Abstract
Context:
Cardiovascular disease (CVD) is the largest cause of mortality in Indians. Insulin resistance and related dyslipidemia of increased triglyceride (TG), small dense low-density lipoprotein (sd-LDL) particles, and decreased high-density lipoprotein-cholesterol (HDL-C) are associated with increased risk of CVD. TG/HDL-C ratio could be a potential surrogate marker for this South Asian phenotype. Data are scarce on the relevance of TG/HDL-C ratio as a useful lipid marker among Indians.
Aims:
To study the prevalence of TG/HDL-C ratio among healthy, young, and middle-aged Indian men (25–44 years) and its relationship with other lipid and nonlipid factors.
Subjects and Methods:
In this cross-sectional analysis, fasting blood samples from 236 healthy participants recruited from an urban community setting were tested for TG/HDL-C ratio, HDL-C, TG, total cholesterol (TC), non-HDL-C, TC/HDL-C, high-sensitivity C-reactive protein, body mass index (BMI), and body fat.
Results:
Mean (standard deviation) age of participants was 34.7 (7.7) years; median (interquartile range) TG/HDL-C ratio was 4 (2.85-5.2). More than half (51.3%) the participants (n = 121) recorded abnormal TG/HDL-C ratio (≥4.0). Across tertiles of TG/HDL-C ratio, there was a significant trend of higher conventional lipid parameters such as non-HDL-C*, TC/HDL-C ratio*, TG*, HDL-C*, TC**; and non-lipid parameters body-fat* and BMI*** (*P < 0.001, **P = 0.015, ***P = 0.002). LDL-C showed moderate and nonsignificant (P = 0.646) increase across tertiles.
Conclusion:
In a sample of apparently healthy, young, and middle-aged Indian men abnormal TG/HDL-C ratio levels were observed among more than half the participants. The TG/HDL-C ratio was closely associated with other lipid parameters and measures of adiposity, such as BMI and body fat, apart from its previously documented unique association with sd-LDL particles. TG/HDL-C ratio should be evaluated in future for risk prediction of incident CVD among Indians.
Keywords: Indian men, lipid fraction, lipid marker, triglyceride/high-density lipoprotein-cholesterol ratio
INTRODUCTION
Cardiovascular diseases (CVD), predominantly coronary heart disease (CHD), is a major public health concern worldwide and is a major contributor to mortality globally.[1,2,3] In India, CVD is the leading cause of death, with a higher incidence compared to the west which has shown a declining trend.[3,4] In addition, CVD has an early age of onset among Indians, largely targeting the younger “working” population.[5,6] This higher risk in Indians is attributed to the rising trends of risk factors including abnormal lipids, insulin resistance, inflammation, and visceral adiposity.[5] Identification of a simple, accurate, and effective cardiovascular risk marker, in healthy individuals at an early stage of life, is vital for efficient prevention and control of heart disease in India.
Insulin resistance characterized by elevated triglycerides (TG), low high-density lipoprotein-cholesterol (HDL-C), and presence of small dense low-density lipoprotein (sd-LDL) particles appears to be the fundamental metabolic defect.[7] In fact, recent research points to sd-LDL particles as being most strongly linked to CVD and the most frequent form of dyslipidemia in premature heart disease.[8] Traditional lipid profiles include LDL-cholesterol (LDL-C) but do not reflect sd-LDL and increased sd-LDL particles are often accompanied by a normal LDL-C. On the other hand, direct measurement of sd-LDL is technically demanding, resource intensive, and not applicable in routine biochemical laboratory.[9] A surrogate marker for sd-LDL is the TG-by-HDL-C ratio (TG/HDL-C ratio).[10] The TG/HDL-C ratio has recently been reported as an independent predictor of insulin resistance.[10,11] In addition, preliminary evidence suggests that TG/HDL-C levels have the strongest association with CHD.[12] Accordingly, TG/HDL-C ratio could emerge as a prominent marker of cardiovascular risk.
