Abstract
Introduction:
Traumatic brain injury (TBI) is an under-recognized cause of hypopituitarism. According to recent data, it could be more frequent than previously known. However, there is a scarcity of data in Indian population.
Aims:
The main aim of the study was to determine the prevalence of pituitary hormone deficiencies in the acute phase of TBI. The secondary objectives were to correlate the severity of trauma with basal hormone levels and to determine whether initial hormone deficiencies predict mortality.
Subjects and Methods:
Forty-nine TBI patients (41 men and 8 women) were included in this study. Pituitary functions were evaluated within 24 h of admission.
Results:
Gonadotropin deficiency was found in 65.3% patient while 46.9% had low insulin-like growth factor-1, 12.24% had cortisol level <7 mcg/dl. Cortisol and prolactin level were positively correlated with the severity of TBI suggestive of stress response. Free triiodothyronine (fT3) and free thyroxine were significantly lower in patients with increasing severity of tuberculosis. Logistic regression analysis revealed that mortality after TBI was unrelated to the basal pituitary hormone levels except low T3 level, which was found to be positively related to mortality.
Conclusions:
Pituitary dysfunction is common after TBI and the most commonly affected axes are growth hormone and gonadotropin axis. Low fT3 correlates best with mortality. During the acute phase of TBI, at least an assessment of cortisol is vital as undetected cortisol deficiency can be life-threatening
Keywords: Pituitary dysfunction, posttraumatic hypopituitarism, traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is a nondegenerative, noncongenital insult to the brain from an external mechanical force causing temporary or permanent neurological dysfunction.[1] It is one of the major causes of morbidity and mortality in both the developed as well as the developing countries. Nearly, 1 million people are disabled due TBI in India annually.[2] TBI is an under-recognized cause of neuroendocrine dysfunction, especially hypopituitarism. The prevalence of hypopituitarism after TBI is at least 25% among long-term survivors with significant impact on morbidities and mortalities.[3,4] In India, we have scarcity of data regarding posttraumatic hypopituitarism.
METHODS
Aims and objectives
The main aim of the study was to determine the prevalence of pituitary hormone deficiencies in the acute phase of TBI. The secondary objectives were to correlate the severity of trauma with basal hormone levels and to determine whether initial hormone deficiencies predict mortality.
Patients
In this cross-sectional, convenient sample study, 49 consecutive patients with TBI (41 men, 8 women; Age range 16–78 years) who were admitted to the trauma care center of tertiary care center in Mumbai were included. The study was approved by our Institutional Ethical Committee and conducted between 2013 and 2014. The level of consciousness of the patients was evaluated according to the Glasgow Coma Scale (GCS) as soon as the patient was admitted to the trauma care center. A score of 13–15 was considered as mild, 9–12 moderate, and ≤8 as severe TBI. Patients who had suffered a prolonged hypotensive episode (Systolic pressure <90 mmHg for more than 30 min), had a history of any known pituitary disorder or on any drug affecting hypothalamo-pituitary function, and pregnant women were excluded from the study.
Basal hormonal evaluation
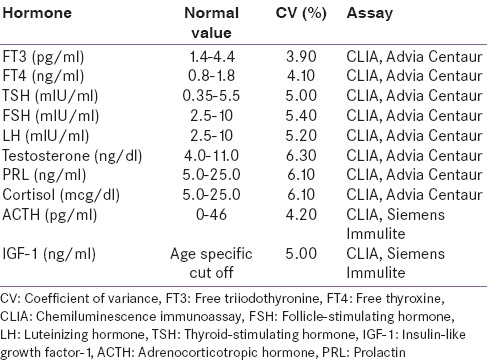
All patients underwent basal hormonal evaluation within the first 24 h of the admission before receiving glucocorticoids and dopamine. Blood samples were taken between 6:00 and 9:00 am. The samples of thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), free thyroxine (fT4), prolactin (PRL), cortisol, adrenocorticotropic hormone (ACTH), follicle stimulating hormone (FSH), luteinizing hormone (LH), Insulin-like growth factor-1 (IGF-I), growth hormone (GH), and total testosterone were collected and estimated on chemiluminescence immunoassay (Advia Centaur CP). The hormonal deficiency was defined as described in Table 1 and the analytic methods to measure hormones with inter- and intra-assay coefficient of variance has been described in Table 2.
Table 1.
