Abstract
Corneal Visualization Scheimpflug Technology (CST) and Ocular Response Analyzer (ORA) measurements were carried out in 105 eyes of 69 patients with primary open-angle glaucoma. All patients had axial length (AL), central corneal thickness (CCT), intraocular pressure (IOP) with Goldmann applanation tonometry (GAT) and eight visual fields (VF)s with the Humphrey Field Analyzer. VF progression was summarized using a time trend analysis of mean total deviation (mTD) and the association between mTD progression rate and a number of ocular parameters (including CST and ORA measurements) was assessed using mixed linear regression analysis. The optimal model of VF progression selected based on the corrected Akaike Information Criteria (AICc) included ORA’s corneal hysteresis (CH) parameter as well as a number of CST measurements: mTD progression rate = 1.2–0.070 * mean GAT + 0.090 * CH–1.5 * highest concavity deformation amplitude with CST + 9.4 * A1 deformation amplitude with CST–0.05 * A2 length with CST (AICc = 125.8). Eyes with corneas that experience deep indentation at the maximum deformation, shallow indentation at the first applanation and wide indentation at the second applanation in the CST measurement are more likely to experience faster rates of VF progression.
Glaucoma is the second leading cause of blindness worldwide, affecting approximately 60 million peoples1. The disease causes irreversible visual field (VF) damage so it is very important to predict its progression and make appropriate interventions as soon as possible. The principal target of glaucoma treatments is to reduce and control intraocular pressure (IOP), which has been shown to reduce VF progression by numerous clinical trials and research studies2,3,4,5,6,7,8,9,10. Tonometry measurements of IOP can be greatly influenced by structural properties of the eye; in particular, IOP measured with Goldmann applanation tonometry (GAT) has been shown to be affected by central corneal thickness (CCT)11,12,13,14,15,16,17,18,19,20,21,22,23. Thus, CCT should be considered when interpreting GAT-measured IOP and making clinical decisions. Furthermore, studies have suggested that CCT is associated with the progression of glaucoma4,24. Other biomechanical properties of the cornea have also been shown to affect the progression of glaucoma. In particular, corneal hysteresis (CH) and corneal resistance factor (CRF), measured with the Ocular Response Analyzer (ORA, Reichert Ophthalmic Instruments, Depew, NY, USA), have been reported to impinge on progression25,26.
The Corneal Visualization Scheimpflug Technology instrument (Corvis ST tonometry: CST; Oculus, Wetzlar, Germany) is a new device, integrated with an ultra-high-speed Scheimpflug camera, to quantitatively measure biomechanical properties of the cornea during the application of a rapid air-puff27. As a result, very detailed corneal movement during the air puff application can be observed, such as velocity of corneal deformation at the first and second applanations and the maximum depth of corneal deformation (Fig. 1). Although CST and ORA both measure the biomechanical properties of the cornea, their mechanisms are completely different, and the relationship between CST-measured corneal parameters and the progression of glaucomatous VF damage has not been reported in detail. Jung et al. have reported the relationship between maximum depth of corneal deformation against peripapillary atrophy area and the disc tilt ratio in patients with glaucoma28, however more detailed investigation has not been reported, such as the relationship between visual field progression rate and various (twelve) CST parameters, including maximum depth of corneal deformation.
