Abstract
Currently available antihyperglycemic agents, despite being effective, provide inadequate glycemic control and/or are associated with side effects or nonadherence. Canagliflozin, a widely used orally active inhibitor of sodium-glucose cotransporter 2 (SGLT2), is a new addition to the therapeutic armamentarium of glucose-lowering drugs. This review summarizes findings from different clinical and observational studies of canagliflozin 300 mg in patients with type 2 diabetes mellitus (T2DM). By inhibiting SGLT2, canagliflozin reduces reabsorption of filtered glucose, thereby increasing urinary glucose excretion in patients with T2DM. Canagliflozin 300 mg has been shown to be effective in lowering glycated hemoglobin, fasting plasma glucose, and postprandial glucose in patients with T2DM. Canagliflozin 300 mg also demonstrated significant reductions in body weight and blood pressure and has a low risk of causing hypoglycemia, when not used in conjunction with insulin and insulin secretagogues. Canagliflozin 300 mg was generally well tolerated in clinical studies. The most frequently reported adverse events include genital mycotic infections, urinary tract infections, osmotic diuresis, and volume depletion-related events.
Keywords: Canagliflozin, glycated hemoglobin, hypoglycemic agents, pleiotropic benefits, sodium-glucose cotransporter 2 inhibitor, type 2 diabetes mellitus
INTRODUCTION
The prevalence of diabetes is growing at an alarming rate globally and is reaching epidemic proportions. The current global prevalence of 415 million is projected to increase to 642 million by 2040 if preventive measures are not put in place.[1] Over 60.0% of the world's population with diabetes resides in Asia,[2] of which India and China contribute the largest.[1] The DiabCare study reports that a vast majority (80.3%) of patients with diabetes in India have poor glycemic control and that type 2 diabetes mellitus (T2DM) begins at an early age.[3] Studies from different parts of India have provided evidence of increasing prevalence of overweight and obesity in India.[4,5,6] According to the recent data published in the Indian Council of Medical Research India Diabetes-3 study, the estimated prevalence of overweight and obesity is projected to increase to 88 million and 395 million, respectively.[7] Increase in obesity increases the risk of diabetes.[6,8]
An array of glucose-lowering agents targeting different tissues such as liver, skeletal muscles, and adipose tissues is available for the management of T2DM [Figure 1].[9,10,11,12,13,14] However, they are unable to restore normal levels of glycated hemoglobin (HbA1c) in the long run[15,16,17] and over half of the patients do not attain the American Diabetes Association-recommended glycemic goal (HbA1c <7.0%).[18,19,20,21] In addition, concerns about weight gain, fear and pain with injections, patient adherence, fluid retention, increased risk of hypoglycemia, congestive heart failure, and gastrointestinal disorders hinder their application in clinical practice.[22,23,24,25,26]
Figure 1.
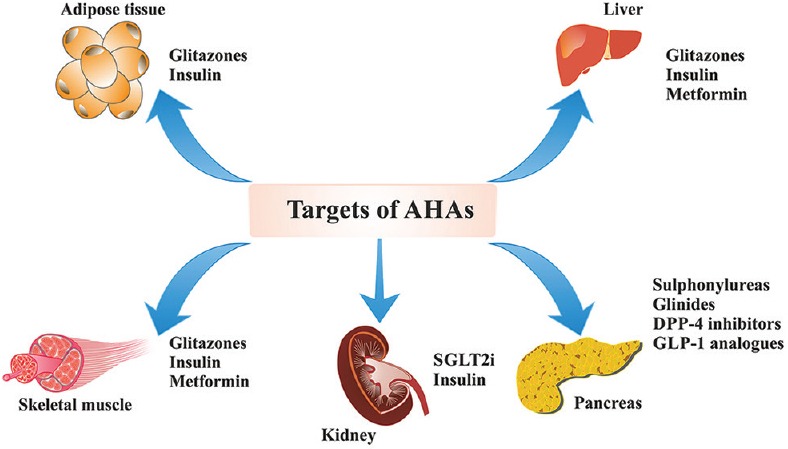
Target organs of antihyperglycemic agents. AHA: Antihyperglycemic agent; DPP-4: Dipeptidyl peptidase-4; GLP-1: Glucagon-like peptide-1; SGLT2i: Sodium-glucose cotransporter 2 inhibitor
The sodium-glucose cotransporter 2 (SGLT2) found in the proximal renal tubule is a low-affinity, high-capacity transporter responsible for the majority (90.0%) of renal glucose reabsorption.[27,28] The SGLT2 inhibitors have a novel mechanism of action as they decrease the amount of glucose reabsorbed. Both national and international guidelines support the use of SGLT2 inhibitors either as monotherapy or as add-on therapy for the management of T2DM.[29,30,31,32,33] Recently, the National Institute for Health and Care Excellence guideline has approved the use of canagliflozin as monotherapy when diet and exercise do not provide adequate glycemic control and metformin was contraindicated or not tolerated or as add-on therapy with antihyperglycemic agents (AHAs), widening the available treatment options for practicing clinicians.[34,35] Among the available SGLT2 inhibitors, canagliflozin is widely used as doses of 100 and 300 mg once daily (OD).[36] Across studies, canagliflozin 300 mg has demonstrated improvement of glycemic and nonglycemic parameters and is generally well tolerated in patients with T2DM.[37,38,39,40] Therefore, the present review summarizes knowledge on canagliflozin 300 mg and the possible differences of canagliflozin 300 mg from other AHAs. This review also aims to understand the additional benefits and risks associated with canagliflozin 300 mg to provide better guidance to the practicing clinicians for recommending canagliflozin 300 mg in patients with T2DM.
REVIEW METHOD
The studies were identified by conducting a literature search from electronic database till August 2016, using PubMed, The Cochrane Library, Google, Google Scholar, and ongoing trials registers at Clinical Trials (http://www.clinicaltrials.gov/). Other data sources included conference posters from International Diabetes Federation, International Society for Pharmacoeconomics and Outcomes Research, and European Association for the Study of Diabetes. The search was made using various Medical Subject Headings terminologies for canagliflozin versus placebo and other comparators to assess its efficacy and safety. These articles were screened and publications considered relevant to the topic were included in the study. Review articles, systematic reviews, and meta-analyses were also included in the review.
CLINICAL PHARMACOLOGY OF CANAGLIFLOZIN
Mechanism of action
Primarily, all the SGLT2 inhibitors act by an insulin-independent mechanism, thereby providing a complementary effect when used in combination with other oral AHAs. Canagliflozin is an orally active, reversible, and selective inhibitor with 250-fold selectivity toward SGLT2 over sodium-glucose cotransporter 1 (SGLT1).[27] By blocking SGLT2, canagliflozin decreases reabsorption of filtered glucose and reduces renal threshold for glucose (RTG), thereby elevating the urinary glucose excretion (UGE) and reducing raised plasma glucose (PG) in patients with T2DM [Figure 2].[41,42,43] The increased UGE causes caloric loss of approximately 300−400 kcal/day with canagliflozin 300 mg,[44] resulting in weight loss.
Figure 2.
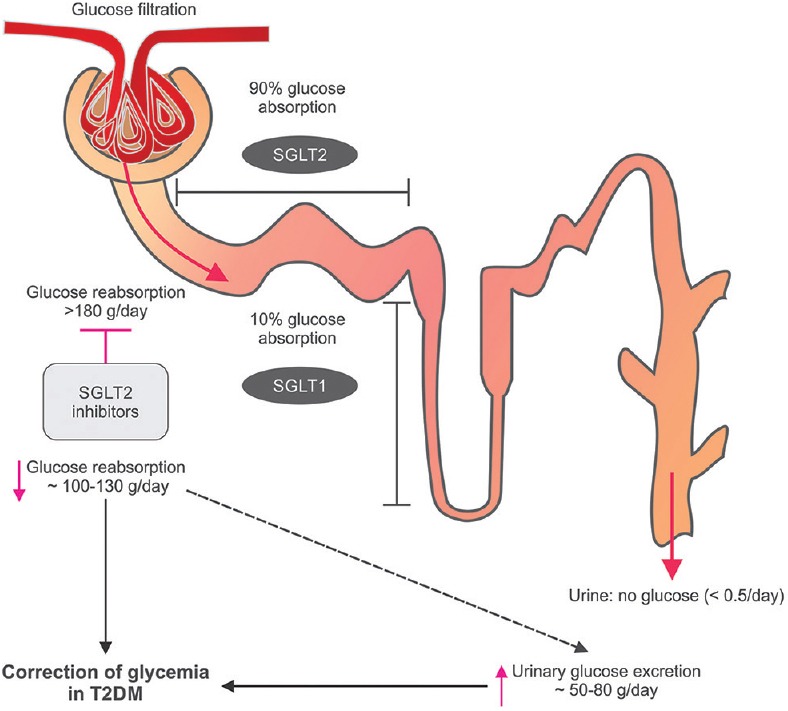
Mechanism of action of sodium-glucose cotransporter 2 inhibitors. T2DM: Type 2 diabetes mellitus; SGLT: Sodium-glucose cotransporter. This figure has been taken from Kalra et al.
