Abstract
Background:
Clinical relevance of association of cabergoline use for hyperprolactinemia and cardiac valvulopathy remains unclear.
Objective:
The aim of the study was to determine the prevalence of valvular heart abnormalities in patients taking cabergoline for the treatment of prolactinoma and to explore any associations with the cumulative dose of drug used.
Design:
A cross-sectional echocardiographic study was performed in patients who were receiving cabergoline therapy for prolactinoma.
Results:
Hundred (61 females, 39 males) prolactinoma cases (81 macroprolactinoma and 19 microprolactinoma) were included in the study. The mean age at presentation was 33.9 ± 9.0 years (range: 16–58 years). The mean duration of treatment was 53.11 ± 43.15 months (range: 12–155 months). The mean cumulative dose was 308.6 ± 290.2 mg (range: 26–1196 mg; interquartile range: 104–416 mg). Mild mitral regurgitation was present in one patient (cumulative cabergoline dose 104 mg). Mild tricuspid regurgitation was present in another two patients (cumulative cabergoline dose 52 mg and 104 mg). Aortic and pulmonary valve functioning was normal in all the cases. There were no cases of significant valvular regurgitation (moderate to severe, Grade 3–4). None of the patients had morphological abnormalities such as thickening, calcification, and restricted mobility of any of the cardiac valves.
Conclusion:
Cabergoline appears to be safe in patients with prolactinoma up to the cumulative dose of ~300 mg. The screening for valvulopathy should be restricted to those with higher cumulative cabergoline exposure.
Keywords: Cabergoline, prolactinoma, valvulopathy
INTRODUCTION
Dopamine agonists (DA) represent first-line therapy for the treatment of prolactinomas. Among the available DA, cabergoline is preferentially used due to its higher efficacy, better tolerability, and favorable pharmacokinetic profile. Cabergoline usage in a dose ranging from 0.5-2.0 mg/week normalizes serum prolactin and achieves significant tumor shrinkage in most of the prolactinoma cases.[1,2] However, drug treatment duration is long lasting, varying from few years to ongoing drug therapy. There have been concerns regarding the safety of ongoing long-term cabergoline therapy.
Zanettini et al. reported 28.6% prevalence of cardiac valvular regurgitation (moderate to severe) in patients with Parkinson's disease (PD) receiving cabergoline. Notably, affected patients were exposed to higher cumulative dose, suggesting a dose-response relationship.[2] Due to serotonin subtype 2B (5-HT2B) agonist activity, cabergoline may cause inappropriate proliferation of valvular endothelial cells and subvalvular apparatus. Cabergoline associated cardiac valvulopathy (CAV) presents as triad of valvular regurgitation, along with restricted and thickened valve.[1,2]
For safety concern, the European medicines agency and the UK medicines and healthcare products regulatory agency recommend that patients receiving cabergoline for prolactinomas should undergo a baseline two-dimensional echocardiography (2D echo) and a repeat study at 3–6 months initially, and at 6–12-month intervals thereafter.[3] Whereas the endocrine society guideline suggests that 2D echo may be required for those on high-dose cabergoline therapy (>3 mg/week).[4]
Over 20 studies (5–27) have examined the frequency of valvular heart disease (VHD) in patients receiving cabergoline for prolactinomas. None of them (except one) have reported a higher frequency of significant valvular regurgitation, in cabergoline exposed prolactinoma patients as compared to healthy controls. There is scanty literature on this subject from the Indian subcontinent. Hence, a cross-sectional study to look for the proposed association was planned in our patient cohort.
MATERIALS AND METHODS
The study was cross-sectional and was approved by institutional review board.
Patients
Medical records (2005–2015) of patients diagnosed with prolactinoma were reviewed. Diagnosis of prolactinoma was based on elevated serum prolactin and visualization of pituitary adenoma on magnetic resonance imaging. Prolactinoma patients receiving ongoing cabergoline treatment (>12 months) were identified and recalled. Patients with known VHD, carcinoid syndrome, history of use of additional drugs associated with cardiac valvulopathy (ergotamine, methysergide, fenfluramine, or dexfenfluramine) and co-secretory pituitary adenomas (prolactin and growth hormone/adrenocorticotropic hormone) were excluded from this study. Patients meeting the inclusion criteria underwent 2D echo study. The cumulative dose of cabergoline was recorded.
