Abstract
The lymphatic vascular system has been minimally explored in the liver despite its essential functions including maintenance of tissue fluid homeostasis. The discovery of specific markers for lymphatic endothelial cells has advanced the study of lymphatics by methods including imaging, cell isolation, and transgenic animal models and has resulted in rapid progress in lymphatic vascular research during the last decade. These studies have yielded concrete evidence that lymphatic vessel dysfunction plays an important role in the pathogenesis of many diseases. This article reviews the current knowledge of the structure, function, and markers of the hepatic lymphatic vascular system as well as factors associated with hepatic lymphangiogenesis and compares liver lymphatics with those in other tissues.
Keywords: VEGF, Inflammation, Cirrhosis, Portal Hypertension
Abbreviations used in this paper: CCl4, carbon tetrachloride; EHE, epithelioid hemangioendothelioma; HA, hyaluronan; HBx Ag, hepatitis B x antigen; HCC, hepatocellular carcinoma; IFN, interferon; IL, interleukin; LSEC, liver sinusoidal endothelial cell; LyEC, lymphatic endothelial cell; LYVE-1, lymphatic vessel endothelial hyaluronan receptor 1; mTOR, mammalian target of rapamycin; NO, nitric oxide; Prox1, prospero homeobox protein 1; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor
Summary.
Research on the lymphatic vascular system has advanced rapidly during the last decade, and lymphatic dysfunction is now implicated in the pathogenesis of multiple diseases. This review provides an overview of the lymphatic vascular system in the liver.
The lymphatic and blood vascular systems together constitute the circulatory system, and both have essential physiological activities. The lymphatic vascular system maintains tissue fluid homeostasis by collecting excess tissue fluid and returning it to the venous circulation. It also plays an essential role in the absorption and transport of dietary fat. Furthermore, lymphatics serve as the main conduits of antigens and antigen-presenting cells from the periphery to lymph nodes and are thus crucial for immune surveillance and acquired immunity.1, 2, 3, 4
Lymphatic vascular research was impeded by a lack of knowledge about the markers and signaling pathways specific to the lymphatic vasculature. From 1995 to 1997, however, it was shown that vascular endothelial growth factor receptor (VEGFR)-3 is expressed in the lymphatic endothelium and that its ligand vascular endothelial growth factor (VEGF)-C promotes lymphangiogenesis.5, 6 This finding identifying signaling pathways specific to the lymphatic vasculature and subsequent discoveries of other specific markers for lymphatic endothelial cells (LyECs), such as lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1),7 prospero homeobox protein 1 (Prox1),8 and podoplanin,9 significantly advanced lymphatic vascular research. As a consequence, it is now recognized that lymphatic vessel dysfunction plays an important role in the pathogenesis of various diseases.
However, in the liver, the lymphatic vascular system has been little explored. This review will provide an overview of the structure, function, and markers of the lymphatic vascular system as well as factors associated with lymphangiogenesis in the liver, highlighting both new findings and areas needing further study.
Structure of the Hepatic Lymphatic Vascular System
This section will address the structure of the lymphatic vascular system in general, followed by structural features specific to the liver. A detailed description of the anatomic structure of the lymphatic and hepatic lymphatic vascular systems is available in other review articles.3, 10, 11, 12
Anatomy of the Lymphatic Vascular System
Lymphatic capillaries
Lymphatic fluid originates from plasma components leaked from blood capillaries into the interstitium and then enters lymphatic capillaries, which are blind-ended, thin-walled vessels consisting of a single layer of LyECs. Lymphatic capillaries are not covered by pericytes or smooth muscle cells and lack basement membranes.13, 14 They are highly permeable, with discontinuous “button-like” junctions through which interstitial fluid, macromolecules, and immune cells can be transported.15 LyECs have anchoring filaments that are mainly composed of emilin-1 and fibrillin and bind LyECs to the surrounding extracellular matrix.14, 16, 17 These filaments keep lymphatic vessel lumens open, facilitating fluid intake in conditions of tissue swelling.
Collecting vessels
Lymphatic capillaries coalesce into collecting vessels, which are covered with smooth muscle cells and have basement membranes.14 Collecting vessels lack the discontinuous junctions typical of lymphatic capillaries and are thus much less permeable. Collecting vessels can be divided into smaller functional units called lymphangions that have unidirectional bicuspid valves at each end.18 The phasic contraction of smooth muscle cells covering lymphangions enables collecting vessels to act as pumps to drive lymphatic flow. Stimulation of smooth muscle cells causes depolarization of cell membrane and opens Ca2+ channels, resulting in Ca2+ influx and smooth muscle cell contraction. Smooth muscle cells also have stretch-activated Ca2+ channels that facilitate phasic contraction.19, 20 On the other hand, LyECs produce the vasodilator nitric oxide (NO) in response to shear stress from fluid flow, counteracting Ca2+-dependent contraction.21, 22 Spatiotemporal alterations of Ca2+ and NO levels are thereby believed to modulate the phasic contraction of lymphangions.23
Lymph nodes and lymph trunks
Collecting vessels connect to 1 or more lymph nodes. Antigen-presenting cells including dendritic cells and macrophages in lymphatic fluid interact with lymphocytes in lymph nodes, facilitating adaptive immune responses. After reaching primary lymph nodes, lymphatic fluid flows to secondary central lymph nodes, tertiary central lymph nodes, and finally lymph trunks.24 Lymphatic fluid from the left side of the body, abdomen, and lower limb ultimately drains into the thoracic duct, the largest lymphatic vessel, which is connected to the left subclavian vein (Figure 1), whereas lymphatic fluid from other parts of the body drains into the right lymph trunk, which is connected to the right subclavian vein.25 Lymphatic fluid that enters the subclavian veins returns to the systemic blood circulation.
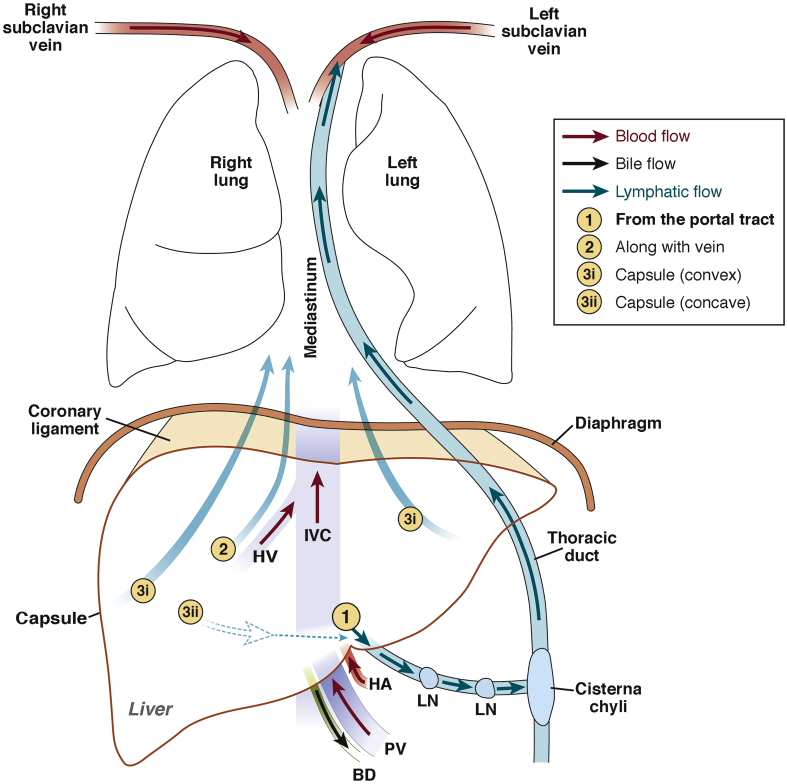
Figure 1.
Schematic diagram of macro-anatomy of hepatic lymphatic vascular system. (1) Lymphatic capillaries in the portal tract coalesce into collecting vessels, which drain to lymph nodes at the hepatic hilum and the lesser omentum. Efferent lymphatic vessels (LV) from these lymph nodes connect to celiac lymph nodes, which drain to the cisterna chyli, the enlarged origin of the thoracic duct. Lymphatic fluid through the thoracic duct drains to the left subclavicular vein and returns to the systemic blood circulation. (2) Lymphatic vessels along the central vein (CV) converge into large lymphatic vessels along the hepatic vein (HV), which then traverse along the inferior vena cava (IVC) through the diaphragm toward mediastinal lymph nodes. (3) Lymphatic fluid running underneath the capsule of the convex surface of the liver (3i) drains to mediastinal lymph nodes through the coronary ligament, whereas that of the concave surface (3ii) drains to lymph nodes of the hepatic hilum and regional lymph nodes. BD, bile duct; HA, hepatic artery; LN, lymph node; PV, portal vein.
Anatomy of the Hepatic Lymphatic Vascular System
A schematic diagram of the hepatic lymphatic system is shown in Figures 1 and 2. Unlike other tissues, the liver has sinusoids instead of capillaries.26 Sinusoids, similar to lymphatic capillaries, are distinct from blood capillaries in that they consist of 1 layer of liver sinusoidal endothelial cells (LSECs) and lack basement membranes. Hepatic lymphatic fluid is thought to originate from plasma components filtered through the fenestrae of LSECs into the space of Disse, the interstitial space between LSECs and hepatocytes.10, 11 Fluid in the space of Disse primarily flows through the space of Mall, a space between the stroma of the portal tract and the outermost hepatocytes,27 into the interstitium of the portal tract and then into lymphatic capillaries. Some portion of the fluid in the space of Disse flows into the interstitium around the central vein, which is located in the center of the liver acinus and connected to the hepatic vein,28 or underneath the hepatic capsule (Figure 2).
