Abstract
High fidelity animal models of human disease are essential for preclinical evaluation of novel gene and protein therapeutics. However, these studies can be complicated by exaggerated immune responses against the human transgene. Here we demonstrate that dogs with a genetic deficiency of the enzyme α-L-iduronidase (IDUA), a model of the lysosomal storage disease mucopolysaccharidosis type I (MPS I), can be rendered immunologically tolerant to human IDUA through neonatal exposure to the enzyme. Using MPS I dogs tolerized to human IDUA as neonates, we evaluated intrathecal delivery of an adeno-associated virus serotype 9 vector expressing human IDUA as a therapy for the central nervous system manifestations of MPS I. These studies established the efficacy of the human vector in the canine model, and allowed for estimation of the minimum effective dose, providing key information for the design of first-in-human trials. This approach can facilitate evaluation of human therapeutics in relevant animal models, and may also have clinical applications for the prevention of immune responses to gene and protein replacement therapies.
Keywords: AAV, gene therapy, intrathecal, MPS I, Neonatal tolerance
Introduction
Mucopolysaccharidosis type I (MPS I) is a rare genetic disease caused by mutations in the gene encoding α-L-iduronidase (IDUA), an enzyme required for the catabolism of ubiquitous glycosaminoglycans (GAGs) in the lysosome. IDUA deficiency results in lysosomal accumulation of GAGs in many tissues, leading to a variety of clinical manifestations including bone and joint deformities, corneal clouding, and cardiac valve insufficiency. MPS I patients also frequently experience neurological complications such as communicating hydrocephalus and spinal cord compression (1–3). The impact of the disease on cognitive function varies; in the attenuated form of MPS I (Scheie syndrome – MIM #607016 or Hurler-Scheie syndrome – MIM #607015) in which there is residual IDUA activity, cognition is affected in only about one third of patients. In the more common severe form of MPS I (Hurler syndrome – MIM #607014), patients universally exhibit rapid cognitive decline in early childhood (4). MPS I is currently treated with intravenous (IV) infusion of the recombinant enzyme, which can be internalized by cells from the circulation via mannose 6-phosphate receptor binding (5, 6). Enzyme replacement improves many disease symptoms, but does not reach the central nervous system (CNS), and therefore has no impact on cognitive function (4). MPS I can also be treated with hematopoietic stem cell transplantation (HSCT), which provides a constant source of circulating IDUA through enzyme secretion by engrafted donor cells. Unlike enzyme replacement, HSCT can improve cognitive outcomes, apparently due to migration of donor-derived cells across the blood-brain barrier, where they serve as a source of secreted enzyme within the CNS. However, HSCT suffers from numerous complications including graft failure, infection, graft versus host disease, and transplant-associated mortality as high as 20% (7–13). After transplant, many patients also exhibit residual cognitive deficits, which may be a consequence of disease progression during the slow engraftment of donor cells in the CNS (14). These shortcomings leave a significant unmet need for a safe and effective therapy for the CNS manifestations of MPS I.
Gene therapy is a promising alternative to HSCT for the treatment of cognitive decline in MPS I patients. Gene transfer has the potential to induce rapid reconstitution of IDUA in the CNS without the adverse effects of HSCT. Successful targeting of even a small number of cells in the CNS could provide a depot of secreted IDUA in the brain, leading to widespread improvement of storage pathology and potentially improving cognitive function. We previously demonstrated that injection of an adeno-associated virus serotype 9 vector (AAV9) into the cerebrospinal fluid (CSF) can efficiently deliver the IDUA gene to cells throughout the CNS, and that the enzyme secreted by transduced cells mediates global resolution of brain storage lesions (15, 16). These proof-of concept studies for intrathecal (IT) AAV9 gene therapy relied on two naturally occurring large animal disease models, the MPS I dog and MPS I cat. The use of these models was critical for evaluating the efficacy of the approach, not only because they accurately reproduce the CNS pathology of MPS I, but also because these large animals better reflect the human CNS anatomy and CSF circulation compared with rodent models, allowing for realistic representation of the clinical route of administration and the resulting vector distribution. While these initial experiments in the MPS I dog and cat were carried out using vectors expressing species-specific transgenes, advancing this approach toward human trials necessitated the evaluation of a clinical candidate vector expressing the human IDUA transgene. However, studies of the clinical candidate vector in the MPS I dog model were complicated by an exaggerated immune response to the human transgene product. Building on our previous finding that neonatal gene transfer could induce persistent tolerance to the transgene product, we applied this approach to induce tolerance to human IDUA in MPS I dogs, which subsequently allowed for accurate evaluation of the efficacy of the human vector in this high fidelity model (15).
Results
Intrathecal AAV9 expressing human IDUA elicits robust transgene-specific immunity in MPS I dogs
The MPS I dog has an IDUA mutation resulting in inclusion of the first intron in the mature mRNA, creating an immediate stop codon. The mutation in MPS I dogs yields no detectable IDUA activity (17–19). In the absence of lysosomal IDUA activity, undegraded GAGs accumulate in the cell (5). This primary GAG storage material in affected tissues can be directly detected histologically by Alcian blue staining (16, 18, 20–25). In addition to the primary GAG storage pathology, lysosomal GAG accumulation leads to a characteristic cascade of cellular abnormalities. The undegraded GAGs cause lysosomal distention, which is visible on histology by increased staining for lysosomal membrane proteins such as LIMP2. Neurons also exhibit secondary accumulation of substances such as gangliosides (e.g. GM3) and un-esterified cholesterol. Lysosomal storage also induces aberrant overexpression of lysosomal enzymes such as hexosaminidase (Hex).