Echocardiographic measurements
Echocardiography studies were performed by an experienced cardiologist, using a Philips iE33 System (Philips Medical Systems, Bothell, WA, USA), equipped with a phased-array transducer (5–1 MHz). ProSolv CardioVascular Analyzer software version 3.5 (Mount International Ultrasound Services Ltd, The Glenmore Centre, Gloucester, UK) was used for reporting, frame-by-frame analysis, and quantification of the lesions.
2D echo and color Doppler were performed according to the recommendations of the American Society of Echocardiography and the European Association of Echocardiography, using a standardized protocol. Assessment included the evaluation of function and morphology of cardiac valves. Valvular regurgitation was graded as absent/trace (Grade 0), mild (Grade 1), moderate (Grade 2), or severe (Grade 3) using multiple parameters. Moderate or severe valvular regurgitation was considered clinically significant dysfunction. Valve leaflet mobility, thickness (>0·5 cm is significant), and calcification were noted. In addition, thickening of the chordae tendineae was also recorded.
RESULTS
Hundred prolactinoma cases (81 macroprolactinoma and 19 microprolactinoma) were included in the study. Among macroprolactinomas, there were 42 females and 39 males, whereas all the microprolactinoma cases were females. The mean age at presentation was 33.9 ± 9.0 years (range: 16–58 years). The mean duration of treatment was 53.11 ± 43.15 months (range: 12–155 months). The mean cumulative dose was 308.6 ± 290.2 mg (range: 26–1196 mg; interquartile range: 104–416 mg). Mild mitral regurgitation (MR) was present in one patient (cumulative cabergoline dose 104 mg). Mild tricuspid regurgitation (TR) was present in another two patients (cumulative cabergoline dose 52 mg and 104 mg). Aortic and pulmonary valve functioning was normal in all cases. There were no cases of significant valvular regurgitation (moderate to severe, Grade 3–4). None of the patients had morphological abnormalities such as thickening, calcification, and restricted mobility of any of the cardiac valves.
DISCUSSION
In our cohort of prolactinoma patients on cabergoline (mean cumulative dose: 308 mg) treatment, clinically significant cardiac valvulopathy was absent.
Our results are in accordance to the previously published studies [Tables 1 and 2]. Approximately, 2000 prolactinoma patients on cabergoline (cumulative dose-mean: 152–465 mg, range: 15–3385 mg) have been studied for cardiac valvulopathy.[5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] None of the studies except one[10] have reported a higher prevalence of significant valvular regurgitation in cabergoline exposed prolactinoma patients compared to controls. In the study described by Colao et al.,[10] cabergoline exposed prolactinoma patients had significantly higher prevalence of moderate TR (54%) than healthy controls (0%). Patients treated with a higher cumulative dose (>280 mg) had higher prevalence of moderate TR (72%) than those treated with a lower dose (36%) (P = 0.023). These finding of this group have been distinctly unique and have not been replicated by other studies. Further Colao et al. in a prospective study of 40 patients followed over 5 years did not found any significant valvular lesions. Wakil et al. (mean cumulative cabergoline exposure 311 mg) reported significantly higher mild TR (11.36%) and mild pulmonary regurgitation (PR) (2.27%) in cabergoline exposed prolactinoma patients compared to controls. Few other studies as shown in Table 1, have reported significantly higher prevalence of mild TR, mild PR, trace MR. Clinical relevance of increased prevalence of these nonsignificant valve diseases is not clear.
Table 1.