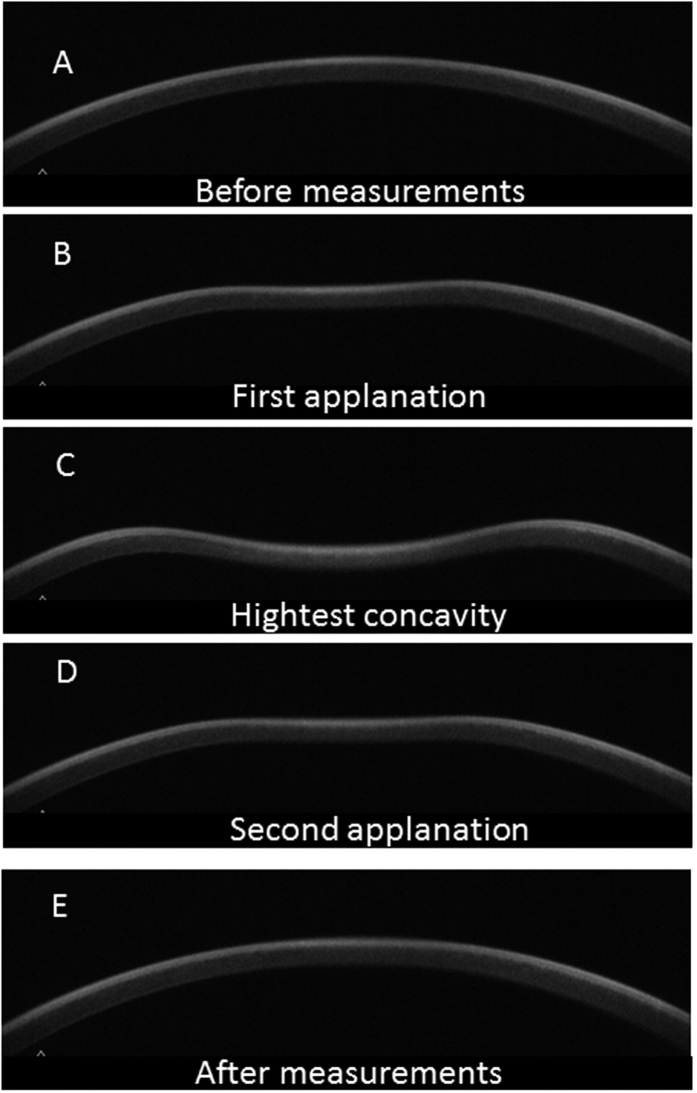
Figure 1. Corneal movement during the CST measurement.
In the CST tonometry measurement, a rapid air puff is applied to cornea and cornea moves inward whereas ORA measures air jet pressure at the events of first and second applanations. The figures show the corneal shape in each phase: (A) prior to air puff applanation, (B) first applanation, (C) highest concavity, (D) second applanation, and (E) posterior to air puff applanation. CST: Corvis ST tonometry, ORA: Ocular Response Analyzer.
Therefore, the purpose of the current study is to investigate the effect of ORA- and CST-measured parameters on the progression of glaucomatous VF damage in patients with primary open angle glaucoma (POAG).
Method
The study was approved by Research Ethics Committee of the Graduated School of Medicine and Faculty of Medicine at The University of Tokyo. Written informed consent was given by patients for their information to be stored in the hospital database and used for research. This study was performed according to the tenets of the Declaration of Helsinki.
Subjects
One hundred and five eyes of 69 POAG patients (36 males and 33 females) were included in this study. POAG was defined as (1) presence of typical glaucomatous changes in the optic nerve head such as a rim notch with a rim width ≤0.1 disc diameters or a vertical cup-to-disc ratio of >0.7 and/or a retinal nerve fiber layer defect with its edge at the optic nerve head margin greater than a major retinal vessel, diverging in an arcuate or wedge shape; and (2) gonioscopically wide open angles of grade 3 or 4 based on the Shaffer classification. All patients had at least 9 VFs measured with the Humphrey Field Analyzer II (HFA, Carl Zeiss Meditec Inc., Dublin, CA, USA), with the SITA standard 24-2 or 30-2 program. Reliable VFs were defined as Fixation loss (FL) rate <20% and False positive (FP) rate <15% following the criteria used in the HFA software; false negative (FN) was not used as an exclusion criterion. Reliable VFs were identified and eyes with at least eight reliable VFs were investigated, excluding the first VF measurement. We chose a minimum of eight VFs because it has recently been reported that this number is needed to precisely analyze VF progression29,30,31,32,33. Eyes that experienced any surgical procedure, including trabeculectomy and cataract surgery, during or prior to this VF series period were excluded. Inclusion criteria were no abnormal eye-related findings except for OAG on biomicroscopy, gonioscopy and funduscopy. Eyes with a history of other ocular disease, such as age-related macular degeneration were also excluded. Eyes with significant cataract which affects VF were carefully excluded. Only subjects aged ≧20 years old were included and eyes with IOP > 25 mmHg or contact lens wearers were excluded. If both eyes satisfied the inclusion criteria, then both were included in the study. Axial length (AL) and CCT were also measured in all patients using the IOL Master, ver. 5.02 (Carl Zeiss Meditec Inc., Dublin, CA, USA) and CST, respectively. CST was performed after the final (8th) VF measurement.