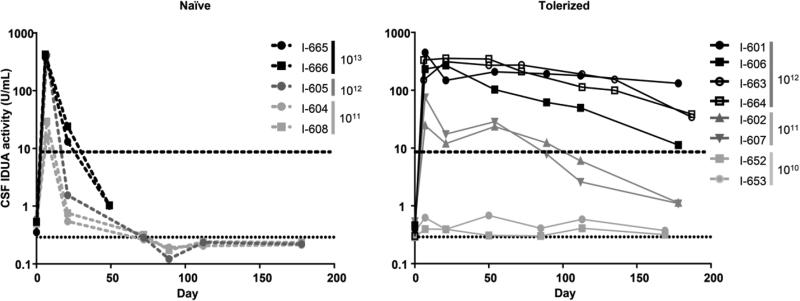
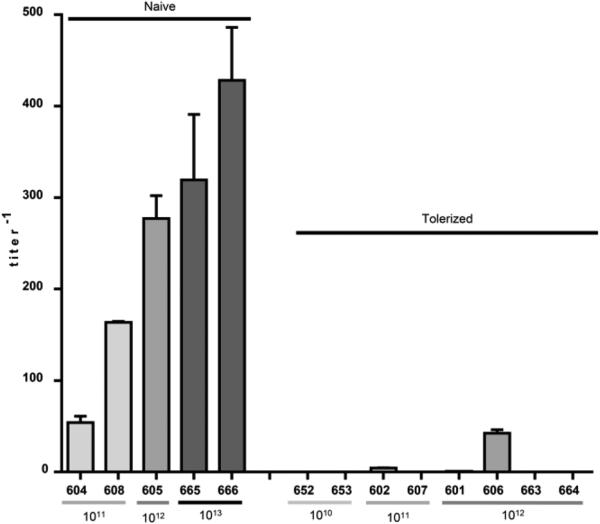
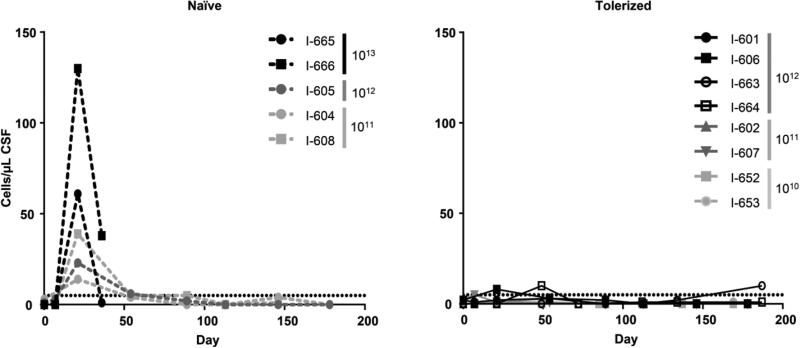
Three MPS I dogs were treated at one month of age with a single intrathecal injection into the cisterna magna of a clinical candidate AAV9 vector expressing human IDUA. Vector doses ranged from 1011 genome copies per kilogram (GC/kg) (n = 2) to 1012 GC/kg (n = 1). The procedure was well tolerated in all subjects. IDUA activity in CSF rapidly increased following vector administration, exceeding that of normal controls by day 7 (Figure 1, Naïve). However, by day 21 post vector administration, CSF IDUA levels fell to baseline, accompanied by an elevation in CSF anti-hIDUA antibody titers (Figure 2, Naïve). Day 21 CSF samples also revealed a lymphocytic pleocytosis in all animals (Figure 3, Naïve). In this cohort, the elevated CSF antibodies and cell counts were not associated with clinical signs or other laboratory abnormalities, and the pleocytosis spontaneously resolved. At the time of necropsy six months after injection, routine histological evaluation revealed no evidence of lesions in the brain or spinal cord. Vector biodistribution demonstrated widespread CNS transduction and persistence of the vector genome (Table S1). Brain hexosaminidase overexpression was reduced relative to untreated MPS I dogs, although it was not normalized at either dose (Figure S1). Histology also showed partial resolution of brain storage lesions by LIMP2 and GM3 immunostaining, which did not appear to be dose dependent (Figure S2).
Figure 1.
CSF IDUA activity in MPS I dogs treated with intrathecal AAV9 expressing human IDUA. Dogs were treated at one month of age with an intrathecal injection of the vector into the cisterna magna. IDUA activity was measured in subsequent CSF samples. Vector doses (GC/kg) are indicated for each animal. The dashed lines represent animals treated with intrathecal vector only (left panel). The solid lines with filled symbols represent animals pretreated on postnatal day 5 with intravenous AAV8 expressing human IDUA from a liver-specific promoter (right panel, filled symbols). Solid lines with open symbols represent animals pretreated on postnatal day 7 and 14 with intravenous infusion of recombinant human IDUA (right panel, open symbols). Animals I-665 and I-666 were euthanized on day 36 due to neurological signs. The horizontal dashed line represents mean CSF IDUA activity in normal dogs. The horizontal dotted line indicates the assay limit of quantification.
Figure 2.
CSF antibody titers against human IDUA. Antibody titers against human IDUA were measured by ELISA in CSF samples collected 50 days post vector administration. CSF samples tested from I-665 and I-666 were collected at the time of necropsy (day 36 post injection). Error bars = SEM. Antibody titers were significantly lower in the animals pre-treated as neonates with AAV8 vector (I-652, I-653, I-602, I-607, I-601, I-606) or recombinant human IDUA (I-663, I-664) compared with controls treated with IT vector alone (I-604, I-608, I-605, I-665, I-666) (p = 0.0016, two-tailed, Mann-Whitney test).
