetylation, promote a more accessible chromatin structure, and universally increase gene expression. However, the complexity of the outcomes of HDAC inhibition is emphasized by a recent study which revealed that inhibition of HDACs can actually promote significant deacetylation of histones [23]. The role of epigenetics in PAH is a novel and active area of investigation, and while epigenetic modifiers (particularly HDAC inhibitors) have been shown to influence PAH in certain experimental models [25–31], the mechanisms involved remain poorly defined. The mechanisms underlying the increased expression of Nox2 and Nox4 in PAH are not yet known and the epigenetic control of Nox expression is, in general, poorly understood.
The RNA-guided endonuclease Cas9 from the microbial CRISPR (clustered regularly interspaced short palindromic repeat) adaptive immune system is a recently adopted and powerful tool for genome editing in mammalian cells and animals [32]. Inactive forms of Cas9 tethered to transcriptional activators and directed by single-guide RNAs (sgRNA) to specific promoter or enhancer regions of genes has been shown to effectively induce gene expression in a process called CRISPR-ON [33]. Studies have shown that the binding of guide RNAs and dCAS9 to target sequences can be influenced by chromatin structure; thus, CRISPR-ON may be a useful tool to explore epigenetic regulation of gene expression [34–36].
Accordingly, the primary goals of this study were to determine the impact of HDAC inhibition on Nox isoform expression, to identify the underlying epigenetic regulatory mechanisms that influence transcriptional activation of Nox enzymes and to determine the significance of this pathway in pulmonary arterial hypertension.
2. Materials and methods
2.1. Cell culture and reagents
COS-7, HEK-293A, promyelocytic leukemia cells (HL-60), THP-1 cells, Caco2, PC-3 cells, human lung fibroblasts were purchased from ATCC and cultured in DMEM as described [3,8,10,37–40]. Human lung microvascular endothelial cells (HLMVEC) were purchased from Lonza and grown in Endothelial Growth Medium-2-Microvessel (EGM-2MV) supplemented with growth factors and 5% FBS (Lonza). Peritoneal macrophages from WT and Nox2−/−mice were isolated and cultured as previously described [40]. In brief, thioglycolate (1 ml) was injected into each mouse and after 3–5 days peritoneal cells were collected via PBS lavage. RBCs were subsequently lysed with hypotonic buffer and isolated macrophages cultured in DMEM medium containing L-glutamine, streptomycin, penicillin, and 10% (v/v) FBS. Cells were grown at 37 °C in a 5% CO2 incubator and used from passage 2–6. COS-7 and HEK-293A cells were transfected using Lipofectamine 3000 re-agent (Invitrogen) as described previously [37,39–42]. In brief, cells were grown on 12-well plates to approximately 90% confluency and transfected using a plasmid DNA-lipid mixture of 1 μg plasmid/well at the recommended ratio of 1 μg DNA: 2 μL lipofectamine 3000. The HDAC inhibitors including scriptaid, suberoylanilide hydroxamic acid (SAHA), trichostatin A (TSA) and valproic acid (VPA) and were purchased from Sigma and Selleck Chemicals. Monocrotaline (MCT) was purchased from Sigma.
2.2. Models and analysis of pulmonary arterial hypertension
Pulmonary hypertension was induced in rats using monocrotaline (MCT). Adult male Sprague-Dawley rats (SDR, 250–300 g) were injected with a single dose of MCT (60 mg/kg, IP) which elicits a progressive, severe and irreversible form of PAH after 2–4 weeks [2,43]. Age-matched male SDR were used as controls. Rats were housed at constant temperature (21–23 °C) with ad libitum access to food and water and 12 h light-dark cycles. Cardiopulmonary parameters reflecting RV hypertrophy and PA remodeling such as RV thickness and velocity time integral (VTI) were measured using non-invasive digital ultrasound micro-imaging system (Vevo 2100, VisualSonics) as previously described [2]. Upon completion of studies, rats were anesthetized (pentobarbital, 50 mg/kg, i.p.), euthanized by thoracotomy and the Fulton index determined and pulmonary arteries isolated.
All procedures and protocols were approved by animal Care and Use Committee at Augusta University, and this study was performed following the guidelines for the Care and Use of Laboratory Animals from the US National Institutes of Health.
2.3. Engineered CRISPR-Cas9 and DNA constructs
The use and design of engineered Cas9 complex and efficient single guide RNA (sgRNA) to induce Nox1/Nox2/Nox4/Nox5 transcriptional activation follows the protocol of Dr. Zhang F [33]. The gRNA primers were annealed and cloned into sgRNA(MS2)-plasmids via BbsI sites. All of the CRISPR constructs were purchased from Addgene (Cat: #61422, 61423 and 61424), and the Nox1 and Nox4 promoter-luciferase constructs were obtained from Dr. Li [44] and Dr. Hart [45] as gifts. The Nox2 promoter-luciferase construct was generated by synthesizing the DNA fragment corresponding to Nox2 promoter region (NOX2 TSS −460 to +9) from GenScript and subcloning into pGL4.20 vector (Promega).
2.4. Analysis of protein and mRNA expression
Pulmonary arteries were dissected down to 4th order from the surrounding pulmonary parenchyma, snap frozen in liquid nitrogen, pulverized and RNA extracted using TRIZOL or proteins solubilized in 2× sample buffer. Cells were lysed directly in TRIZOL as described [2]. Total RNA (tRNA) extracted from PA (Direct-zol) and cells and used to synthesize cDNA using the iScriptcDNA Synthesis Kit (Bio-Rad). Relative gene expression was determined using real time RT-PCR (Bio-Rad iQ SYBR Green) with the following primers: Mouse Nox2: GCTGGGATCACAGGAATTGT (forward), GGTGATGACCACCTTTTGCT (reverse). Mouse GAPDH: AGGTCATCCCAGAGCTGAACG (forward), CACCCTGTTGCTGTAGCCGTAT (reverse). Human Nox1: AAGCCGACAGGCCACAGAT (forward), GTCACATACTCCACTGTCGTGTTTC (reverse). Human Nox2: GCAGCCTGCCTGAATTTCA (forward), TGAGCAGCACGCACTGGA (reverse). Human Nox4: CTTCCGTTGGTTTGCAGATT (forward), TGGGTCCACAACAGAAAACA (reverse). Human Nox5 AAGACTCCATCACGGGGCTGCA (forward), CCCTTCAGCACCTTGGCCAGAG (reverse). Human GAPDH: AGAAGGCTGGGGCTCATTTG (forward), AGGGGCCATCCACAGTCTTC (reverse). Western blotting was performed as described previously [9,46–49] using anti-Nox2 (BD, Sigma, Abcam), p300 (Active Motif) and anti-GAPDH antibodies (Santa Cruz Biotechnology).
2.5. Measurement of superoxide and hydrogen peroxide
NADPH oxidase-derived superoxide was measured by L-012 as described previously [9,37,39,40,42,50–52]. In brief, cells were plated into white tissue culture-treated 96-well plates (Thermo Lab systems) at a density of approximately 4–6 × 104 cells/well in phenol free DMEM (Sigma) with L-012 at the concentration of 400 μM (Wako) for 10 min and luminescence was quantified over time using a Lumistar Galaxy (BMG) luminometer. The relative light units (RLU) reflect changes in the superoxide produced and are completely inhibited by 100 U/ml SOD. Hydrogen peroxide production was measured using the Amplex Red assay with excitation of 530–560 nm and emission detection at ~590 nm. Cells were incubated at 37 °C with 50 μM Amplex Red and 0.125 U/ml horseradish peroxidase in phenol-free Dulbecco's modified Eagle's medium (Sigma) for 10 min. Relative light units were calculated after subtraction of negative controls.