Literature on valvular abnormalities in prolactinoma patients on cabergoline (studies with controls)
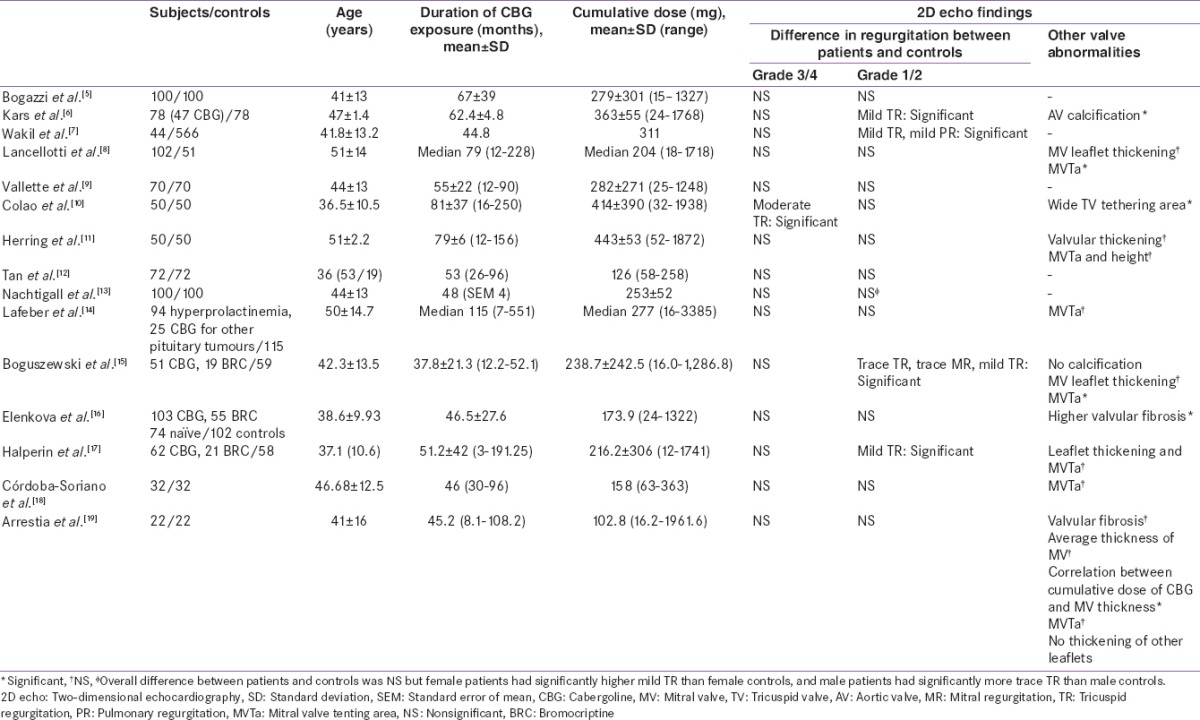
Table 2.
Literature on valvular abnormalities in prolactinoma patients on cabergoline (studies without controls)
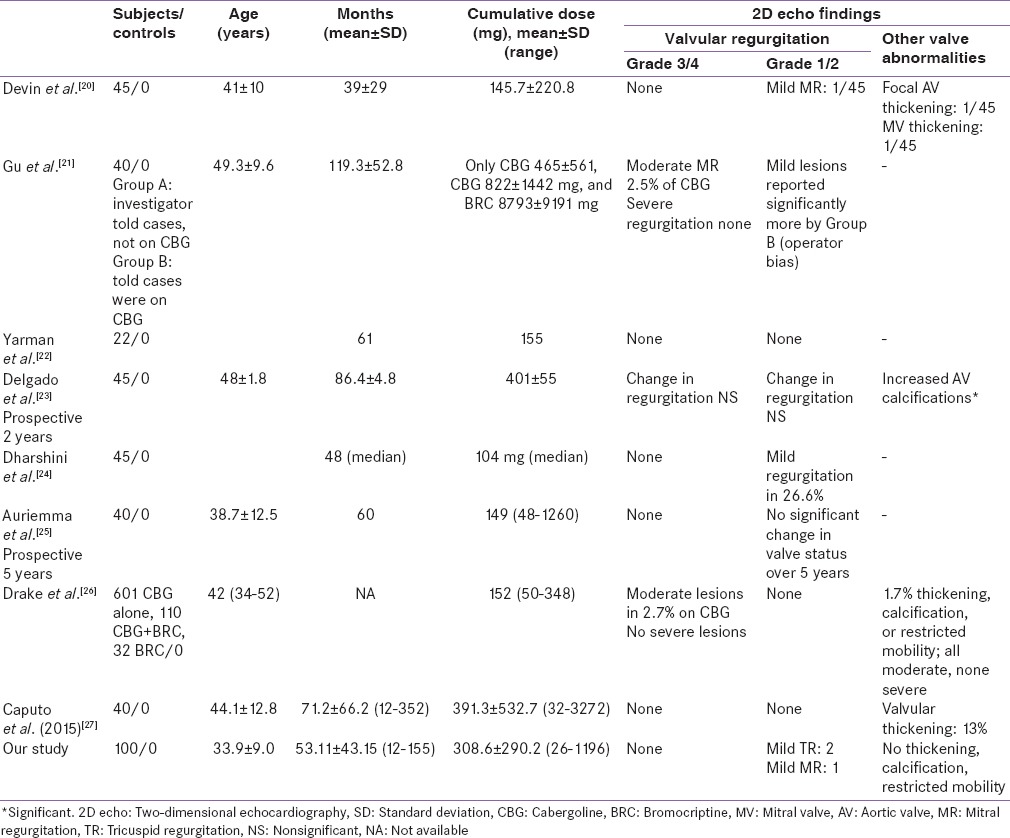
Most studies have focused on the prevalence of any valvular lesion as detected by 2D echo, without distinguishing morphological changes of CAV. Only two cases of morphologically proven CAV are reported in literature. Cawood et al., reported a case study of a 59-year-old man with prolactinoma, on cabergoline therapy (cumulative dose of 252 mg over 3·5 years). This patient had severe MR associated with thickening of valve due to a fibrous proliferation (proven on histopathology). Moreover, this patient had a baseline normal 2D echo at the time of initiating cabergoline.[28] Another case reported by Gu et al. had moderate MR associated with restriction of the posterior mitral leaflet and thickening of the tips of both leaflets and the chordae, but with no commissural fusion. These appearances were consistent with CAV as well.[21] Hence, it is important to take into account the morphological features. Notably, none of the patients from our cohort had morphological abnormalities such as thickening, calcification, and restricted mobility of any of the cardiac valve. Few studies have reported significantly higher prevalence of valvular abnormalities such as valve calcification,[7,18] higher tricuspid valve tethering area,[11] and higher mitral valve tenting area.[9,19] Implications of such findings need to prove by larger prospective studies with higher cumulative doses.
Overall normal 2D echo finding in carbergoline treated prolactinoma patients is plausible as, most of the prolactinoma patients are on ~1–2 mg/week cabergoline. To extrapolate, it will take >30 years to reach a cumulative cabergoline dose of 3000 mg, a dose after at which cardiac valvulopathy is reported in PD patients. Cabergoline is a relatively recent drug in prolactinoma management. Long-term follow-up of these patients when they reach comparable cumulative dose exposure as that of PD will help in addressing CAV in prolactinoma patients. Large prospective controlled studies are required to make evidence-based recommendations.
A limited number of patients, retrospective study design, and lack of control arm can be considered as limitations of our study. However, as none of the subjects had significant valve disease, the lack of control arm is unlikely to have altered the results.