Figure 3.
CSF nucleated cell counts following intrathecal AAV9 injection. Total nucleated cell counts were measured in CSF samples from naïve dogs treated with intrathecal AAV9 (left panel) as well as animals treated as neonates with systemic recombinant human IDUA (I-663 and I-664) or an AAV8 vector expressing IDUA before receiving intrathecal AAV9 (right panel). Nucleated cell counts were significantly elevated on day 21 after vector injection in the naïve animals compared with those pre-treated as neonates with AAV8 vector or recombinant human IDUA (p = 0.0008, two-tailed, Mann-Whitney test).
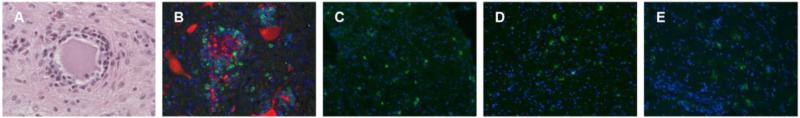
Based on the favorable safety profile observed in these dogs, two additional MPS I dogs were treated with a 10-fold higher dose of vector (1013 GC/kg). These dogs developed CSF pleocytosis with similar kinetics to the animals treated at the lower two doses (Figure 3, Naïve); however, in these two subjects the response was more pronounced, and the pleocytosis was temporally associated with the onset of neurological signs. Beginning 21 days after vector administration, the animals exhibited hyporeflexia and weakness of the hind limbs, and pain upon flexion of the neck. Pain and CSF pleocytosis began to resolve following treatment with analgesics and corticosteroids; however, the hind limb weakness persisted, and the animals were euthanized two weeks after the onset of clinical signs. Histopathology demonstrated robust transduction of spinal motor neurons, particularly in the lumbar spinal cord, and lymphocytic infiltrates surrounding transduced neurons (Figure 4A). Systematic evaluation of sections throughout the brain and spinal cord confirmed that the lesions were primarily localized to the lumbar spinal cord, although occasional infiltrates were observed in the brain (Table S2). Immunostaining revealed that the infiltrating mononuclear cells consisted primarily of B cells, with occasional CD4 and CD8 T cells in the associated spinal nerves (Figure 4 B-E). The clinical signs of hind limb weakness correlated with the histological evidence for high levels of transgene expression in the lumbar spinal motor neurons and selective lymphocytic infiltration at this level. This suggested that neurological toxicity was driven by a transgene-specific immune response targeting heavily transduced motor neurons. An interferon gamma ELISPOT assay performed on peripheral blood mononuclear cells (PBMCs) collected at necropsy was negative for T cell responses to the transgene or the vector capsid (data not shown) although these results may be confounded by the preceding course of corticosteroids.
Figure 4.
Lymphocyte infiltration of the lumbar spinal cord in MPS I dogs exhibiting hind limb weakness following human IDUA gene transfer. Representative sections of the lumbar spinal cord (A, B) and lumbar spinal nerves (C-E) are shown for the two naïve dogs (I-665 and I-666) treated with the highest dose of AAV9 expressing human IDUA. Sections were stained with H&E (A) or immunostained for human IDUA (red) and CD20 (green) (B, C), CD4 (D), or CD8 (E). Immunostained sections were counterstained with DAPI (blue) to show nuclei.
Neonatal exposure to human IDUA through hepatic gene transfer induces tolerance to subsequent intrathecal gene transfer
To evaluate the AAV9 vector expressing human IDUA in the absence of an exaggerated immune response to the transgene, we attempted to induce immunological tolerance to the human protein through neonatal exposure. On postnatal day 5, six MPS I dogs were treated with a single intravenous injection of an AAV serotype 8 vector (AAV8) expressing human IDUA from a liver specific promoter. At one month of age the animals were treated with an intrathecal injection into the cisterna magna of different doses of the AAV9 vector expressing human IDUA in three cohorts (n = 2 animals per cohort) as follows: 1010, 1011 and 1012 GC/kg. All animals exhibited a dose-dependent elevation in CSF IDUA activity similar to the non-tolerized dogs (Figure 1); however, in this cohort CSF enzyme expression persisted throughout the six-month study (Figure 1, Tolerized). CSF antibody responses were blunted compared with those observed when naïve (i.e., non-tolerized) animals were dosed with intrathecal vector; only two animals in the tolerized cohorts (I-602 and I-606) exhibited detectable titers, which were approximately 20-fold lower than naïve animals treated with an equivalent vector dose (Figure 2). Only the dog with the highest antibody titer in this cohort (I-606) exhibited elevated CSF lymphocytes at day 21, albeit at lower levels than in the naïve animals (Figure 3). There were no clinical adverse events in these cohorts.