In this study, we investigated the prevalence of the TG/HDL-C ratio among apparently healthy, young, and middle-aged Indian men and assessed its relationship with other lipid fractions, inflammatory marker high-sensitivity C-reactive protein (hs-CRP), body mass index (BMI), and body fat as measured by bioimpedance.
SUBJECTS AND METHODS
Design and participants
The present study was conducted among 236 apparently healthy, young, and middle-aged Indian men (aged 25–44 years) recruited from an urban setting in Delhi-NCR, namely, executives from a corporate setup and students, academicians, staff from a higher education institute. All consecutive subjects seen in July 2013 and between June and September 2014 were recruited. The socioeconomic status of the participants ranged from middle-income group to upper-middle income group (MIG to U-MIG), based on the level of education, occupation, and monthly family income.[13] The exclusion criteria included dyslipidemia on treatment, diabetes, or hypertension, which resulted in the exclusion of six subjects (n = 6 of 242).
The study received ethics approval from the University of Delhi. In addition, at the site of data collection, the study specifications were shared with the concerned authorities, and an independent approval was taken. Each participant provided an informed written consent for participation.
Blood measurements
Participants were apprised of the protocol to be followed a day before and the morning of their blood sampling. Strict instructions were given to avoid alcohol, to restrict vigorous exercise, and to not eat or drink anything except plain water for a minimum of 10 h before the blood sampling. Fasting venous blood samples were drawn using vacutainers. The samples were subsequently centrifuged, aliquoted, and transferred to the laboratory for biochemical analysis. The analytical procedures were either performed on the same day or the aliquots were stored at −70°C for analysis at a later stage.
Direct measurements included serum HDL-C by Immunoinhibition method, serum TG by GPO-PAP enzymatic method, and serum total cholesterol (TC) by CHOD-PAP enzymatic method[14] using test kit (Dialab, Austria) on a Roche Modular-P analyzer (Roche Diagnostics, Indianapolis, IN, USA). Serum levels of hs-CRP were measured by enzyme-linked immunosorbent assay method[15] using commercially available kit (BioCheck, California). The TG/HDL-C ratio was calculated with the measured values of serum TG and HDL-C. LDL-C concentration was derived from the Friedewald equation.[16] Non-HDL cholesterol (non-HDL-C) concentration was calculated as TC minus HDL-C. The TC/HDL-C ratio was calculated with the measured values of TC and HDL-C.
Anthropometric measurements
Weight (to nearest 0.1 kg) and body fat percentage were measured using a regularly calibrated Tanita analyzer (BC-420MA; Tanita Corporation, Japan) based on bioelectrical impedance. The measurement was done in fasting state, clothes weight (a standard value kept constant for all participants during a specific season) was deducted, and metallic items were removed. BMI was calculated automatically on the Tanita analyzer as per the standard formula (BMI = weight [kg]/height2 [m2][17]). For this calculation, the height (in centimeters) had to be manually fed in the machine for each person. It was measured using a stature meter. The participants were barefoot, and the hair was made flat. They were told to keep their feet together, with heels, buttocks, and shoulders touching the wall. They were made to stand erect, looking straight ahead with the hands hanging by the sides in a natural manner. The head was positioned in the Frankfurt plane.
Statistical analysis
Data of the participants are presented as mean ± standard deviation (SD) or median (interquartile range) for all continuous variables. Participants were stratified into tertiles of TG/HDL-C ratio. The variability in lipid parameters including HDL-C, TG, TC, LDL-C, non-HDL-C, TC/HDL-C ratio; and nonlipid parameters including hs-CRP, BMI, and body fat were examined across tertiles, and the difference between groups were assessed using one-way ANOVA. Post hoc analysis for multiple comparisons was done using Bonferroni correction in P value. Wilcoxon signed-rank test and Kruskal–Wallis equality-of-populations rank test was used when the variables had a nonnormal distribution. The data analysis was conducted using STATA version 12 (Stata Corp., College Station, TX, USA), P < 0.05 was considered statistically significant.