ORA measurements
ORA records two applanation pressure measurements, prior to and following indentation by a rapid jet of air. Due to its viscoelastic property, the cornea resists the air puff, resulting in delays in the inward and outward applanation events, which causes a measureable difference in air puff values. This difference is called CH, while CRF represents the resistance of cornea34.
Both ORA and CST were carried out three times (on the same day) prior to the GAT-IOP measurement and within 180 days from the eight VF measurement. The average value of the three measurements was used in the analyses. The order of ORA and CST measurements was decided randomly. All data were of sufficient quality, as guaranteed by analyzing only eyes with a quality index >7.5.
Corvis ST tonometer measurements
The principles of CST are described in detail elsewhere27. In short, the instrument’s camera records a sequence of images that capture corneal deformation following a rapid air puff. The device is capable of capturing 4,330 images per second that are analyzed to quantify CCT, deformation amplitude, applanation length and corneal velocity. Each measurement is further distinguished as follows: ‘A1/A2 time’ is the length of time from the initiation of the air puff to the first (cornea moves inwards) or second applanation (cornea moves outwards); ‘A1/2 length’ is the length of the flattened cornea at the first or second applanation; ‘A1/2 velocity’ is the velocity of the movement of cornea during the first or second applanation; ‘A1/2 deformation amplitude’ is the movement of the corneal apex of the flattened cornea at the first or second applanation; ‘peak distance’ is the distance between the two surrounding peaks of the cornea at the highest concavity; ‘highest concavity deformation amplitude’ is the magnitude of movement of the corneal apex from before deformation to its highest concavity: ‘highest concavity time’ is the length of the time taken to reach highest concavity from pre-deformation of the cornea; ‘radius’ is the central curvature radius at the point of highest concavity.
CST (software version; 1.2r1092) was performed three separate times, on the same day, with at least a one minute interval between each repeat measurement of ORA and CST. Averages of CST parameters were calculated for the three repeated tests. All CST measurements were considered reliable according to the “OK” quality index displayed on the device monitor.
Other measurements
The mean and standard deviation (SD) of all GAT-IOP measurements during the follow up period were calculated.
VF data
The mean total deviation (mTD) of the 52 test points used in the 24-2 HFA VF test pattern was calculated, for both of 24-2 and 30-2 HFA VFs. The progression rate of mTD was calculated using the eight VFs collected from each eye, similarly to the MD trend analysis employed in the HFA.
Statistical analysis
The relationship between ORA (CH and CRF), CST parameters (A 1/2 time, A 1/2 length, A 1/2 velocity, A 1/2 deformation amplitude, highest deformation amplitude, highest concavity time, peak distance, and radius) and other ocular/systemic parameters (age, mean GAT, SD of GAT, CCT, AL, and mTD in the initial VF) against mTD progression rate was investigated using a linear mixed model with patient as a random effect (because one or two eyes of a patient were included). The optimal linear mixed model (modelbasic) to describe mTD progression rate using ocular/systemic parameters was selected according to the second order bias corrected Akaike Information Criterion (AICc) index. Two further models were selected adding only ORA parameters and also both ORA and CST parameters (modelORA and modelORA_CST, respectively). The AICc is the corrected form of the common statistical measure of AIC. AICc gives an accurate estimation even when the sample size is small35. In a multivariate regression model, degrees of freedom decreases as the number of variables increases, hence it is recommended to use model selection methods to improve the model fit by removing redundant variables36,37. Any magnitude of reduction in AICc suggests an improvement of the model, and the probability that one particular model is the model that minimizes ‘information loss’ can be calculated; when there are n candidate models and the AICc values of those models are AIC1, AIC2, AIC3, …, AICn. If AICmin is the minimum of these values then exp((AICmin − AICi)/2) describes the relative probability that the ith model minimizes the information loss (i.e. is the ‘optimal model’)38. Relative probabilities were calculated among all candidate models. All statistical analyses were performed using the statistical programming language ‘R’ (R version 3.2.3; The foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the study subjects are summarized in Table 1. The mean ± standard deviation (SD) [range] age was 63.2 ± 9.7 [43 to 85], 36 patients were male and 33 patients were female. Eight VFs were measured over an average period of 2412.2 ± 868.9 [630 to 6881] days. GAT was conducted 29.0 ± 7.1 [18 to 69] times during the follow up period (between the initial VF and the eighth VF). Mean GAT-IOP was 13.5 ± 2.2 [8.9 to 20.2] mmHg with an SD value of 1.5 ± 0.47 [0.79 to 3.6].