Criterion to define deficiency of various hormones
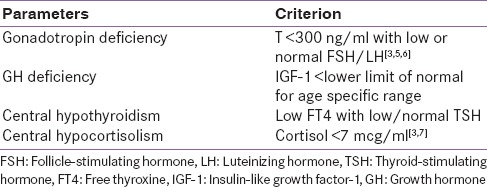
Table 2.
Analytic methods to measure hormones in this study
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences 17.0 (Chicago: SPSS Inc) program. All data were subjected to the Kolmogorov–Smirnov test for normality and are presented as mean ± standard deviation. Normally distributed values between two variables were compared by unpaired t-test. More than two variables were compared using one-way ANOVA test. In addition, we used Pearson's correlation analysis to determine whether significant correlations existed between chosen variables. A multifactorial logistic regression model was used to determine which variables independently predicted the development of mortality; P < 0.05 was considered statistically significant.
RESULTS
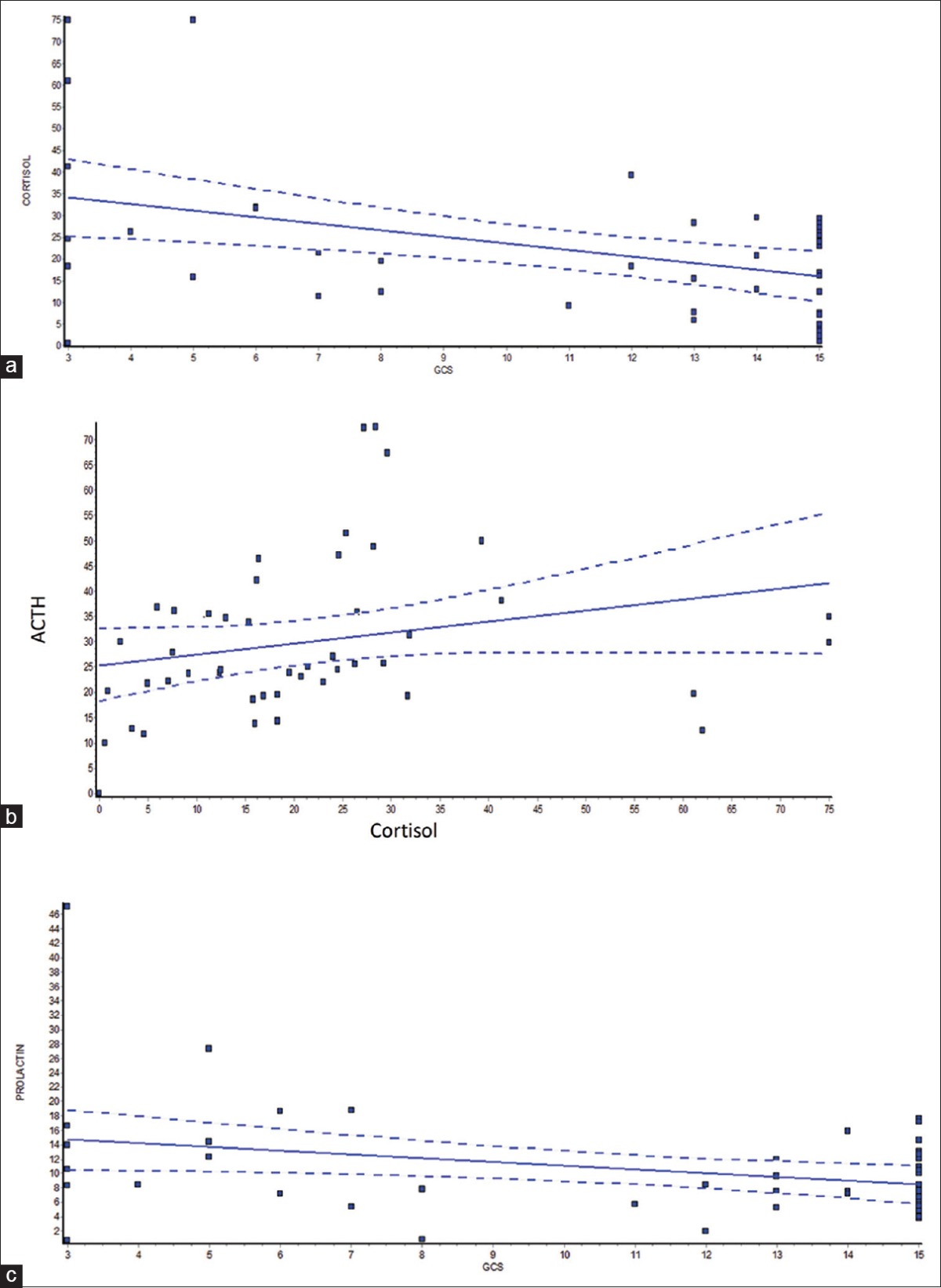
The most common mode of injury was a road traffic accident (55%) followed by a fall (34%), railway accident (6.1%), and assault (4.08%). Twenty-nine (59.18%) patients had mild, 4 (8.16%) had moderate, and 16 (32.65%) had severe TBI. Comparison between mild, moderate, and severe TBI groups in acute phase is described in Table 3. Ten of 49 patients died during the acute phase, 7 of them had severe TBI.