Intrathecal AAV9-mediated hIDUA expression effects dose-dependent correction of brain biochemical abnormalities and storage lesions
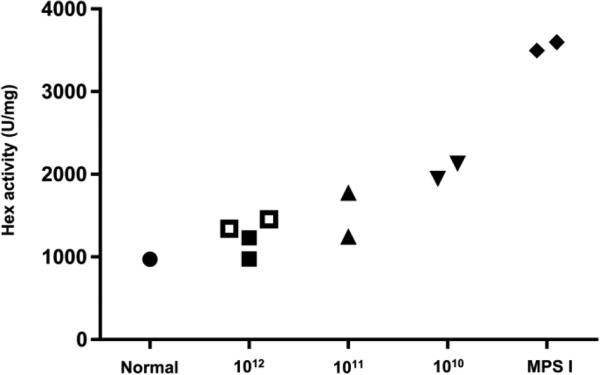
The six MPS I dogs tolerized to human IDUA through neonatal gene transfer were sacrificed 6 months post intrathecal AAV9 injection. Brain lysates demonstrated complete normalization of hexosaminidase activity at the highest vector dose, with partial correction at the lowest dose (Figure 5). Hexosaminidase activity was normalized in CSF at all vector doses (Figure S3). The thickening of the meninges, which can contribute to spinal cord compression in MPS I patients, was reversed in animals treated at all doses [Figure S4; measurements at the cervical spinal cord level are presented in Figure S4; analysis of meninges in brain, thoracic, and lumbar spinal cords showed comparable results (data not shown)]. Histological evaluation of the brain revealed dose-dependent decreases in storage lesions in the hIDUA tolerant dogs (Figure 6). Animals treated with the highest vector dose exhibited LIMP2 and GM3 staining similar to normal controls; at the lowest dose, there were measurable improvements in some markers (LIMP2 and Hex), whereas GM3 accumulation was not clearly reduced compared with untreated MPS I dogs. The low dose of 1010 GC/kg therefore appeared to be the minimum effective dose (MED). Scaled to the 45 g brain mass of a one-month old dog, and with an average body weight of 2 kg, this dose would correspond to an MED of 4.4 × 108 GC/g brain mass, or approximately 6.2 × 1011 GC in an adult human.
Figure 5.
Normalization of brain hexosaminidase activity in human IDUA tolerant MPS I dogs treated with intrathecal AAV9. Hex activity was measured in samples collected from 6 brain regions (frontal cortex, temporal cortex, occipital cortex, hippocampus, medulla, and cerebellum). The mean activity is shown for 1 normal control dog, 2 untreated MPS I dogs, and the 8 hIDUA tolerant dogs treated with intrathecal AAV9 expressing human IDUA. Open symbols indicate animals tolerized with infusion of recombinant human IDUA. Hex activity was significantly reduced in the high dose cohort compared to untreated controls (p = 0.014, Kruskal-Wallis test followed by Dunn's multiple comparisons test).
Figure 6.
Dose-dependent correction of brain storage lesions in human IDUA tolerant dogs treated with intrathecal AAV9. Brains were sectioned and stained for LIMP2 and GM3 (A). Meningeal GAG accumulation was imaged using Alcian blue staining. Automated quantification of GM3 (B) and LIMP2 (C) positive cells was performed on cortical brain images (n = 10 per animal). Open symbols indicate animals tolerized with infusion of recombinant human IDUA. GM3 and LIMP2 were significantly reduced in the high dose cohort compared to untreated controls (p = 0.0002 and 0.0026, respectively, Kruskal-Wallis test followed by Dunn's multiple comparisons test).
Infusion of recombinant hIDUA in newborn MPS I dogs is sufficient to induce tolerance to intrathecal AAV9-mediated hIDUA expression
To determine whether hepatic expression of human IDUA was necessary for tolerance induction, we treated two MPS I dogs (I-663 and I-664) with infusions of recombinant human IDUA (0.58 mg/kg) on postnatal day 7 and 14 before intrathecal AAV9 injection at one month of age. Similar to dogs treated as newborns with a vector expressing human IDUA, following intrathecal gene therapy the enzyme-treated dogs exhibited persistently high levels of CSF IDUA activity (Figure 1) and minimal antibody response against human IDUA (Figure 2) or CSF pleocytosis (Figure 3). Brain hexosaminidase activity was reduced (Figure 5) and storage lesions were effectively cleared in both animals (Figure 6).
Materials and Methods
Experimental Design
This study included 13 MPS I dogs. A subset of the dogs was treated on postnatal day 5 with an intravenous injection of an AAV8 vector expressing human IDUA from a liver-specific thyroid hormone binding globulin (TBG) promoter (n = 6). Two animals were treated with infusions of recombinant human IDUA on postnatal day 7 and 14. These 8 MPS I dogs, as well as 5 naïve MPS I dogs, were treated with an intrathecal vector injection at one month of age. IDUA activity was measured in CSF throughout the study. Subjects were sacrificed six months after vector injection, except where noted in the text. Brain storage lesions were measured by LIMP2 and GM3 immunostaining. Study personnel were not blinded to the treatment group. Histological analyses were performed by a blinded reviewer.
Vector production
A codon-optimized human IDUA cDNA was cloned into an expression construct bearing the cytomegalovirus immediate early enhancer, chicken beta-actin promoter and rabbit globin polyadenylation sequence flanked by AAV2 inverted terminal repeats. The construct was packaged in an AAV9 capsid, purified and titered as previously described (41).
Animal procedures
The MPS I dog colony was maintained at the University of Pennsylvania School of Veterinary Medicine under NIH and USDA guidelines for the care and use of animals in research. For infusions of recombinant human IDUA, laronidase (Genzyme, Cambridge, MA) was diluted 5–fold in saline immediately before use. Infusions were performed through a peripheral venous catheter over two hours. Vector injections, serum and CSF collection, and euthanasia were performed as previously described (15).
Enzyme assays
IDUA and Hex activity were measured in tissue lysates and CSF as previously described (16).