Table 1. Subject demographics.
| Variables | Value |
|---|---|
| age, (mean ± SD) [range], years old | 63.2 ± 9.7 [43 to 85] |
| Male/Female | 36/33 |
| Right/Left | 50/55 |
| GAT, (mean ± SD) [range], mmHg | 13.5 ± 2.2 [8.9 to 20.2] |
| AL, (mean ± SD) [range], mm | 25.1 ± 1.6 [22.3 to 29.2] |
| CCT, (mean ± SD) [range], μm | 530.9 ± 35.6 [458.3 to 624.3] |
| mTD, (mean ± SD) [range], dB | −6.8 ± 6.5 [−27.0 to 3.9] |
sd: standard deviation, GAT: intraocular pressure measured with Goldmann tonometry, AL: axial length, CCT: central corneal thickness, mTD: mean of total deviation values.
Summary statistics of CST and CH measurements are shown in Table 2. The modelled relationships between mTD progression rate and mean GAT, SD of GAT, ORA parameters and CST parameters are shown in Table 3. A significant relationship was observed for age and CH (p = 0.032 and 0.049, respectively, linear mixed model).
Table 2. CST and ORA parameters.
| Parameter | Value (mean ± sd) [range] | |
|---|---|---|
| ORA | CH (mmHg) | 9.2 ± 1.1 [6.5 to 11.8] |
| CRF (mmHg) | 8.4 ± 1.4 [4.9 to 13.0] | |
| CST | A1 time (ms) | 7.2 ± 0.3 [6.5 to 8.4] |
| A1 length (mm) | 1.7 ± 0.072 [1.4 to 1.8] | |
| A1 velocity (m/s) | 0.16 ± 0.014 [0.10 to 0.20] | |
| A1 deformation amplitude (mm) | 0.12 ± 0.0083 [0.11 to 0.16] | |
| A2 time (ms) | 21.9 ± 0.46 [20.9 to 23.2] | |
| A2 length (mm) | 1.7 ± 0.23 [0.83 to 2.2] | |
| A2 velocity (m/s) | −0.39 ± 0.079 [−0.16 to −0.63] | |
| A2 deformation amplitude (mm) | 0.41 ± 0.072 [0.25 to 0.57] | |
| highest deformation amplitude (mm) | 1.1 ± 0.11 [0.82 to 1.3] | |
| highest concavity time (ms) | 16.9 ± 0.57 [15.4 to 18.4] | |
| Peak distance (mm) | 3.4 ± 0.88 [2.1 to 5.5] | |
| Radius (mm) | 7.5 ± 0.85 [6.2 to 10.3] |
sd: standard deviation, CCT: central corneal thickness, CH: corneal hysteresis, CRF: corneal resistant factor.
Table 3. The relationship between CST/ORA parameters and various ocular parametetrs, and visual field progression rate.
| Coefficient | Standard error | p value | AICc | |
|---|---|---|---|---|
| age (years old) | 0.0094 | 0.0043 | 0.032 | 132.3 |
| mean GAT (mmHG) | −0.017 | 0.019 | 0.38 | 136.2 |
| SD of GAT (mmHG) | −0.076 | 0.092 | 0.41 | 136.3 |
| CCT (mm) | 0.00070 | 0.00120 | 0.52 | 136.5 |
| axial length (mm) | 0.019 | 0.026 | 0.47 | 136.5 |
| mTD in the initial VF | 0.0057 | 0.0057 | 0.40 | 136.2 |
| A1 time (ms) | 0.21 | 0.15 | 0.16 | 134.9 |
| A1 length (mm) | −0.20 | 0.60 | 0.74 | 136.9 |
| A1 velocity (m/s) | −2.7 | 3.1 | 0.37 | 136.3 |
| A1 deformation amplitude (mm) | 6.7 | 5.2 | 0.20 | 135.1 |
| A2 time (ms) | −0.026 | 0.094 | 0.78 | 136.9 |
| A2 length (mm) | −0.18 | 0.19 | 0.33 | 136.1 |
| A2 velocity (m/s) | 0.72 | 0.55 | 0.20 | 135.1 |
| A2 deformation amplitude (mm) | −0.11 | 0.60 | 0.86 | 136.9 |
| highest deformation amplitude (mm) | −0.59 | 0.39 | 0.13 | 134.7 |
| highest concavity time (ms) | 0.0019 | 0.075 | 0.98 | 137.0 |
| Peak distance (mm) | −0.053 | 0.051 | 0.30 | 135.4 |
| Radius (mm) | 0.072 | 0.050 | 0.15 | 134.8 |
| CH (mmHg) | 0.076 | 0.038 | 0.049 | 132.8 |
| CRF (mmHg) | 0.045 | 0.030 | 0.14 | 134.6 |
Bold characters represent p < 0.05.