2.6. Chromatin immunoprecipitation (ChIP)- quantitative PCR (qPCR)
ChIP was performed using the ChIP-IT Express Enzymatic Magnetic Chromatin Immunoprecipitation Kit & Enzymatic Shearing Kit (Active Motif) according to the manufacturer's instructions. Macrophages or human lung fibroblasts were treated with vehicle (DMSO) or scriptaid (3 μg/ml) for 3 h and then fixed with 1% formaldehyde for 10 min. Chromatin was then prepared by enzymatic shearing and optimized according to the manufacturer's instructions. ChIP was performed using ChIP-IT Express on sheared chromatin from approximately 7.5 × 105 cells using a negative control IgG, an anti-RNA pol II antibody, an anti-H3K4me3 antibody, an anti-H3K9ac antibody, and an anti-p300 antibody (Active Motif). Real-time PCR was performed on DNA isolated from each of the ChIP reactions using specific primer pairs for the mouse Nox2 promoter regions located −75 to −1 upstream of Nox2 transcriptional start site (TSS), human Nox4 promoter regions located +185 to +344 downstream of Nox4 transcriptional start site (TSS), and the Nox5 promoter regions located −307 to −452 upstream of Nox5 transcriptional start site (TSS). The delta Ct was calculated by the relative fold enrichment for each antibody used versus IgG negative control.
2.7. Promoter activity assays
Cells were transfected and the total amount of expression plasmid transfected per well was balanced with varying amounts of a control vector. Luciferase reporter plasmids and control luciferase plasmid (Guassia luciferase) were co-transfected into the cells, and 24 h post-transfection, cells were treated with an HDAC inhibitor for another 24 h. Cells were eventually processed in lysis buffer (Promega, New England Biolabs), and promoter activity was measured by a dual luciferase system using firefly luciferase normalized to Gaussia luciferase (Promega, New England Biolabs).
2.8. Statistical analysis
Data is presented as mean ± SE. Statistical analysis were performed using Instat software (GraphPad Software Inc., San Diego, CA). An unpaired student's t-test and an ANOVA analysis with a Bonferroni post hoc test were used for single and multiple comparisons between two or more groups respectively. p<0.05 was considered statistically significant.
3. Results
3.1. Structurally distinct inhibitors of HDAC dose-dependently suppress superoxide production from Nox2 in immune cells from humans and mice
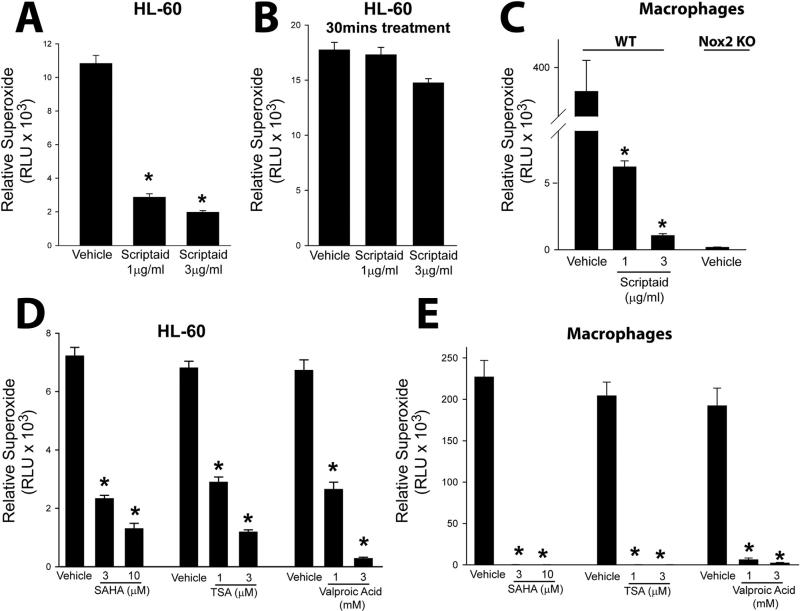
To determine the effect of HDAC inhibition on NADPH oxidase activity and ROS production, we incubated human HL-60 cells and murine macrophages with increasing concentrations of the HDAC inhibitor scriptaid for different periods of time. We found that long-term (24 h) treatment with scriptaid significantly decreased superoxide production (Fig. 1A). To assess whether the HDAC inhibitor has non-specific effects that could influence superoxide levels (i.e. anti-oxidant activity) cells were incubated with scriptaid over the short-term (30 min). Short-term exposure of scriptaid did not modify the cellular superoxide levels (Fig. 1B). In macrophages, scriptaid was also very effective at reducing superoxide levels in WT macrophages. Superoxide could not be detected in macrophages isolated from Nox2−/− mice (Fig. 1C). The ability to suppress superoxide was also observed with other HDAC inhibitors. In HL-60 cells and macrophages, superoxide release was significantly reduced in cells treated with the HDAC inhibitors suberoylanilide hydroxamic acid (SAHA), trichostatin A (TSA) and valproic acid (VPA) (Fig. 1D-E).
Fig. 1.
Inhibition of histone deacetylase (HDAC) suppresses Nox2 activity in immune cells. (A-B) Neutrophil-differentiated, HL-60 cells were treated with vehicle (DMSO) or different doses of the HDAC inhibitor, scriptaid, for long-term (24 h) or short term (30 min) inhibition, and superoxide production was measured using L-012 chemiluminescence. (C) Macrophages isolated from C57BL/6 and Nox2−/− mice were treated with vehicle (DMSO) or different doses of scriptaid for 24 h, and superoxide production was measured using L-012 chemiluminescence. (D-E) HL-60 cells and macrophages were treated with increasing concentrations of the structurally distinct HDAC inhibitors, SAHA, TSA or Valproic Acid (VPA) for 24 h. Superoxide production was measured using L-012 chemiluminescence. Data are expressed as means ± S.E., *P<0.05 versus vehicle. (n=4–6).