In addition, canagliflozin 300 mg transiently inhibits SGLT1, an important intestinal glucose transporter, due to its locally high intestinal concentrations shortly after dosing.[45] However, circulating concentrations of canagliflozin did not meaningfully inhibit SGLT1 (based on the free [unbound] plasma concentrations of canagliflozin and the in vitro inhibitory concentration 50.0%) and studies have shown no glucose malabsorption with canagliflozin as virtually all of the ingested glucose was absorbed during the full 6 h period.[41,45,46] In healthy individuals, canagliflozin 300 mg provided greater reductions in postprandial glucose (PPG) and insulin excursions that could be explained by the increase in UGE due to renal SGLT2 inhibition and delayed absorption of ingested glucose due to intestinal SGLT1 inhibition.[45,47]
Pharmacokinetic and pharmacodynamic properties
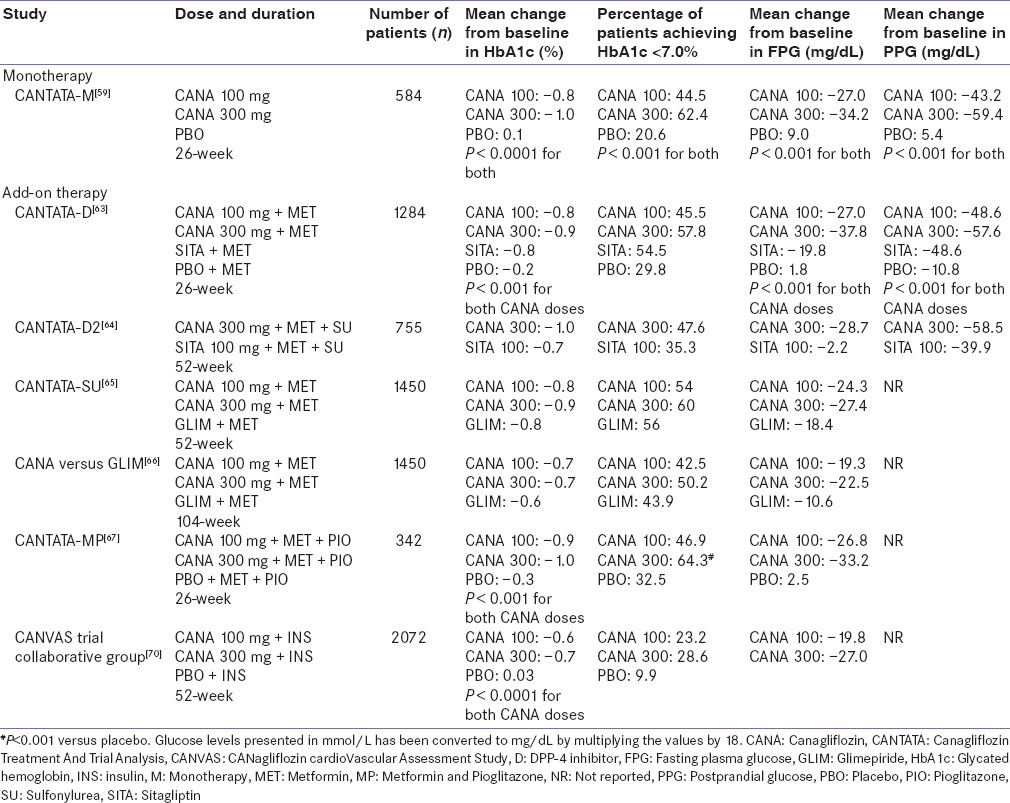
The pharmacokinetic (PK) properties of canagliflozin are similar in healthy individuals and patients with T2DM and are independent of age, gender, body weight, and ethnicity.[36] Dose-dependent increase in maximum plasma canagliflozin concentration (Cmax), area under the plasma concentration-time curve (AUC), and UGE and decrease in RTG were demonstrated in healthy individuals.[48,49,50] The time to achieve Cmax(tmax) of canagliflozin 300 mg was 1.5 h and elimination half-life (t1/2) was 12.6 h in healthy individuals, which supports OD dosing.[50] In patients with T2DM, the mean Cmax was achieved 1–2 h after administration and steady-state concentration was reached after 4 days administration of canagliflozin 100–300 mg OD. The apparent canagliflozin elimination t1/2 and tmax were independent of the dose [Table 1].[41] Canagliflozin is rapidly absorbed and its mean absolute oral bioavailability is nearly 65.0% for a single 300 mg dose.[51] The plasma protein binding of canagliflozin is 99.0% and has no clinically relevant drug–drug interactions, which is therapeutically desired.[52,53] Canagliflozin is metabolized into three inactive O-glucuronidation metabolites: M7, M5, and M9.[51,52] It is predominantly (~60.0%) excreted via the fecal route, the remainder (33.0%) is excreted in urine, and <1.0% is excreted as unchanged drug in the urine [Table 2].[36,52,54,55,56]
Table 1.
Summary of mean pharmacokinetic/pharmacodynamic parameters after single- and multiple-dose of canagliflozin in patients with type 2 diabetes mellitus
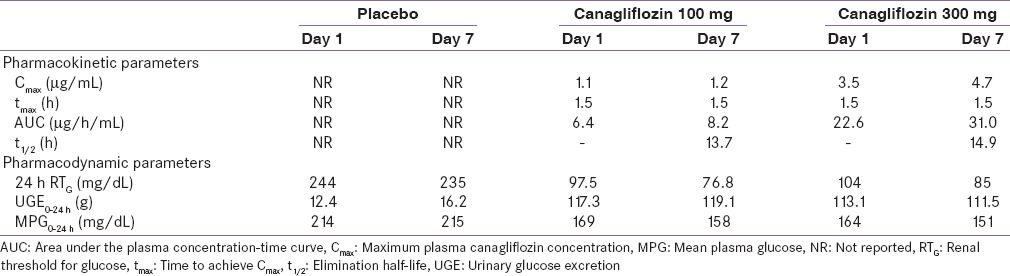
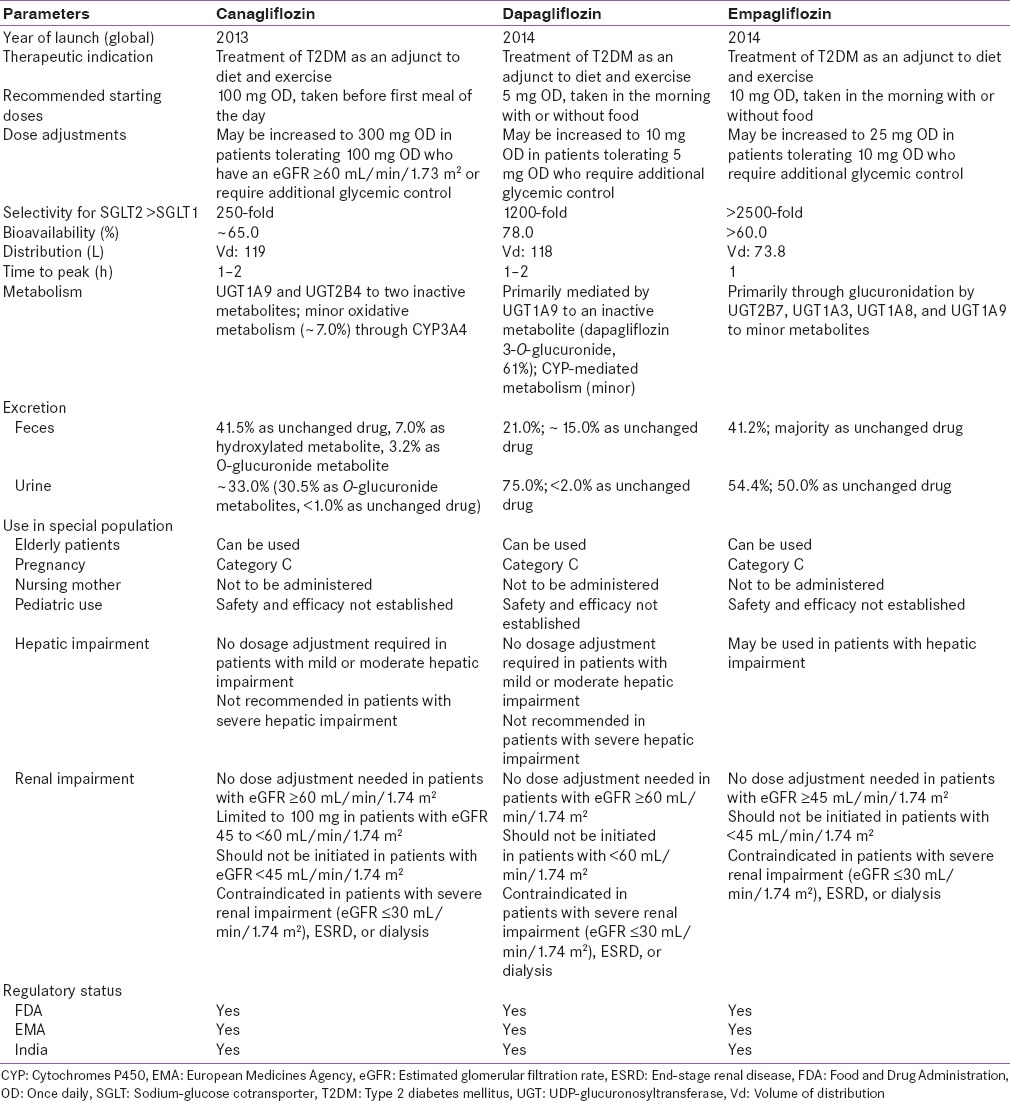
Table 2.
Comparison between sodium-glucose cotransporter 2 inhibitors
Following single- and multiple-daily dose administration of canagliflozin in patients with T2DM, dose-dependent reduction in RTG, with maximal suppression of RTG from a baseline of ~240 to ~70–90 mg/dL, was observed with canagliflozin 300 mg, suggesting a low risk for treatment-induced hypoglycemia. Canagliflozin was also associated with dose-dependent increase in UGE, mean AUC, and Cmax and reduction in 24 h mean PG values.[41,51] A PK/pharmacodynamic (PD) model predicting 24 h RTG profile for canagliflozin 100 and 300 mg demonstrated that 300 mg dose provided near-maximal reduction in RTG for the full 24 h dosing interval, while a modest suppression of this effect was observed in the overnight period with 100 mg dose.[52] Thus, canagliflozin 300 mg appears to have better PK/PD profiles.