CST: Corvis ST tonometry, ORA: ocular response analyzer.
As a result of model (parameter) selection, the optimum equation for modelbasic was: mTD progression rate = 0.32 (intercept) −0.0094 * age (AICc = 132.3); thus all other variables (mean GAT, SD of GAT, CCT, AL and mTD in the initial VF) were not deemed to improve the model. The equation for modelORA was: mTD progression rate = −0.35 −0.0083 * age + 0.065 * CH (AICc = 131.4). The equation for modelORA_CST was: mTD progression rate = 1.2–0.070 * mean GAT + 0.090 * CH + −1.5 * highest deformation amplitude + 9.4 * A1 deformation amplitude −0.05 * A2 length (AICc = 125.8). The probability that modelORA minimizes information loss compared to modelbasic was 36.2%. The probability that modelORA_CST minimizes information loss compared to modelORA and modelbasic was 93.9% and 96.1%, respectively.
Discussion
In the current study CST and ORA measurements were carried out in 105 eyes of 69 patients with POAG. In models describing the progression rate of mTD, the inclusion of CH and CST parameters resulted in the most favorable model. This model included mean GAT-IOP (higher IOP leads to faster progression) and CH (lower CH indicates faster progression) agreeing with previous studies4,25,26,39,40,41, as well as highest concavity deformation amplitude (the higher the amplitude, the faster progression), A1 deformation amplitude (lower amplitude suggests faster progression), A2 length (larger length leads to faster progression).
Interestingly, modelbasic did not include mean GAT-IOP, despite its undoubted influence on VF progression3,42,43,44. However, this does not deny the importance of IOP control in the management of glaucoma. The current study analyzed clinical data from a real world setting where IOP reduction interventions would be intensified if VF progression was identified. This result aligns with our recent findings from a multi-central study in which the real world clinical data was analyzed and a relationship between mean IOP and the progression of glaucoma was not found45. In the same study, however, the SD of IOP measurements was related to the progression of glaucoma45. These findings are probably because patients’ VF progression is effectively controlled by reducing mean IOP, but SD of IOP measurements is less considered in the clinical treatment decision.
Only the age variable was selected in modelbasic. Age has been reported elsewhere to be an independent risk factor of the progression of glaucoma4,39,40,41. Surprisingly, age was not included in modelORA_CST, however, this model did include CH, which may act as a proxy for age since CH decreases as age increases46. Further, CST parameters are also included in modelORA_CST and some CST parameters are also related to age; in particular, highest deformation amplitude increases as age increases. Thus, the current results may suggest that the accelerating effect of age on VF progression may actually be driven a change in CH and/or highest deformation amplitude. This is clinically very important because an eye with a low CH and a large highest deformation amplitude has a greater risk of progression at any age.
CCT has also been reported to be associated with the progression of glaucoma4,11,13,24,47,48, however, CCT was not selected in any of our models in the current study. Recent studies have reported that CH is a stronger risk factor for the progression of glaucoma than CCT26, and indeed, in the current study, CH was included in both modelORA and modelORA_CST. Baseline VF damage may4,39,49 or may not40,50,51 be related to faster VF progression; however, in the current study, mTD in the initial VF was not included in any of the optimal models.
Interestingly, the AICc of modelORA_CST was significantly smaller than the AICc of modelORA (according to their AIC values, modelORA_CST is deemed to be the better model with a probability of 93.9%). Thus, it may be advantageous to use CST, in addition to ORA, to better interpret VF progression in glaucoma patients. In particular, modelORA_CST suggests that eyes with corneas experiencing a deep indentation following the CST air-puff are likely to progress at a faster rate. The hysteresis of a viscoelastic material is defined as the amount of energy absorption during the ‘loading/unloading’ stress/strain cycle and the magnitude of the energy absorption can be calculated as the area surrounded by the loading and unloading curves52, which is thought to reflect less compliance of the lamina cribrosa and thus provide further information about glaucoma risk25,53. A deeper highest deformation amplitude indicates the change of shape of the loading/unloading curves. Its effect on the progression of glaucoma has not been examined in detail so a further study should be carried out to shed light on this finding. Further, a wide applanated area at the second applanation (A2 length) may be related to a deeper indentation at the maximum deformation. Smaller movements of the corneal apex at the first applanation were related to faster progression in the current study. A smaller movement of the corneal apex at the first applanation may indicate that the absorption of the projected energy is finished at the phase of the first applanation. An eye exhibiting this ‘poor’ absorption of energy could be indented more deeply at the highest deformation. Again, a further study is needed to investigate these findings in more detail.