Anti-hIDUA ELISA
Polystyrene ELISA plates were coated overnight at 4 degrees with recombinant human IDUA (Genzyme) diluted to 5 μg/mL in phosphate buffer pH 5.8. The plate was washed and blocked in 2% BSA in pH 5.8 phosphate buffer. The plate was incubated 1 hour at room temperature with CSF samples diluted 1:50 in PBS. The plate was washed and bound antibody detected with HRP conjugated anti-canine IgG (Pierce, Rockford, IL) diluted 1:10,000 in phosphate buffer with 2% BSA. The ELISA was developed with tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO) substrate for 15 minutes, then stopped with 2 N sulfuric acid and absorbance was measured at 450 nm. Titers were calculated from a standard curve of a serially diluted positive sample.
Histology
Tissue processing, GM3 and LIMP2 immunostaining, and quantification were performed as previously described (15). Combined immunofluorescence staining for CD20 and hIDUA was performed on paraffin sections following the protocol for LIMP2 staining but using instead rabbit antibodies against CD20 (Life Technologies, Grand Island, NY) and sheep antibodies specific for hIDUA (R&D Systems, Minneapolis, MN) followed by corresponding FITC- or TRITC-labeled secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Staining for CD4 and CD8 was performed on acetone-fixed cryosections with monoclonal antibodies CA13.1E4 and YCATE55.9 (AbD Serotec, Raleigh, NC), respectively, followed by FITC-labeled secondary antibodies (Jackson ImmunoResearch). Quantification of thickness of the cervical meninges was performed on H&E stained sections of the cervical spinal cord using ImageJ software (W. S. Rasband, National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/). Fifteen measurements of total meningeal thickness were made per slide at 300 μm intervals.
Biodistribution
Vector biodistribution was evaluated as described (15).
Statistics
Data were evaluated using Kruskal-Wallis test followed by Dunn's test or Mann-Whitney test as appropriate. P <0.05 was considered statistically significant. All statistical analyses were performed using Prism 6.0 (GraphPad Software, San Diego, CA). Sample sizes, P values, and the specific statistical test performed for each experiment are included in the figure legends.
Study approval
All study protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Discussion
Evaluating the efficacy of intrathecal AAV9 delivery for the treatment of MPS I required assessment of both the vector distribution that could be achieved via injection into the CSF, and the impact of that degree of transduction on disease-specific markers. These studies necessitated the use of an animal model that could accurately reflect the disease pathophysiology while also having sufficiently similar size and anatomy to allow for meaningful evaluation of the clinical delivery method and the resulting vector distribution. The canine model of MPS I faithfully replicates the human phenotype, exhibiting not only the same biochemical and histological lesions, but also many of the same clinical manifestations (17, 20, 21, 26–29). Due to the phenotypic similarity to MPS I in humans, MPS I dogs were used extensively in the development of enzyme replacement therapy for the treatment of systemic disease (21, 30). MPS I dogs also mimic CNS manifestations of the disease, sporadically developing spinal cord compression and hydrocephalus (27, 28, 31). Though cognitive studies have not been reported for MPS I dogs, the histological and biochemical manifestations in the brain have been well characterized, and faithfully recapitulate the findings in humans with the severe form of the disease (20, 26, 32). MPS I dog brains demonstrate accumulation of lysosomal membrane proteins (LIMP2) and gangliosides (GM3), and upregulation of lysosomal enzymes such as hexosaminidase (Hex). Ganglioside accumulation correlates with cognitive function in MPS I and other lysosomal storage diseases, and thus is a critical marker for evaluating disease severity and therapeutic outcomes (33, 34). MPS I dogs also exhibit changes in neuronal morphology similar to those identified in patients (32). These striking similarities made this a compelling model for the evaluation of intrathecal AAV delivery as a novel therapy for the CNS manifestations of MPS I in humans. The capacity of large animal models to replicate the route of administration that would be used clinically for IT AAV9 delivery, as well as the resulting vector distribution in the CNS, further supported the relevance of the MPS I dog for these studies.
Although the MPS I dog appeared to be an excellent model for evaluation of the clinical vector, the immune response to human IDUA presented a critical obstacle. From previous studies it is clear that the immune response to human IDUA in MPS I dogs is much more extreme than that observed in patients. Intravenous delivery of the protein in both dogs and MPS I patients often elicits antibodies; however in dogs these responses are more robust, less likely to decline upon continued administration, and more often associated with anaphylactic responses to subsequent infusions (21, 35). The difference in immune response to human IDUA in the CNS is even more striking; MPS I dogs treated with intrathecal infusions of the enzyme showed evidence of meningitis as well as antibody responses detectable in CSF. In contrast, for both pediatric and adult MPS I patients treated with repeated IT infusions of the protein, there have been no similar adverse effects, and in the 5 patients that have been tested for CSF antibodies against IDUA only one has been positive (25, 27, 28, 36–40). Interestingly, MPS I dogs also develop CSF antibodies against normal canine IDUA, albeit at lower levels than to the human enzyme, suggesting that this model has a greater overall tendency toward immunity to IDUA, which is exacerbated by the use of the non-species-specific protein (15). These marked differences in the outcome of both intravenous and intrathecal delivery of human IDUA in MPS I dogs and patients indicate a consistently exaggerated immune response to human IDUA in MPS I dogs, and suggest that preventing this response will be necessary to replicate the anticipated vector activity in humans. Inducing tolerance to the protein through neonatal exposure allowed for the evaluation of the efficacy of the human vector in this model without the interference of the exaggerated immune response. This provided critical information, allowing for the accurate determination of a minimum effective dose—an essential factor in the design of first-in-human gene therapy trials—in the most relevant animal model. Without this approach, the only options would be to extrapolate efficacy data from vectors with species-specific transgenes, which could have important differences in potency, or move studies to a less representative animal model that is more immune tolerant to the human protein. Pharmacologic immune suppression can also be employed in this setting, although the neonatal tolerance-induction protocol has the clear advantage of avoiding secondary consequences of the immune-suppressing drugs.