Table 3.
Comparison between mild, moderate, and severe traumatic brain injury groups in acute phase
Based on the criterion defined in Table 1, 63.5% patient had gonadotropin deficiency, 46.9% had low IGF-1 level, 12.24% had cortisol level <7 mcg/dl, 6.1% had low fT4 level, and PRL level was high in 4% of patients. The correlations between GCS and basal hormones were analyzed.
Cortisol levels were significantly higher in patients with low GCS (r = −0.42; P = 0.0027) and cortisol levels were positively correlated with ACTH levels (r = 0.43, P = 0.002) suggestive of hypothalamo–pituitary–adrenal axis activation.
IGF-1 did not correlated with severity of injury while GH is higher in patients with severe compared to mild TBI (P = 0. 02). PRL levels negatively correlated with GCS (r= −0.32; P = 0. 023). The fT4 and fT3 positively correlated with GCS (r = 0.31; P = 0.041, r = 0.49; P = 0.0003, respectively). In our male cohort, there was a positive correlation between total testosterone and GCS in male, but the correlation was not significant (r = 0. 21; P = 0. 19). The significance is summarized in Figure 1a–c.
Figure 1.
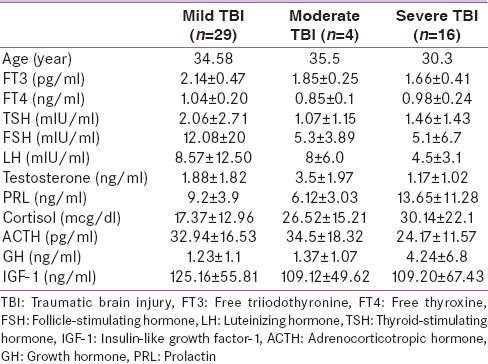
Significant correlations Glasgow Coma Scale and serum cortisol level (a), serum adrenocorticotropic hormone and serum cortisol levels (b), between Glasgow Coma Scale and serum prolactin level (c)
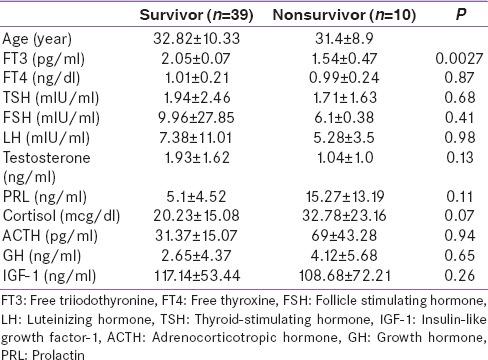
Mean age, GCS, and basal hormone levels of the patients who died after TBI no survivors (NS), 10 patients, were compared with the living patients survivors (S), 39 patients [Table 4]. The fT3 level (NS: 2.05 ± 1.6, S: 1.54 ± 30.47; P = 0.0027) were significantly lower in NSG. There was no significant difference in mean fT4, TSH, PRL cortisol, ACTH, FSH, LH, IGF-I, GH, and total testosterone between the NS and S. Logistic regression analysis revealed that mortality after TBI was unrelated to the basal pituitary hormone levels except low T3 level, which was found related to mortality.
Table 4.