Use in special population
Safety and efficacy of canagliflozin have not been established in children and pregnant women and it is not recommended in nursing women. Canagliflozin is considered safe to be used in patients with mild to moderate hepatic failure patients.[36] It is recommended that the dose of canagliflozin be limited to 100 mg in patients with estimated glomerular filtration rate (eGFR) 45 to <60 mL/min/1.73 m2 and dose may be increased to 300 mg in patients tolerating 100 mg who have an eGFR ≥60 mL/min/1.73 m2 or require additional glycemic control.[36] However, it is contraindicated in patients with eGFR <45 mL/min/1.73 m2 and in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2), patients with end-stage renal disease, or patients on dialysis [Table 2].[36]
THERAPEUTIC INDICATIONS AND REGULATORY STATUS
The US Food and Drug Administration (US FDA) in 2013 approved the use of canagliflozin as an adjunct to diet and exercise to improve glycemic control in patients with T2DM.[36] The recommended starting dose of canagliflozin is 100 mg OD, taken before the first meal of the day. In patients tolerating canagliflozin 100 mg OD who have an eGFR ≥60 mL/min/1.73 m2 and require additional glycemic control, the dose can be increased to 300 mg OD.[36] In the same year, the European Medicines Agency approved the use of canagliflozin 100 and 300 mg as monotherapy when diet and exercise do not provide adequate glycemic control and metformin was contraindicated or not tolerated or as add-on therapy with AHAs,[57] while the Central Drugs Standard Control Organization permitted the use of canagliflozin 100 and 300 mg in 2014 in India as an adjunct to diet and exercise to improve glycemic control in patients with T2DM.[58] Other SGLT2 inhibitors such as dapagliflozin, and empagliflozin are also approved for the treatment of T2DM [Table 2].[54,55,56]
THERAPEUTIC EFFICACY OF CANAGLIFLOZIN 300 MG
Monotherapy
The 26-week monotherapy study Canagliflozin Treatment And Trial Analysis-Monotherapy (CANTATA-M) comparing canagliflozin 100 and 300 mg with placebo in patients with T2DM had mean baseline HbA1c levels ranging from 8.0% to 10.6%.[59] In this study, canagliflozin 300 mg demonstrated significant improvements in HbA1c, fasting PG (FPG), and PPG levels from baseline versus placebo (all parameters P < 0.001) [Figure 3].[59] Proportion of patients achieving HbA1c <7.0% was higher in the canagliflozin 300 mg than placebo group (62.4% vs. 20.6%) [Table 3].[59] Furthermore, the glucose-lowering effect of canagliflozin 300 mg was maintained over a 52-week extension phase [Figure 3].[60]
Figure 3.
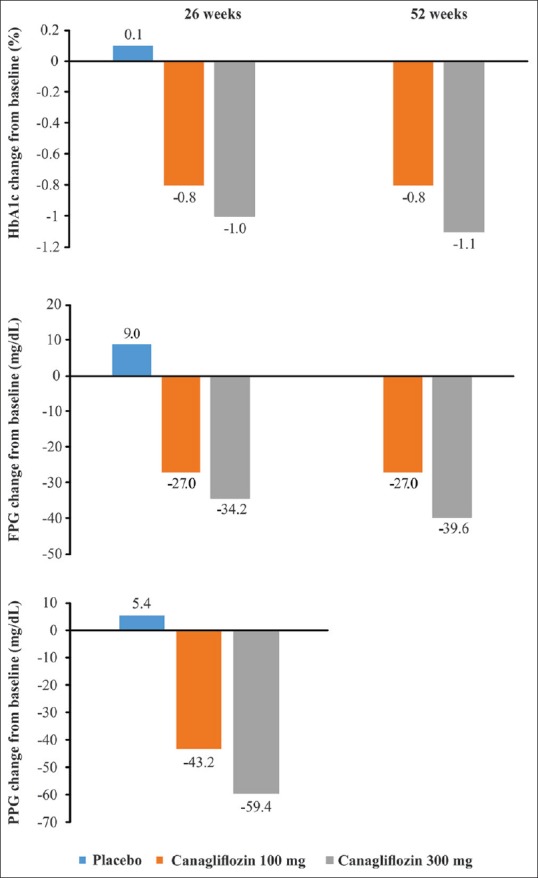
Mean change in glycated hemoglobin, fasting plasma glucose, and postprandial glucose in clinical studies with canagliflozin monotherapy versus placebo. HbA1c: Glycated hemoglobin; FPG: Fasting plasma glucose; PPG: Postprandial glucose
Table 3.
Comparison of canagliflozin as monotherapy, combination therapy and with insulin in different clinical studies
Add-on therapy
The effectiveness of combination therapy with canagliflozin and AHAs was examined in several randomized controlled studies [Table 3].[61,62,63,64,65,66,67,68,69,70] The studies were of 26−104 weeks duration, with mean baseline HbA1c levels ranging from ≥7.0% to ≤10.5%. Across studies, patients received canagliflozin (100 and 300 mg), sitagliptin (100 mg), glimepiride (6 and 8 mg), metformin (≥1500−≥2000 mg/day), and insulin (≥50 IU/day). In a 52-week dual-therapy study comparing canagliflozin 300 mg against sitagliptin 100 mg; canagliflozin 300 mg was superior to sitagliptin in reducing HbA1c levels (−0.9% vs. −0.7%); difference (95% confidence interval [CI]) versus sitagliptin was −0.15% (−0.27, −0.03) for canagliflozin 300 mg [Figure 4].[63] As an adjunct to metformin and sitagliptin 100 mg, canagliflozin 300 mg (doses pooled) caused greater reductions in HbA1c than placebo (−0.91% vs. −0.01%; P < 0.001).[71] Similar results were demonstrated in another 52-week triple therapy study of canagliflozin 300 mg versus sitagliptin 100 mg, wherein canagliflozin 300 mg was once again superior to sitagliptin in lowering HbA1c (−1.03% vs. −0.66%) and FPG levels (−28.7 vs. −2.2 mg/dL, P < 0.001) [Figure 4].[64] Likewise, canagliflozin 300 mg demonstrated a superior HbA1c reduction versus glimepiride (−0·12%; 95% CI: −0·22 to − 0·02) in another 52-week study with add-on metformin therapy [Figure 4].[65] In the follow-up study, HbA1c reduction was maintained over 104 weeks with canagliflozin 300 mg (−0.74%) but increased with glimepiride (−0.55%).[66] In the CANTATA-MSU study, canagliflozin 300 mg led to significant reductions in HbA1c (−1.1% vs. −0.1%, P < 0.001), FPG (−30.6 vs. 3.6 mg/dL, P < 0.001), and PPG (−55.8 vs. −19.8, P = not reported) versus placebo in patients with T2DM uncontrolled with background metformin + sulfonylurea (SU).[68] Proportion of patients achieving HbA1c <7.0% was higher in the canagliflozin 300 mg than placebo group (56.6% vs. 18.0%)[68] [Figure 5][59,63,64,65,70] and was maintained over the 52-week treatment period.[68] The efficacy of canagliflozin 300 mg in HbA1c level reduction was also confirmed in the CANTATA-MP.[66] These findings suggest that canagliflozin 300 mg is a novel option as an add-on therapy to metformin and/or SU in controlling HbA1c levels in patients with T2DM.[66]
Figure 4.
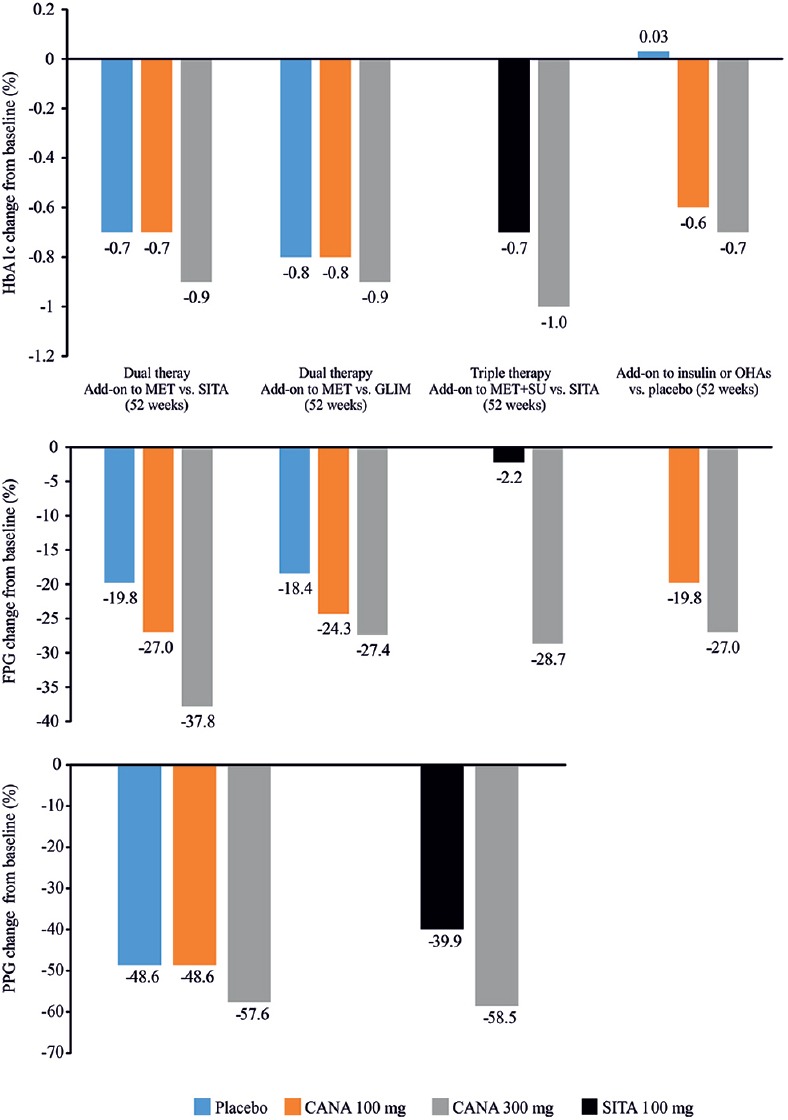
Mean change in glycated hemoglobin, fasting plasma glucose, and postprandial glucose in clinical studies with canagliflozin as add-on therapy versus placebo/active comparators, sitagliptin, glimepiride, and insulin. HbA1c: Glycated hemoglobin; CANA: Canagliflozin; FPG: Fasting plasma glucose; GLIM: Glimepiride; MET: Metformin; OHAs: Oral hypoglycemic agents; PPG: Postprandial glucose; SITA: Sitagliptin; SU: Sulfonylurea
Figure 5.