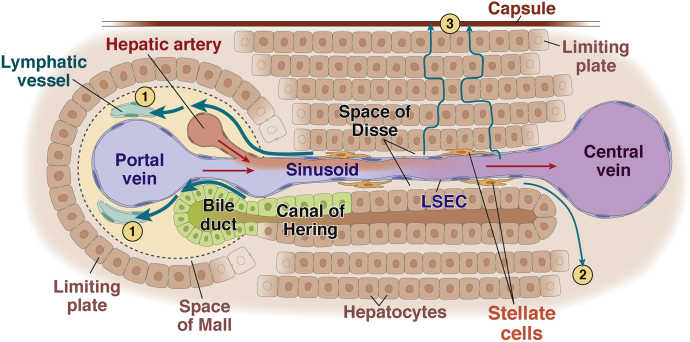
Figure 2.
Schematic diagram of the micro-anatomy of the hepatic lymphatic vascular system. Blood flow (red arrows) from the portal vein (PV) and hepatic artery (HA) enters the liver. Plasma components are filtered through LSECs into the space of Disse, the interstitial space between LSECs and hepatocytes, and are regarded as the source of lymphatic fluid. Lymphatic fluid in the space of Disse mostly flows through the space of Mall, the space between the stroma of the portal tract and the outermost hepatocytes, into the interstitium of the portal tract and then into lymphatic capillaries (1). Some portion of the lymphatic fluid in the space of Disse flows into the interstitium around the central vein (2) or underneath the hepatic capsule (3).
Lymphatic capillaries in the portal tract coalesce into collecting vessels and drain to lymph nodes at the hepatic hilum, whereas lymphatic vessels along the central vein converge into 5–6 large lymphatic vessels that traverse along the inferior vena cava through the diaphragm toward posterior mediastinal lymph nodes. Lymphatic fluid running underneath the capsule of the convex surface of the liver drains to mediastinal lymph nodes through the coronary ligament, whereas that fluid running along the concave surface drains to lymph nodes in the hepatic hilum and to regional lymph nodes (Figure 1).10, 11, 12, 29 On the basis of their locations, lymphatic vessels along the portal tract and the central vein are called the deep lymphatic system, and those along the hepatic capsule are called the superficial lymphatic system.10, 11, 12, 29
Markers of Lymphatic Vessels
Lymphatic vessel markers generally refer to those specific to LyECs. The markers LYVE-1,7, 30, 31 podoplanin,9, 32 Prox1,8, 33, 34, 35 and VEGFR-35 are most commonly used for microscopic imaging of lymphatic vessels.36 Identification of more specific markers for the liver is needed because the most common LyEC markers, LYVE-1 and Prox1, are also expressed in LSECs and hepatocytes, respectively. Table 1 summarizes LyEC markers histologically examined in the liver.
Table 1.
Lymphatic Markers
| Marker | Postnatal expression except for lymphatic vessels |
Hepatic expression in pathologic conditions | Reference | |
|---|---|---|---|---|
| Liver | Other organs/cells | |||
| LYVE-1 | Sinusoidal endothelial cells | A portion of macrophages, pulmonary capillaries, epididymal adipose tissue, mesentery, eye (cornea, sclera, choroid, iris, and retina), wounded skin, and malignant tumors (melanoma and insulinoma) | In chronic hepatitis and liver cirrhosis in humans, LYVE-1(+) lymphatic vessels increase, but LYVE-1(+) sinusoidal endothelial cells decrease. | 38, 40, 41, 42, 43, 105, 159, 160, 161, 162, 163, 164 |
| Prox1 | Hepatocytes | Adrenal medulla, megakaryocytes, and platelets | Intrahepatic CCC, ductular cells in cirrhotic livers, and HCC in humans. | 8, 52, 58, 59 |
| Podoplanin | Cholangiocytes | Inflammatory macrophages, mesothelial cells, cardiomyocytes, FRCs, follicular dendritic cells, TH17 cells, and osteoblasts | Podoplanin(+) lymphatic vessels increase in decompensated cirrhosis in humans. Podoplanin(+) FRCs increase in livers of primary biliary cirrhosis patients. EHE and angiomyolipoma in humans. | 32, 72, 75, 76, 77, 78, 79, 165, 166 |
| VEGFR-3 | Cholangiocytes | A portion of macrophages, proliferating blood vessels, and fenestrated capillaries in endocrine glands, choroid plexus, kidney, and small intestine | HBx Ag–positive HCC and hepatic progenitor cells in primary biliary cirrhosis in humans. | 80, 83, 84, 85, 102, 167, 168 |
| CCL21 | Sinusoidal endothelial cells | A portion of dendritic cells, HEVs of lymph nodes and Peyer’s patches, T-cell areas of spleen, lymph nodes, and Peyer’s patches | Lymphoid tissue in primary biliary cirrhosis and primary sclerosing cholangitis in humans. | 169, 170, 171 |
| MMR1 | Sinusoidal endothelial cells and Kupffer cells | A portion of macrophages, sinusoidal endothelial cells in bone marrow and spleen, perivascular microglia, and glomerular mesangial cells | Unknown | 172, 173, 174, 175 |
| Desmoplakin | Basolateral plasma membrane of hepatocytes and cholangiocytes | Esophagus, intestine, colon, salivary gland, mammary gland, sweat gland, thymus, and endocervix | Entire plasma membrane of HCC cells | 174, 176, 177, 178, 179 |
| Integrin α9 | Hepatocytes | Airway epithelial cells, keratinocytes, muscle cells (smooth/skeletal/cardiac), neutrophils, osteoclasts, and oocytes | Unknown | 174, 180 |
CCC, cholangiocellular carcinoma; CCL21, C-C motif chemokine ligand 21; FRC, fibroblastic reticular cell; HEV, high endothelial venules; MMR, macrophage mannose receptor 1.
Lymphatic vessel endothelial hyaluronan receptor 1
LYVE-1 is a lymphatic vessel endothelial hyaluronan (HA) receptor, a homolog of the CD44 HA receptor,7 that belongs to the superfamily of Link proteins containing a conserved HA-binding domain known as the Link module.37 Its structural features suggest that LYVE-1 may be involved in the transport of HA across the lymphatic endothelium. LYVE-1 is strongly expressed on the entire luminal and abluminal surfaces of LyECs, even on the fine filopodia of growing vessels during lymphangiogenesis.
No definite alterations in lymphatic vessel structure and function were reported for LYVE-1–/– mice.38 However, diphtheria toxin-induced LYVE-1 depletion in mice caused acute loss of lymphatic lacteals in intestinal villi and lymphatic vessels in systemic lymph nodes. These changes resulted in the structural distortion of blood capillaries and villi, leading to death due to sepsis within 60 hours after LYVE-1 depletion.39 These observations indicate that LYVE-1 plays an important role in the maintenance of the lymphatic vascular system, especially lacteals in intestinal villi and lymph nodes. Compensatory mechanisms in the setting of congenital loss of LYVE-1 may explain the relatively mild phenotype of these mice.
In the liver, LYVE-1 is expressed not only in LyECs but also in LSECs, as shown in mice40 and humans.40, 41, 42, 43 However, LYVE-1 positivity in LSECs was reported to diminish in inflamed human livers such as those of chronic hepatitis and cirrhosis.40, 42 Expression levels of LYVE-1 in human hepatocellular carcinoma (HCC) negatively correlated with the overall survival of patients.44
Prospero homeobox protein 1
Prox1, a homolog of the Drosophila melanogaster homeobox gene prospero, is a transcription factor and regulates genes related to LyECs, including VEGFR-3 and podoplanin.45, 46 Prox1 is essential for the development of the lymphatic vascular system8 and also plays a role in the development of other tissues, including the lens,34, 47 retina,48 heart,49 central nervous system,50 pancreas,51 and liver.52, 53 Prox1 is expressed in the nucleus in contrast to other lymphatic markers that are expressed in the cytoplasm or on the plasma membrane.
Prox1 is essential for budding of lymphatic endothelial sacs8; Prox1–/– mice lack a lymphatic vascular system and die at approximately E14.5. Prox1 heterozygote mice die a few days after their birth and demonstrate dysfunction of lymphatic vessels with chylous ascites.8, 33, 47 Several lines of Prox1 promoter-directed reporter mice have recently been established as research tools (GFP,54 mOrange,55 and tdTomato56, 57, 58).
In the early endoderm, Prox1 expression is restricted to the primordia of the liver and pancreas.51 Prox1 regulates hepatocyte migration during liver morphogenesis51 and is expressed in postnatal hepatocytes, although not in postnatal pancreas.52 In humans, cholangiocytes of normal livers were negative for Prox1 expression, but intrahepatic cholangiocarcinoma and ductular cells in fibrotic septa of cirrhotic livers and HCC were positive.59 In addition, expression levels of Prox1 (like LYVE1) in human HCC negatively correlated with the overall survival of patients.60 Prox1 acts together with nuclear receptors, such as hepatocyte nuclear factor 4α,61 estrogen-related receptor α,62, 63 liver receptor homolog-1,64 and retinoic acid-related orphan receptors α/γ,65 and regulates bile acid synthesis64 and circadian metabolism in the liver.63, 65
Podoplanin
Podoplanin is a type I transmembrane glycoprotein essential for the development of the heart,66, 67, 68, 69 lung,70 spleen, and lymph nodes.71 Its expression is regulated by Prox1.45 Podoplanin is also a ligand of C-type lectin receptor CLEC-2, which is highly expressed in platelets and immune cells and promotes platelet aggregation and activation.72
Podoplanin–/– mice die at birth as a result of respiratory failure. These mice have congenital lymphedema caused by lymphatic vessel defects, although blood vessel formation is normal.32 Podoplanin heterozygote mice are healthy and fertile, with a partial incomplete lymphatic vessel network.32 Keratinocyte-specific podoplanin-deficient mice73 and a tamoxifen-inducible podoplanin depletion mouse model (Pdpnf/f, CagCre)74 have recently been developed.
