Abstract
Aim:
Diabetic retinopathy (DR) is the most common preventable cause of blindness where early detection and treatment can be sight-saving. Search for biomarkers of the disease has been relentless. We aimed to determine whether lipoproteins apolipoproteins A1 and B1 (Apo-A1 and Apo-B1) have stronger associations with DR in contrast to conventionally measured low-density lipoprotein (LDL) and high-density lipoprotein cholesterol levels.
Materials and Methods:
We performed a cross-sectional study and studied 117 patients. Serum lipid profile was assessed by autoanalyzer. Serum Apo-A1 and Apo-B were measured using immunoturbidimetric kit on an autoanalyzer. Apo-B/A1 ratio was calculated. Retinopathy was graded from the digital retinal photographs, taken with nonmydriatic auto fundus camera and classified according to International Clinical DR Disease Severity Scale.
Results:
Mean Apo-A1 for mild, moderate, severe retinopathy, and proliferative DR (PDR) shows a significant negative correlation (P = 0.001) with severity of retinopathy. Mean Apo-B for mild, moderate, severe, PDR displayed a significant positive correlation with severity of retinopathy (P = 0.001). Mean Apo-B/A1 for mild, moderate, severe, PDR showed highly significant positive correlation with severity of retinopathy (P < 0.001). In contrast, mean LDL for mild, moderate, severe, PDR showed insignificant association with severity of DR (P = 0.081).
Conclusion:
Apo-A1 and Apo-B have a stronger association with the development of DR than traditional lipids and can thus facilitate early detection and treatment of the disease.
Keywords: Biomarkers, diabetic retinopathy, serum apolipoprotein
INTRODUCTION
Diabetic retinopathy (DR) is a well-recognized sight-threatening chronic microvascular complication of diabetes. Despite decades of research and effective therapies, DR remains one of the leading causes of new onset blindness. However, with appropriate medical and ophthalmologic care, it can be prevented in 90% of cases.[1]
Diabetes duration, hyperglycemia, and hypertension are established risk factors of DR. Earlier lipid profile was believed to have a direct correlation with DR but in contrast to this widely accepted view, previous studies have not shown a consistent relationship between lipid profile and DR.
Recently, there has been an interest in the relationship of apolipoprotein A1 (Apo-A1) and Apo-B with DR.[2,3,4] Apo-A1 is an high-density lipoprotein (HDL) constituent and Apo-B is present in very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), LDL, and lipoprotein(a). Both Apo are not affected by prandial status.[5] Because Apo-A1 better reflects lipid accumulation in peripheral tissue and Apo-B is present in the retina of human eyes with DR, they may be more directly relevant to the biophysiological changes associated with DR than the traditional lipids (e.g., HDL and LDL cholesterol and triglycerides).[6] However, the extent to which Apo measures are useful to identify individuals at risk of DR remains unknown. In this study, the associations of Apo and traditional lipid parameters with DR were evaluated and compared in adults with diabetes.
MATERIALS AND METHODS
Study design
This study was a cross-sectional type of study. Diabetes patients of age group 16–75 years, who were suffering from DR, were selected on the basis of inclusion and exclusion criteria.
Inclusion criteria
Diabetes patients of age group 16–75 years with DR.
Exclusion criteria
History of glaucoma
History of epilepsy
Had undergone vitreous surgery
Cataract on eye examination
Any other preexisting retinal disease/retinopathy from any other cause
Patients on lipid profile modifying drugs.
This study included 117 patients.
Detailed history, clinical examination, anthropometric measurements, and biochemical indices were assessed for all the selected patients.
Serum lipid profile was obtained by autoanalyzer and included HDL, LDL, VLDL, triglycerides, and total cholesterol level.
Serum Apo-A1 and Apo-B were measured using immunoturbidimetric kit, SGM ITALY, on a fully autoanalyzer machine. The ratio of Apo-B/A1 was calculated. Retinopathy was graded from the digital retinal photographs, taken with NonMydriatic Auto Fundus Camera AFC 230/210 (for 7 visual fields) and classified according to International Clinical DR Disease Severity Scale. All findings were confirmed by the same ophthalmologist.
Statistical analysis
The analysis was performed using SPSS (version 10.1 for windows, IBM in 2009). Baseline characteristics of the participants with DR were compared using a Chi-square test for proportions, t-test for means. Correlation matrix of variables was used to assess the association between serum lipids and Apo and DR.
RESULTS
Mean LDL values for mild NPDR (119.74 mg/dl), moderate NPDR (124.76 mg/dl), severe NPDR (132.60 mg/dl), and PDR (128.11 mg/dl) were observed. On statistical analysis, their correlation with severity of DR was found to be poor (P = 0.081).
Mean HDL values for mild NPDR (39.96 mg/dl), moderate NPDR (36.74 mg/dl), severe NPDR (32.95 mg/dl), and PDR (29.33 mg/dl) were observed. On statistical analysis, they were found to be significantly negatively correlated to the severity of DR (P ≤ 0.001).