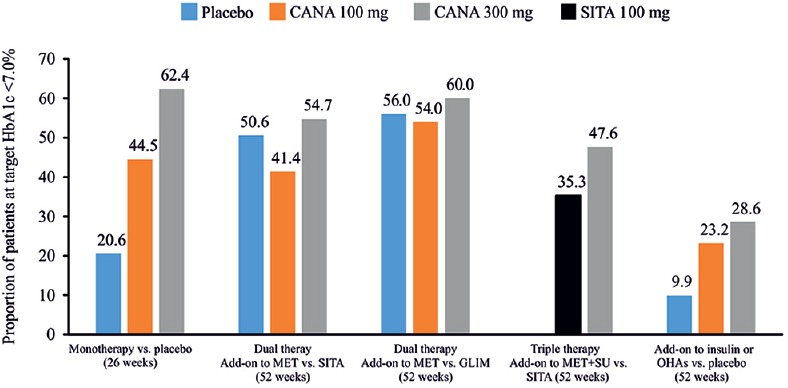
Proportion of patients achieving glycated hemoglobin <7.0% in clinical studies with canagliflozin as add-on therapy versus placebo/active comparators, sitagliptin, glimepiride and insulin. HbA1c: Glycated hemoglobin; CANA: Canagliflozin; GLIM: Glimepiride; MET: Metformin; OHAs: Oral hypoglycemic agents; SITA: Sitagliptin; SU: Sulfonylurea
Canagliflozin 300 mg added-on to dipeptidyl peptidase-4 inhibitors (DPP-4is) or glucagon-like peptide-1 (GLP-1) receptor agonists improved HbA1c control better than DPP-4is (−0.75%, 95% CI: −0.95, −0.54) or GLP-1 receptor agonists (−1.06%, 95% CI: −1.43, −0.69).[72] As an adjunct to insulin ± oral hypoglycemic agents (OHAs) [Figure 4],[69,70] canagliflozin 300 mg caused greater reductions in HbA1c (−0.9% vs. −0.2%) and FPG (−42.3 vs. 8.6 mg/dL) than placebo.[69] Similar improvements in glycemic parameters were observed with canagliflozin 300 mg in patients with T2DM from India[73,74] and Japan.[75]
In elderly patients with T2DM uncontrolled on stable regimen of OHAs, administration of canagliflozin 300 mg showed significantly improved HbA1c (−0.7% vs. −0.03%, P < 0.001) and FPG (−20.3 vs. 7.4 mg/dL, P < 0.001) levels than placebo.[76] Significant efficacy was sustained over 104 weeks, suggesting effectiveness of canagliflozin 300 mg in this age group.[77] Because canagliflozin acts independently of insulin secretion and action, it can be used at any stage of the disease regardless of baseline HbA1c or the duration of diabetes.[78]
Indirect comparison of canagliflozin 300 mg with other antihyperglycemic agents
In the absence of head-to-head study data, indirect comparisons based on Bayesian network meta-analysis had been used to compare glycemic benefits of canagliflozin with other AHAs. Across studies, patients received canagliflozin (100 and 300 mg), dapagliflozin (10 mg), empagliflozin (10 and 25 mg), exenatide (5, 10, and 20 µg), liraglutide (1.2 and 1.8 mg), and pioglitazone 30 mg. Canagliflozin 300 mg as add-on therapy achieved more effective glycemic control versus DPP-4is,[79,80,81] exenatide (5 and 10 µg),[80] and liraglutide (1.2 mg)[82] and was similar to liraglutide (1.8 mg) and exenatide (20 µg).[80,81,83]
PLEOTROPIC EFFECTS OF CANAGLIFLOZIN 300 MG
Besides glucose control, canagliflozin 300 mg directly or indirectly exhibits additional benefits on nonglycemic parameters.
Body weight
In the clinical development program of canagliflozin (CANTATA), canagliflozin 100 and 300 mg doses were evaluated for body weight reduction. Unlike some AHAs, canagliflozin 300 mg is not associated with weight gain but rather demonstrates a weight loss effect that can be attributed to caloric loss amounting to 300–400 kcal/day.[44] Change in body weight from baseline to study end ranged from −2.5 to −4.7 kg with canagliflozin 300 mg [Figure 6].[59,60,63,64,65,68,70] Across all phase 3 studies, canagliflozin consistently reduced body weight when used as mono- or dual- or triple-therapy; canagliflozin 300 mg caused numerically greater reductions in body weight than canagliflozin 100 mg.[59] Dual-energy X-ray absorptiometry analysis revealed that this reduction in body weight was majorly due to loss of body fat mass, rather than a loss of fluid or lean mass.[65] The weight-reducing effect of canagliflozin is an important therapeutic consideration for patients with T2DM who are overweight or obese. Interestingly, in a 12-week, randomized, double-blinded study of 376 obese and overweight patients without diabetes, canagliflozin 300 mg significantly reduced body weight versus placebo (P < 0.05).[84]
Figure 6.
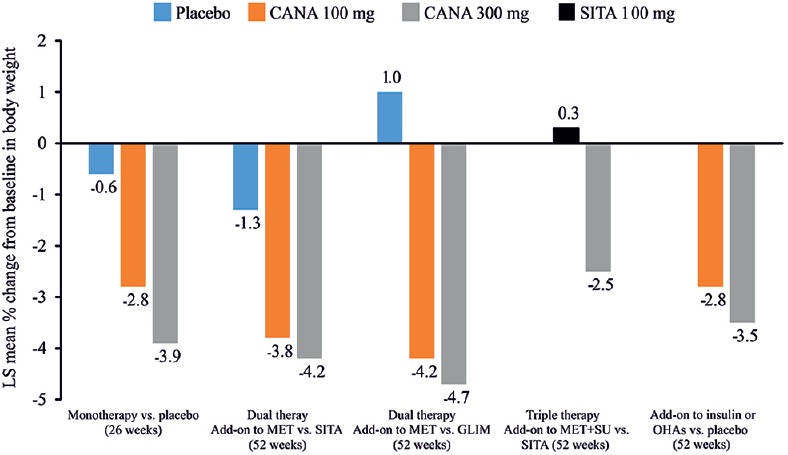
Percent change in body weight in clinical studies with canagliflozin as monotherapy or add-on therapy versus placebo/active comparators, sitagliptin, glimepiride and insulin. CANA: Canagliflozin; GLIM: Glimepiride; LS: Least squares; MET: Metformin; OHAs: Oral hypoglycemic agents; SITA: Sitagliptin; SU: Sulfonylurea
Blood pressure and cardiovascular safety
Canagliflozin caused clinically meaningful reduction in blood pressure (BP) than placebo/active comparators [Figure 7].[59,63,64,65,70] When assessed in a pooled placebo-controlled population, average systolic BP (SBP) and diastolic BP (DBP) reductions were −4.7 and −1.9 mmHg, respectively, with canagliflozin 300 mg versus placebo.[85] This effect might be ascribed to increased osmotic diuresis and sodium excretion and weight loss.[86,87] However, the BP reduction with canagliflozin 300 mg did not result in increased heart rate.[88] Thus, the pleiotropic benefits of canagliflozin in terms of weight loss and BP reductions can be optimally utilized in countries such as India where T2DM with its associated comorbidities such as hypertension and obesity is increasing at an alarming pace.
Figure 7.
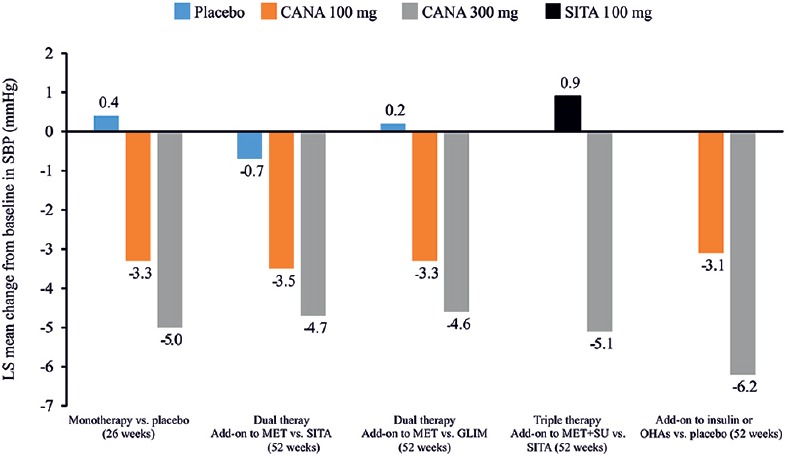
Change in systolic blood pressure in clinical studies with canagliflozin as monotherapy or add-on therapy versus placebo/active comparators, sitagliptin, glimepiride and insulin. CANA: Canagliflozin; GLIM: Glimepiride; LS: Least squares; MET: Metformin; OHAs: Oral hypoglycemic agents; SITA: Sitagliptin; SU: Sulfonylurea; SBP: Systolic blood pressure
Although canagliflozin 300 mg appears to have a beneficial effect on cardiovascular (CV) risk factors such as HbA1c, body weight, and BP, there is paucity of data on clinical outcomes such as stroke, myocardial infarction, and CV death. In a pooled meta-analysis of phase 2 and 3 studies, composite primary end-point (nonfatal stroke, nonfatal myocardial infarction, time to event of CV death, and unstable angina requiring hospitalization) showed that canagliflozin does not increase the CV risk relative to comparators.[89] An evaluation of long-term effects of canagliflozin on CV outcomes is underway in the large-scale, double-blind, placebo-controlled Canagliflozin Cardiovascular Assessment Study (CANVAS)[90] and a pooled analysis with another CV outcome study of similar design and in a similar population, CANVAS-R,[91] will be completed and submitted to the Health Authorities in 2017.