Histologic analysis of normal mouse livers showed expression of podoplanin in cholangiocytes in addition to LyECs.75 In humans, podoplanin-positive lymphatic vessels were increased in the livers of patients with decompensated cirrhosis,76 and podoplanin-positive fibroblastic reticular cells were increased in livers of patients with primary biliary cirrhosis.77 Podoplanin has proven to be a useful histologic marker for diagnosing patients who have vascular tumors with lymphatic differentiation, such as epithelioid hemangioendotheliomas (EHEs)78 and angiomyolipomas.79
Vascular endothelial growth factor receptor
VEGFR-3 is a membrane-anchored tyrosine kinase and the receptor for VEGF-C and VEGF-D. It plays a crucial role in lymphangiogenesis. In early embryogenesis before LyEC differentiation, VEGFR-3 is expressed in most endothelial cells, but in the later stages of development, its expression becomes mostly restricted to the lymphatic endothelium.5
VEGFR-3–/– mice have lymphatic vessel defects and die at approximately E10.5,80 whereas VEGFR-3 heterozygous mice present with leaky lymphatic vessels and transient chylous ascites.80, 81 A mouse line (Vegfr3EGFPLuc) in which a dual reporter for fluorescence and luminescence is expressed under VEGFR-3-promoter was established recently, enabling luminescence imaging of tumor-induced lymphangiogenesis.82
VEGFR-3 is expressed by cholangiocytes in normal rat livers and is increased in cholestatic rat livers after bile duct ligation.83 Hepatic progenitor cells were also found to express VEGFR-3 in patients with primary biliary cirrhosis.84 Hepatitis B x antigen (HBx Ag), one of the antigens of hepatitis B virus, promotes hepatocarcinogenesis by upregulating expression of genes associated with proliferation of hepatocytes; upregulation of VEGFR-3 expression was observed in HBx Ag–positive human HCC, and the prognosis of patients with VEGFR-3–positive HCC was worse than for those with VEGFR-3–negative HCC.85
Lymphangiogenesis
This section addresses the mechanism of lymphangiogenesis in the postnatal stage and factors that affect lymphangiogenesis, including inflammatory cells, in the lymphatic system in general and then summarizes the implications of lymphangiogenesis in the pathophysiology of liver diseases.
Factors Associated With Lymphangiogenesis
In the postnatal stage, lymphatic vessels are mostly quiescent, and lymphangiogenesis generally occurs in pathologic conditions such as tissue repair, inflammation, and tumor-related conditions.86 Many cytokines and growth factors have been reported to promote lymphangiogenesis or inhibit lymphangiogenesis, as summarized in Table 2. The extent and duration of lymphangiogenesis are determined by the balance between pro- and anti- lymphangiogenic factors.87, 88
Table 2.
Lymphangiogenic and Anti-lymphangiogenic Factors
| Experimental model | Remarks | Reference | |
|---|---|---|---|
| Lymphangiogenic factors | |||
| VEGF-A | Mouse corneal lymphangiogenesis | VEGF-A recruits macrophages, which promote lymphangiogenesis by secreting VEGF-C/VEGF-D. | 103 |
| Mouse subcutaneous immunization model | VEGF-A expression is upregulated concomitantly with lymphangiogenesis in LNs of immunized mice. | 117 | |
| Oxazolone sensitized delayed-type hypersensitivity in mouse ear | Systemic blockade of VEGF-A attenuates lymphangiogenesis in draining LNs. | 181 | |
| HSV-1 infection of cornea | HSV-1 causes lymphangiogenesis by promoting infected cells to secrete VEGF-A. | 182 | |
| VEGF-C, VEGF-D | VEGF-C transgenic mouse | VEGF-C promotes LyEC proliferation and LV enlargement in the skin. | 6 |
| Isolated LyEC | VEGF-C stimulates survival, growth, and migration of LyEC. | 91 | |
| FGF-2–induced corneal lymphangiogenesis | VEGFR-3 blockade cancels lymphangiogenesis. | 183 | |
| Chronic airway inflammation | VEGFR-3 blockade cancels lymphangiogenesis. | 184 | |
| LPS-induced peritonitis | VEGF-C and VEGF-D promote lymphangiogenesis in diaphragm. | 185 | |
| Ang 2 | Mouse corneal lymphangiogenesis | Ang 2 is upregulated in inflamed cornea, and Ang2 blockade inhibits inflammatory lymphangiogenesis. | 186 |
| Mouse corneal lymphangiogenesis | Ang 2 is expressed in lymphatic vessels and macrophages in inflamed cornea. Inflammatory lymphangiogenesis of cornea is suppressed in Ang2 knockout mice. Ang2 blockade inhibits LyEC proliferation and capillary tube formation. | 187 | |
| HGF | Canine primary LyEC, rat tail lymphedema | HGF promotes proliferation and migration of LyEC. Weekly HGF gene transfer improves lymphedema in vivo. | 188 |
| LT | CCL21 transgenic mouse, RAG knockout mouse defective in T and B cell | LT overexpression by CCL21 transgene promotes lymphangiogenesis in thyroid. T-cell depletion cancels this phenomenon. | 189 |
| LTα knockout mouse, LTα transgenic mouse | LTα gene deletion decreases LV. Ectopic LTα expression causes lymphangiogenesis in tertiary lymphoid organs. | 190 | |
| IL1β | Mouse corneal lymphangiogenesis | IL1β promotes lymphangiogenesis by upregulating expression of VEGF-A, VEGF-C, and VEGF-D. | 191 |
| IL7 | Breast cancer cell lines, subcutaneous injection of Matrigel and/or IL7 and/or breast cancer cell lines | IL7 promotes VEGF-D expression of cell lines in vitro and promotes lymphangiogenesis in vivo. | 192 |
| HECV cell line (originated from human umbilical cord), subcutaneous injection of Matrigel and/or IL7 and/or HECV cell | IL7 promotes expression of Prox1, LYVE-1, and podoplanin and proliferation, migration, and tubular formation of LyEC via upregulation of VEGF-D. | 193 | |
| IL8 | Human primary LyEC, IL8 transgenic mouse and Prox1-GFP mouse | IL8 promotes proliferation, migration, and tube formation of LyEC. IL8 overexpression promotes lymphangiogenesis in vivo. | 194 |
| IL17 | Cornea micro pocket assay, autoimmune ocular disease mouse | IL17 promotes proliferation of LyEC via upregulation of VEGF-D. Blockade of IL17 decreases corneal lymphangiogenesis. | 195 |
| IL20 | Human telomerase-transfected dermal LyEC | IL20 promotes proliferation, migration, and tubular formation of LyEC via PI3K and mTOR pathways. | 196 |
| Anti-lymphangiogenic factors | |||
| TGF-β | Human dermal lymphatic microvascular endothelial cells | TGF-β inhibits LyEC proliferation, cord formation, migration, expression of lymphatic markers (LYVE-1, Prox1), and lymphangiogenesis by VEGF-A/C via TGF-β type I receptor. | 197 |
| Mouse tail skin excision and lymphatic vessel ligation | TGF-β1 inhibition promotes lymphatic vessel regeneration. TGF-β1 inhibits LyEC proliferation and fibrosis. | 198 | |
| Biopsy specimens from limbs of secondary lymphedema patients and mouse tail skin excision | TGF-β1 positive cells increase 3-fold in human lymphedema specimens. TGF-β1 inhibition decreases fibrosis, increases lymphangiogenesis and lymphatic function. | 199 | |
| BMP2 | Zebrafish BMP2 transgenic model | BMP2 inhibits LyEC differentiation from cardinal veins via inhibition of Prox1 expression. | 200 |
| IFN-α, IFN-γ | LyEC isolated from pig thoracic duct | IFN-α or IFN-γ decreases LyEC proliferation and migration. Treatment with both IFN-α and IFN-γ promotes LyEC apoptosis. | 201 |
| Cervical LNs of T-cell–deprived mouse | T cells inhibit lymphangiogenesis in LNs by secreting IFN-γ. | 116 | |
| IL4, IL13 | Mouse LyEC isolated from LNs, human dermal LyEC, mouse asthma model | IL4 and IL13 inhibit expression of Prox1 and LYVE-1 and tube formation of LyEC. Blockade of IL4 and/or IL13 increases the density and function of lung LVs in asthma model. | 202 |
| IL27 | Human dermal lymphatic microvascular endothelial cells | IL27 inhibits LyEC proliferation and migration via STAT1/CXCL10, CXCL-11 axis. | 203 |
| Activin A | Subcutaneous injection of melanoma cell line to mouse | Activin A reduces lymphangiogenesis in melanoma model and inhibits sprouting of LyEC via phosphorylation of SMAD2. | 204 |
FGF-2, fibroblast growth factors-2; HGF, hepatocyte growth factor; HSV-1, herpes simplex virus 1; LN, lymph node; LPS, lipopolysaccharide; LT, lymphotoxin; LV, lymphatic vessel; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; STAT, signal transducer and activator of transcription; TGF, tumor growth factor.
Intracellular Signaling Pathways in Lymphangiogenesis
Signaling pathways in lymphangiogenesis have largely been determined in studies of developmental lymphangiogenesis. Signaling via VEGF-C/D and VEGFR-3 is the most well-known pathway for lymphangiogenesis (Figure 3).6 VEGF-C or VEGF-D binding to VEGFR-3 results in autophosphorylation of multiple C-terminal tyrosine residues in VEGFR-3,89 which transduces signals through the Ras/Raf/MEK/ERK pathway.90 Signal transduction also occurs through the PI3K/Akt pathway,91 which causes phosphorylation of Akt, thereby activating mammalian target of rapamycin (mTOR) and Rac1.92 Activation of these signaling pathways facilitates LyEC proliferation and migration, ie, lymphangiogenesis.91 Chronic inflammation and malignant tumors in the liver induce several pro-lymphangiogenic growth factors including VEGF-C/D. However, a direct link between these increased pro-lymphangiogenic growth factors and lymphangiogenesis in these pathologic conditions remains to be demonstrated (Figure 3). Excellent review articles are available detailing signaling pathways in lymphangiogenesis.93, 94, 95
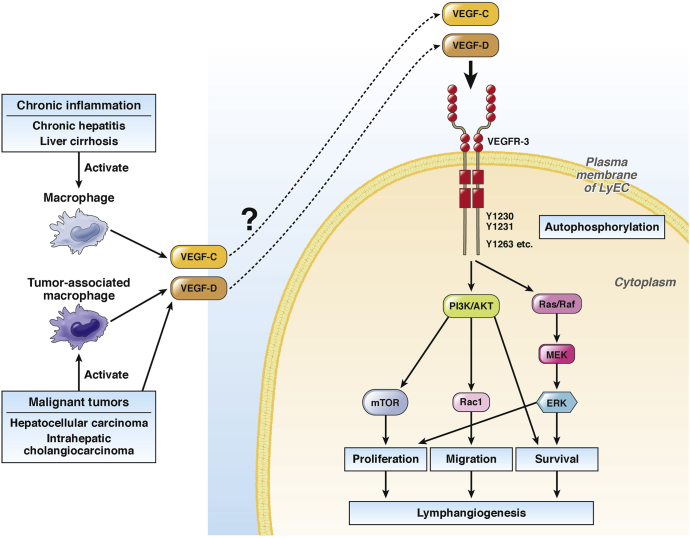
Figure 3.