Comparison between survivors and nonsurvivors of traumatic brain injury
DISCUSSION
TBI in acute phase can lead to pituitary dysfunction, results in a temporary increase or decrease in pituitary hormonal levels. In our study, suppression of the hypothalamo–pituitary–gonadal axis was most common which was concordant with other studies.[3] According to the previous studies, acute phase of TBI associated with low or high GH with low IGF-1.[8] We found low IGF-1 in 46.9% of patients, but it was not correlating with severity which is also demonstrated by Tanriverdi et al.[7] GH was high in patients with severe TBI compared to mild TBI (P = 0.02). This could be due to more GH resistance in patients with severe TBI. Gonadotroph and somatotroph cells are located in the vascular territory of the long hypophyseal system which can be easily affected by TBI, which explains most common involvement of these axes.[1]
As described in various studies[3,9,10] cortisol level was found to increase immediately following TBI, and it was concordant with ACTH level suggestive of activation of hypothalamic–pituitary–adrenal axis. Cortisol level correlated positively with the severity. Low cortisol (<7 mcg/dl) was found in 12.24% of patients, suggestive of posttraumatic damage at the hypothalamic-pituitary level. PRL level correlated positively with the severity of the TBI, which may represent stress response to TBI. We found fT3, fT4 were significantly lower in patient with increasing severity of TBI. Previous studies have shown that low thyroid hormones correlated with worst prognosis.[7,11] Similarly, our study showed, low fT3 could be risk marker for mortality.
CONCLUSION
Pituitary hormone deficiencies were identified in a substantial proportion of patients with acute TBI. Gonadotropin and growth axes were most commonly affected. Increased level of PRL and low level of fT3, fT4, and cortisol correlated significantly with the severity of TBI. During acute phase of TBI at least assessment of cortisol is vital as undetected cortisol deficiency can be life-threatening. The fT3 could be one of the predictors of an adverse outcome. Our future plan is to follow this cohort of patients for the assessment of long-term hormonal deficiency which could be factor for improvement in quality of life.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bondanelli M, Ambrosio MR, Zatelli MC, De Marinis L, degli Uberti EC. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679–91. doi: 10.1530/eje.1.01895. [DOI] [PubMed] [Google Scholar]
- 2.Gururaj G. Epidemiology of traumatic brain injuries: Indian scenario. Neurol Res. 2002;24:24–8. doi: 10.1179/016164102101199503. [DOI] [PubMed] [Google Scholar]
- 3.Agha A, Thompson CJ. Anterior pituitary dysfunction following traumatic brain injury (TBI) Clin Endocrinol (Oxf) 2006;64:481–8. doi: 10.1111/j.1365-2265.2006.02517.x. [DOI] [PubMed] [Google Scholar]
- 4.Agha A, Rogers B, Mylotte D, Taleb F, Tormey W, Phillips J, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf) 2004;60:584–91. doi: 10.1111/j.1365-2265.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- 5.Hohl A, Mazzuco TL, Coral MH, Schwarzbold M, Walz R. Hypogonadism after traumatic brain injury. Arq Bras Endocrinol Metabol. 2009;53:908–14. doi: 10.1590/s0004-27302009000800003. [DOI] [PubMed] [Google Scholar]
- 6.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 7.Tanriverdi F, Senyurek H, Unluhizarci K, Selcuklu A, Casanueva FF, Kelestimur F. High risk of hypopituitarism after traumatic brain injury: A prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J Clin Endocrinol Metab. 2006;91:2105–11. doi: 10.1210/jc.2005-2476. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Miell J, Freeman E, Jones J, Matthews D, Preece M, et al. Critically ill patients have high basal growth hormone levels with attenuated oscillatory activity associated with low levels of insulin-like growth factor-I. Clin Endocrinol (Oxf) 1991;35:47–54. doi: 10.1111/j.1365-2265.1991.tb03495.x. [DOI] [PubMed] [Google Scholar]
- 9.Barton RN, Stoner HB, Watson SM. Relationships among plasma cortisol, adrenocorticotrophin, and severity of injury in recently injured patients. J Trauma. 1987;27:384–92. doi: 10.1097/00005373-198704000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Feibel J, Kelly M, Lee L, Woolf P. Loss of adrenocortical suppression after acute brain injury: Role of increased intracranial pressure and brain stem function. J Clin Endocrinol Metab. 1983;57:1245–50. doi: 10.1210/jcem-57-6-1245. [DOI] [PubMed] [Google Scholar]
- 11.Woolf PD, Lee LA, Hamill RW, McDonald JV. Thyroid test abnormalities in traumatic brain injury: Correlation with neurologic impairment and sympathetic nervous system activation. Am J Med. 1988;84:201–8. doi: 10.1016/0002-9343(88)90414-7. [DOI] [PubMed] [Google Scholar]