CONCLUSION
Cabergoline appears to be safe in patients with prolactinoma up to the cumulative dose of ~300 mg. The screening for valvulopathy should be restricted to those with higher cumulative cabergoline exposure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Valassi E, Klibanski A, Biller BM. Clinical Review#: Potential cardiac valve effects of dopamine agonists in hyperprolactinemia. J Clin Endocrinol Metab. 2010;95:1025–33. doi: 10.1210/jc.2009-2095. [DOI] [PubMed] [Google Scholar]
- 2.Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson's disease. N Engl J Med. 2007;356:39–46. doi: 10.1056/NEJMoa054830. [DOI] [PubMed] [Google Scholar]
- 3.Medicines and Healthcare Products Regulatory Agency. Drug Safety Update: Ergot-Derived Dopamine Agonists: Risk of Fibrotic Reactions in Chronic Endocrine Uses. Vol. 2. London: Drug Safety Update; 2008. Oct, p. 2. [Google Scholar]
- 4.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:273–88. doi: 10.1210/jc.2010-1692. [DOI] [PubMed] [Google Scholar]
- 5.Bogazzi F, Buralli S, Manetti L, Raffaelli V, Cigni T, Lombardi M, et al. Treatment with low doses of cabergoline is not associated with increased prevalence of cardiac valve regurgitation in patients with hyperprolactinaemia. Int J Clin Pract. 2008;62:1864–9. doi: 10.1111/j.1742-1241.2008.01779.x. [DOI] [PubMed] [Google Scholar]
- 6.Kars M, Delgado V, Holman ER, Feelders RA, Smit JW, Romijn JA, et al. Aortic valve calcification and mild tricuspid regurgitation but no clinical heart disease after 8 years of dopamine agonist therapy for prolactinoma. J Clin Endocrinol Metab. 2008;93:3348–56. doi: 10.1210/jc.2007-2658. [DOI] [PubMed] [Google Scholar]
- 7.Wakil A, Rigby AS, Clark AL, Kallvikbacka-Bennett A, Atkin SL. Low dose cabergoline for hyperprolactinaemia is not associated with clinically significant valvular heart disease. Eur J Endocrinol. 2008;159:R11–4. doi: 10.1530/EJE-08-0365. [DOI] [PubMed] [Google Scholar]
- 8.Lancellotti P, Livadariu E, Markov M, Daly AF, Burlacu MC, Betea D, et al. Cabergoline and the risk of valvular lesions in endocrine disease. Eur J Endocrinol. 2008;159:1–5. doi: 10.1530/EJE-08-0213. [DOI] [PubMed] [Google Scholar]
- 9.Vallette S, Serri K, Rivera J, Santagata P, Delorme S, Garfield N, et al. Long-term cabergoline therapy is not associated with valvular heart disease in patients with prolactinomas. Pituitary. 2009;12:153–7. doi: 10.1007/s11102-008-0134-2. [DOI] [PubMed] [Google Scholar]
- 10.Colao A, Galderisi M, Di Sarno A, Pardo M, Gaccione M, D’Andrea M, et al. Increased prevalence of tricuspid regurgitation in patients with prolactinomas chronically treated with cabergoline. J Clin Endocrinol Metab. 2008;93:3777–84. doi: 10.1210/jc.2007-1403. [DOI] [PubMed] [Google Scholar]
- 11.Herring N, Szmigielski C, Becher H, Karavitaki N, Wass JA. Valvular heart disease and the use of cabergoline for the treatment of prolactinoma. Clin Endocrinol (Oxf) 2009;70:104–8. doi: 10.1111/j.1365-2265.2008.03458.x. [DOI] [PubMed] [Google Scholar]
- 12.Tan T, Cabrita IZ, Hensman D, Grogono J, Dhillo WS, Baynes KC, et al. Assessment of cardiac valve dysfunction in patients receiving cabergoline treatment for hyperprolactinaemia. Clin Endocrinol (Oxf) 2010;73:369–74. doi: 10.1111/j.1365-2265.2010.03827.x. [DOI] [PubMed] [Google Scholar]
- 13.Nachtigall LB, Valassi E, Lo J, McCarty D, Passeri J, Biller BM, et al. Gender effects on cardiac valvular function in hyperprolactinaemic patients receiving cabergoline: A retrospective study. Clin Endocrinol (Oxf) 2010;72:53–8. doi: 10.1111/j.1365-2265.2009.03608.x. [DOI] [PubMed] [Google Scholar]
- 14.Lafeber M, Stades AM, Valk GD, Cramer MJ, Teding van Berkhout F, Zelissen PM. Absence of major fibrotic adverse events in hyperprolactinemic patients treated with cabergoline. Eur J Endocrinol. 2010;162:667–75. doi: 10.1530/EJE-09-0989. [DOI] [PubMed] [Google Scholar]