Intracellular signaling pathways in lymphangiogenesis. Signaling via VEGF-C/D and VEGFR-3 is the most well-known pathway for lymphangiogenesis. VEGF-C or VEGF-D binds to its receptor VEGFR-3 in the plasma membrane of LyECs, which facilitates signal transduction through various intracellular signaling pathways, leading to lymphangiogenesis. In the liver, activated macrophages in chronic inflammatory conditions, such as chronic hepatitis and liver cirrhosis, secrete VEGF-C and/or VEGF-D. Malignant liver tumors, such as HCC and intrahepatic cholangiocarcinoma, also secrete VEGF-C and/or VEGF-D. Furthermore, these malignant tumors activate tumor-associated macrophages, which also secrete VEGF-C and/or VEGF-D. Secreted VEGF-C and VEGF-D are likely related to lymphangiogenesis in liver diseases through VEGFR-3–mediated pathways.
Role of Immune Cells
Adaptive immune responses are initiated by the migration of immune cells to inflamed sites where they phagocytose pathogens and transmigrate through lymphatic vessels to lymph nodes to present antigens to T cells. However, immune cells not only migrate through lymphatic vessels but also interact with lymphatic vessels and promote lymphangiogenesis.96 An increase in lymphatic vessels helps infiltrating immune cells exit inflamed sites via lymphatic vessels and accelerates resolution of inflammation.97, 98, 99
Macrophages
Among the various immune cells, macrophages interact most with lymphatic vessels. LyECs secrete chemotactic factors, such as C10, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1, to attract macrophages.100 Macrophages in turn secrete lymphangiogenic cytokines such as VEGF-C, VEGF-D, and VEGF-A,101 which promote tumor-associated lymphangiogenesis102 and inflammation-induced lymphangiogenesis, as shown in the cornea,103 skin,98 and tail.104 Macrophages were recently suggested to have the ability to transdifferentiate to LyECs.105, 106, 107 However, this is controversial and requires further investigation.
Dendritic cells
Upregulation of inflammatory cytokines such as tumor necrosis factor-α and interleukin (IL) 1β in inflamed tissues promotes expression of chemokines (eg, CCL21/CCL19 and CXCL12) and their receptors (eg, CCR7 and CXCR-4) in LyECs and dendritic cells,108, 109, 110, 111 which enhances transmigration of dendritic cells through LyECs.112, 113 Inflammatory cytokines also increase expression of adhesion molecules such as intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin in LyECs and promote dendritic cell transmigration to lymphatic vessels.114 Dendritic cells have also been reported to secrete VEGF-C and promote lymphangiogenesis.115
T cells
In a mouse model of tail lymphedema, nude mice exhibited less edema than wild-type mice, concomitant with decreased lymphangiogenic cytokines and increased anti-lymphangiogenic cytokines. The balance between these cytokines was modulated by T-cell–mediated inflammation.88 T cells negatively regulated lymph node lymphangiogenesis by secreting interferon (IFN)-γ in mice.116
B cells
B cells promote lymphangiogenesis in inflamed lymph nodes by secreting a robust amount of VEGF-A in mice given keyhole-limpet hemocyanin emulsified in complete Freund’s adjuvant (an experimental model of inflamed lymph nodes).117 Interestingly, VEGF-C was not detected in this study. Another study that used transgenic mice overexpressing VEGF-A specifically in B cells showed increased lymphangiogenesis as well as angiogenesis.118
Neutrophils
Neutrophils are reported to contribute to lymphangiogenesis by modulating the bioavailability and bioactivity of VEGF-A and by secreting VEGF-D.119 The bioavailability of VEGF-A is increased by the secretion of matrix metalloproteinases 9 and heparanase. Depletion of neutrophils in mice resulted in skin inflammation in response to immunization or contact hypersensitization, and lymphangiogenesis was decreased in these mice with increased local inflammation, suggesting that neutrophils play a role in lymphangiogenesis and that lymphangiogenesis is helpful for reducing inflammation.
Lymphangiogenesis in the Liver
Because 25%–50% of lymph passing through the thoracic duct originates in the liver,1, 120 the liver can be considered the most important organ for lymphatic fluid production. However, the lymphatic vascular system in the liver has been minimally explored. A small number of studies have reported on hepatic lymphangiogenesis in pathologic conditions such as chronic hepatitis, liver fibrosis/cirrhosis, portal hypertension, malignant tumors, and post-transplantation. This section summarizes these studies.
Chronic hepatitis, liver fibrosis, and cirrhosis
Resistance to sinusoidal blood flow increases in cirrhotic livers because of architectural deformations including around the portal and central venules. Consequently, sinusoidal hydrostatic pressure is elevated, and plasma components filtrated through sinusoids (which form lymphatic fluid) increase. In cirrhotic patients, lymphatic fluid produced in the liver increases up to 30-fold,121, 122, 123, 124, 125 and liver surface lymphatic vessels dilate, as shown by peritoneoscopic observation.126
Ascites formation in association with cirrhosis is one of the most recognized clinical manifestations of lymphatic vascular disorders. How ascites is formed still remains to be elucidated. Although several theories have been put forward,127, 128, 129 the most accepted currently is “the peripheral arterial vasodilation theory”, also known as “the forward theory”.130, 131, 132 According to this theory, splanchnic arterial vasodilation caused by portal hypertension results in underfilling of the splanchnic arterial circulation (hypovolemia). In moderate stages, the hypovolemia is compensated for by renal retention of sodium and water. However, in severe portal hypertension with splanchnic arterial vasodilation, sodium and water retention is persistent and leads to leakage of fluid into the peritoneal cavity. When its amount exceeds the absorption capacity of lymphatic vessels, ascites results.129, 133
On a related note, impaired lymphatic drainage in the splanchnic and peripheral regions was reported in cirrhotic rats with ascites. This was accompanied by increased activity of endothelial NO synthase and production of NO by LyECs in these regions.134 In addition, smooth muscle cell coverage of lymphatic vessels in these regions was significantly decreased. Treatment of these cirrhotic rats with an NO synthase inhibitor improved lymphatic drainage, decreased ascites volume, and increased smooth muscle cell coverage. This study thus demonstrates a role for NO in the impairment of lymphatic vessels in splanchnic and peripheral regions and in the development of ascites. It is not known whether lymphatic vessels in human cirrhotic livers show similar pathologic features.
The occurrence of hepatic lymphangiogenesis was reported for the first time in liver fibrosis and cirrhosis by Vollmar et al135 in 1997. They found lymphatic vessels to be increased and enlarged in rat liver cirrhosis induced by carbon tetrachloride (CCl4). These observations were confirmed the following year in patients with chronic viral hepatitis/cirrhosis.136
Microarray analysis demonstrated a 4-fold increase in VEGF-D expression in endothelial cells from CCl4-induced cirrhotic rat livers as compared with control rat livers. Because VEGF-D is a well-known lymphangiogenic factor that binds to VEGFR-3,137 which is also highly expressed in the LyECs of these cirrhotic rats,5 increased VEGF-D could be associated with the lymphangiogenesis observed in liver cirrhosis (Figure 3).
Lymphangiogenesis also occurs in idiopathic portal hypertension in human patients.138 It was presumed that increased lymph production that was due to increased portal pressure caused lymphangiogenesis. In 2 rat models of portal hypertension (portacaval shunt and portal vein ligation), upregulation of Vegfr-3 expression was observed, leading us to speculate the occurrence of lymphangiogenesis.139 However, the significance and mechanism of hepatic lymphangiogenesis, including in chronic hepatitis and liver fibrosis and cirrhosis, remain unknown.
Malignant tumors
Lymphatic vessels play a pivotal role in the pathogenesis of malignant tumors by serving as a pathway through which tumor cells metastasize. The incidence of lymph node metastasis differs among tumors. For example, it is 5.1% in HCC and 45.1% in intrahepatic cholangiocarcinoma. The prognosis of tumor-bearing patients with lymph node metastasis is worse than in cases without such metastasis.140, 141 Many malignant tumors secrete lymphangiogenic factors such as VEGF-C and VEGF-D and promote lymphangiogenesis in their adjacent tissues, which helps tumor cells to metastasize to lymph nodes,142 and many studies have demonstrated that tumor-associated macrophages play a vital role in lymphangiogenesis in malignant tumors by secreting VEGF-C and VEGF-D.102, 143, 144, 145 In intrahepatic cholangiocarcinoma, the lymphatic vessel density of surgically resected tumors was positively correlated with the incidence of lymphatic metastasis.146 In HCC, VEGF-C expression was positively correlated with the size of tumors and the number of extrahepatic metastases and was negatively correlated with disease-free survival time.147 Thus, blockade of VEGF-C may be a potential therapeutic strategy against malignant tumors. A VEGF-C neutralizing antibody (VGX-100) is now the subject of a Phase I clinical trial for adult patients with advanced or metastatic solid tumors (NCT01514123).148
Post-transplant lymphangiogenesis
In solid organ transplants, lymphatic vessel connections between the graft and the recipient are interrupted. Because lymphatic vessels are essential for adaptive immunity, the association between lymphangiogenesis and graft rejection has received considerable attention. Post-transplant lymphangiogenesis in grafts was associated with acute cellular graft rejection in transplants of various organs (kidney,149, 150, 151 heart,152 and lung153) in humans. However, the pathologic role of post-transplant lymphangiogenesis in graft rejection remains unclear.151 Post-transplant lymphangiogenesis could be detrimental if newly formed lymphatic vessels promote antigen presentation in draining lymph nodes and provoke alloimmune responses that result in graft rejection. On the other hand, these newly formed lymphatic vessels could be beneficial if they efficiently clear immune cells. In a rat model of liver transplantation, post-transplant lymphangiogenesis in grafts was associated with long-term survival of recipients for more than 90 days.154 In addition, rats that had failed grafting by 11 days with acute cellular rejection and antibody-mediated rejection showed disappearance of lymphatic vessels from severely rejected areas, suggesting that lymphatic vessels have an important role in mitigation of inflammation at least in the early stage of transplantation. Further investigations to determine the mechanism and the time course of clearance of infiltrating immune cells by lymphatic vessels, especially in the early post-transplant period, may help increase transplant success.