Abnormal level of the TG/HDL-C ratio was defined as ≥4.0. While ATP-III recommendations identify a normal TG/HDL-C ratio as <3.8, based on recommended levels of normal fasting TG (<150 mg/dL) and normal HDL-C (≥40 mg/dL), the ratio cutoff used in practice varies slightly with different populations, ethnicities, and medical history.[9,12,18] In addition, keeping in view the measurement errors that are likely to be there and the greater day-to-day variation in TG,[19] a value of 4 would unequivocally indicate risk. Furthermore, a cut-off value of 4 has been validated by other authors.[12,20]
RESULTS
Demographic, anthropometric, and biochemical characteristics
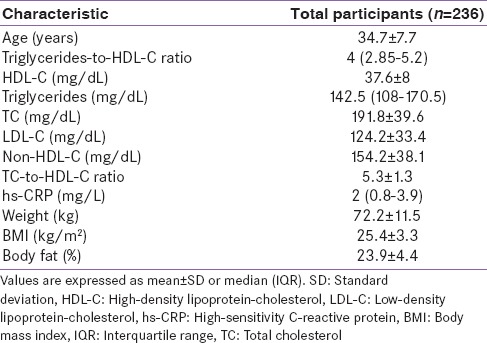
Table 1 displays various characteristics of the 236 study participants. The mean (SD) age of the participants was 34.7 (7.7) years and composed of 100% men. The median (interquartile range [IQR]) TG/HDL-C ratio was 4 (2.85–5.2). In addition, an abnormally high value of TG/HDL-C ratio (TG/HDL-C ratio ≥ 4.0) was recorded among 51.3% of the participants (n = 121 of 236). Other parameters of the biochemical profile including TC, LDL-C, and non-HDL-C recorded normal mean values, although not optimal as per ATP-III guidance. However, three nonlipid metrics including inflammatory marker hs-CRP, BMI, and body fat percentage were aberrant, which was in reasonable concordance with the elevated average value of TG/HDL-C ratio.
Table 1.
Biochemical and anthropometric profile of the participants
Relation of triglyceride/high-density lipoprotein-cholesterol ratio tertiles to other biochemical and anthropometric variables
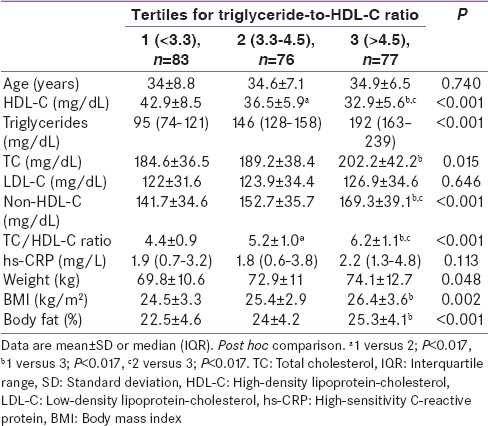
Across the tertiles of TG/HDL-C ratio [Table 2], there was a significant trend of increasing risk-in lipid parameters non-HDL-C*, TC/HDL-C ratio*, TG*, HDL-C*, TC**; and the nonlipid parameters of body fat percentage* and BMI*** (*P < 0.001, **P = 0.015, ***P = 0.002). LDL-C showed a nonsignificant (P = 0.646) and only a moderate increase across the TG/HDL-C ratio tertiles. There was minimal difference in age (P = 0.740) and a modest increase in hs-CRP (P = 0.113), across the three groups.
Table 2.
Biochemical and anthropometric markers of participants according to tertiles of the triglyceride-to-high-density lipoprotein-cholesterol ratio
DISCUSSION
In the present study, a markedly high average level of TG/HDL-C ratio was observed. More than half the participants recorded an abnormal value (≥4.0) indicating high prevalence. Further, the TG/HDL-C ratio demonstrated a close relationship with other lipid parameters, BMI and body fat, apart from its previously documented unique association with sd-LDL particles. The evidence suggests that TG/HDL-C ratio is an important lipid fraction in apparently healthy, young, and middle-aged Indian males.