SAFETY AND TOLERABILITY OF CANAGLIFLOZIN 300 MG
Hypoglycemia
Hypoglycemia is a potential side effect of some OHAs and insulin therapy. However, canagliflozin when used as monotherapy or combination therapy does not stimulate insulin release and event rate range does not contribute to the risk of hypoglycemia. This is because the observed RTG values with canagliflozin treatment are above the usual threshold for hypoglycemia (≤70.0 mg/dL), a level that is above the PG concentration at which hypoglycemic symptoms occur.[36] Proportion of hypoglycemia episodes (mild to moderate) with canagliflozin 300 mg was lower than glimepiride (5% vs. 34%)[65] and comparable with sitagliptin (6.8% vs. 4.1%).[63] There were no episodes of severe hypoglycemia reported in most of the studies.[60,63,65] Hypoglycemia rates may be increased when canagliflozin 300 mg was used in combination with insulin or insulin secretagogues and the doses of which may need to be suitably reduced to avoid the risk of hypoglycemia.[36]
Other adverse events
The overall incidence of adverse events (AEs) was similar with canagliflozin 100 and 300 mg [Table 4].[92] Canagliflozin 300 mg was well tolerated in the treatment of patients with T2DM. The most frequently reported AEs with canagliflozin 300 mg were genital mycotic infections (GMIs), urinary tract infections (UTIs), osmotic diuresis (thirst or frequent urination), and volume depletion-related events (hypotension, postural dizziness, and orthostatic hypotension).[92] A pooled analysis of four phase 3 studies showed that canagliflozin 300 mg dose was associated with more frequent occurrences of GMIs versus placebo in women (11.4% vs. 3.2%) and men (3.7% vs. 0.6%).[92] The GMIs mostly occurred during the first 3 months of canagliflozin treatment initiation and declined over time in both men and women with T2DM.[93,94] There was no difference in the incidence of GMI in patients ≥65 years versus <65 years of age.[95] Most GMIs were generally mild to moderate in severity and could be managed with topical or oral antifungal drugs.[95]
Table 4.
Summary of overall safety and selected adverse events in the overall population
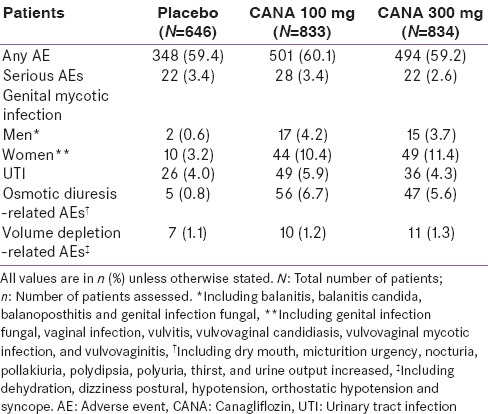
The incidence of UTIs was higher in the canagliflozin treatment groups, albeit minimal increases than control, nondose-dependent, similar in severity, and no difference of upper UTI.[96] The UTIs occurred more frequently in female patients, and most diagnosed infections were generally considered to be mild to moderate in nature and responded to standard antimicrobial treatment.[92] Incidence of UTIs was higher with canagliflozin 100 mg versus canagliflozin 300 mg and placebo in patients <65 years and comparable across patients ≥65 years, indicating no dose relationship to the AE.[95] One serious UTI was reported in patients <65 years but not in >65 years patients; none led to study discontinuation in both age-group categories.[95] Mild to moderate UTIs with canagliflozin 300 mg were comparable with sitagliptin 100 mg[63,64] and were only slightly higher compared with glimepiride.[66,67] In postmarketing surveillance by the US FDA, serious events of UTIs including pyelonephritis, requiring hospitalization, have been reported in patients receiving canagliflozin.[36] Hence, it is recommended that patients treated with canagliflozin should be evaluated for signs and symptoms of UTIs, and if confirmed, appropriate medical attention should be sought immediately.
The osmotic diuresis-related AEs occurred more frequently with canagliflozin 300 mg than placebo (5.6% vs. 0.8%)[92] and were greater compared with glimepiride (6.6% vs. 2.1%)[66] and sitagliptin 100 mg (3.0% vs. 0.5%).[63] The AEs related to volume depletion increased in a dose-dependent manner with canagliflozin doses and were greater than with placebo/active comparators, in individuals ≥75 years of age,[95] in patients with an eGFR of <60 mL/min/1.73 m2, and in patients using loop diuretics.[36] These AEs predominantly occurred in the first 3 months of canagliflozin treatment and declined subsequently.[95] Because of osmotic diuretic effect of canagliflozin, mild and transient changes in eGFR, albumin-creatinine ratio, and blood urea nitrogen were noted with canagliflozin 300 mg. However, these parameters trended toward baseline levels at the 26-week treatment period. In post-marketing surveillance by the US FDA, serious events of acute kidney injury (AKI), requiring hospitalization and dialysis, were reported in patients receiving canagliflozin.[36] Patients treated with canagliflozin should be evaluated for factors that may predispose them to AKI (such as hypovolemia and chronic renal insufficiency). If AKI is confirmed, canagliflozin should be discontinued immediately and appropriate remedial treatment initiated.[36] Long-term outcomes of canagliflozin treatment on renal function are being evaluated in the ongoing Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation study (CREDENCE).[97]
Recently, the US FDA issued a drug safety communication indicating that in patients with T2DM, SGLT2 inhibitors may be associated with diabetic ketoacidosis (DKA) perhaps as a consequence of their noninsulin-dependent glucose clearance, negative fluid and sodium balance, hypoinsulinemia, hyperglucagonemia, glucosuria, decrease in sodium reabsorption, and alteration in insulin-glucagon ratio.[98,99] Mildly elevated blood glucose (<200 mg%), high anion gap metabolic acidosis, ketonuria/ketonemia, reduced carbohydrate intake, infection, acute illness, and alcohol use can also predispose a patient to DKA.[99] The US FDA AE Reporting System database identified 20 cases of DKA in patients with T2DM treated with SGLT2 inhibitors from March 2013 to June 6, 2014.[100] In a pooled analysis of 17,596 patients with T2DM in clinical studies on canagliflozin, the overall incidence of DKA and related events was low with canagliflozin 300 mg (0.1%) and similar across canagliflozin and noncanagliflozin treatment groups.[101] Future long-term studies are required to clarify the potential association of SGLT2 inhibitor with DKA in patients with T2DM, but clinicians need to consider the potential risk when prescribing these drug in patients with T2DM. Patients treated with canagliflozin presenting with symptoms of severe metabolic acidosis should be evaluated for ketoacidosis, irrespective of presenting blood glucose levels (<250 mg/dL). If ketoacidosis is confirmed, canagliflozin should be discontinued immediately and appropriate remedial measures should be undertaken.[36,102] Patients receiving canagliflozin should avoid alcohol consumption and low carbohydrate diets, and should be counseled for self-management of diabetes.[99]
Evidence of an increased frequency of bone fractures was reported in patients treated with canagliflozin. In the CANVAS study, a nondose-dependent increase in fractures was reported with canagliflozin versus placebo (doses pooled 4.0% vs. 2.6%).[103] Fractures were reported 12 weeks after treatment initiation and were more likely to be from low trauma and affect the upper extremities (e.g. hand and wrist).[36] The increase in fractures may be mediated by falls or other extrinsic factors in the high-risk population, but the causes of bone fractures in patients exposed to canagliflozin are unknown. No distinct changes in bone mineral density were observed with canagliflozin over 104 weeks.[77]
Foot complications, including leg and toes amputations, are the common complications in patients with T2DM. An interim analysis of the ongoing CANVAS study (median exposure 4.5 years) conducted by the Independent Data Monitoring Committee demonstrated higher incidence of lower limb amputation, primarily of the toe, with canagliflozin treatment versus placebo (100 mg: 7/1000, 300 mg: 5/1000 vs. placebo: 3/1000 patient-year).[104] However, the same risk was not observed across 12 other completed phase 3 or 4 studies in the development program (>8000 patients) or in post-marketing safety surveillance.[104] Although the underlying mechanism linking canagliflozin treatment and an increased risk of amputation is currently unknown, as a precautionary measure, healthcare professionals (HCPs) should also counsel patients about the importance of routine preventive foot care, to notify their HCPs if ulceration, discoloration, new lower extremity pain, tenderness, or gangrene develops and encourage them to remain well hydrated.[104,105]
TREATMENT ADHERENCE AND PERSISTENCE OF CANAGLIFLOZIN 300 MG
In addition to the beneficial effects of canagliflozin 300 mg, adherence data play an important role in validating the acceptability of long-term use since better treatment adherence can lead to better glycemic control and clinical outcomes in patients with T2DM.[106] In a 12-month follow-up study of treatment adherence, proportion of days covered (PDC) was 71.0% and medication possession ratio (MPR) was 76.0% in patients with T2DM (N = 881) receiving canagliflozin. The corresponding median PDC was 83.0% and MPR value was 88.0%, indicating good treatment adherence with canagliflozin 300 mg.[107] Furthermore, persistence with AHAs is an important predictor of outcomes in these patients. Low medication persistence has been reported with metformin, SUs, or metformin + SU combination therapy in patients with T2DM.[108,109] Proportion of patients with medication persistency was noted to be higher with canagliflozin 300 mg (65.0%) versus DPP-4is (sitagliptin: 51.0%, linagliptin: 30.2%) and GLP-1 agonists (exenatide: 24.3%, liraglutide: 40.3%, P < 0.0001 for all comparisons).[110] In another analysis of treatment persistence in patients (N = 38,083) with T2DM, greater proportion of patients remained persistent on canagliflozin 300 mg (67.0%) versus DPP-4is (47.0% to 53.0%) and GLP-1 receptor agonists (26.0% to 50.0%), indicating more likely consistent use of canagliflozin 300 mg than other AHAs.[111] Gastrointestinal-related side effects of GLP-1 agonists and weight-neutral effects of DPP-4i treatment were cited as the reasons for low persistence with these drugs.[112,113,114] In addition, DPP-4i and GLP-1 agonists may lose their efficacy over time as insulin resistance worsens and β-cell function deteriorates.[115]
REAL-WORLD EXPERIENCE OF CANAGLIFLOZIN 300 MG
Several real-world studies have explored the efficacy of canagliflozin in patients with T2DM with inadequate glycemic control at baseline. In a retrospective study of this patient population, canagliflozin (doses pooled) treatment significantly reduced mean HbA1c levels from 8.54% at baseline to 7.76% at follow-up (P < 0.001).[116] Proportion of patients achieving HbA1c <7.0% was higher in canagliflozin 300 mg (35.3%), and the patients used fewer AHAs (including insulin) during the 3-month follow-up period.[116] In other retrospective, observational studies in similar patient populations (baseline HbA1c ≥7.0%), glycemic goals improved remarkably following canagliflozin (doses pooled) treatment and proportion of patients with HbA1c ≥9.0% at baseline (33.0% was reduced to almost half (16.0%).[117,118,119] This indicates that prior to canagliflozin treatment initiation, the target HbA1c goal was not achieved despite treatment with multiple AHAs in patients with T2DM. In comparison with DPP-4i, canagliflozin 300 mg was associated with significant reductions in HbA1c (between-treatment difference: −0.37%, P = 0.002). Higher proportion of patients achieved HbA1c goals (<7.0% or <8.0%) with canagliflozin 300 mg versus DPP-4i (HbA1c <7.0% OR: 1.48 vs. HbA1c <8.0% OR: 1.49, both P = 0.003).[120] These findings corroborate those of the phase 3 clinical studies.[66,67] A beneficial effect of canagliflozin was noted in patients with SBP/DBP of ≥140/90 mmHg at baseline; >50.0% patients achieved BP goals after 3 months.[118]
COST-EFFECTIVENESS OF CANAGLIFLOZIN 300 MG
To date, there is a paucity of data regarding the cost-effectiveness of canagliflozin 300 mg in the management of T2DM in India. However, in specific payer settings, canagliflozin 300 mg has shown to be cost-effective versus liraglutide (1.2 and 1.8 mg) and sitagliptin (100 mg).[121,122,123]
SUMMARY
Canagliflozin 300 mg is efficacious and improved HbA1c, FPG, and PPG levels when administered as monotherapy and in combination with other AHAs as dual or triple therapy in patients with T2DM. Canagliflozin 300 mg showed superiority to sitagliptin and glimepiride and caused greater HbA1c reductions compared with DPP-4i and was comparable with GLP-1 agonists. Improvements in glycemic control with canagliflozin 300 mg were also observed in elderly patients with T2DM. Canagliflozin 300 mg decreases BP levels and shows a significant weight losing effect with low risk of hypoglycemia. Canagliflozin 300 mg was generally well tolerated in patients with T2DM. Thus, canagliflozin 300 mg could be a viable treatment option for a range of patients with T2DM.