Conclusions and Perspective
The lymphatic vascular system has been poorly studied in the liver. To drive research in this area, it is essential to identify better markers for LyECs that do not overlap with markers for LSECs, hepatocytes, and other liver cells. The development of experimental models for studying the lymphatic vascular system in postnatal livers will be important in examining its role and molecular mechanisms in physiological and pathophysiological conditions. Although this field is wide open, it may be helpful to identify specific questions particularly in need of study.
First, the mechanism of hepatic lymphangiogenesis is largely unknown. The VEGF-C/VEGFR-3 axis is considered the most potent signaling pathway that regulates lymphangiogenesis in other organs.95 However, cellular sources of VEGF-C and VEGFR-3 have not been fully identified in the liver. Furthermore, as shown in Table 2, many other molecules are reported to regulate lymphangiogenesis. These molecules are mostly observed in the liver in physiological and pathophysiological conditions. It would be worth characterizing these molecules in relation to hepatic lymphangiogenesis.
Second, although the relationship between the lymphatic vascular system and metastasis is well-known and the growth of lymphatic capillaries in liver tumors has been observed, the role of lymphatic capillary growth in the development and progression of liver tumors is largely unknown. As for angiogenesis, it would be interesting to investigate lymphangiogenesis in liver cancer.
Third, inflammation is closely related to the development of many liver diseases, and infiltrating immune cells are drained to lymphatic vessels. Thus, it would be interesting to examine lymphangiogenesis in relation to inflammation in the liver. It is also unknown how immune cells recognize lymphatic vessels at the time of migration. Elucidation of these mechanisms may help in the development of anti-inflammatory strategies that facilitate immune cell clearance.
Fourth, although LyECs are derived from cardinal veins8, 81 and LSECs are derived from the septum transversum,155 LyECs and LSECs have many similarities. Both LyECs and LSECs express LYVE-1.40, 41, 42, 43 VAP-1, a type II transmembrane protein that supports leukocyte adhesion, and reelin, a glycoprotein that is associated with embryonic development, are also expressed in both LyECs and LSECs.156, 157 Furthermore, under normal conditions, neither LyECs nor LSECs are associated with basement membranes. Examining the similarities and differences between these 2 types of endothelial cells could help to understand endothelial cell–related liver function.
In summary, the lymphatic vascular system in the liver is a large open area for investigation.158 More research will significantly advance our understanding of liver physiology and pathophysiology and in turn contribute to the development of new therapeutic strategies for many liver diseases.
Acknowledgments
The authors thank Dr Teruo Utsumi for his careful review of the manuscript and helpful suggestions.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by National Institutes of Health grants R01 DK082600 and R21AA023599 and Connecticut DPH grant #2015-0901 (Y.I.) and a research fellowship of the Uehara Memorial Foundation and a grant-in-aid of the International Research Fund for Subsidy of Kyushu University School of Medicine Alumni (M.T.).
References
- 1.Chung C., Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol. 2013;19:99–104. doi: 10.3350/cmh.2013.19.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tammela T., Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 3.Schulte-Merker S., Sabine A., Petrova T.V. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607–618. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koltowska K., Betterman K.L., Harvey N.L. Getting out and about: the emergence and morphogenesis of the vertebrate lymphatic vasculature. Development. 2013;140:1857–1870. doi: 10.1242/dev.089565. [DOI] [PubMed] [Google Scholar]
- 5.Kaipainen A., Korhonen J., Mustonen T. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeltsch M., Kaipainen A., Joukov V. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science (New York, NY) 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 7.Banerji S., Ni J., Wang S.X. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wigle J.T., Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 9.Breiteneder-Geleff S., Soleiman A., Kowalski H. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trutmann M., Sasse D. The lymphatics of the liver. Anat Embryol (Berl) 1994;190:201–209. doi: 10.1007/BF00234299. [DOI] [PubMed] [Google Scholar]
- 11.Ohtani O., Ohtani Y. Lymph circulation in the liver. Anat Rec. 2008;291:643–652. doi: 10.1002/ar.20681. [DOI] [PubMed] [Google Scholar]
- 12.Pupulim L.F., Vilgrain V., Ronot M. Hepatic lymphatics: anatomy and related diseases. Abdom Imaging. 2015;40:1997–2011. doi: 10.1007/s00261-015-0350-y. [DOI] [PubMed] [Google Scholar]
- 13.Alitalo K., Tammela T., Petrova T.V. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 14.Maby-El Hajjami H., Petrova T.V. Developmental and pathological lymphangiogenesis: from models to human disease. Histochem Cell Biol. 2008;130:1063–1078. doi: 10.1007/s00418-008-0525-5. [DOI] [PubMed] [Google Scholar]
- 15.Baluk P., Fuxe J., Hashizume H. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danussi C., Spessotto P., Petrucco A. Emilin1 deficiency causes structural and functional defects of lymphatic vasculature. Mol Cell Biol. 2008;28:4026–4039. doi: 10.1128/MCB.02062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solito R., Alessandrini C., Fruschelli M. An immunological correlation between the anchoring filaments of initial lymph vessels and the neighboring elastic fibers: a unified morphofunctional concept. Lymphology. 1997;30:194–202. [PubMed] [Google Scholar]
- 18.Breslin J.W. Mechanical forces and lymphatic transport. Microvasc Res. 2014;96:46–54. doi: 10.1016/j.mvr.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirasawa Y., Benoit J.N. Stretch-induced calcium sensitization of rat lymphatic smooth muscle. Am J Physiol Heart Circ Physiol. 2003;285:H2573–H2577. doi: 10.1152/ajpheart.00002.2003. [DOI] [PubMed] [Google Scholar]
- 20.Davis M.J., Scallan J.P., Wolpers J.H. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol. 2012;303:H795–H808. doi: 10.1152/ajpheart.01097.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohlen H.G., Gasheva O.Y., Zawieja D.C. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol. 2011;301:H1897–H1906. doi: 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shyy J.Y., Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 23.Kunert C., Baish J.W., Liao S. Mechanobiological oscillators control lymph flow. Proc Natl Acad Sci U S A. 2015;112:10938–10943. doi: 10.1073/pnas.1508330112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forster R., Braun A., Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 2012;33:271–280. doi: 10.1016/j.it.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Jeltsch M., Tammela T., Alitalo K. Genesis and pathogenesis of lymphatic vessels. Cell Tissue Res. 2003;314:69–84. doi: 10.1007/s00441-003-0777-2. [DOI] [PubMed] [Google Scholar]
- 26.Wake K., Sato T. “The sinusoid” in the liver: lessons learned from the original definition by Charles Sedgwick Minot (1900) Anat Rec (Hoboken) 2015;298:2071–2080. doi: 10.1002/ar.23263. [DOI] [PubMed] [Google Scholar]
- 27.Mall F.P. A study of the structural unit of the liver. American Journal of Anatomy. 1906;5:227–308. [Google Scholar]
- 28.Munoz S.J., Fenkel J.M., Kiley K. The liver in circulatory failure. In: Schiff E.R., Maddrey W.C., Sorrell M.F., editors. Schiff's diseases of the liver. Wiley-Blackwell; Hoboken: 2011. pp. 924–933. [Google Scholar]
- 29.Ross M.H. 3rd ed. Lippincott Williams and Wilkins; Philadelphia: 1995. Histology: a text and atlas. [Google Scholar]
- 30.Prevo R., Banerji S., Ferguson D.J. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 31.Jackson D.G. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526–538. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- 32.Schacht V., Ramirez M.I., Hong Y.-K. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wigle J.T., Harvey N., Detmar M. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncan M.K., Cui W., Oh D.-J. Prox1 is differentially localized during lens development. Mech Dev. 2002;112:195–198. doi: 10.1016/s0925-4773(01)00645-1. [DOI] [PubMed] [Google Scholar]
- 35.Wilting J., Papoutsi M., Christ B. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J. 2002;16:1271–1273. doi: 10.1096/fj.01-1010fje. [DOI] [PubMed] [Google Scholar]
- 36.Baluk P., McDonald D.M. Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann N Y Acad Sci. 2008;1131:1–12. doi: 10.1196/annals.1413.001. [DOI] [PubMed] [Google Scholar]
- 37.Neame P.J., Barry F.P. The link proteins. Experientia. 1993;49:393–402. doi: 10.1007/BF01923584. [DOI] [PubMed] [Google Scholar]
- 38.Gale N.W., Prevo R., Espinosa J. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang J.Y., Koh Y.J., Lee S.H. Conditional ablation of LYVE-1+ cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood. 2013;122:2151–2161. doi: 10.1182/blood-2013-01-478941. [DOI] [PubMed] [Google Scholar]
- 40.Mouta Carreira C., Nasser S.M., di Tomaso E. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- 41.Lalor P.F., Lai W.K., Curbishley S.M. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J Gastroenterol. 2006;12:5429–5439. doi: 10.3748/wjg.v12.i34.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arimoto J., Ikura Y., Suekane T. Expression of LYVE-1 in sinusoidal endothelium is reduced in chronically inflamed human livers. J Gastroenterol. 2010;45:317–325. doi: 10.1007/s00535-009-0152-5. [DOI] [PubMed] [Google Scholar]
- 43.Nonaka H., Tanaka M., Suzuki K. Development of murine hepatic sinusoidal endothelial cells characterized by the expression of hyaluronan receptors. Dev Dyn. 2007;236:2258–2267. doi: 10.1002/dvdy.21227. [DOI] [PubMed] [Google Scholar]