While there has been consistent growth in evidence on the potential value of TG/HDL-C ratio for cardiovascular risk assessment,[10,12] the relevance of this lipoprotein ratio to Indians had been inadequately addressed. Literature that found a strong association with premature CHD[20] further highlighted its potential value for the Indian population. The high average value of TG/HDL-C ratio in the present sample of Indian males is an important observation. The median (IQR) TG/HDL-C ratio of 4 (2.85–5.2) is noticeably higher than the (gender-specific) cutoff points proposed by others: 2.75,[21] 3.5,[10] and 3.75.[19] While these cutoff points are largely applied to determine individual-level risk, this finding observed in an apparently healthy and relatively younger group is notable. The high TG/HDL-C ratio value may be explained to an extent by the known racial/ethnic susceptibility of the Indian populations to display very commonly a form of dyslipidemia that is characterized by substantially higher TG and lower HDL-C levels.[22] Hadaegh et al.[23] reported a TG/HDL-C ratio median (IQR) value of 4.34 (2.69–6.75) in a sample of apparently healthy urban male adults of Asian origin (Iranian), whereas the median/mean values of TG/HDL-C ratio ranged from only 1.3 to 2.9[21,24,25] in apparently healthy study populations of Caucasian origin. This attribute of a high TG/HDL-C ratio in the Indian/Asian populations could potentially be one of the mediating factors or an explanatory risk factor underlying the high incidence of CHD in India.
Another key finding of this study was that more than half the participants recorded an abnormally high value of TG/HDL-C ratio (≥4.0). This was a remarkably large proportion of participants to have signs of potential cardiometabolic abnormality, especially in view of the disease-free status and lower age range (mean age = years 34.7 ± 7.7) of this group. The TG/HDL-C ratio is known to indicate abnormality in people who may not have yet developed high blood glucose or hypertension,[26] unlike the diagnosis of metabolic syndrome (also linked to insulin resistance) wherein two of the five criteria would capture participants only with known disease. This suggests that TG/HDL-C ratio may be an ‘early detector’ of lipid abnormality and could be used to identify individuals without clinical signs yet. In addition, the increase in TG/HDL-C ratio levels was independent of change in age [Table 2] within this younger age group (25–44 years). In contrast, a notably higher age and even higher BMI has been reported in persons diagnosed with metabolic syndrome.[26]
With CVD operating on a continuum,[27] any potential parameter that displays a capability of indicating relativity in risk level will hold high value to therapeutics and public health. Suggestive evidence on risk stratifying attribute of TG/HDL-C ratio has emerged in a few recent studies conducted among other geographical and racial groups including Iranian men[23] and US adults,[25] but this research is at a nascent stage. In the current sample, the TG/HDL-C ratio variability was an indicator of variability in almost the entire group of the conventional lipid parameters and anthropometric parameters while showing sensitivity to the degree of derangement at a single point in time. This finding further contributes to growing evidence that TG/HDL-C ratio could be a better lipid fraction. In addition, the present analysis noted an exception to the observed gradient. The LDL-C, which is currently the main CHD risk marker in clinical guidelines and the goal of therapy as per the widely followed ATP-III guidance,[19] showed a lack of significant change even in the backdrop of increasing overall abnormality in conventional lipid fractions and anthropometry. This suggests that the TG/HDL-C ratio could be a better marker linked to CHD risk – even beyond the LDL-C. This similar pattern in LDL values was observed across increasing TG/HDL-C ratio levels in another published study.[25] Several authors have explained that while LDL-C values may remain unchanged in a population, a potentially increased cardiometabolic risk is related to an increasing density and decreasing size of the LDL particles.[28,29] It is the increase in these sd-LDL particles, which has powerful atherogenic risk potential, possibly due to their increased permeability into the arterial wall, increased oxidation (since decreased interaction with LDL receptors), and robust binding to proteoglycans in the intimal matrix (thus more readily retained).[8] This insight gives a reasonable clarification to the lack of significant change in LDL-C. The observed trends across increasing TG/HDL-C ratio tertiles are further understood by accumulating evidence on the robust positive association of sd-LDL particles to TG/HDL-C ratio.[9,18] This strengthens the position of TG/HDL-C ratio over LDL-C as a refined lipid marker in the present sample.