3.2. Transcriptional regulation of NADPH oxidases by HDAC inhibitors
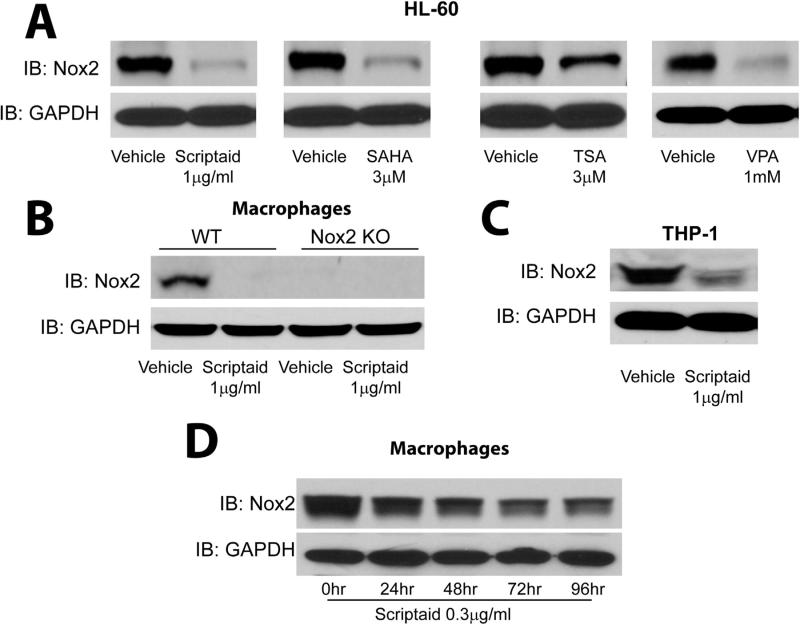
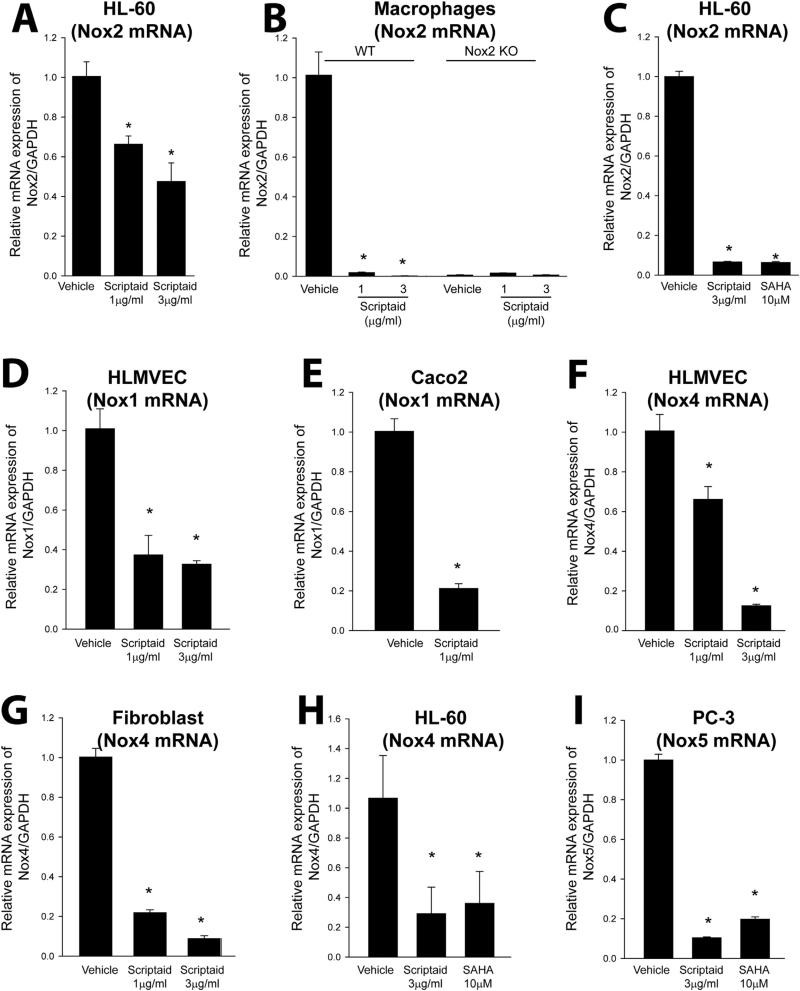
As chronic but not acute exposure to HDAC inhibitors reduces superoxide levels, we next investigated the effect of HDAC inhibitors on the expression level of Nox2 in both human and murine cells. We found that HDAC inhibitors potently reduce the protein expression of Nox2 in the human immune cell lines, HL-60 and THP-1, as well as in murine macrophages (Fig. 2A-C and Supplemental Fig. 1A-C). The absence of Nox2 expression in macrophages isolated from Nox2−/− mice confirmed the specificity of the Nox2 antibody (Fig. 2B). Low dose (0.3 μg/ml) scriptaid reduced Nox2 protein expression in a time-dependent manner in macrophages with a maximum effect observed at 96 h (Fig. 2D and Supplemental Fig. 1D). To determine whether HDAC inhibitors reduce Nox expression via transcriptional or post-translational mechanisms, we next measured the levels of mRNA using real-time PCR. Exposure of human HL-60 cells and mouse macrophages to scriptaid and SAHA significantly reduced the levels of Nox2 mRNA (Fig. 3A-C). The ability of scriptaid to decrease Nox2 mRNA expression in both cell types was dose-dependent. There was no amplification in Nox2−/− macrophages which confirmed the specificity of Nox2 primers in real-time PCR measurements (Fig. 3B). We next investigated whether HDAC inhibitors influence the transcription of other NADPH oxidase isoforms. In addition to Nox2, we found that scriptaid was also effective at inhibiting Nox1 mRNA expression in HLMVEC and in a human colon epithelial cancer cell line, Caco-2 where Nox1 is expressed at high levels (Fig. 3D-E). Expression levels of Nox4 mRNA in HLMVEC, lung fibroblasts and HL-60 cells were also reduced in the presence of scriptaid and SAHA (Fig. 3F-H). We also assessed the expression of Nox5 in a human prostate cancer cell line (PC-3) that expresses high levels of Nox5 which has been shown to be important for increased proliferative potential [53]. Exposure of PC-3 cells to either scriptaid or SAHA robustly downregulated Nox5 mRNA expression (Fig. 3I).
Fig. 2.
Structurally distinct HDAC inhibitors decrease Nox2 protein expression. (A-C) HL-60 cells, macrophages, and THP-1 cells were treated with vehicle (DMSO) or different inhibitors of HDACs (scriptaid, SAHA, TSA or VPA) for 24 h, and cell lysates were immunoblotted for Nox2 and GAPDH (as a loading control). (D) Macrophages were treated with a low concentration of scriptaid (0.3 μM) for 0, 24, 48, 72 and 96 h, and lysates were immunoblotted for Nox2 and GAPDH. Results are representative of at least 3–5 separate experiments.
Fig. 3.
HDAC inhibition downregulates expression of Nox 1, 2, 4 and 5 mRNA in immune, vascular and cancer cells. (A-C) HL-60 cells, and WT and Nox2−/− macrophages, were treated with or without HDAC inhibitors (scriptaid or SAHA) for 24 h, and Nox2 mRNA levels were determined by real-time PCR relative to GAPDH. (D-E) HLMVEC and Caco2 cells were treated with scriptaid for 24 h, and Nox1 mRNA expression was determined by real-time PCR. (F-H) HLMVEC, fibroblasts and HL-60 cells were treated with or without the HDAC inhibitors scriptaid or SAHA for 24 h, and Nox4 mRNA expression was determined by real-time PCR. (I) Human prostate cancer cells (PC-3) which endogenously express Nox5 were treated with or without scriptaid or SAHA for 24 h, and Nox5 mRNA levels were determined by real-time PCR relative to GAPDH. Data are expressed as means ± S.E., *P<0.05 versus vehicle. (n=4–6).