- 44.Kitagawa K., Nakajima G., Kuramochi H. Lymphatic vessel endothelial hyaluronan receptor-1 is a novel prognostic indicator for human hepatocellular carcinoma. Mol Clin Oncol. 2013;1:1039–1048. doi: 10.3892/mco.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong Y.K., Harvey N., Noh Y.H. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 46.Ordonez N.G. Immunohistochemical endothelial markers: a review. Adv Anat Pathol. 2012;19:281–295. doi: 10.1097/PAP.0b013e3182691c2a. [DOI] [PubMed] [Google Scholar]
- 47.Wigle J.T., Chowdhury K., Gruss P. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- 48.Dyer M.A., Livesey F.J., Cepko C.L. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- 49.Risebro C.A., Searles R.G., Melville A.A. Prox1 maintains muscle structure and growth in the developing heart. Development. 2009;136:495–505. doi: 10.1242/dev.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lavado A., Oliver G. Prox1 expression patterns in the developing and adult murine brain. Dev Dyn. 2007;236:518–524. doi: 10.1002/dvdy.21024. [DOI] [PubMed] [Google Scholar]
- 51.Burke Z., Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev. 2002;118:147–155. doi: 10.1016/s0925-4773(02)00240-x. [DOI] [PubMed] [Google Scholar]
- 52.Dudas J., Elmaouhoub A., Mansuroglu T. Prospero-related homeobox 1 (Prox1) is a stable hepatocyte marker during liver development, injury and regeneration, and is absent from “oval cells”. Histochem Cell Biol. 2006;126:549–562. doi: 10.1007/s00418-006-0191-4. [DOI] [PubMed] [Google Scholar]
- 53.Sosa-Pineda B., Wigle J.T., Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Genet. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- 54.Choi I., Chung H.K., Ramu S. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood. 2011;117:362–365. doi: 10.1182/blood-2010-07-298562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagerling R., Pollmann C., Kremer L. Intravital two-photon microscopy of lymphatic vessel development and function using a transgenic Prox1 promoter-directed mOrange2 reporter mouse. Biochem Soc Trans. 2011;39:1674–1681. doi: 10.1042/BST20110722. [DOI] [PubMed] [Google Scholar]
- 56.Bianchi R., Teijeira A., Proulx S.T. A transgenic Prox1-Cre-tdTomato reporter mouse for lymphatic vessel research. PLoS One. 2015;10:e0122976. doi: 10.1371/journal.pone.0122976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Truman L.A., A-Gonzalez N., Bentley K.L. Lymphatic vessel function in head and neck inflammation. Lymphat Res Biol. 2013;11:187–192. doi: 10.1089/lrb.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Truman L.A., Bentley K.L., Smith E.C. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am J Pathol. 2012;180:1715–1725. doi: 10.1016/j.ajpath.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dudas J., Mansuroglu T., Moriconi F. Altered regulation of Prox1-gene-expression in liver tumors. BMC Cancer. 2008;8:1–15. doi: 10.1186/1471-2407-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimoda M., Takahashi M., Yoshimoto T. A homeobox protein, prox1, is involved in the differentiation, proliferation, and prognosis in hepatocellular carcinoma. Clin Cancer Res. 2006;12:6005–6011. doi: 10.1158/1078-0432.CCR-06-0712. [DOI] [PubMed] [Google Scholar]
- 61.Song K.H., Li T., Chiang J.Y. A Prospero-related homeodomain protein is a novel co-regulator of hepatocyte nuclear factor 4alpha that regulates the cholesterol 7alpha-hydroxylase gene. J Biol Chem. 2006;281:10081–10088. doi: 10.1074/jbc.M513420200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charest-Marcotte A., Dufour C.R., Wilson B.J. The homeobox protein Prox1 is a negative modulator of ERR{alpha}/PGC-1{alpha} bioenergetic functions. Genes Dev. 2010;24:537–542. doi: 10.1101/gad.1871610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dufour C.R., Levasseur M.P., Pham N.H. Genomic convergence among ERRalpha, PROX1, and BMAL1 in the control of metabolic clock outputs. PLoS Genet. 2011;7:e1002143. doi: 10.1371/journal.pgen.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin J., Gao D.M., Jiang Q.F. Prospero-related homeobox (Prox1) is a corepressor of human liver receptor homolog-1 and suppresses the transcription of the cholesterol 7-alpha-hydroxylase gene. Mol Endocrinol. 2004;18:2424–2439. doi: 10.1210/me.2004-0009. [DOI] [PubMed] [Google Scholar]
- 65.Takeda Y., Jetten A.M. Prospero-related homeobox 1 (Prox1) functions as a novel modulator of retinoic acid-related orphan receptors alpha- and gamma-mediated transactivation. Nucleic Acids Res. 2013;41:6992–7008. doi: 10.1093/nar/gkt447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin-Villar E., Scholl F.G., Gamallo C. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 67.Mahtab E.A., Wijffels M.C., Van Den Akker N.M. Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: correlation with abnormal epicardial development. Dev Dyn. 2008;237:847–857. doi: 10.1002/dvdy.21463. [DOI] [PubMed] [Google Scholar]
- 68.Mahtab E.A., Vicente-Steijn R., Hahurij N.D. Podoplanin deficient mice show a RhoA-related hypoplasia of the sinus venosus myocardium including the sinoatrial node. Dev Dyn. 2009;238:183–193. doi: 10.1002/dvdy.21819. [DOI] [PubMed] [Google Scholar]
- 69.Douglas Y.L., Mahtab E.A., Jongbloed M.R. Pulmonary vein, dorsal atrial wall and atrial septum abnormalities in podoplanin knockout mice with disturbed posterior heart field contribution. Pediatr Res. 2009;65:27–32. doi: 10.1203/PDR.0b013e31818bc11a. [DOI] [PubMed] [Google Scholar]
- 70.Ramirez M.I., Millien G., Hinds A. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol. 2003;256:61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- 71.Bekiaris V., Withers D., Glanville S.H. Role of CD30 in B/T segregation in the spleen. J Immunol. 2007;179:7535–7543. doi: 10.4049/jimmunol.179.11.7535. [DOI] [PubMed] [Google Scholar]
- 72.Astarita J.L., Acton S.E., Turley S.J. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol. 2012;3:283. doi: 10.3389/fimmu.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baars S., Bauer C., Szabowski S. Epithelial deletion of podoplanin is dispensable for re-epithelialization of skin wounds. Exp Dermatol. 2015;24:785–787. doi: 10.1111/exd.12781. [DOI] [PubMed] [Google Scholar]
- 74.Herzog B.H., Fu J., Wilson S.J. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–109. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y., Wang J., Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci U S A. 2013;110:2324–2329. doi: 10.1073/pnas.1214136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yokomori H., Oda M., Kaneko F. Lymphatic marker podoplanin/D2-40 in human advanced cirrhotic liver: re-evaluations of microlymphatic abnormalities. BMC Gastroenterol. 2010;10:131. doi: 10.1186/1471-230X-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Link A., Hardie D.L., Favre S. Association of T-zone reticular networks and conduits with ectopic lymphoid tissues in mice and humans. Am J Pathol. 2011;178:1662–1675. doi: 10.1016/j.ajpath.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fujii T., Zen Y., Sato Y. Podoplanin is a useful diagnostic marker for epithelioid hemangioendothelioma of the liver. Mod Pathol. 2008;21:125–130. doi: 10.1038/modpathol.3800986. [DOI] [PubMed] [Google Scholar]
- 79.Xian Z.H., Cong W.M., Lu X.Y. Angiogenesis and lymphangiogenesis in sporadic hepatic angiomyolipoma. Pathol Res Pract. 2011;207:403–409. doi: 10.1016/j.prp.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Dumont D.J., Jussila L., Taipale J. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 81.Karkkainen M.J., Haiko P., Sainio K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Corral I., Olmeda D., Dieguez-Hurtado R. In vivo imaging of lymphatic vessels in development, wound healing, inflammation, and tumor metastasis. Proc Natl Acad Sci U S A. 2012;109:6223–6228. doi: 10.1073/pnas.1115542109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaudio E., Barbaro B., Alvaro D. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 84.Franchitto A., Onori P., Renzi A. Expression of vascular endothelial growth factors and their receptors by hepatic progenitor cells in human liver diseases. Hepatobiliary Surg Nutr. 2013;2:68–77. doi: 10.3978/j.issn.2304-3881.2012.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lian Z., Liu J., Wu M. Hepatitis B x antigen up-regulates vascular endothelial growth factor receptor 3 in hepatocarcinogenesis. Hepatology. 2007;45:1390–1399. doi: 10.1002/hep.21610. [DOI] [PubMed] [Google Scholar]
- 86.Paupert J., Sounni N.E., Noel A. Lymphangiogenesis in post-natal tissue remodeling: lymphatic endothelial cell connection with its environment. Mol Aspects Med. 2011;32:146–158. doi: 10.1016/j.mam.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Kelley P.M., Steele M.M., Tempero R.M. Regressed lymphatic vessels develop during corneal repair. Lab Invest. 2011;91:1643–1651. doi: 10.1038/labinvest.2011.121. [DOI] [PubMed] [Google Scholar]
- 88.Zampell J.C., Avraham T., Yoder N. Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti-lymphangiogenic cytokines. Am J Physiol Cell Physiol. 2012;302:C392–C404. doi: 10.1152/ajpcell.00306.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dixelius J., Makinen T., Wirzenius M. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem. 2003;278:40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- 90.Ichise T., Yoshida N., Ichise H. H-, N- and Kras cooperatively regulate lymphatic vessel growth by modulating VEGFR3 expression in lymphatic endothelial cells in mice. Development. 2010;137:1003–1013. doi: 10.1242/dev.043489. [DOI] [PubMed] [Google Scholar]
- 91.Makinen T., Veikkola T., Mustjoki S. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vanhaesebroeck B., Stephens L., Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 93.Zheng W., Aspelund A., Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. 2014;124:878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coso S., Bovay E., Petrova T.V. Pressing the right buttons: signaling in lymphangiogenesis. Blood. 2014;123:2614–2624. doi: 10.1182/blood-2013-12-297317. [DOI] [PubMed] [Google Scholar]