A key strength of the current study is that it generated vital evidence on the prevalence of the lipid marker TG/HDL-C ratio among Indians, a racial group for which there was insignificant data. This addressed an important gap in literature as recent global evidence pointed to TG/HDL-C ratio as the strongest marker of CHD as well as its potential status of a surrogate marker to the South Asian phenotype of high TG, sd-LDL, and low HDL-C.[12,20] Further, we believe that as a marker which can be conveniently measured through simple laboratory testing, TG/HDL-C ratio has substantial value in population level assessments. Given that this finding is preliminary in Indian population, we suggest that similar studies should be conducted to assess this lipid marker in a larger population that includes women – the present study is limited to a male sample as inclusion of females would have warranted a much larger scale to enable analysis by gender.[30,31]
CONCLUSION
In the present sample of apparently healthy, young, and middle-aged Indian men, there was high prevalence of abnormal TG/HDL-C ratio. The TG/HDL-C ratio emerged as a simple and effective early detector of lipid abnormality in the absence of any clinical signs of disease. In addition, apart from its previously documented unique association with sd-LDL particles, the TG/HDL-C ratio demonstrated a close relationship with other conventional lipid parameters and measures of adiposity. It was an indicator of abnormality represented by these variables while showing sensitivity to the degree of derangement at a single point in time. Evidence from the present analysis also suggested its superiority over LDL-C as a refined lipid marker in younger Indians with an otherwise disease-free status. TG/HDL-C ratio has potential use in primary prevention. Future research should investigate the responsiveness of TG/HDL-C ratio to interventions to establish its functional use in public health. In addition, long-term cohort studies should evaluate its predictive value for metabolic manifestations including incident CHD.
Financial support and sponsorship
This research was financially supported by the Department of Science and Technology, Government of India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank Dr. D. Prabhakaran for the valuable comments and editing of manuscript.
REFERENCES
- 1.Reddy KS. Cardiovascular disease in non-Western countries. N Engl J Med. 2004;350:2438–40. doi: 10.1056/NEJMp048024. [DOI] [PubMed] [Google Scholar]
- 2.Reddy KS. Cardiovascular diseases in the developing countries: Dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2002;5:231–7. doi: 10.1079/phn2001298. [DOI] [PubMed] [Google Scholar]
- 3.Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in India: Current epidemiology and future directions. Circulation. 2016;133:1605–20. doi: 10.1161/CIRCULATIONAHA.114.008729. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Kelly BB. Board for Global Health. Promoting Cardiovascular Health in Developing World: A Critical Challenge to Achieve Global Health. Washington, DC: Institutes of Medicine; 2010. [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). WHO Global Report 2005 – Preventing Chronic Diseases: A Vital Investment. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 6.Leeder S, Raymond S, Greenberg H, Liu H, Esson K. A Race Against Time: The Challenge of Cardiovascular Disease in Developing Economies. New York: Columbia University; 2004. [Google Scholar]
- 7.Reaven GM, Chen YD, Jeppesen J, Maheux P, Krauss RM. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest. 1993;92:141–6. doi: 10.1172/JCI116541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwiterovich PO., Jr Clinical relevance of the biochemical, metabolic, and genetic factors that influence low-density lipoprotein heterogeneity. Am J Cardiol. 2002;90:30i–47i. doi: 10.1016/s0002-9149(02)02749-2. [DOI] [PubMed] [Google Scholar]
- 9.Hanak V, Munoz J, Teague J, Stanley A, Jr, Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94:219–22. doi: 10.1016/j.amjcard.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 11.Liu A, Reaven GM. Is measurement of non-HDL cholesterol an effective way to identify the metabolic syndrome? Nutr Metab Cardiovasc Dis. 2013;23:1122–7. doi: 10.1016/j.numecd.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Luz PL, Favarato D, Faria-Neto JR, Jr, Lemos P, Chagas AC. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics (Sao Paulo) 2008;63:427–32. doi: 10.1590/S1807-59322008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar N, Gupta N, Kishore J. Kuppuswamy's socioeconomic scale: Updating income ranges for the year 2012. Indian J Public Health. 2012;56:103–4. doi: 10.4103/0019-557X.96988. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC)/National Center for Health Statistics (NCHS). Laboratory Procedure Manual: Total Cholesterol, Direct HDL, Precipitated HDL, Triglycerides, and LDL. Lab Protocol for NHANES 2003-2004 Data. [Last cited on 2015 Jun 19]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l13_c_met_lipids.pdf .