Financial support and sponsorship
This work was funded by Johnson and Johnson Pvt. Ltd., India.
Conflicts of interest
Dr. K. M. Prasanna Kumar and Dr. Sujoy Ghosh have received honoraria from Johnson and Johnson Pvt. Ltd., India. Dr. William Canovatchel is an employee of Janssen Research and Development, LLC. Dr. Nishant Garodia and Dr. Sujith Rajashekar are employees of Johnson and Johnson Pvt. Ltd, India. We have no other relevant affiliations or financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
We thank Palash Kumar Das, Ph. D. for writing assistance and Madhavi Patil, Ph. D. (both SIRO Clinpharm Pvt. Ltd.) for additional editorial support for the development of this manuscript.
REFERENCES
- 1.International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015. [Last accessed on 2016 Jul 15]. Available from: http://www.diabetesatlas.org/resources/2015-atlas.html . [Google Scholar]
- 2.Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3:110–7. doi: 10.4239/wjd.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohan V, Shah SN, Joshi SR, Seshiah V, Sahay BK, Banerjee S, et al. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: Results from the DiabCare India 2011 Study. Indian J Endocrinol Metab. 2014;18:370–8. doi: 10.4103/2230-8210.129715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalra S, Unnikrishnan AG. Obesity in India: The weight of the nation. J Med Nutr Nutraceuticals. 2012;1:37–41. [Google Scholar]
- 5.Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP, et al. Prevalence of generalized and abdominal obesity in urban and rural India – the ICMR-INDIAB Study (Phase-I) [ICMR- NDIAB-3] Indian J Med Res. 2015;142:139–50. doi: 10.4103/0971-5916.164234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meshram II, Vishnu Vardhana Rao M, Sudershan Rao V, Laxmaiah A, Polasa K. Regional variation in the prevalence of overweight/obesity, hypertension and diabetes and their correlates among the adult rural population in India. Br J Nutr. 2016;115:1265–72. doi: 10.1017/S0007114516000039. [DOI] [PubMed] [Google Scholar]
- 7.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 8.Little M, Humphries S, Patel K, Dodd W, Dewey C. Factors associated with glucose tolerance, pre-diabetes, and type 2 diabetes in a rural community of South India: A cross-sectional study. Diabetol Metab Syndr. 2016;8:21. doi: 10.1186/s13098-016-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrannini E. The target of metformin in type 2 diabetes. N Engl J Med. 2014;371:1547–8. doi: 10.1056/NEJMcibr1409796. [DOI] [PubMed] [Google Scholar]
- 10.Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 11.Groop LC. Sulfonylureas in NIDDM. Diabetes Care. 1992;15:737–54. doi: 10.2337/diacare.15.6.737. [DOI] [PubMed] [Google Scholar]
- 12.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 13.Swinnen SG, Hoekstra JB, DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S253–9. doi: 10.2337/dc09-S318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicasio J, McFarlane SI. Early insulin therapy and the risk of cardiovascular disease in Type 2 diabetes. Therapy. 2005;2:685–8. [Google Scholar]
- 15.Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000. doi: 10.2337/diacare.28.5.995. [DOI] [PubMed] [Google Scholar]
- 16.Riedel AA, Heien H, Wogen J, Plauschinat CA. Secondary failure of glycemic control for patients adding thiazolidinedione or sulfonylurea therapy to a metformin regimen. Am J Manag Care. 2007;13:457–63. [PubMed] [Google Scholar]
- 17.Riedel AA, Heien H, Wogen J, Plauschinat CA. Loss of glycemic control in patients with type 2 diabetes mellitus who were receiving initial metformin, sulfonylurea, or thiazolidinedione monotherapy. Pharmacotherapy. 2007;27:1102–10. doi: 10.1592/phco.27.8.1102. [DOI] [PubMed] [Google Scholar]
- 18.Mitka M. More patients get good diabetes control, but only a minority meet all goals. JAMA. 2013;309:1335–6. doi: 10.1001/jama.2013.2414. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence DB, Ragucci KR, Long LB, Parris BS, Helfer LA. Relationship of oral antihyperglycemic (sulfonylurea or metformin) medication adherence and hemoglobin HbA1c goal attainment for HMO patients enrolled in a diabetes disease management program. J Manag Care Pharm. 2006;12:466–71. doi: 10.18553/jmcp.2006.12.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract. 2011;65:314–22. doi: 10.1111/j.1742-1241.2010.02544.x. [DOI] [PubMed] [Google Scholar]
- 21.Braga MF, Casanova A, Teoh H, Gerstein HC, Fitchett DH, Honos G, et al. Poor achievement of guidelines-recommended targets in type 2 diabetes: Findings from a contemporary prospective cohort study. Int J Clin Pract. 2012;66:457–64. doi: 10.1111/j.1742-1241.2012.02894.x. [DOI] [PubMed] [Google Scholar]
- 22.Cernea S, Raz I. Therapy in the early stage: Incretins. Diabetes Care. 2011;34(Suppl 2):S264–71. doi: 10.2337/dc11-s223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 24.Fowler MJ. Diabetes treatment, part 2: Oral agents for glycemic management. Clin Diabetes. 2007;25:131–4. [Google Scholar]
- 25.Korytkowski M. When oral agents fail: Practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18–24. doi: 10.1038/sj.ijo.0802173. [DOI] [PubMed] [Google Scholar]
- 26.Nakar S, Yitzhaki G, Rosenberg R, Vinker S. Transition to insulin in type 2 diabetes: Family physicians’ misconception of patients’ fears contributes to existing barriers. J Diabetes Complications. 2007;21:220–6. doi: 10.1016/j.jdiacomp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335–80. doi: 10.2147/DDDT.S50773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–94. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 29.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21:438–47. doi: 10.4158/EP15693.CS. [DOI] [PubMed] [Google Scholar]
- 30.Standards of medical care in diabetes-2015: Summary of revisions. Diabetes Care. 2015;38(Suppl):S4. doi: 10.2337/dc15-S003. [DOI] [PubMed] [Google Scholar]
- 31.Madhu S, Saboo B, Makkar BM, Reddy GC, Jana J, Panda JK, et al. RSSDI clinical practice recommendations for management of type 2 diabetes mellitus, 2015. Int J Diabetes Dev Ctries. 2015;35:1–71. [Google Scholar]
- 32.Harper W, Clement M, Goldenberg R, Hanna A, Main A, et al. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Policies, guidelines and consensus statements: Pharmacologic management of type 2 diabetes-2015 interim update. Can J Diabetes. 2015;39:250–2. doi: 10.1016/j.jcjd.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 33.International Diabetes Federation. Managing Older People with Type 2 Diabetes Global Guideline. 2013. [Last accessed on 2016 Jul 15]. Available from: http://www.idf.org/guidelines-older-people-type-2-diabetes .
- 34.National Institute for Health and Care Excellence. Canagliflozin, Dapagliflozin and Empagliflozin as Monotherapies for Treating Type 2 Diabetes. 2016. [Last accessed on 2016 Jul 15]. Available from: http://www.nice.org.uk/guidance/ta390 .
- 35.National Institute for Health and Care Excellence. Canagliflozin in Combination Therapy for Treating Type 2 Diabetes. 2014. [Last accessed on 2016 Jul 15]. Available from: http://www.nice.org.uk/guidance/ta315 .
- 36.INVOKANA® (Canagliflozin) Tablets. Highlights of Prescribing Information. 2016. [Last accessed on 2016 Oct 05]. Available from: https://www.icanimagine.com/file-resource/be183ed7-5830-4369-b0c0-35220e3eecdc .