Though efficacy assessment was confounded by the immune response and loss of circulating IDUA in the non-tolerized dogs treated with the human vector, some useful data can be derived from these animals. While the strong immune response is not likely to represent the immune response in humans, it could inform monitoring plans for first-in-human studies by demonstrating key characteristics of immune-mediated toxicity. In this case, we observed that immune-mediated toxicity was dose dependent, the peak of the immune response occurred 3 weeks after vector administration, presented with focal motor symptoms likely due to high transduction of spinal motor neurons, and was accompanied by CSF pleocytosis. These findings could be directly integrated into the phase 1 trial protocol, with intensive monitoring for immune-mediated toxicity and neurological symptoms extending for several weeks after vector administration, and CSF analysis for pleocytosis occurring 2-4 weeks after injection. If neurological symptoms accompanied by pleocytosis appeared with similar kinetics in a human study subject, the findings in naïve dogs would suggest that the toxicity is due to an immune response (as opposed to overexpression toxicity, for example) and could guide therapeutic decisions.
A strong correlation emerged between vector dose, CSF enzyme levels, and correction of brain storage lesions in MPS I dogs that were tolerized to human IDUA. The relationship between IDUA activity in the CSF and correction of brain pathology could be a valuable observation as this approach advances into human trials, where IDUA activity detected in CSF may be a useful predictor of clinical response. Even more useful would be the identification of CSF markers that directly reflect the severity of CNS storage pathology. CSF biomarkers would be a valuable tool for evaluating correction of the underlying CNS pathology in MPS I patients, and the canine model could be an ideal system for identification of such markers. In this study, we evaluated one potential CSF biomarker, the enzyme hexosaminidase. While substantially elevated in brain tissue of MPS I dogs, Hex activity was only modestly elevated in the CSF. CSF Hex was normalized in all treated animals, regardless of the degree of tissue response. CSF Hex may therefore be useful to confirm vector activity in clinical studies, but is not likely to predict a therapeutic response. Future studies using the MPS I dog model may allow for evaluation of additional CSF markers and their correlation with brain storage lesions, which could ultimately yield powerful new tools to non-invasively evaluate the severity of CNS involvement in MPS I and the impact of novel therapeutics.
The present findings indicate that neonatal exposure to human IDUA can induce tolerance using two different sources of the enzyme. While we had previously found that AAV-mediated expression could induce transgene-specific tolerance in neonates, here we found that infusion of the recombinant enzyme could also induce tolerance. If this approach is generalizable to other proteins, it could be useful for more accurate preclinical evaluation of many human therapeutics in animal models. Further, if a similar approach could induce tolerance to foreign proteins in human neonates, it could have enormous potential to improve the efficacy of protein replacement therapies for diseases in which antibody responses to the normal protein limit efficacy. While most MPS I patients appear to tolerate intrathecal IDUA infusions, the vast majority develop serum antibodies against intravenous enzyme replacement, and these antibodies can diminish the response to therapy. Combining neonatal tolerance induction with a gene or protein replacement therapy may substantially improve patient outcomes. The availability of an approved recombinant enzyme makes MPS I an excellent candidate for an initial human trial of this approach. If human neonates exhibit the same window of 1-2 weeks for tolerance induction, newborn screening would be essential for identifying patients early enough for successful intervention. The ongoing implementation of newborn screening for MPS I and other lysosomal storage diseases will therefore be critically important for clinical evaluation of a neonatal tolerance-induction protocol.
Supplementary Material
Highlights.
MPS I dogs treated with gene therapy develop immune response against human IDUA
MPS I dogs exposed to human IDUA as newborns develop tolerance to the protein
Tolerance induction allows for accurate evaluation of human vector in canine model
Important approach for preclinical evaluation of gene or protein therapeutics
Potential to induce tolerance to therapeutic proteins for recessive diseases
Acknowledgments
We would like to acknowledge the support of the Penn Vector and Cell Morphology Cores of the Gene Therapy Program (Philadelphia, PA, USA). This work was supported by a grant from REGENXBIO [to J.M.W.]; and National Institutes of Health grants [P40-OD010939 to M.E.H. and M.L.C., DK54481 to M.E.H. and M.L.C.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
J.M. Wilson is an advisor to REGENXBIO, Dimension Therapeutics, and Solid Gene Therapy, and is a founder of, holds equity in, and has a sponsored research agreement with REGENXBIO and Dimension Therapeutics; in addition, he is a consultant to several biopharmaceutical companies and is an inventor on patents licensed to various biopharmaceutical companies. The remaining authors have declared that no conflict of interest exists.