A limitation of the current study is that we could not control for the effect of anti-glaucomatous eye drops on corneal biomechanical properties54,55,56,57. As all patients were recruited from real world glaucoma clinics this could not be avoided, but it could have a non-negligible effect on the study findings. In addition, ORA and CST measured values may change over a long-term follow-up. As there is no study which investigated the variability of CST parameters in long-term follow-up, it should be investigated in a future study. Also, it is the mTD (or mean deviation) slope trend analysis most frequently used at the clinical settings, and hence we used this method to evaluate VF progression. However it is also true mTD slope analysis may miss focal progression. There is no gold standard method to evaluate the focal progression, but it may be the best way to divide VF into small clusters and evaluate the influence of CST parameters in each sector, which should be carried out in a following study. Also, it would be needed to further confirm the current result using an independent population.
In conclusion, it is advantageous to carry out CST tonometry in addition to ORA when assessing the progression of glaucomatous VF change. Careful glaucoma management is required in eyes with any of the following characteristics: a low CH, a large highest concavity deformation amplitude, a large A2 length, or, a small A1 deformation amplitude since these eyes are at greatest risk of VF progression.
Additional Information
How to cite this article: Matsuura, M. et al. The usefulness of CorvisST Tonometry and the Ocular Response Analyzer to assess the progression of glaucoma. Sci. Rep. 7, 40798; doi: 10.1038/srep40798 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was partially supported by Japan Science and Technology Agency (JST) -CREST and Grant 26462679 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Author Contributions M.M. and K.H. prepared the material. M.M. and R.A. wrote the main manuscript text prepared figures. M.M., K.H., H.M., S.M., Y.K., and R.A. reviewed the manuscript.
References
- Quigley H. A. Glaucoma. Lancet 377, 1367–1377 (2011). [DOI] [PubMed] [Google Scholar]
- Heijl A. et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 120, 1268–1279 (2002). [DOI] [PubMed] [Google Scholar]
- Garway-Heath D. F. et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 385, 1295–1304 (2015). [DOI] [PubMed] [Google Scholar]
- Leske M. C. et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 114, 1965–1972 (2007). [DOI] [PubMed] [Google Scholar]
- Holmin C., Thorburn W. & Krakau C. E. Treatment versus no treatment in chronic open angle glaucoma. Acta Ophthalmol (Copenh) 66, 170–173 (1988). [DOI] [PubMed] [Google Scholar]
- Pajic B., Pajic-Eggspuehler B. & Hafliger I. O. Comparison of the effects of dorzolamide/timolol and latanoprost/timolol fixed combinations upon intraocular pressure and progression of visual field damage in primary open-angle glaucoma. Curr Med Res Opin 26, 2213–2219 (2010). [DOI] [PubMed] [Google Scholar]
- Migdal C., Gregory W. & Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology 101, 1651–1656; discussion 1657 (1994). [DOI] [PubMed] [Google Scholar]
- Jay J. L. & Murray S. B. Early trabeculectomy versus conventional management in primary open angle glaucoma. Br J Ophthalmol 72, 881–889 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch D. C., Gillespie B. W., Lichter P. R., Niziol L. M. & Janz N. K. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology 116, 200–207 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol 130, 429–440 (2000). [DOI] [PubMed] [Google Scholar]
- Whitacre M. M. & Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol 38, 1–30 (1993). [DOI] [PubMed] [Google Scholar]
- Kotecha A., Elsheikh A., Roberts C. R., Zhu H. & Garway-Heath D. F. Corneal thickness- and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Invest Ophthalmol Vis Sci 47, 5337–5347 (2006). [DOI] [PubMed] [Google Scholar]