- 15.Gan SD, Patel KR. Enzyme immunoassay and enzyme-linked immunosorbent assay. J Invest Dermatol. 2013;133:e12. doi: 10.1038/jid.2013.287. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147–53. [PubMed] [Google Scholar]
- 18.Bhalodkar NC, Blum S, Enas EA. Accuracy of the ratio of triglycerides to high-density lipoprotein cholesterol for predicting low-density lipoprotein cholesterol particle sizes, phenotype B, and particle concentrations among Asian Indians. Am J Cardiol. 2006;97:1007–9. doi: 10.1016/j.amjcard.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 19.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 20.da Luz PL, Cesena FH, Favarato D, Cerqueira ES. Comparison of serum lipid values in patients with coronary artery disease at <50, 50 to 59, 60 to 69, and >70 years of age. Am J Cardiol. 2005;96:1640–3. doi: 10.1016/j.amjcard.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 21.Cordero A, Laclaustra M, León M, Casasnovas JA, Grima A, Luengo E, et al. Comparison of serum lipid values in subjects with and without the metabolic syndrome. Am J Cardiol. 2008;102:424–8. doi: 10.1016/j.amjcard.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 22.Enas EA, Garg A, Davidson MA, Nair VM, Huet BA, Yusuf S. Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart J. 1996;48:343–53. [PubMed] [Google Scholar]
- 23.Hadaegh F, Khalili D, Ghasemi A, Tohidi M, Sheikholeslami F, Azizi F. Triglyceride/HDL-cholesterol ratio is an independent predictor for coronary heart disease in a population of Iranian men. Nutr Metab Cardiovasc Dis. 2009;19:401–8. doi: 10.1016/j.numecd.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Ford ES, Meng YX, Mokdad AH, Reaven GM. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc Diabetol. 2008;7:4. doi: 10.1186/1475-2840-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quispe R, Manalac RJ, Faridi KF, Blaha MJ, Toth PP, Kulkarni KR, et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: The Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis. 2015;242:243–50. doi: 10.1016/j.atherosclerosis.2015.06.057. [DOI] [PubMed] [Google Scholar]
- 26.Flowers E, Molina C, Mathur A, Reaven GM. Use of plasma triglyceride/high-density lipoprotein cholesterol ratio to identify increased cardio-metabolic risk in young, healthy South Asians. Indian J Med Res. 2015;141:68–74. doi: 10.4103/0971-5916.154506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, et al. The cardiovascular disease continuum validated: Clinical evidence of improved patient outcomes: Part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease) Circulation. 2006;114:2850–70. doi: 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 28.Mudd JO, Borlaug BA, Johnston PV, Kral BG, Rouf R, Blumenthal RS, et al. Beyond low-density lipoprotein cholesterol: Defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;50:1735–41. doi: 10.1016/j.jacc.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 29.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol. 2002;90:22i–9i. doi: 10.1016/s0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- 30.Taking sex into account in medicine. Lancet. 2011;378:1826. doi: 10.1016/S0140-6736(11)61795-9. [DOI] [PubMed] [Google Scholar]
- 31.Salazar MR, Carbajal HA, Espeche WG, Leiva Sisnieguez CE, Balbín E, Dulbecco CA, et al. Relation among the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio, insulin resistance, and associated cardio-metabolic risk factors in men and women. Am J Cardiol. 2012;109:1749–53. doi: 10.1016/j.amjcard.2012.02.016. [DOI] [PubMed] [Google Scholar]