References
- 1.Taccone A, Donati PT, Marzoli A, Dellacqua A, Gatti R, Leone D. Mucopolysaccharidosis - thickening of dura-mater at the craniocervical junction and other CT/MRI findings. Pediatr. Radiol. 1993;23:349–352. doi: 10.1007/BF02011954. [DOI] [PubMed] [Google Scholar]
- 2.Kachur E, Del Maestro R. Mucopolysaccharidoses and spinal cord compression: Case report and review of the literature with implications of bone marrow transplantation. Neurosurgery. 2000;47:223–228. doi: 10.1097/00006123-200007000-00046. [DOI] [PubMed] [Google Scholar]
- 3.Vijay S, Wraith JE. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediatr. 2005;94:872–877. doi: 10.1111/j.1651-2227.2005.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 4.Wraith JE, Beck M, Lane R, van der Ploeg A, Shapiro E, Xue Y, Kakkis ED, Guffon N. Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: Results of a multinational study of recombinant human alpha-L-iduronidase (Laronidase). Pediatrics. 2007;120:E37–E46. doi: 10.1542/peds.2006-2156. [DOI] [PubMed] [Google Scholar]
- 5.Sando GN, Neufeld EF. Recognition and receptor-mediated uptake of a lysosomal enzyme, α-l-iduronidase, by cultured human fibroblasts. Cell. 1977;12:619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- 6.Dahms NM, Lobel P, Kornfeld S. Mannose 6-phosphate receptors and lysosomal-enzyme targeting. J. Biol. Chem. 1989;264:12115–12118. [PubMed] [Google Scholar]
- 7.Aldenboven M, Boelens F, de Koning TF. The clinical outcome of Hurler syndrome after stem cell transplantation. Biol. Blood Marrow Tr. 2008;14:485–498. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Boelens JJ, Wynn RF, O'Meara A, Veys P, Bertrand Y, Souillet G, Wraith JE, Fischer A, Cavazzana-Calvo M, Sykora KW, et al. Outcomes of hematopoietic stem cell transplantation for Hurler's syndrome in Europe: a risk factor analysis for graft failure. Bone Marrow Transpl. 2007;40:225–233. doi: 10.1038/sj.bmt.1705718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braunlin EA, Stauffer NR, Peters CH, Bass JL, Berry JM, Hopwood JJ, Krivit W. Usefulness of bone marrow transplantation in the Hurler syndrome. Am. J. Cardiol. 2003;92:882–886. doi: 10.1016/s0002-9149(03)00909-3. [DOI] [PubMed] [Google Scholar]
- 10.de Ru MH, Boelens JJ, Das AM, Jones SA, van der Lee JH, Mahlaoui N, Mengel E, Offringa M, O'Meara A, Parini R, et al. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J. Rare Dis. 2011;6:9. doi: 10.1186/1750-1172-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souillet G, Guffon N, Maire I, Pujol M, Taylor P, Sevin F, Bleyzac N, Mulier C, Durin A, Kebaili K, et al. Outcome of 27 patients with Hurler's syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transpl. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- 12.Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood S, Wenger DA, Rubinstein P, et al. Cord-Blood Transplants from Unrelated Donors in Patients with Hurler's Syndrome. New Engl. J. Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 13.Whitley CB, Belani KG, Chang PN, Summers CG, Blazar BR, Tsai MY, Latchaw RE, Ramsay NKC, Kersey JH. Long-term outcome of hurler syndrome following bone-marrow transplantation. Am. J.Med. Genet. 1993;46:209–218. doi: 10.1002/ajmg.1320460222. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- 15.Hinderer C, Bell P, Louboutin JP, Zhu Y, Yu H, Lin G, Choa R, Gurda BL, Bagel J, O'Donnell P, et al. Neonatal Systemic AAV Induces Tolerance to CNS Gene Therapy in MPS I Dogs and Nonhuman Primates. Mol. Ther. 2015;23:1298–1307. doi: 10.1038/mt.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, Wilson JM. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinderer C, Bell P, Gurda BL, Wang Q, Louboutin J-P, Zhu Y, Bagel J, O'Donnell P, Sikora T, Ruane T, et al. Intrathecal Gene Therapy Corrects CNS Pathology in a Feline Model of Mucopolysaccharidosis I. Mol. Ther. 2014;22:2018–2027. doi: 10.1038/mt.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon KP, Tieu PT, Neufeld EF. Architecture of the canine IDUA gene and mutation underlying canine mucopolysaccharidosis I. Genomics. 1992;14:763–768. doi: 10.1016/s0888-7543(05)80182-x. [DOI] [PubMed] [Google Scholar]
- 19.Terlato NJ, Cox GF. Can mucopolysaccharidosis type I disease severity be predicted based on a patient's genotype? A comprehensive review of the literature. Genet. Med. 2003;5:286–294. doi: 10.1097/01.GIM.0000078027.83236.49. [DOI] [PubMed] [Google Scholar]
- 20.He XX, Li CM, Simonaro CM, Wan Q, Haskins ME, Desnick RJ, Schuchman EH. Identification and characterization of the molecular lesion causing mucopolysaccharidosis type I in cats. Mol. Genet. Metab. 1999;67:106–112. doi: 10.1006/mgme.1999.2860. [DOI] [PubMed] [Google Scholar]
- 21.Shull RM, Helman RG, Spellacy E, Constantopoulos G, Munger RJ, Neufeld EF. Morphologic and biochemical studies of canine mucopolysaccharidosis I. Am. J. Pathol. 1984;114:487–495. [PMC free article] [PubMed] [Google Scholar]