- 95.Secker G.A., Harvey N.L. VEGFR signaling during lymphatic vascular development: from progenitor cells to functional vessels. Dev Dyn. 2015;244:323–331. doi: 10.1002/dvdy.24227. [DOI] [PubMed] [Google Scholar]
- 96.Kim H., Kataru R.P., Koh G.Y. Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest. 2014;124:936–942. doi: 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Serhan C.N., Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 98.Kataru R.P., Jung K., Jang C. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 99.Kataru R.P., Lee Y.G., Koh G.Y. Interactions of immune cells and lymphatic vessels. Adv Anat Embryol Cell Biol. 2014;214:107–118. doi: 10.1007/978-3-7091-1646-3_9. [DOI] [PubMed] [Google Scholar]
- 100.Mancardi S., Vecile E., Dusetti N. Evidence of CXC, CC and C chemokine production by lymphatic endothelial cells. Immunology. 2003;108:523–530. doi: 10.1046/j.1365-2567.2003.01613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ji R.C. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell Mol Life Sci. 2012;69:897–914. doi: 10.1007/s00018-011-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schoppmann S.F., Birner P., Stockl J. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cursiefen C., Chen L., Borges L.P. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan A., Avraham T., Zampell J.C. Mechanisms of lymphatic regeneration after tissue transfer. PLoS One. 2011;6:e17201. doi: 10.1371/journal.pone.0017201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maruyama K., Ii M., Cursiefen C. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kerjaschki D., Huttary N., Raab I. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nature Medicine. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 107.Lee S., Choi I., Hong Y.K. Heterogeneity and plasticity of lymphatic endothelial cells. Semin Thromb Hemost. 2010;36:352–361. doi: 10.1055/s-0030-1253457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vigl B., Aebischer D., Nitschke M. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood. 2011;118:205–215. doi: 10.1182/blood-2010-12-326447. [DOI] [PubMed] [Google Scholar]
- 109.Kabashima K., Shiraishi N., Sugita K. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol. 2007;171:1249–1257. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Teijeira A., Rouzaut A., Melero I. Initial afferent lymphatic vessels controlling outbound leukocyte traffic from skin to lymph nodes. Front Immunol. 2013;4:433. doi: 10.3389/fimmu.2013.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnson L.A., Jackson D.G. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis. 2014;17:335–345. doi: 10.1007/s10456-013-9407-0. [DOI] [PubMed] [Google Scholar]
- 112.Saeki H., Moore A.M., Brown M.J. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- 113.Tal O., Lim H.Y., Gurevich I. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208:2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson L.A., Clasper S., Holt A.P. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gagliostro V., Seeger P., Garrafa E. Pro-lymphangiogenic properties of IFN-gamma-activated human dendritic cells. Immunol Lett. 2016;173:26–35. doi: 10.1016/j.imlet.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 116.Kataru R.P., Kim H., Jang C. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34:96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 117.Angeli V., Ginhoux F., Llodra J. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 118.Shrestha B., Hashiguchi T., Ito T. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J Immunol. 2010;184:4819–4826. doi: 10.4049/jimmunol.0903063. [DOI] [PubMed] [Google Scholar]
- 119.Tan K.W., Chong S.Z., Wong F.H. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood. 2013;122:3666–3677. doi: 10.1182/blood-2012-11-466532. [DOI] [PubMed] [Google Scholar]
- 120.Cain J.C., Grindlay J.H. Lymph from liver and thoracic duct; an experimental study. Surg Gynecol Obstet. 1947;85:558–562. [PubMed] [Google Scholar]
- 121.Nix J.T., Flock E.V., Bollman J.L. Influence of cirrhosis on proteins of cisternal lymph. Am J Physiol. 1951;164:117–118. doi: 10.1152/ajplegacy.1950.164.1.117. [DOI] [PubMed] [Google Scholar]
- 122.Dumont A.E., Mulholland J.H. Flow rate and composition of thoracic-duct lymph in patients with cirrhosis. N Engl J Med. 1960;263:471–474. doi: 10.1056/NEJM196009082631001. [DOI] [PubMed] [Google Scholar]
- 123.Dumont A.E., Mulholland J.H. Alterations in thoracic duct lymph flow in hepatic cirrhosis: significance in portal hypertension. Ann Surg. 1962;156:668–675. doi: 10.1097/00000658-196210000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Witte C.L., Witte M.H., Dumont A.E. Lymph imbalance in the genesis and perpetuation of the ascites syndrome in hepatic cirrhosis. Gastroenterology. 1980;78:1059–1068. [PubMed] [Google Scholar]
- 125.Barrowman J.A., Granger D.N. Effects of experimental cirrhosis on splanchnic microvascular fluid and solute exchange in the rat. Gastroenterology. 1984;87:165–172. [PubMed] [Google Scholar]
- 126.Shimada Y. Observations on hepatic superficial lymph flow. Lymphology. 1979;12:11–13. [PubMed] [Google Scholar]
- 127.Atkinson M., Losowsky M.S. The mechanism of ascites formation in chronic liver disease. Q J Med. 1961;30:153–166. [PubMed] [Google Scholar]
- 128.Lieberman F.L., Ito S., Reynolds T.B. Effective plasma volume in cirrhosis with ascites: evidence that a decreased value does not account for renal sodium retention, a spontaneous reduction in glomerular filtration rate (GFR), and a fall in GFR during drug-induced diuresis. J Clin Invest. 1969;48:975–981. doi: 10.1172/JCI106078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schrier R.W., Arroyo V., Bernardi M. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 130.De Franchis R., Salerno F. Pathogenesis of ascites and predictors of resistance to therapy. J Gastroenterol Hepatol. 2002;17(Suppl 3):S242–S247. doi: 10.1046/j.1440-1746.17.s3.7.x. [DOI] [PubMed] [Google Scholar]
- 131.Arroyo V., Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38(Suppl 1):S69–S89. doi: 10.1016/s0168-8278(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 132.Gordon F.D. Ascites. Clin Liver Dis. 2012;16:285–299. doi: 10.1016/j.cld.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 133.Sanyal A.J., Bosch J., Blei A. Portal hypertension and its complications. Gastroenterology. 2008;134:1715–1728. doi: 10.1053/j.gastro.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 134.Ribera J., Pauta M., Melgar-Lesmes P. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut. 2013;62:138–145. doi: 10.1136/gutjnl-2011-300703. [DOI] [PubMed] [Google Scholar]
- 135.Vollmar B., Wolf B., Siegmund S. Lymph vessel expansion and function in the development of hepatic fibrosis and cirrhosis. Am J Pathol. 1997;151:169–175. [PMC free article] [PubMed] [Google Scholar]
- 136.Yamauchi Y., Michitaka K., Onji M. Morphometric analysis of lymphatic and blood vessels in human chronic viral liver diseases. Am J Pathol. 1998;153:1131–1137. doi: 10.1016/S0002-9440(10)65657-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Achen M.G., Jeltsch M., Kukk E. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oikawa H., Masuda T., Sato S. Changes in lymph vessels and portal veins in the portal tract of patients with idiopathic portal hypertension: a morphometric study. Hepatology. 1998;27:1607–1610. doi: 10.1002/hep.510270621. [DOI] [PubMed] [Google Scholar]
- 139.Guerin F., Wagner M., Line A. Hepatic proliferation and angiogenesis markers are increased after portal deprivation in rats: a study of molecular, histological and radiological changes. PLoS One. 2015;10:e0125493. doi: 10.1371/journal.pone.0125493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun H.C., Zhuang P.Y., Qin L.X. Incidence and prognostic values of lymph node metastasis in operable hepatocellular carcinoma and evaluation of routine complete lymphadenectomy. J Surg Oncol. 2007;96:37–45. doi: 10.1002/jso.20772. [DOI] [PubMed] [Google Scholar]
- 141.Yamamoto M., Takasaki K., Yoshikawa T. Lymph node metastasis in intrahepatic cholangiocarcinoma. Jpn J Clin Oncol. 1999;29:147–150. doi: 10.1093/jjco/29.3.147. [DOI] [PubMed] [Google Scholar]
- 142.Das S., Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci. 2008;1131:235–241. doi: 10.1196/annals.1413.021. [DOI] [PubMed] [Google Scholar]
- 143.Skobe M., Hamberg L.M., Hawighorst T. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iwata C., Kano M.R., Komuro A. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67:10181–10189. doi: 10.1158/0008-5472.CAN-07-2366. [DOI] [PubMed] [Google Scholar]
- 145.Schoppmann S.F., Fenzl A., Nagy K. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139:839–846. doi: 10.1016/j.surg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 146.Thelen A., Scholz A., Weichert W. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 2010;105:1123–1132. doi: 10.1038/ajg.2009.674. [DOI] [PubMed] [Google Scholar]
- 147.Yamaguchi R., Yano H., Nakashima O. Expression of vascular endothelial growth factor-C in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:152–160. doi: 10.1111/j.1440-1746.2005.04217.x. [DOI] [PubMed] [Google Scholar]
- 148.Tampellini M., Sonetto C., Scagliotti G.V. Novel anti-angiogenic therapeutic strategies in colorectal cancer. Expert Opin Investig Drugs. 2016;25:1–14. doi: 10.1517/13543784.2016.1161754. [DOI] [PubMed] [Google Scholar]
- 149.Kerjaschki D., Regele H.M., Moosberger I. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 150.Stuht S., Gwinner W., Franz I. Lymphatic neoangiogenesis in human renal allografts: results from sequential protocol biopsies. Am J Transplant. 2007;7:377–384. doi: 10.1111/j.1600-6143.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 151.Vass D.G., Hughes J., Marson L.P. Restorative and rejection-associated lymphangiogenesis after renal transplantation: friend or foe? Transplantation. 2009;88:1237–1239. doi: 10.1097/TP.0b013e3181c1afa7. [DOI] [PubMed] [Google Scholar]