3.3. Epigenetic regulation of Nox transcription by histone modification
We next determined whether inhibition of HDACs affects Nox promoter activity. We used Nox1, Nox2 and Nox4 promoter luciferase reporter plasmids containing the proximal 5′ reporter region upstream of the transcription start site [44,45]. Despite prominent effects on endogenous gene expression, the relative activity of the Nox1, Nox2 and Nox4 proximal promoters was unchanged in the presence of HDAC inhibitor SAHA (Supplemental Fig. 2). Given that differences likely exist in the accessibility of plasmid DNA versus native chromatinized DNA, we next assessed the role of epigenetic mechanisms in macrophages and lung fibroblasts. ChIP-qPCR experiments were employed to ascertain epigenetic changes at the Nox2/Nox4/Nox5 promoter regions. As shown in Fig. 4A-B, the binding of RNA polymerase II to the Nox2 promoter region (−75 to −1) upstream of the transcription start site was reduced by scriptaid, consistent with reduced transcription. Furthermore, marks of open chromatin structures including trimethylation of lysine 4 of histone H3 (H3K4me3), acetylation of lysine 9 of histone H3 (H3K9ac), and p300 binding at the Nox2 promoter region were significantly reduced in the presence of scriptaid. As shown in Fig. 4C-E, RNA polymerase II and p300 binding, and H3K9ac, at the Nox4 and Nox5 promoter regions were also reduced in scriptaid-treated fibroblasts, consistent with reduced transcription. Moreover, the total protein expression of p300 was significantly reduced in scriptaid-treated macrophages and lung fibroblasts compared to control (Fig. 4F-G).
Fig. 4.
Epigenetic regulation of Nox transcription by HDAC inhibition. (A) Agarose gel analysis of enzymatic shearing of chromatin from macrophages. L represents the 100 kb to 1000 kb DNA ladder, S1 and S2 represent sheared chromatin following enzymatic digestion for 5 and 10 min. (B) Macrophages treated with vehicle (DMSO) or scriptaid (3 μg/ml) were fixed with 1% formaldehyde for 10 mins and chromatin prepared by enzymatic shearing for 10 min. ChIP was performed on isolated sheared chromatin using a negative control IgG and ChIP grade antibodies to RNA pol II, H3K4 trimethylation (H3K4me3), H3K9 acetylation (H3K9ac) or p300. Real-time PCR was performed on DNA purified from each of the ChIP reactions using primers specific for the Nox2 promoter regions located −75 to −1 upstream of Nox2 transcriptional start site (TSS). (C) Agarose gel analysis of enzymatic shearing of chromatin from human lung fibroblasts. S1 and S2 represent sheared chromatin following enzymatic digestion for 10 min. (D-E) Human lung fibroblasts treated with vehicle (DMSO) or scriptaid (3 μg/ml) were fixed with 1% formaldehyde for 10 min and chromatin prepared by enzymatic shearing for 10 min. ChIP was performed on isolated sheared chromatin using a negative control IgG and ChIP grade antibodies to RNA pol II, H3K9 acetylation (H3K9ac) or p300. Real-time PCR was performed on DNA purified from each of the ChIP reactions using primers specific for the Nox4 and Nox5 promoter regions located +185 to +344 downstream, and −307 to −452 upstream, of the TSS, respectively. (F-G) Macrophages or human lung fibroblasts were treated with vehicle (DMSO) or scriptaid (3 μg/ml) for 24 h, and cell lysates were immunoblotted for p300 and GAPDH as a loading control. Data are expressed as mean ± S.E., *P<0.05 versus vehicle. (n=4–6). Results are representative of at least 3 separate experiments.
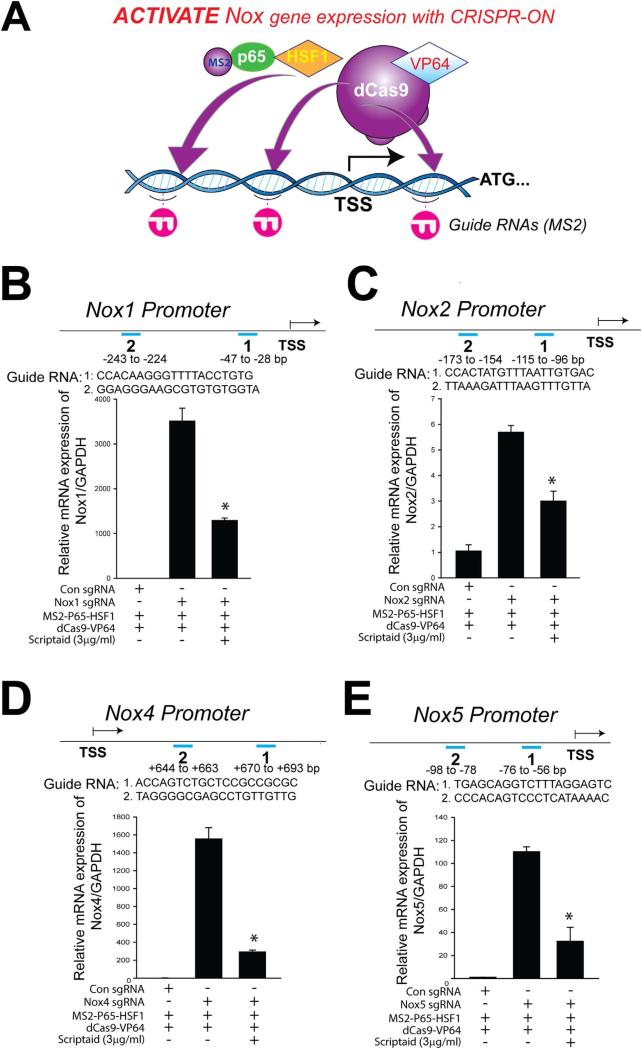
CRISPR-ON refers to the ability of an engineered, inactive Cas9 enzyme that is fused to a transcriptional activation complex and guided by RNA to activate the promoter regions of select genes [33]. As a tool to further dissect the functional consequences of the epigenetic regulatory landscape that regulates Nox enzyme expression, we adopted a CRISPR-ON strategy. As shown in Fig. 5A-E, CRISPR-ON targeted to the Nox1/Nox2/Nox4/Nox5 promoter regions robustly increased the expression of the respective endogenous genes. The ability of CRISPR-ON to drive Nox1/Nox2/Nox4/Nox5 gene transcription as well as ROS production was attenuated in the presence of scriptaid (Fig. 5B-E and Supplemental Fig. 3).
Fig. 5.
Stimulation of endogenous Nox1/2/4/5 expression using CRISPR-ON: effects of HDAC inhibition. (A) Schematic of the CRISPR-ON strategy to activate Nox gene expression. (B-E) Guide RNA (gRNA) sequences and binding regions on the Nox1/Nox2/Nox4/Nox5 promoters. HEK-293 cells were transfected with either a control gRNA or gRNAs targeting the proximal Nox1/Nox2/Nox4/Nox5 promoter regions in the presence or absence of scriptaid (3 μg/ml) for 24 h. Nox1/Nox2/Nox4/Nox5 mRNA levels were determined by real-time PCR relative to GAPDH. Data are expressed as means ± S.E., *P<0.05 versus control group. (n=4–6).