- Feltgen N., Leifert D. & Funk J. Correlation between central corneal thickness, applanation tonometry, and direct intracameral IOP readings. Br J Ophthalmol 85, 85–87 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers N. & Bramsen T. Importance of corneal thickness in applanation tonometry [proceedings]. Acta Ophthalmol Suppl 32 (1975). [DOI] [PubMed] [Google Scholar]
- Bhan A. et al. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest Ophthalmol Vis Sci 43, 1389–1392 (2002). [PubMed] [Google Scholar]
- Foster P. J. et al. Central corneal thickness and intraocular pressure in a Mongolian population. Ophthalmology 105, 969–973 (1998). [DOI] [PubMed] [Google Scholar]
- Gunvant P. et al. Effect of corneal parameters on measurements using the pulsatile ocular blood flow tonograph and Goldmann applanation tonometer. Br J Ophthalmol 88, 518–522 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. et al. Relationship between corneal thickness and measured intraocular pressure in a general ophthalmology clinic. Ophthalmology 106, 2154–2160 (1999). [DOI] [PubMed] [Google Scholar]
- Shimmyo M., Ross A. J., Moy A. & Mostafavi R. Intraocular pressure, Goldmann applanation tension, corneal thickness, and corneal curvature in Caucasians, Asians, Hispanics, and African Americans. Am J Ophthalmol 136, 603–613 (2003). [DOI] [PubMed] [Google Scholar]
- Stodtmeister R. Applanation tonometry and correction according to corneal thickness. Acta Ophthalmol Scand 76, 319–324 (1998). [DOI] [PubMed] [Google Scholar]
- Tonnu P. A. et al. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br J Ophthalmol 89, 851–854 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs R. C. et al. Distribution of central corneal thickness and its association with intraocular pressure: The Rotterdam Study. Am J Ophthalmol 123, 767–772 (1997). [DOI] [PubMed] [Google Scholar]
- Liu J. & Roberts C. J. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg 31, 146–155 (2005). [DOI] [PubMed] [Google Scholar]
- Jonas J. B. & Holbach L. Central corneal thickness and thickness of the lamina cribrosa in human eyes. Invest Ophthalmol Vis Sci 46, 1275–1279 (2005). [DOI] [PubMed] [Google Scholar]
- Wells A. P. et al. Corneal hysteresis but not corneal thickness correlates with optic nerve surface compliance in glaucoma patients. Invest Ophthalmol Vis Sci 49, 3262–3268 (2008). [DOI] [PubMed] [Google Scholar]
- Lascaratos G. et al. Intraocular pressure (IOP) measured with the Ocular Response Analyzer is a better predictor of glaucoma progression than Goldmann IOP in the United Kingdom Glaucoma TreatmentStudy (UKGTS). http://iovs.arvojournals.org/article.aspx?articleid=2266497&resultClick=1 (2014).
- Koprowski R. Automatic method of analysis and measurement of additional parameters of corneal deformation in the Corvis tonometer. Biomed Eng Online 13, 150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Park H. Y. & Park C. K. Association between Corneal Deformation Amplitude and Posterior Pole Profiles in Primary Open-Angle Glaucoma. Ophthalmology 123, 959–964 (2016). [DOI] [PubMed] [Google Scholar]
- Taketani Y., Murata H., Fujino Y., Mayama C. & Asaoka R. How Many Visual Fields Are Required to Precisely Predict Future Test Results in Glaucoma Patients When Using Different Trend Analyses? Invest Ophthalmol Vis Sci 56, 4076–4082 (2015). [DOI] [PubMed] [Google Scholar]
- Krakau C. E. A statistical trap in the evaluation of visual field decay. Acta Ophthalmol Suppl 173, 19–21 (1985). [DOI] [PubMed] [Google Scholar]
- Spry P. G., Bates A. B., Johnson C. A. & Chauhan B. C. Simulation of longitudinal threshold visual field data. Invest Ophthalmol Vis Sci 41, 2192–2200 (2000). [PubMed] [Google Scholar]
- Bengtsson B., Patella V. M. & Heijl A. Prediction of glaucomatous visual field loss by extrapolation of linear trends. Arch Ophthalmol 127, 1610–1615 (2009). [DOI] [PubMed] [Google Scholar]
- Holmin C. & Krakau C. E. Regression analysis of the central visual field in chronic glaucoma cases. A follow-up study using automatic perimetry. Acta Ophthalmol (Copenh) 60, 267–274 (1982). [DOI] [PubMed] [Google Scholar]