- 37.Plosker GL. Canagliflozin: A review of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74:807–24. doi: 10.1007/s40265-014-0225-5. [DOI] [PubMed] [Google Scholar]
- 38.Seufert J. SGLT2 inhibitors-an insulin-independent therapeutic approach for treatment of type 2 diabetes: Focus on canagliflozin. Diabetes Metab Syndr Obes. 2015;8:543–54. doi: 10.2147/DMSO.S90662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatia J, Gamad N, Bharti S, Arya DS. Canagliflozin-current status in the treatment of type 2 diabetes mellitus with focus on clinical trial data. World J Diabetes. 2014;5:399–406. doi: 10.4239/wjd.v5.i3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parveen R, Agarwal NB, Kaushal N, Mali G, Raisuddin S. Efficacy and safety of canagliflozin in type 2 diabetes mellitus: Systematic review of randomized controlled trials. Expert Opin Pharmacother. 2016;17:105–15. doi: 10.1517/14656566.2016.1109629. [DOI] [PubMed] [Google Scholar]
- 41.Devineni D, Curtin CR, Polidori D, Gutierrez MJ, Murphy J, Rusch S, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013;53:601–10. doi: 10.1002/jcph.88. [DOI] [PubMed] [Google Scholar]
- 42.Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–72. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 43.Kalra S, Singh V, Nagrale D. Sodium-glucose cotransporter-2 inhibition and the glomerulus: A review. Adv Ther. 2016;33:1502–18. doi: 10.1007/s12325-016-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh SK, Gupta A. SGLT2 inhibitors for treatment of type 2 diabetes mellitus: Focus on canagliflozin. Muller J Med Sci Res. 2014;5:166. [Google Scholar]
- 45.Polidori D, Sha S, Mudaliar S, Ciaraldi TP, Ghosh A, Vaccaro N, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: Results of a randomized, placebo-controlled study. Diabetes Care. 2013;36:2154–61. doi: 10.2337/dc12-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One. 2012;7:e30555. doi: 10.1371/journal.pone.0030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein P, Berg JK, Morrow L, Polidori D, Artis E, Rusch S, et al. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces post-meal glucose excursion in patients with type 2 diabetes by a non-renal mechanism: Results of a randomized trial. Metabolism. 2014;63:1296–303. doi: 10.1016/j.metabol.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Devineni D, Manitpisitkul P, Murphy J, Stieltjes H, Ariyawansa J, Di Prospero NA, et al. Effect of food on the pharmacokinetics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, and assessment of dose proportionality in healthy participants. Clin Pharmacol Drug Dev. 2015;4:279–86. doi: 10.1002/cpdd.151. [DOI] [PubMed] [Google Scholar]
- 49.Devineni D, Vaccaro N, Polidori D, Stieltjes H, Wajs E. Single- and multiple-dose pharmacokinetics and pharmacodynamics of canagliflozin, a selective inhibitor of sodium glucose co-transporter 2, in healthy participants. Int J Clin Pharmacol Ther. 2015;53:129–38. doi: 10.5414/CP202218. [DOI] [PubMed] [Google Scholar]
- 50.Devineni D, Polidori D, Curtin C, Stieltjes H, Tian H, Wajs E. Single- and multiple-dose pharmacokinetics and pharmacodynamics of canagliflozin, a selective inhibitor of sodium glucose co-transporter 2, in healthy participants. Int J Clin Pharmacol Ther. 2015;53:129–38. doi: 10.5414/CP202218. [DOI] [PubMed] [Google Scholar]
- 51.Devineni D, Murphy J, Wang SS, Stieltjes H, Rothenberg P, Scheers E, et al. Absolute oral bioavailability and pharmacokinetics of canagliflozin: A microdose study in healthy participants. Clin Pharmacol Drug Dev. 2015;4:95–304. doi: 10.1002/cpdd.162. [DOI] [PubMed] [Google Scholar]
- 52.Devineni D, Polidori D. Clinical pharmacokinetic, pharmacodynamic, and drug-drug interaction profile of canagliflozin, a sodium-glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2015;54:1027–41. doi: 10.1007/s40262-015-0285-z. [DOI] [PubMed] [Google Scholar]
- 53.Devineni D, Manitpisitkul P, Murphy J, Skee D, Wajs E, Mamidi RN, et al. Effect of canagliflozin on the pharmacokinetics of glyburide, metformin, and simvastatin in healthy participants. Clin Pharmacol Drug Dev. 2015;4:226–36. doi: 10.1002/cpdd.166. [DOI] [PubMed] [Google Scholar]
- 54.FARXIGA® (Dapagliflozin) Tablets. Highlights of Prescribing Information. 2014. [Last accessed on 2016 Oct 05]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202293s003lbl.pdf .
- 55.JARDIANCE® (Empagliflozin) Tablets. Highlights of Prescribing Information. 2014. [Last accessed on 2016 Oct 05]. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf .
- 56.Haas B, Eckstein N, Pfeifer V, Mayer P, Hass MD. Efficacy, safety and regulatory status of SGLT2 inhibitors: Focus on canagliflozin. Nutr Diabetes. 2014;4:e143. doi: 10.1038/nutd.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.European Medicines Agency. Invokana 100 and 300 mg Filmcoated Tablets: Summary of Product Characteristics. 2013. [Last accessed on 2016 Jul 15]. Accessed from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_._Product_Information/human/002649/WC500156456.pdf .
- 58.Central Drugs Standard Control Organization. List of FDC and new drugs approved for marketing in India. [Last accessed on 2016 Jul 15]. Available from: http://www.cdsco.nic.in/forms/list.aspx?lid=2056 and Id=11 .
- 59.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–82. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stenlöf K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: Findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163–75. doi: 10.1185/03007995.2013.850066. [DOI] [PubMed] [Google Scholar]
- 61.Yang T, Lu M, Ma L, Zhou Y, Cui Y. Efficacy and tolerability of canagliflozin as add-on to metformin in the treatment of type 2 diabetes mellitus: A meta-analysis. Eur J Clin Pharmacol. 2015;71:1325–32. doi: 10.1007/s00228-015-1923-y. [DOI] [PubMed] [Google Scholar]
- 62.Sun YN, Zhou Y, Chen X, Che WS, Leung SW. The efficacy of dapagliflozin combined with hypoglycaemic drugs in treating type 2 diabetes mellitus: Meta-analysis of randomised controlled trials. BMJ Open. 2014;4:e004619. doi: 10.1136/bmjopen-2013-004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia. 2013;56:2582–92. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: A 52-week randomized trial. Diabetes Care. 2013;36:2508–15. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–50. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 66.Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis DA, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: A randomized, double.blind, phase 3 study. Diabetes Care. 2015;38:355–64. doi: 10.2337/dc13-2762. [DOI] [PubMed] [Google Scholar]
- 67.Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467–77. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilding JP, Charpentier G, Hollander P, González-Gálvez G, Mathieu C, Vercruysse F, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: A randomised trial. Int J Clin Pract. 2013;67:1267–82. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Devineni D, Morrow L, Hompesch M, Skee D, Vandebosch A, Murphy J, et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539–45. doi: 10.1111/j.1463-1326.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 70.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–11. doi: 10.2337/dc14-1237. [DOI] [PubMed] [Google Scholar]
- 71.Rodbard HW, Seufert J, Aggarwal N, Cao A, Fung A, Pfeifer M, et al. Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin. Diabetes Obes Metab. 2016;18:812–9. doi: 10.1111/dom.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fulcher G, Matthews DR, Perkovic V, de Zeeuw D, Mahaffey KW, Mathieu C, et al. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:82–91. doi: 10.1111/dom.12589. [DOI] [PubMed] [Google Scholar]
- 73.Kumar KM, Mohan V, Sethi B, Gandhi P, Bantwal G, Xie J, et al. PO422 efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus from India. Diabetes Res Clin Pract. 2014;106(Suppl 1):S260–1. [Google Scholar]
- 74.Prasanna Kumar KM, Mohan V, Sethi B, Gandhi P, Bantwal G, Xie J, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus from India. Indian J Endocrinol Metab. 2016;20:372–80. doi: 10.4103/2230-8210.179996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15:1136–45. doi: 10.1111/dom.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: A randomized trial. Hosp Pract. 2013;41:72–84. doi: 10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- 77.Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294–303. doi: 10.1111/dom.12428. [DOI] [PubMed] [Google Scholar]
- 78.Wilding JP, Blonde L, Leiter LA, Cerdas S, Tong C, Yee J, et al. Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. J Diabetes Complications. 2015;29:438–44. doi: 10.1016/j.jdiacomp.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 79.Pacou M, Taieb V, Abrams KR, Diels J, Van Sanden S, Garg M, et al. Bayesian network meta-analysis to assess the relative efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin. Value Health. 2013;; 16:A609. [Google Scholar]
- 80.Schroeder M, Taieb V, Pacou M, Ho S, Nielsen AT, Schubert A, et al. Stockholm, Sweden: 2015. Sep 14-18, A Network meta-analysis to assess options for treatment intensification for patients with type 2 diabetes inadequately controlled on dual therapy. Poster presented at the 51st annual meeting of the European Association for the Study of Diabetes (EASD) [Google Scholar]
- 81.Pacou M, Taieb V, Abrams KR, Diels J, Van Sanden S, Garg M, et al. Bayesian network meta-analysis to assess the relative efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus (T2DM) inadequately controlled on metformin and sulphonylurea (MET+SU) Value Health. 2013;16:A431. [Google Scholar]
- 82.Taieb V, Pacou M, Schroeder M, Nielsen AT, Schubert A, Neslusan C. Milan, Italy: 2015. Nov 7-11, A Network meta-analysis (NMA) to assess the longer-term relative efficacy of canagliflozin in patients with type 2 diabetes inadequately controlled on metformin. Poster presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 18th Annual European Congress. [Google Scholar]
- 83.van Sanden S, Diels J, Guillon P, Nielsen AT. Bayesian network meta-analysis (NMA) to assess relative efficacy of canagliflozin (CANA) versus glucagon-like peptide-1 (GLP-1) agonists in dual and triple therapy in patients with type 2 diabetes mellitus (T2DM) Value Health. 2015;18:A54. [Google Scholar]
- 84.Bays HE, Weinstein R, Law G, Canovatchel W. Effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring) . 2014;22:1042–9. doi: 10.1002/oby.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weir MR, Januszewicz A, Gilbert RE, Vijapurkar U, Kline I, Fung A, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich) 2014;16:875–82. doi: 10.1111/jch.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seufert J. SGLT-2 inhibition with canagliflozin: A new option for the treatment of type 2 diabetes. Dtsch Med Wochenschr. 2014;139(Suppl 2):S52–8. doi: 10.1055/s-0033-1359991. [DOI] [PubMed] [Google Scholar]
- 87.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: A systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262–75.e9. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Mikhail N. Place of sodium-glucose co-transporter type 2 inhibitors for treatment of type 2 diabetes. World J Diabetes. 2014;5:854–9. doi: 10.4239/wjd.v5.i6.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Summary of Product Characteristics of INVOKANA. UK: Janssen-Cilag Ltd; 2015. [Last accessed on 2016 Jul 151]. Available from: https://www.medicines.org.uk/emc/medicine/28401 . [Google Scholar]
- 90.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS) – a randomized placebo-controlled trial. Am Heart J. 2013;166:217–23.e11. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 91.CANVAS-R. [Last accessed on 2016 Jul 15]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01989754?term=canvas-r and rank=1 .