3.4. Pulmonary arterial hypertension (PAH) is associated with increased vascular expression of HDACs and Nox enzymes, and HDAC inhibition reduces Nox expression and mitigates PAH
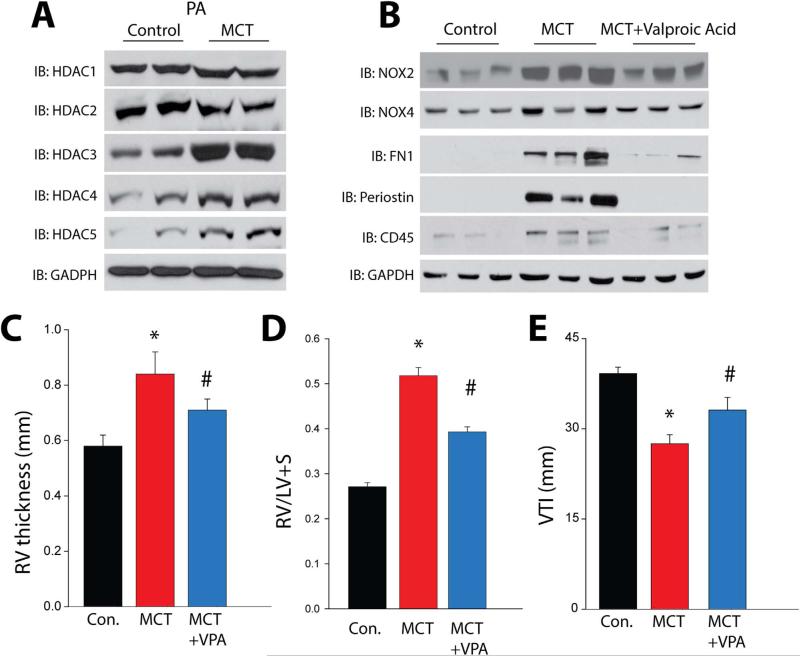
To determine the significance of HDACs in pulmonary arterial hypertension (PAH), we first assessed the expression level of HDACs in pulmonary blood vessels using real time PCR and Western blotting in the rat MCT model of PAH. We found increased mRNA and protein level of HDACs 3, 4 and 5 in isolated pulmonary arteries (PA) (Fig. 6A and Supplemental Fig. 4A and 5). We also observed that Nox2 and Nox4 levels were increased in hypertensive PA (Fig. 6B and Supplemental Fig. 4B) and reduced in the presence of the HDAC inhibitor Valproic acid (VPA). In addition, cellular fibronectin (FN1), periostin and CD45 were increased in PA from 4wk MCT rats versus control and decreased in the presence of VPA (Fig. 6B and Supplemental Fig. 4B). Non-invasive assessment of PAH parameters in 4-week MCT-treated rats using the vevo2100 digital ultrasound revealed a significant increase in RV thickness and decreased velocity time integral (VTI) which were corroborated post-mortem by an increase in the Fulton index. Treatment of MCT-treated rats with the HDAC inhibitor VPA reduced RV thickness, the Fulton index and increased VTI (an index of vessel stiffness and remodeling), consistent with improved hemodynamics and reduced pulmonary hypertension (Fig. 6C-E).
Fig. 6.
Pulmonary arterial hypertension is associated with increased HDAC expression in pulmonary arteries, and HDAC inhibition reduces Nox expression, markers of fibrosis and inflammation, and indices of pulmonary hypertension in the MCT-rat model. (A) Western blot analysis of HDACs 1–5 versus GAPDH in isolated PA from control and 4week MCT-rats. (B) Western blot of Nox2, Nox4 and fibronectin1, periostin and CD45 in PA isolated from control, MCT and MCT-treated rats treated without or with the HDAC inhibitor Valproic Acid (VPA) for 4 weeks. (C-E) Non-invasive assessment using the Vevo 2100 reveals significant increases in RV thickness in 4 week MCT-treated rats, which was confirmed by the Fulton index post mortem, and increased PA stiffness as determined by the velocity time integral (VTI). Administration of the HDAC inhibitor, VPA for 4weeks reduced RV hypertrophy and increased VTI in 4-week MCT-treated rats. *Significantly different from Vehicle, # significantly different from MCT, p<0.05 (n=5–6).
4. Discussion
The aims of this study were to determine whether HDAC inhibition influences the expression of Nox enzymes and the production of ROS and whether this pathway plays an important role in PAH. We found that multiple HDAC inhibitors have the ability to dose-dependently decrease superoxide production from macrophages and immune cell lines concomitant with the down-regulation of Nox2 mRNA and protein expression. In addition to Nox2, we also found that the mRNA expression of other Nox isoforms that are expressed in blood vessels (Nox1, Nox4 and Nox5), were significantly reduced in the presence of HDAC inhibitors. Mechanistically, HDAC inhibitors did not alter the activity of transiently expressed Nox promoter-luciferase constructs but greatly reduced transcription of the endogenous Nox2/Nox4/Nox5 genes. ChIP experiments revealed decreased levels of RNA polymerase II, H3K4me3, H3K9ac and p300 at Nox promoter sites, indicating that HDAC inhibitors alter chromatin accessibility. Consistent with this, the ability of CRISPR-ON to stimulate transcription of the endogenous Nox1/Nox2/Nox4/Nox5 genes was reduced in the presence of HDAC inhibitors. In a rat MCT model of pulmonary hypertension, we found that multiple HDAC isoforms are upregulated in isolated PAs along with increased expression of Nox2 and Nox4. Administration of HDAC inhibitors to MCT-treated rats reduces the expression of Nox2 and 4 and blunts indices of PAH. Collectively, these data suggest that in PAH, increased expression or activity of HDACs facilitates the transcriptional activation of Nox genes via histone modifications, and that HDAC inhibitors reverse these changes to decrease Nox expression and improve pulmonary hypertension.
Mammalian DNA is organized and condensed through the winding of DNA around a spool of eight histone proteins to form a nucleosome which is then packed even more tightly into chromatin [54]. The dense packing of chromatin can limit the accessibility of DNA binding proteins, including transcription factors, until DNA is partially unwound or relaxed. Studies have shown that this dynamic state of chromatin is associated with post-translational modifications on the amino-terminal tails of histones with changes in the acetylation, methylation and phosphorylation of specific amino acids [55]. Acetylation of lysine residues on histone tails neutralizes the positive charge of lysine and leads to weaker binding to DNA, whereas deacetylation has the opposite effect [56]. The ability of histone acetylation to relax DNA has been proposed as a mechanism linking histone acetylation with increased transcription. In our study we found that HDAC inhibitors, which theoretically should increase histone acetylation and gene transcription, instead resulted in a dramatic decrease in the expression of Nox mRNA and protein. Our results are instead consistent with microarray studies showing that HDAC inhibitors promote roughly equal proportions of upregulated and down-regulated genes in a subset of the transcriptome [23,24]. The ability of HDAC inhibition to modify gene expression can also be influenced by the type of HDAC inhibitor, the concentration used and the cell type. In our study, we found that multiple HDAC inhibitors including scriptaid, SAHA, trichostatin and VPA produced consistent inhibitory effects on Nox expression over multiple doses, in different cell types and different species both in vitro and in vivo. A limitation of our study is the absence of data showing that HDAC inhibitors can reduce HDAC activity. However this is likely based on the use of multiple commercially obtained inhibitors at a range of concentrations that are well above the published IC50s. The inhibitory effect of HDAC inhibitors was also consistent among Nox family members, including Nox1, Nox2, Nox4 and Nox5.