- Terai N., Raiskup F., Haustein M., Pillunat L. E. & Spoerl E. Identification of biomechanical properties of the cornea: the ocular response analyzer. Curr Eye Res 37, 553–562 (2012). [DOI] [PubMed] [Google Scholar]
- Burnham K. P. & DR. A. Multimodel inference: understanding: AIC and BIC in model selection. Sociological Methods & Research 33, 261–304 (2004). [Google Scholar]
- Tibshirani R. J. & Taylor J. Degrees of freedom in lasso problems. Annals of Statistics 40, 1198–1232 (2012). [Google Scholar]
- Mallows C. Some comments on Cp. Technometrics 15, 661–675 (1973). [Google Scholar]
- Burnham K. P. & Anderson D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (2nd ed.). (Springer-Verlag, 2002). [Google Scholar]
- Lichter P. R. et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 108, 1943–1953 (2001). [DOI] [PubMed] [Google Scholar]
- Gordon M. O. et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 120, 714–720; discussion 829–730 (2002). [DOI] [PubMed] [Google Scholar]
- De Moraes C. G. et al. Risk factors for visual field progression in the low-pressure glaucoma treatment study. Am J Ophthalmol 154, 702–711 (2012). [DOI] [PubMed] [Google Scholar]
- The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol 126, 498–505 (1998). [DOI] [PubMed]
- Kass M. A. et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 120, 701–713; discussion 829–730 (2002). [DOI] [PubMed] [Google Scholar]
- Ederer F., Gaasterland D. E., Sullivan E. K. & Investigators A. The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials 15, 299–325 (1994). [DOI] [PubMed] [Google Scholar]
- Fujino Y. et al. Evaluation of glaucoma progression in large-scale clinical data: the Japanese Archive of Multicentral Databases in Glaucoma (JAMDIG). Invest Ophthalmol Vis Sci (in press). [DOI] [PubMed]
- Kotecha A. et al. Biomechanical parameters of the cornea measured with the Ocular Response Analyzer in normal eyes. BMC Ophthalmol 14, 11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha A., White E. T., Shewry J. M. & Garway-Heath D. F. The relative effects of corneal thickness and age on Goldmann applanation tonometry and dynamic contour tonometry. Br J Ophthalmol 89, 1572–1575 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre M. M., Stein R. A. & Hassanein K. The effect of corneal thickness on applanation tonometry. Am J Ophthalmol 115, 592–596 (1993). [DOI] [PubMed] [Google Scholar]
- Lee J. M. et al. Baseline prognostic factors predict rapid visual field deterioration in glaucoma. Invest Ophthalmol Vis Sci 55, 2228–2236 (2014). [DOI] [PubMed] [Google Scholar]
- Gordon M. O. et al. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology 114, 10–19 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros F. A. et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol 123, 1351–1360 (2005). [DOI] [PubMed] [Google Scholar]
- Ewing J. A. On hysteresis in the relation of strain to stress. British Association Reports, 502–504 (1889). [Google Scholar]
- Lesk M. R., Hafez A. S. & Descovich D. Relationship between central corneal thickness and changes of optic nerve head topography and blood flow after intraocular pressure reduction in open-angle glaucoma and ocular hypertension. Arch Ophthalmol 124, 1568–1572 (2006). [DOI] [PubMed] [Google Scholar]
- Zhong Y., Shen X., Yu J., Tan H. & Cheng Y. The comparison of the effects of latanoprost, travoprost, and bimatoprost on central corneal thickness. Cornea 30, 861–864 (2011). [DOI] [PubMed] [Google Scholar]
- Nielsen C. B. & Nielsen P. J. Effect of alpha- and beta-receptor active drugs on corneal thickness. Acta Ophthalmol (Copenh) 63, 351–354 (1985). [DOI] [PubMed] [Google Scholar]
- Inoue K., Okugawa K., Oshika T. & Amano S. Influence of dorzolamide on corneal endothelium. Jpn J Ophthalmol 47, 129–133 (2003). [DOI] [PubMed] [Google Scholar]
- Kaminski S. et al. Influence of dorzolamide on corneal thickness, endothelial cell count and corneal sensibility. Acta Ophthalmol Scand 76, 78–79 (1998). [DOI] [PubMed] [Google Scholar]