- 15.Boguszewski CL, dos Santos CM, Sakamoto KS, Marini LC, de Souza AM, Azevedo M. A comparison of cabergoline and bromocriptine on the risk of valvular heart disease in patients with prolactinomas. Pituitary. 2012;15:44–9. doi: 10.1007/s11102-011-0339-7. [DOI] [PubMed] [Google Scholar]
- 16.Elenkova A, Shabani R, Kalinov K, Zacharieva S. Increased prevalence of subclinical cardiac valve fibrosis in patients with prolactinomas on long-term bromocriptine and cabergoline treatment. Eur J Endocrinol. 2012;167:17–25. doi: 10.1530/EJE-12-0121. [DOI] [PubMed] [Google Scholar]
- 17.Halperin I, Aller J, Varela C, Mora M, Abad A, Doltra A, et al. No clinically significant valvular regurgitation in long-term cabergoline treatment for prolactinoma. Clin Endocrinol (Oxf) 2012;77:275–80. doi: 10.1111/j.1365-2265.2012.04349.x. [DOI] [PubMed] [Google Scholar]
- 18.Córdoba-Soriano JG, Lamas-Oliveira C, Hidalgo-Olivares VM, Tercero-Martínez A, Barambio-Ruíz M, Salas-Nieto J. Valvular heart disease in hyperprolactinemic patients treated with low doses of cabergoline. Rev Esp Cardiol (Engl Ed) 2013;66:410–2. doi: 10.1016/j.rec.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Arrestia D, Florio L, Gutierrez M, Elhordoy M, Seoane E, Alonso C, et al. Valvular heart disease in patients with prolactinomas on cabergoline treatment. Endocrinol Metab Int J. 2015;2:30. [Google Scholar]
- 20.Devin JK, Lakhani VT, Byrd BF, 3rd, Blevins LS., Jr Prevalence of valvular heart disease in a cohort of patients taking cabergoline for management of hyperprolactinemia. Endocr Pract. 2008;14:672–7. doi: 10.4158/EP.14.6.672. [DOI] [PubMed] [Google Scholar]
- 21.Gu H, Luck S, Carroll PV, Powrie J, Chambers J. Cardiac valve disease and low-dose dopamine agonist therapy: An artefact of reporting bias? Clin Endocrinol (Oxf) 2011;74:608–10. doi: 10.1111/j.1365-2265.2010.03973.x. [DOI] [PubMed] [Google Scholar]
- 22.Yarman S, Kurtulmus N, Bilge A. Optimal effective doses of cabergoline and bromocriptine and valvular leasions in men with prolactinomas. Neuro Endocrinol Lett. 2012;33:340–6. [PubMed] [Google Scholar]
- 23.Delgado V, Biermasz NR, van Thiel SW, Ewe SH, Marsan NA, Holman ER, et al. Changes in heart valve structure and function in patients treated with dopamine agonists for prolactinomas, a 2-year follow-up study. Clin Endocrinol (Oxf) 2012;77:99–105. doi: 10.1111/j.1365-2265.2011.04326.x. [DOI] [PubMed] [Google Scholar]
- 24.Dharshini K, Somasundaram N, Senevirathna HM, Kumar SR. No link to cardiac valvulopathy was seen in cabergoline treated patients. Sri Lanka J Diabetes Endocrinol Metab. 2012;2:69–72. [Google Scholar]
- 25.Auriemma RS, Pivonello R, Perone Y, Grasso LF, Ferreri L, Simeoli C, et al. Safety of long-term treatment with cabergoline on cardiac valve disease in patients with prolactinomas. Eur J Endocrinol. 2013;169:359–66. doi: 10.1530/EJE-13-0231. [DOI] [PubMed] [Google Scholar]
- 26.Drake WM, Stiles CE, Howlett TA, Toogood AA, Bevan JS, Steeds RP UK Dopamine Agonist Valvulopathy Group. A cross-sectional study of the prevalence of cardiac valvular abnormalities in hyperprolactinemic patients treated with ergot-derived dopamine agonists. J Clin Endocrinol Metab. 2014;99:90–6. doi: 10.1210/jc.2013-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caputo C, Prior D, Inder WJ. The need for annual echocardiography to detect cabergoline-associated valvulopathy in patients with prolactinoma: A systematic review and additional clinical data. Lancet Diabetes Endocrinol. 2015;3:906–13. doi: 10.1016/S2213-8587(14)70212-8. [DOI] [PubMed] [Google Scholar]
- 28.Cawood TJ, Bridgman P, Hunter L, Cole D. Low-dose cabergoline causing valvular heart disease in a patient treated for prolactinoma. Intern Med J. 2009;39:266–7. doi: 10.1111/j.1445-5994.2009.01920.x. [DOI] [PubMed] [Google Scholar]