- 152.Geissler H.J., Dashkevich A., Fischer U.M. First year changes of myocardial lymphatic endothelial markers in heart transplant recipients. Eur J Cardiothorac Surg. 2006;29:767–771. doi: 10.1016/j.ejcts.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 153.Dashkevich A., Heilmann C., Kayser G. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann Thorac Surg. 2010;90:406–411. doi: 10.1016/j.athoracsur.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 154.Ishii E., Shimizu A., Kuwahara N. Lymphangiogenesis associated with acute cellular rejection in rat liver transplantation. Transplant Proc. 2010;42:4282–4285. doi: 10.1016/j.transproceed.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 155.Enzan H., Himeno H., Hiroi M. Development of hepatic sinusoidal structure with special reference to the Ito cells. Microsc Res Tech. 1997;39:336–349. doi: 10.1002/(SICI)1097-0029(19971115)39:4<336::AID-JEMT4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 156.Salmi M., Jalkanen S. Cell-surface enzymes in control of leukocyte trafficking. Nat Rev Immunol. 2005;5:760–771. doi: 10.1038/nri1705. [DOI] [PubMed] [Google Scholar]
- 157.Ikeda Y., Terashima T. Expression of reelin, the gene responsible for the reeler mutation, in embryonic development and adulthood in the mouse. Dev Dyn. 1997;210:157–172. doi: 10.1002/(SICI)1097-0177(199710)210:2<157::AID-AJA8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 158.Iwakiri Y. The lymphatic system: a new frontier in hepatology. Hepatology. 2016;64:706–707. doi: 10.1002/hep.28650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Favre C.J., Mancuso M., Maas K. Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. Am J Physiol Heart Circ Physiol. 2003;285:H1917–H1938. doi: 10.1152/ajpheart.00983.2002. [DOI] [PubMed] [Google Scholar]
- 160.Kaser-Eichberger A., Schroedl F., Bieler L. Expression of lymphatic markers in the adult rat spinal cord. Front Cell Neurosci. 2016;10:23. doi: 10.3389/fncel.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cho C.H., Koh Y.J., Han J. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100:e47–e57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 162.Zheng M., Kimura S., Nio-Kobayashi J. Three types of macrophagic cells in the mesentery of mice with special reference to LYVE-1-immunoreactive cells. Biomed Res. 2014;35:37–45. doi: 10.2220/biomedres.35.37. [DOI] [PubMed] [Google Scholar]
- 163.Xu H., Chen M., Reid D.M. LYVE-1-positive macrophages are present in normal murine eyes. Invest Ophthalmol Vis Sci. 2007;48:2162–2171. doi: 10.1167/iovs.06-0783. [DOI] [PubMed] [Google Scholar]
- 164.Schledzewski K., Falkowski M., Moldenhauer G. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 165.Lua I., Li Y., Zagory J.A. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J Hepatol. 2016;64:1137–1146. doi: 10.1016/j.jhep.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peters A., Pitcher L.A., Sullivan J.M. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kamba T., Tam B.Y., Hashizume H. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 168.Witmer A.N., van Blijswijk B.C., van Noorden C.J. In vivo angiogenic phenotype of endothelial cells and pericytes induced by vascular endothelial growth factor-A. J Histochem Cytochem. 2004;52:39–52. doi: 10.1177/002215540405200105. [DOI] [PubMed] [Google Scholar]
- 169.Gunn M.D., Kyuwa S., Tam C. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Heydtmann M., Hardie D., Shields P.L. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J Immunol. 2006;177:729–738. doi: 10.4049/jimmunol.177.1.729. [DOI] [PubMed] [Google Scholar]
- 171.Grant A.J., Goddard S., Ahmed-Choudhury J. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160:1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee S.J., Evers S., Roeder D. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 173.Takahashi K., Donovan M.J., Rogers R.A. Distribution of murine mannose receptor expression from early embryogenesis through to adulthood. Cell Tissue Res. 1998;292:311–323. doi: 10.1007/s004410051062. [DOI] [PubMed] [Google Scholar]
- 174.Petrova T.V., Makinen T., Makela T.P. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Linehan S.A., Martinez-Pomares L., Stahl P.D. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gigi-Leitner O., Geiger B. Antigenic interrelationship between the 40-kilodalton cytokeratin polypeptide and desmoplakins. Cell Motil Cytoskeleton. 1986;6:628–639. doi: 10.1002/cm.970060611. [DOI] [PubMed] [Google Scholar]
- 177.Sawa Y., Shibata K., Braithwaite M.W. Expression of immunoglobulin superfamily members on the lymphatic endothelium of inflamed human small intestine. Microvasc Res. 1999;57:100–106. doi: 10.1006/mvre.1998.2132. [DOI] [PubMed] [Google Scholar]
- 178.Cao Y., Chang H., Li L. Alteration of adhesion molecule expression and cellular polarity in hepatocellular carcinoma. Histopathology. 2007;51:528–538. doi: 10.1111/j.1365-2559.2007.02820.x. [DOI] [PubMed] [Google Scholar]
- 179.Ebata N., Nodasaka Y., Sawa Y. Desmoplakin as a specific marker of lymphatic vessels. Microvasc Res. 2001;61:40–48. doi: 10.1006/mvre.2000.2280. [DOI] [PubMed] [Google Scholar]
- 180.Hoye A.M., Couchman J.R., Wewer U.M. The newcomer in the integrin family: integrin alpha9 in biology and cancer. Adv Biol Regul. 2012;52:326–339. doi: 10.1016/j.jbior.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 181.Halin C., Tobler N.E., Vigl B. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158–3167. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wuest T.R., Carr D.J. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kubo H., Cao R., Brakenhielm E. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baluk P., Tammela T., Ator E. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim K.E., Koh Y.J., Jeon B.H. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol. 2009;175:1733–1745. doi: 10.2353/ajpath.2009.090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yan Z.X., Jiang Z.H., Liu N.F. Angiopoietin-2 promotes inflammatory lymphangiogenesis and its effect can be blocked by the specific inhibitor L1-10. Am J Physiol Heart Circ Physiol. 2012;302:H215–H223. doi: 10.1152/ajpheart.00895.2011. [DOI] [PubMed] [Google Scholar]
- 187.Yuen D., Grimaldo S., Sessa R. Role of angiopoietin-2 in corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2014;55:3320–3327. doi: 10.1167/iovs.13-13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Saito Y., Nakagami H., Morishita R. Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis. Circulation. 2006;114:1177–1184. doi: 10.1161/CIRCULATIONAHA.105.602953. [DOI] [PubMed] [Google Scholar]
- 189.Furtado G.C., Marinkovic T., Martin A.P. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci U S A. 2007;104:5026–5031. doi: 10.1073/pnas.0606697104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mounzer R.H., Svendsen O.S., Baluk P. Lymphotoxin-alpha contributes to lymphangiogenesis. Blood. 2010;116:2173–2182. doi: 10.1182/blood-2009-12-256065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Watari K., Nakao S., Fotovati A. Role of macrophages in inflammatory lymphangiogenesis: enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem Biophys Res Commun. 2008;377:826–831. doi: 10.1016/j.bbrc.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 192.Al-Rawi M.A., Watkins G., Mansel R.E. Interleukin 7 upregulates vascular endothelial growth factor D in breast cancer cells and induces lymphangiogenesis in vivo. Br J Surg. 2005;92:305–310. doi: 10.1002/bjs.4832. [DOI] [PubMed] [Google Scholar]
- 193.Al-Rawi M.A., Watkins G., Mansel R.E. The effects of interleukin-7 on the lymphangiogenic properties of human endothelial cells. Int J Oncol. 2005;27:721–730. [PubMed] [Google Scholar]
- 194.Choi I., Lee Y.S., Chung H.K. Interleukin-8 reduces post-surgical lymphedema formation by promoting lymphatic vessel regeneration. Angiogenesis. 2013;16:29–44. doi: 10.1007/s10456-012-9297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chauhan S.K., Jin Y., Goyal S. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4634. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hammer T., Tritsaris K., Hubschmann M.V. IL-20 activates human lymphatic endothelial cells causing cell signalling and tube formation. Microvasc Res. 2009;78:25–32. doi: 10.1016/j.mvr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 197.Oka M., Iwata C., Suzuki H.I. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood. 2008;111:4571–4579. doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- 198.Clavin N.W., Avraham T., Fernandez J. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol. 2008;295:H2113–H2127. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]
- 199.Avraham T., Daluvoy S., Zampell J. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010;177:3202–3214. doi: 10.2353/ajpath.2010.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dunworth W.P., Cardona-Costa J., Bozkulak E.C. Bone morphogenetic protein 2 signaling negatively modulates lymphatic development in vertebrate embryos. Circ Res. 2014;114:56–66. doi: 10.1161/CIRCRESAHA.114.302452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shao X., Liu C. Influence of IFN- alpha and IFN- gamma on lymphangiogenesis. J Interferon Cytokine Res. 2006;26:568–574. doi: 10.1089/jir.2006.26.568. [DOI] [PubMed] [Google Scholar]
- 202.Shin K., Kataru R.P., Park H.J. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat Commun. 2015;6:6196. doi: 10.1038/ncomms7196. [DOI] [PubMed] [Google Scholar]
- 203.Nielsen S.R., Hammer T., Gibson J. IL-27 inhibits lymphatic endothelial cell proliferation by STAT1-regulated gene expression. Microcirculation. 2013;20:555–564. doi: 10.1111/micc.12055. [DOI] [PubMed] [Google Scholar]
- 204.Heinz M., Niederleithner H.L., Puujalka E. Activin A is anti-lymphangiogenic in a melanoma mouse model. J Invest Dermatol. 2015;135:212–221. doi: 10.1038/jid.2014.328. [DOI] [PubMed] [Google Scholar]