Mean Apo-B for mild NPDR (74.98 mg/dl), moderate NPDR (96.45 mg/dl), severe NPDR (101.00 mg/dl), and PDR (108.11 mg/dl) were observed. On statistical analysis, Apo-B has significant positive correlation with severity of DR (P ≤ 0.001) [Graph 1].
Graph 1.
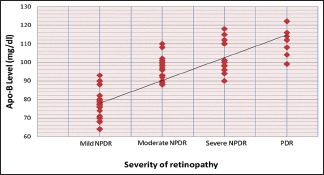
Distribution of cases according to apolipoprotein-B in relation to severity of retinopathy
Mean Apo-A1 for mild NPDR (148.86 mg/dl), moderate NPDR (124.39 mg/dl), severe NPDR (110.30 mg/dl), and proliferative DR (103.22 mg/dl) were observed. On statistical analysis, Apo-A1 has significant negative correlation with severity of DR (P ≤ 0.001) [Graph 2].
Graph 2.
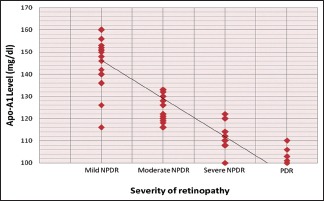
Distribution of cases according to apolipoprotein-A1 in relation to severity of retinopathy
Graph 3 shows the distribution of cases according to Apo-B/A1 in relation to severity of retinopathy. In Apo-B/A1 0.40–0.50 group, totally 19 patients were found, and they all belonged to mild NPDR group. In 0.51–0.60 Apo-B/A1 group, totally 29 patients were found, and they all also belonged to mild NPDR group. In Apo-B/A1 group 0.61–0.70, totally 6 patients were found and out of them, 1 and 5 had mild and moderate NPDR, respectively. In Apo-B/A1 group 0.71–0.80, total 22 patients were found and out of them 1, 19, and 2 had mild, moderate, and severe NPDR, respectively. In Apo-B/A1 group 0.81–0.90 totally 17 patients were found and out of them 14, 3 had moderate and severe NPDR, respectively. In Apo-B/A1 group 0.91–1.00, totally 17 patients were found and out of them, 14 and 3 had severe NPDR and PDR retinopathy, respectively. While in Apo-B/A1 group >1.00, 7 patients were found and out of them, 1 and 6 had severe NPDR and PDR retinopathy, respectively.
Graph 3.
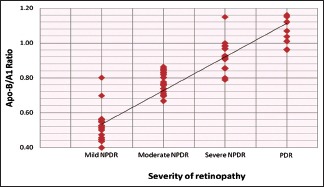
Distribution of cases according to apolipoprotein-B/A1 in relation to severity of retinopathy
Mean Apo-B/A1 for mild NPDR (0.51), moderate NPDR (0.78), severe NPDR (0.92), and PDR (1.05) were observed. Thus, the ratio of Apo-B/Apo-A1 is a better marker of DR with stronger correlation than each molecule alone (P ≤ 0.001).
DISCUSSION
In our study, it was found that HDL correlated well with the severity of DR, bearing an inverse relationship with the same. Lesser values of HDL were found in advanced forms of retinopathy. LDL, however, showed an inconsistent relationship with severity of DR.
Apo-A1 and Apo-B showed a consistent relationship with severity of DR. Apo-A1 was inversely related to the severity of DR while Apo-B was directly related to the severity of DR. The ratio Apo-B/Apo-A1 showed a direct relationship with severity of DR with severity increasing as the ratio increased.
Lyons et al. in their study, showed an inverse relationship of HDL with the severity of DR.[2] Zoppini et al. in their study, showed that TG/HDL-C ratio was positively associated with DR and other microvascular complications.[7] Our study also supports these findings regarding the protective role of HDL in DR.
HDL-C itself is a complex of several lipoproteins, cholesterol, and triglycerides and has several postulated mechanisms of vascular action, notably reverse cholesterol transport as well as anti-inflammatory and antioxidant properties. Furthermore, there is emerging evidence that HDL-C improves glycemic control by modulating glucose uptake into skeletal muscle and protects against islet β-cell dysfunction. Although the exact putative mechanism for protection is unknown, it is known that HDL-C exerts beneficial effects on many of the pathways known to be detrimental to the vascular biology.
Sinav et al. found significantly higher levels of LDL and total serum cholesterol in patients with DR.[8]
Wu et al., in their research, showed that quantitative dyslipidemia is associated with the initiation and progression of DR.[6] They hypothesized that in a manner analogs to atherogenesis, extravasation of LDL from retinal capillaries and its subsequent oxidation may be implicated in the progression of DR. However, lipid profile has shown an inconsistent relationship with DR in many of the other previous studies. Similar to our findings, data from the DCCT did not show an association with total cholesterol levels. The Multi-Ethnic Study of Atherosclerosis showed no associations of serum lipids with DR.
Apo-A1 is the main HDL structural protein. HDL itself is a vasoprotective lipoprotein. Apo-A1 is produced by the liver and intestine and is essential for the reverse transport of cholesterol from peripheral tissue to the liver. In addition to possessing an anti-inflammatory and anti-oxidant actions, Apo-A1 is also involved in intraretinal lipid transport indicating its importance in preventing microvascular ocular complication of diabetes.