ChIP analysis revealed that HDAC inhibition is associated with reduced binding of acetylated histones to the proximal promoters of multiple Nox isoforms, concomitant with reduced binding of RNA polymerase II. This data together with reduced mRNA expression levels is consistent with decreased transcription of Nox genes. The reduced acetylation of histones in the presence of HDAC inhibitors was unexpected but is consistent with recent reports showing widespread deacetylation of histones in response to HDAC inhibitors [23,57]. The mechanisms by which HDAC inhibitors promote histone deacetylation are not yet fully understood. It has been proposed, at least for some genes, to be due to reduced expression and decreased binding of the histone acetyltransferase, EP300/CREBBP to the promoter regions [23]. Whether EP300/CREBBP regulates Nox expression via increased acetylation of histones at the promoter region has not been reported. In ChIP experiments we found that HDAC inhibition with scriptaid reduced the binding of p300 to the Nox2, Nox4 and Nox5 promoters. This data suggests that inhibition of HDACs paradoxically promotes reduced binding of p300, leading to reduced acetylation of histones and ultimately impaired chromatin accessibility at the promoter regions of Nox genes. The mechanism underlying the reduced binding of p300 is likely to be due to the decreased total protein expression of p300 found in cells treated with scriptaid.
The ability of HDAC inhibitors to suppress Nox isoform expression is consistent with prior observations that scriptaid significantly decreases Nox4 mRNA expression in human umbilical vein endothelial cells [58]. However, our findings are in contrast to a study from Zhao et al. who reported that the HDAC inhibitor trichostatin A (TSA) increased both Nox2 expression and the production of ROS in myocardium [59]. The reasons underlying this discrepancy are not known but may be due to a cell type-dependent effect or a specific or non-specific effect of the HDAC inhibitor, TSA. We found that multiple HDAC inhibitors effectively reduce Nox expression at a range of doses, in multiple cells in vitro and in pulmonary blood vessels in vivo and this effect was conserved in mice, rats and humans. TSA and VPA are specific inhibitors of HDAC classes I and II, whereas scriptaid and SAHA are selective for HDAC class I [60–63]. Class I HDACs include HDAC 1, 2, 3 and 8. We found increased mRNA expression of HDAC 4, 5 and 7 and increased protein expression of HDACs 3, 4 and 5 in isolated PA from rats with pulmonary hypertension. Whether increased expression of these HDACs contributes to the increased Nox expression either directly or indirectly is not yet known.
A surprising finding of our study was that the mRNA levels of Nox1, Nox2, Nox4 and Nox5 are all reduced following HDAC inhibition. The genes for each Nox isoform contain distinct promoter sequences that are regulated by different types and combinations of transcription factors [64]. Therefore, it is unlikely that the effect of HDAC inhibitors on the expression of Nox isoforms occurs by altering the levels or activity of a specific set of transcription factors. This hypothesis is supported by the inability of the HDAC inhibitors to alter the activity of transiently expressed Nox1, Nox2 and Nox4 promoter-luciferase constructs. Analogous to our findings, others have shown that eNOS mRNA and protein expression are reduced by HDAC inhibitors but promoter-luciferase activity is increased [65]. Analysis of promoter activity using reporters, while useful to provide information on relevant transcription factor activity, can yield results contradictory to the effects of the epigenetic regulation of the endogenous gene as transiently expressed plasmid DNA is not bound to histones in the same way as genomic DNA [58,65]. To assess the factors influencing chromatin state in the region of the Nox promoters we employed ChIP assays. Using this approach, we found that HDAC inhibitors reduced the presence of histones bearing activation marks (H3K4me3 and H3K9ac) at the Nox 2, 4 and 5 promoter regions. These results, along with the reducing binding of RNA polymerase II, suggest that chromatin accessibility in the region of the Nox promoters is reduced by HDAC inhibition. To functionally test whether accessibility to the proximal promoter regions of the Nox genes is altered by HDAC inhibitors, we employed CRISPR-ON which utilizes an RNA guided, cleavage deficient form of Cas9 fused to a transcriptional activator [25]. Previous studies have shown that the ability of RNA guides and inactive Cas9 to bind DNA, and thus the efficiency of CRISPR-ON, can be influenced by chromatin remodeling [26–28]. Using guide RNAs to target CRISPR-ON to the proximal promoters of Nox1, Nox2, Nox4 and Nox5, we found that the HDAC inhibitor scriptaid reduced the ability of CRISPR-ON to increase mRNA expression and Nox-derived ROS. These data suggest that inhibition of HDACs promotes chromatin remodeling in the Nox1, Nox2, Nox4 and Nox5 promoter regions, restricting access to regulatory factors, including transcription factors and RNA polymerase II. Collectively, these results are consistent with the reduced expression of Nox enzymes observed in human fibroblasts and immune cells, mouse macrophages and rat pulmonary arteries treated with HDAC inhibitors.
Pulmonary arterial hypertension (PAH) results from aberrant pulmonary vascular remodeling and elevated pulmonary vascular resistance [66,67] which promotes right ventricular hypertrophy and ultimately right heart failure. Increased levels of Nox2 and 4 have been reported in lung tissues from both animal models and humans with PAH [2,68,69], and genetic deletion or pharmacological inhibition of Nox enzymes reduces pulmonary hypertension [2,15,17,18]. The mechanisms underlying the increased expression of Nox enzymes in PAH are poorly understood, and epigenetic reprogramming has been implicated [70,71]. In an animal model of PAH, we found changes in the expression of several HDACS, notably the increased expression of HDAC 3, 4, 5 and 7 in isolated PAs. In hypertensive MCT-treated rats, inhibition of HDACs with VPA reversed the increased expression of Nox2 and Nox4 and these results are in agreement with those obtained in isolated cells. VPA also reduced the expression of markers of fibrosis (cellular fibronectin, FN1 and periostin) and inflammation (CD45) and reduced indices of PAH including right ventricular hypertrophy and PA stiffening.
In summary, our study reveals a novel epigenetic mechanism that controls the transcription of NADPH oxidases in vitro and in vivo. In specific, we have found that HDAC inhibitors potently reduce ROS production as well as mRNA and protein expression of Nox 1, 2, 4 and 5 in multiple cell types. HDAC inhibitors promote the deacetylation of histones through the decreased binding of histone acetyltransferase p300 to the promoter regions of the Nox2/Nox4/Nox5 genes, reducing histone acetylation and the accessibility of RNA polymerase II, which ultimately reduces transcription. Further, in a rat model of PAH, HDAC inhibitors reduced the expression of Nox isoforms in PA and mitigated indices of PAH. Thus HDAC inhibitors potently reduce the expression of Nox genes and maybe useful in the treatment of diseases that result from the overproduction of ROS, such as PAH.
Supplementary Material
Acknowledgements
The authors thank Louise Meadows for technical assistance and the late Dr. Tapan Chatterjee for his expertise in epigenetic regulation and insightful suggestions. This work was supported by NIH grants R01HL125926-01A1 (DF, SB), 1R01HL124773-01A1 (DS, DF), P01 HL101902-01A1 (DF), R01HL112640 and R01HL126949 (NLW), and NSFC grant 81400033 (FC).