- 92.Usiskin K, Kline I, Fung A, Mayer C, Meininger G. Safety and tolerability of canagliflozin in patients with type 2 diabetes mellitus: Pooled analysis of phase 3 study results. Postgrad Med. 2014;126:16–34. doi: 10.3810/pgm.2014.05.2753. [DOI] [PubMed] [Google Scholar]
- 93.Sobel JD, Goldenberg RM, Khunti K, Davies MJ, Vijapurkar U, Meininger G. Incidence of genital mycotic infections decreases over time in patients with type 2 diabetes mellitus treated with canagliflozin over 2 years. Diabetes. 2015;64(Suppl 1):A1178. [Google Scholar]
- 94.Goldenberg RM, Sobel JD, Khunti K, Davies MJ, Vijapurkar U, Meininger G. Vancouver, BC, Canada: 2015. Incidence of genital mycotic infections and urinary tract infections in patients with type 2 diabetes mellitus treated with canagliflozin over 2 years. Poster presented at the 23rd World Diabetes Congress of the International Diabetes Federation (IDF); 30 November-4 December. [Google Scholar]
- 95.Sinclair A, Bode B, Harris S, Vijapurkar U, Mayer C, Fung A, et al. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: A pooled analysis of clinical studies. BMC Endocr Disord. 2014;14:37. doi: 10.1186/1472-6823-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nicolle LE, Capuano G, Fung A, Usiskin K. Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Postgrad Med. 2014;126:7–17. doi: 10.3810/pgm.2014.01.2720. [DOI] [PubMed] [Google Scholar]
- 97.Janssen Research and Development LLC. Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (CREDENCE). US National Institutes of Health. 2014. [Last accessed on 2016 Jul 15]. Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT02065791 .
- 98.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA Warns that SGLT2 Inhibitors for Diabetes May Result in a Serious Condition of Too Much Acid in the Blood. [Last accessed on 2016 Jul 15]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm .
- 99.Soni H. Euglycemic ketoacidosis and sodium glucose co-transporter 2 inhibitors: Is this novel class safe for the diabetic patients? MOJ Toxicol. 2015;1:21. [Google Scholar]
- 100.Kalra S, Sahay R, Gupta Y. Sodium glucose transporter 2 (SGLT2) inhibition and ketogenesis. Indian J Endocrinol Metab. 2015;19:524–8. doi: 10.4103/2230-8210.157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38:1680–6. doi: 10.2337/dc15-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22:753–62. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]
- 103.Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:157–66. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Food and Drug Administration. FDA Drug Safety Communication: Interim Clinical Trial Results Find Increased Risk of Leg and Foot Amputations, Mostly Affecting the Toes, with the Diabetes Medicine Canagliflozin (Invokana, Invokamet); FDA to Investigate. [Last accessed on 2016 Jul 15]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm500965.htm .
- 105.Drug Safety Alert for Canagliflozin. [Last accessed on 2016 Jul 15]. Available from: http://www.scpod.org/news/drug-safety-alert-for-canagliflozin/
- 106.Santoleri F, Sorice P, Lasala R, Costantini A. Liraglutide vs. exenatide: Patient adherence, medication persistence and economic evaluation in the treatment of type 2 diabetes mellitus. Pharmacol Pharm. 2014;5:332–9. [Google Scholar]
- 107.Jain R, Cai J, Fu AC, Chow W, Tan H. Real world (RW) glycemic control and medication adherence among patients with type 2 diabetes mellitus (T2DM) initiated on canagliflozin. Poster Presentation. American Diabetes Association, 76th scientific sessions. New Orleans, Louisiana: 2016. p. 1161. [Google Scholar]
- 108.Jermendy G, Wittmann I, Nagy L, Kiss Z, Rokszin G, Abonyi-Tóth Z, et al. Persistence of initial oral antidiabetic treatment in patients with type 2 diabetes mellitus. Med Sci Monit. 2012;18:CR72–7. doi: 10.12659/MSM.882459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stephens JM, Blak BT, Gold KF, Botteman MF, Pashos CL, Walkinshaw CS, et al. Persistence patterns with oral hypoglycemic agents in type 2 diabetes. JCOM. 2002;9:491–9. [Google Scholar]
- 110.Diels J, Neslusan C. Comparative persistency with newer agents used to treat type 2 diabetes (T2DM) in the United States: Canagliflozin versus dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. Value Health. 2015;18:A68–9. [Google Scholar]
- 111.Cai J, Wang Y, Baser O, Xie L, Diels J, Neslusan C, et al. Comparative persistence with antihyperglycemic agents (AHA) used to treat type 2 diabetes mellitus (T2DM) in the real world. Poster Presentation. American Diabetes Association, 76th scientific sessions. New Orleans, Louisiana: 2016. p. 1155. [Google Scholar]
- 112.Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: A retrospective cohort study. Adv Ther. 2015;32:341–55. doi: 10.1007/s12325-015-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilke T, Groth A, Berg B, Sikirica M, Martin AA, Fuchs A, et al. Non-adherence and non-persistence related to Glp-1 therapy in patients with diabetes mellitus type 2 (T2dm): Analysis of a large german claims-based dataset and comparison to oral anti-diabetics. Value Health. 2014;17:A359. doi: 10.1016/j.jval.2014.08.778. [DOI] [PubMed] [Google Scholar]
- 114.Foley JE, Jordan J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: Mechanistic basis and clinical experience. Vasc Health Risk Manag. 2010;6:541–8. doi: 10.2147/vhrm.s10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Campbell RK, Cobble ME, Reid TS, Shomali ME. Distinguishing among incretin-based therapies. Pathophysiology of type 2 diabetes mellitus: Potential role of incretin-based therapies. J Fam Pract. 2010;59(9 Suppl 1):S5–9. [PubMed] [Google Scholar]
- 116.Buysman EK, Chow W, Henk HJ, Rupnow MF. Characteristics and short-term outcomes of patients with type 2 diabetes mellitus treated with canagliflozin in a real-world setting. Curr Med Res Opin. 2015;31:137–43. doi: 10.1185/03007995.2014.982750. [DOI] [PubMed] [Google Scholar]
- 117.Chow W, Buysman EK, Rupnow MF, Henk HJ. PDB16. Real-world treatment patterns of antihyperglycemic agents among patients with type 2 diabetes mellitus (T2DM) initiated on canagliflozin. Value Health. 2015;18:A55. [Google Scholar]
- 118.Lefebvre P, Pilon D, Robitaille MN, Lafeuille MH, Chow W, Pfeifer M, et al. Glycated hemoglobin (HbA1c) control in patients with type 2 diabetes mellitus (T2DM) treated with canagliflozin in a real-world setting. Value Health. 2015;18:A57. [Google Scholar]
- 119.Meckley LM, Miyasato G, Kokkotos F, Bailey RA. Real-world canagliflozin utilization: Impact on glycemic control in patients with type 2 diabetes mellitus. Value Health. 2015;18:A54–5. [Google Scholar]
- 120.Thayer S, Chow W, Korrer S, Aguilar R. Real-world evaluation of glycemic control among patients with type 2 diabetes mellitus treated with canagliflozin versus dipeptidyl peptidase-4 inhibitors. Curr Med Res Opin. 2016;32:1087–96. doi: 10.1185/03007995.2016.1159954. [DOI] [PubMed] [Google Scholar]
- 121.Bacon T, Willis M, Johansen P, Neslusan C, Nuhoho S, Worbes-Cerezo M. The cost-effectiveness of canagliflozin verse liraglutide in patients with type 2 diabetes (T2dm) failing to achieve glycaemic control on metformin monotherapy in Ireland. Value Health. 2014;17:A345. doi: 10.1016/j.jval.2014.08.700. [DOI] [PubMed] [Google Scholar]
- 122.Troelsgaard A, Pitcher A, Granados D, Hemels M, Lloyd A. The Cost-effectiveness of canagliflozin compared with liraglutide in patients with type 2 diabetes inadequately controlled with metformin and sulfonylurea in France. Value Health. 2014;17:A346–7. doi: 10.1016/j.jval.2014.08.707. [DOI] [PubMed] [Google Scholar]
- 123.Neslusan C, Teschemaker A, Johansen P, Willis M, Valencia-Mendoza A, Puig A. Cost-effectiveness of canagliflozin versus sitagliptin as add-on to metformin in patients with type 2 diabetes mellitus in Mexico. Value Health Reg Issues. 2015;8C:8–19. doi: 10.1016/j.vhri.2015.01.002. [DOI] [PubMed] [Google Scholar]