Higher Apo-A1 levels in vitreous fluid and retinal pigment epithelium among individuals with diabetes than in those without diabetes suggest protective mechanisms within the retina through Apo-A1 against lipid deposition and inflammation induced lipotoxicity that can otherwise lead to DR. On the contrary, Apo-B is a major structural protein for VLDL, IDL, and LDL and is responsible for delivering lipids from the liver and intestine to peripheral tissue. Total Apo-B levels may thus reflect atherogenic potential.
Simo et al. in their study, compared Apo-A1 expression in retina of diabetic and nondiabetic donors.[3] They found that Apo-A1 overexpression is an early event in the retina of diabetic patients and can be involved in the pathophysiology of DR. One study, by Vinodhini et al., however, failed to document any association between Apo-A1 and Apo-B and severity of DR.[9] The major limitation of their study, as stated by them, was the small size of the study groups. There was widespread use of statins in both the study and control groups. Probably these reasons might have accounted for their result.
First, Apo and components of conventional basic lipid profile (including HDL and LDL) are not one and the same. Apo are protein moieties with structural, enzymatic, and receptor binding functions, and HDL cholesterol and LDL cholesterol refer to the cholesterol lipid content of HDL and LDL only. Using only HDL and LDL ignores the contribution from other lipoproteins, resulting in the inconsistent association.
Second, the metabolic environment of a diabetic patient differs from a normal person. The diabetic environment induces changes such as nonenzymatic glycation, oxidation, and advanced glycation end product modification of lipoproteins which may affect the assay results. Similarly, there may be differences in the precision of measurement of lipoproteins and potential for differential effects of sample handling and nonenzymatic glycation and oxidation on the different assays. On the contrary, Apo-A1 and Apo-B levels are more stable than lipid levels, particularly in individuals with diabetes and are not affected significantly by prandial status.
These reasons may have accounted for our findings showing highly significant association of Apo-A1 and Apo-B in comparison to lipid profile.
Strengths of this study include an appreciable sample size and study of both lipid profile as well as Apo in our study. However, cross-sectional design accounts for our shortcomings as further prospective studies will be needed for better understanding of the association and risk factors. Second, lack of control population is also one shortcoming of our study. Finally, it was not possible to determine the strength of association of Apo-B, Apo-A1, and Apo-B/A1 ratio was independent of other factors such as duration of diabetes, the level of glycemic control, hypertension.
Therefore, although further studies are needed to establish the result, we propose these Apo as the better and stronger biomarkers of DR as compared to HDL and LDL in relation with DR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Brownlee M, Aiello LP, Cooper ME, Vinik AI, Nesto RW, Boulton AJ. In: Complications of diabetes mellitus. Williams Textbook of Endocrinology. 12th ed. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Philadelphia: Elsevier Saunders; 2011. pp. 1485–92. [Google Scholar]
- 2.Lyons TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT, et al. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45:910–8. doi: 10.1167/iovs.02-0648. [DOI] [PubMed] [Google Scholar]
- 3.Simó R, García-Ramírez M, Higuera M, Hernández C. Apolipoprotein A1 is overexpressed in the retina of diabetic patients. Am J Ophthalmol. 2009;147:319–25.e1. doi: 10.1016/j.ajo.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Simó R, Higuera M, García-Ramírez M, Canals F, García-Arumí J, Hernández C. Elevation of apolipoprotein A-I and apolipoprotein H levels in the vitreous fluid and overexpression in the retina of diabetic patients. Arch Ophthalmol. 2008;126:1076–81. doi: 10.1001/archopht.126.8.1076. [DOI] [PubMed] [Google Scholar]
- 5.Davidson MH. Apolipoprotein measurements: Is more widespread use clinically indicated? Clin Cardiol. 2009;32:482–6. doi: 10.1002/clc.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M, Chen Y, Wilson K, Chirindel A, Ihnat MA, Yu Y, et al. Intraretinal leakage and oxidation of LDL in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:2679–85. doi: 10.1167/iovs.07-1440. [DOI] [PubMed] [Google Scholar]
- 7.Zoppini G, Negri C, Stoico V, Casati S, Pichiri I, Bonora E. Triglyceride-high-density lipoprotein cholesterol is associated with microvascular complications in type 2 diabetes mellitus. Metabolism. 2012;61:22–9. doi: 10.1016/j.metabol.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Sinav S, Onelge MA, Onelge S, Sinav B. Plasma lipids and lipoproteins in retinopathy of type I (insulin-dependent) diabetic patients. Ann Ophthalmol. 1993;25:64–6. [PubMed] [Google Scholar]
- 9.Vinodhini VM, Gnaneswaran S, Ebenezer WW, Kumar JS, Jeevanathan A. A study on the pattern of lipid profile and apolipoproteins in patients with diabetic retinopathy. Int J Pharm Clin Res. 2013;5:1–3. [Google Scholar]