- 22.Shull RM, Kakkis ED, McEntee MF, Kania SA, Jonas AJ, Neufeld EF. Enzyme replacement in a canine model of Hurler-syndrome. P. Natl. Acad. Sci. USA. 1994;91:12937–12941. doi: 10.1073/pnas.91.26.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke LA, Wraith JE, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Sidman M, Kakkis ED, et al. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics. 2009;123:229–240. doi: 10.1542/peds.2007-3847. [DOI] [PubMed] [Google Scholar]
- 24.Ellinwood NM, Colle MA, Well MA, Casal ML, Vite CH, Wiemelt S, Hasson CW, O'Malley TM, He XX, Prociuk U, et al. Bone marrow transplantation for feline mucopolysaccharidosis I. Mol. Genet. Metab. 2007;91:239–250. doi: 10.1016/j.ymgme.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haskins ME, Aguirre GD, Jezyk PF, Desnick RJ, Patterson DF. The pathology of the feline model of mucopolysaccharidosis I. Am. J. Pathol. 1983;112:27. [PMC free article] [PubMed] [Google Scholar]
- 26.Chen A, Vogler C, McEntee M, Hanson S, Ellinwood NM, Jens J, Snella E, Passage M, Le S, Guerra C, et al. Glycosaminoglycan storage in neuroanatomical regions of mucopolysaccharidosis I dogs following intrathecal recombinant human iduronidase. Apmis. 2011;119:513–521. doi: 10.1111/j.1600-0463.2011.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciron C, Desmaris N, Colle MA, Raoul S, Joussemet B, Verot L, Ausseil J, Froissart R, Roux F, Cherel Y, et al. Gene therapy of the brain in the dog model of Hurler's syndrome. Ann. Neurol. 2006;60:204–213. doi: 10.1002/ana.20870. [DOI] [PubMed] [Google Scholar]
- 28.Dickson P, Ellinwood NM, Dierenfeld A, Kline K, Parkes J, Hanson S, Vite C, Mlikotic A, Chen A, Gross W, et al. Intrathecal enzyme replacement therapy treats meningeal storage and spinal cord compression in MPS I dogs. Mol. Genet. Metab. 2010;99:S15–S15. [Google Scholar]
- 29.Dickson PI, Ellinwood NM, Hanson S, Vite C, Passage M, Le S, Guerra C. Intrathecal enzyme replacement therapy may stabilize or reverse signs of spinal cord compression in MPS I dogs. Mol. Genet. Metab. 2009;98:70–70. [Google Scholar]
- 30.Traas AM, Wang P, Ma X, Tittiger M, Schaller L, O'Donnell P, Sleeper MM, Vite C, Herati R, Aguirre GD, et al. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol. Ther. 2007;15:1423–1431. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 31.Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J. Clin. Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vite CH, Nestrasil I, Mlikotic A, Jens JK, Snella EM, Gross W, Shapiro EG, Kovac V, Provenzale JM, Chen S, et al. Features of Brain MRI in Dogs with Treated and Untreated Mucopolysaccharidosis Type I. Comparative Med. 2013;63:163–173. [PMC free article] [PubMed] [Google Scholar]
- 33.Walkley SU, Haskins ME, Shull RM. Alterations in neuron morphology in mucopolysaccharidosis type I. Acta Neuropathol. 1988;75:611–620. doi: 10.1007/BF00686207. [DOI] [PubMed] [Google Scholar]
- 34.Walkley SU, Vanier MT. Secondary lipid accumulation in lysosomal disease. Biochim. Biophys. Acta. 2009;1793:726–736. doi: 10.1016/j.bbamcr.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Constantopoulos G, Iqbal K, Dekaban AS. Mucopolysaccharidosis Types IH, IS, II and IIIA: Glycosaminoglycans and Lipids of Isolated Brain Cells and Other Fractions from Autopsied Tissues. J. Neurochem. 1980;34:1399–1411. doi: 10.1111/j.1471-4159.1980.tb11220.x. [DOI] [PubMed] [Google Scholar]
- 36.Kakkis E, Lester T, Yang R, Tanaka C, Anand V, Lemontt J, Peinovich M, Passage M. Successful induction of immune tolerance to enzyme replacement therapy in canine mucopolysaccharidosis I. Proc. Natl. Acad. Sci. USA. 2004;101:829–834. doi: 10.1073/pnas.0305480101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickson PI, Hanson S, McEntee MF, Vite CH, Vogler CA, Mlikotic A, Chen AH, Ponder KP, Haskins ME, Tippin BL, et al. Early versus late treatment of spinal cord compression with long-term intrathecal enzyme replacement therapy in canine mucopolysaccharidosis type I. Mol. Genet. Metab. 2010;101:115–122. doi: 10.1016/j.ymgme.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickson PI, Naylor D, Mlikotic A, Victoroff A, Chen A, Passage M, Le S, Collaborat MPSIR. Intrathecal recombinant human a-L-iduronidase alleviates spinal cord compression symptoms and is well-tolerated in attenuated MPS I patients. Mol. Genet. Metab. 2008;93:247–247. [Google Scholar]
- 39.Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, Mobley W, Dickson P, Hanson S, Passage M. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol. Genet. Metab. 2004;83:163–174. doi: 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Lund TC, Miller W, Polgreen L, Baso L, Raymond G, Tolar J, Orchard P. Improvement in biomarkers after intrathecal iduronidase for children with MPS IH. Mol. Genet. Metab. 2014;111:S74. [Google Scholar]
- 41.Vera M, Le S, Kan SH, Garban H, Naylor D, Mlikotic A, Kaitila I, Harmatz P, Chen A, Dickson P. Immune response to intrathecal enzyme replacement therapy in mucopolysaccharidosis I patients. Pediatr. Res. 2013;74:712–720. doi: 10.1038/pr.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.