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.freeradbiomed.2016.08.003.
References
- 1.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 2.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2014;34:1704–1715. doi: 10.1161/ATVBAHA.114.303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F, Barman S, Yu Y, Haigh S, Wang Y, Black SM, Rafikov R, Dou H, Bagi Z, Han W, Su Y, Fulton DJ. Caveolin-1 is a negative regulator of NADPH oxidase-derived reactive oxygen species. Free Radic. Biol. Med. 2014;73:201–213. doi: 10.1016/j.freeradbiomed.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 6.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur. Respir. J. 1998;11:745–757. [PubMed] [Google Scholar]
- 7.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Yu Y, Haigh S, Johnson J, Lucas R, Stepp DW, Fulton DJ. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PloS One. 2014;9:e88405. doi: 10.1371/journal.pone.0088405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD, Fulton DJ. Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1919–H1928. doi: 10.1152/ajpheart.00910.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Haigh S, Barman S, Fulton DJ. From form to function: the role of Nox4 in the cardiovascular system. Front. Physiol. 2012;3:412. doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid. Redox Signal. 2009;11:2443–2452. doi: 10.1089/ars.2009.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim BY, Han MJ, Chung AS. Effects of reactive oxygen species on proliferation of Chinese hamster lung fibroblast (V79) cells. Free Radic. Biol. Med. 2001;30:686–698. doi: 10.1016/s0891-5849(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 13.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab. Investig. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 14.Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, Steinhorn RH. Increased p22(phox)/Nox4 expression is involved in remodeling through hydrogen peroxide signaling in experimental persistent pulmonary hypertension of the newborn. Antioxid. Redox Signal. 2013;18:1765–1776. doi: 10.1089/ars.2012.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am. J. Respir. Cell Mol. Biol. 2012;47:718–726. doi: 10.1165/rcmb.2011-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demarco VG, Whaley-Connell AT, Sowers JR, Habibi J, Dellsperger KC. Contribution of oxidative stress to pulmonary arterial hypertension. World J. Cardiol. 2010;2:316–324. doi: 10.4330/wjc.v2.i10.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norton CE, Broughton BR, Jernigan NL, Walker BR, Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid. Redox Signal. 2013;18:1777–1788. doi: 10.1089/ars.2012.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 19.Webster AL, Yan MS, Marsden PA. Epigenetics and cardiovascular disease. Can. J. Cardiol. 2013;29:46–57. doi: 10.1016/j.cjca.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Paluch BE, Naqash AR, Brumberger Z, Nemeth MJ, Griffiths EA. Epigenetics: a primer for clinicians. Blood Rev. 2016 doi: 10.1016/j.blre.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boffa LC, Vidali G, Mann RS, Allfrey VG. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J. Biol. Chem. 1978;253:3364–3366. [PubMed] [Google Scholar]
- 22.Galletti M, Cantoni S, Zambelli F, Valente S, Palazzini M, Manes A, Pasquinelli G, Mai A, Galie N, Ventura C. Dissecting histone deacetylase role in pulmonary arterial smooth muscle cell proliferation and migration. Biochem. Pharmacol. 2014;91:181–190. doi: 10.1016/j.bcp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Rafehi H, Balcerczyk A, Lunke S, Kaspi A, Ziemann M, Kn H, Okabe J, Khurana I, Ooi J, Khan AW, Du XJ, Chang L, Haviv I, Keating ST, Karagiannis TC, El-Osta A. Vascular histone deacetylation by pharmacological HDAC inhibition. Genome Res. 2014;24:1271–1284. doi: 10.1101/gr.168781.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol. Cancer Ther. 2003;2:151–163. [PubMed] [Google Scholar]
- 25.Zhao L, Chen CN, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, Wilkins MR. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation. 2012;126:455–467. doi: 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan B, Hayama E, Kawaguchi N, Furutani Y, Nakanishi T. Therapeutic efficacy of valproic acid in a combined monocrotaline and chronic hypoxia rat model of severe pulmonary hypertension. PloS One. 2015;10:e0117211. doi: 10.1371/journal.pone.0117211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Saren G, Jiang H. HDAC inhibition: a novel therapeutic target for attenuating pulmonary hypertension by regulating Tregs. Int. J. Cardiol. 2015;198:176–177. doi: 10.1016/j.ijcard.2015.06.172. [DOI] [PubMed] [Google Scholar]
- 28.Huang YY, Fang ZF, Hu XQ, Zhou SH. HDAC inhibition: a novel therapeutic target for pulmonary hypertension by reducing right ventricular hypertrophy through diverse pathological mechanisms. Int. J. Cardiol. 2015;196:125–126. doi: 10.1016/j.ijcard.2015.05.170. [DOI] [PubMed] [Google Scholar]
- 29.De Raaf MA, Hussaini AA, Gomez-Arroyo J, Kraskaukas D, Farkas D, Happe C, Voelkel NF, Bogaard HJ. Histone deacetylase inhibition with trichostatin A does not reverse severe angioproliferative pulmonary hypertension in rats (2013 Grover Conference series). Pulm Circ. 2014;4:237–243. doi: 10.1086/675986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nozik-Grayck E, Woods C, Stearman RS, Venkataraman S, Ferguson BS, Swain K, Bowler RP, Geraci MW, Ihida-Stansbury K, Stenmark KR, McKinsey TA, Domann FE. Histone deacetylation contributes to low extra-cellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;00263:02015. doi: 10.1152/ajplung.00263.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stratton MS, McKinsey TA. Acetyl-lysine erasers and readers in the control of pulmonary hypertension and right ventricular hypertrophy. Biochem. Cell Biol. 2015;93:149–157. doi: 10.1139/bcb-2014-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, Nureki O, Zhang F. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Scott DA, Kriz AJ, Chiu AC, Hsu PD, Dadon DB, Cheng AW, Trevino AE, Konermann S, Chen S, Jaenisch R, Zhang F, Sharp PA. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat. Biotechnol. 2014;32:670–676. doi: 10.1038/nbt.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeder ML, Angstman JF, Richardson ME, Linder SJ, Cascio VM, Tsai SQ, Ho QH, Sander JD, Reyon D, Bernstein BE, Costello JF, Wilkinson MF, Joung JK. D.N.A. Targeted, demethylation and activation of endogenous genes using programmable TALE-TET1 fusion proteins. Nat. Biotechnol. 2013;31:1137–1142. doi: 10.1038/nbt.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, Fulton DJ. An inhibitor of protein arginine methyltransferases, 7,7′-carbonylbis(azanediyl)bis(4-hydroxynaphthalene-2-sulfonic acid (AMI-1), is a potent scavenger of NADPH-oxidase-derived superoxide. Mol. Pharmacol. 2010;77:280–287. doi: 10.1124/mol.109.061077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen F, Kumar S, Yu Y, Aggarwal S, Gross C, Wang Y, Chakraborty T, Verin AD, Catravas JD, Lucas R, Black SM, Fulton DJ. PKC-dependent phosphorylation of eNOS at T495 regulates eNOS coupling and endothelial barrier function in response to G+-toxins. PloS One. 2014;9:e99823. doi: 10.1371/journal.pone.0099823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen F, Pandey D, Chadli A, Catravas JD, Chen T, Fulton DJ. Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxid. Redox Signal. 2011;14:2107–2119. doi: 10.1089/ars.2010.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen F, Yu Y, Qian J, Wang Y, Cheng B, Dimitropoulou C, Patel V, Chadli A, Rudic RD, Stepp DW, Catravas JD, Fulton DJ. Opposing actions of heat shock protein 90 and 70 regulate nicotinamide adenine dinucleotide phosphate oxidase stability and reactive oxygen species production. Arterioscler. Thromb. Vasc. Biol. 2012;32:2989–2999. doi: 10.1161/ATVBAHA.112.300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elms S, Chen F, Wang Y, Qian J, Askari B, Yu Y, Pandey D, Iddings J, Caldwell RB, Fulton DJ. Insights into the arginine paradox: evidence against the importance of subcellular location of arginase and eNOS. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H651–H666. doi: 10.1152/ajpheart.00755.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey D, Chen F, Patel A, Wang CY, Dimitropoulou C, Patel VS, Rudic RD, Stepp DW, Fulton DJ. SUMO1 negatively regulates reactive oxygen species production from NADPH oxidases. Arterioscler. Thromb. Vasc. Biol. 2011;31:1634–1642. doi: 10.1161/ATVBAHA.111.226621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 44.Valente AJ, Zhou Q, Lu Z, He W, Qiang M, Ma W, Li G, Wang L, Banfi B, Steger K, Krause KH, Clark RA, Li S. Regulation of NOX1 expression by GATA, HNF-1alpha, and Cdx transcription factors. Free Radic. Biol. Med. 2008;44:430–443. doi: 10.1016/j.freeradbiomed.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 45.Lu X, Murphy TC, Nanes MS, Hart CM. PPAR{gamma} regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-{kappa}B. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:L559–L566. doi: 10.1152/ajplung.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen F, Dang YH, Yan CX, Liu YL, Deng YJ, Fulton DJ, Chen T. Sequence-length variation of mtDNA HVS-I C-stretch in Chinese ethnic groups. J. Zhejiang Univ. Sci. B. 2009;10:711–720. doi: 10.1631/jzus.B0920140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YJ, Liu YL, Zhong Q, Yu YF, Su HL, Toque HA, Dang YH, Chen F, Xu M, Chen T. Tetrahydropalmatine protects against methamphetamine-induced spatial learning and memory impairment in mice. Neurosci. Bull. 2012;28:222–232. doi: 10.1007/s12264-012-1236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y, Xing B, Dang YH, Qu CL, Zhu F, Yan CX. Microinjection of valproic acid into the ventrolateral orbital cortex enhances stress-related memory formation. PloS One. 2013;8:e52698. doi: 10.1371/journal.pone.0052698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma XC, Jiang D, Jiang WH, Wang F, Jia M, Wu J, Hashimoto K, Dang YH, Gao CG. Social isolation-induced aggression potentiates anxiety and depressive-like behavior in male mice subjected to unpredictable chronic mild stress. PloS One. 2011;6:e20955. doi: 10.1371/journal.pone.0020955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen F, Lucas R, Fulton D. The subcellular compartmentalization of arginine metabolizing enzymes and their role in endothelial dysfunction. Front. Immunol. 2013;4:184. doi: 10.3389/fimmu.2013.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandey D, Fulton DJ. Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H1336–H1344. doi: 10.1152/ajpheart.01163.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian J, Chen F, Kovalenkov Y, Pandey D, Moseley MA, Foster MW, Black SM, Venema RC, Stepp DW, Fulton DJ. Nitric oxide reduces NADPH oxidase 5 (Nox5) activity by reversible S-nitrosylation. Free Radic. Biol. Med. 2012;52:1806–1819. doi: 10.1016/j.freeradbiomed.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holl M, Koziel R, Schafer G, Pircher H, Pauck A, Hermann M, Klocker H, Jansen-Durr P, Sampson N. ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Mol. Carcinog. 2016;55:27–39. doi: 10.1002/mc.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meier K, Brehm A. Chromatin regulation: how complex does it get? Epigenetics. 2014;9:1485–1495. doi: 10.4161/15592294.2014.971580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 56.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu. Rev. Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 57.Ooi JY, Tuano NK, Rafehi H, Gao XM, Ziemann M, Du XJ, El-Osta A. HDAC inhibition attenuates cardiac hypertrophy by acetylation and deacetylation of target genes. Epigenetics. 2015;10:418–430. doi: 10.1080/15592294.2015.1024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siuda D, Zechner U, El Hajj N, Prawitt D, Langer D, Xia N, Horke S, Pautz A, Kleinert H, Forstermann U, Li H. Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res. Cardiol. 2012;107:283. doi: 10.1007/s00395-012-0283-3. [DOI] [PubMed] [Google Scholar]
- 59.Zhao TC, Zhang LX, Cheng G, Liu JT. gp-91 mediates histone deacetylase inhibition-induced cardioprotection. Biochim. Biophys. Acta. 2010;1803:872–880. doi: 10.1016/j.bbamcr.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blackwell L, Norris J, Suto CM, Janzen WP. The use of diversity profiling to characterize chemical modulators of the histone deacetylases. Life Sci. 2008;82:1050–1058. doi: 10.1016/j.lfs.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 62.Damaskos C, Karatzas T, Nikolidakis L, Kostakis ID, Karamaroudis S, Boutsikos G, Damaskou Z, Kostakis A, Kouraklis G. Histone Deacetylase (HDAC) inhibitors: current evidence for therapeutic activities in pancreatic cancer. Anticancer Res. 2015;35:3129–3135. [PubMed] [Google Scholar]
- 63.Cavasin MA, Demos-Davies K, Horn TR, Walker LA, Lemon DD, Birdsey N, Weiser-Evans MC, Harral J, Irwin DC, Anwar A, Yeager ME, Li M, Watson PA, Nemenoff RA, Buttrick PM, Stenmark KR, McKinsey TA. Selective class I histone deacetylase inhibition suppresses hypoxia-induced cardiopulmonary remodeling through an antiproliferative mechanism. Circ. Res. 2012;110:739–748. doi: 10.1161/CIRCRESAHA.111.258426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manea SA, Constantin A, Manda G, Sasson S, Manea A. Regulation of Nox enzymes expression in vascular pathophysiology: focusing on transcription factors and epigenetic mechanisms. Redox Biol. 2015;5:358–366. doi: 10.1016/j.redox.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rossig L, Li H, Fisslthaler B, Urbich C, Fleming I, Forstermann U, Zeiher AM, Dimmeler S. Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ. Res. 2002;91:837–844. doi: 10.1161/01.res.0000037983.07158.b1. [DOI] [PubMed] [Google Scholar]
- 66.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 67.Cool CD, Groshong SD, Oakey J, Voelkel NF. Pulmonary hypertension: cellular and molecular mechanisms. Chest. 2005;128:565S–571S. doi: 10.1378/chest.128.6_suppl.565S. [DOI] [PubMed] [Google Scholar]
- 68.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ. Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 69.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am. J. Respir. Cell Mol. Biol. 2009;40:601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J. Immunol. 2011;187:2711–2722. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang JB, Liang J, Zhao XF, Wu WS, Zhang F. Epigenetics: novel mechanism of pulmonary hypertension. Lung. 2013;191:601–610. doi: 10.1007/s00408-013-9505-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.