Abstract
To enhance the immunogenicity of the Influenza H5N1 vaccine, we developed an oil-in-water nanoemulsion (NE) adjuvant. NE displayed good temperature stability and maintained particle size. More importantly, it significantly enhanced IL-6 and MCP-1 production to recruit innate cells, including neutrophils, monocytes/macrophages and dendritic cells to the local environment. Furthermore, NE enhanced dendritic cell function to induce robust antigen-specific T and B cell immune responses. NE-adjuvanted H5N1 vaccine not only elicited significantly higher and long-lasting antibody responses, but also conferred enhanced protection against homologous clade 1 as well as heterologous clade 2 H5N1 virus challenge in young as well as in aged mice. The pre-existing immunity to seasonal influenza did not affect the immunogenicity of NE-adjuvanted H5N1 vaccine.
Keywords: oil-in-water nanoemulsion, adjuvant, H5N1 vaccine, innate immunity, adaptive immunity, influenza
Background
Since the first report of human infections of avian influenza subtype H5N1 in China in 1997, H5N1 has spread to several countries in Asia, Europe and Africa with approximately a 53% fatality rate and continues to pose a pandemic threat1. Vaccination is the best preventative strategy against a potential avian influenza pandemic. However, in general, the current H5N1 vaccines, both inactivated and a live attenuated H5N2, are poorly immunogenic2,3. The first FDA-approved H5N1 vaccine only achieved acceptable levels of sero-protection at two doses of 90 µg each4, which raises concerns that current flu vaccine manufacturing facilities which may not be able to produce enough pandemic flu vaccine at this high dose level. Adjuvants have been used to overcome poor immunogenicity for more than 80 years5. However, alum-adjuvanted inactivated influenza H5N1 vaccines had little effect on the immunogenicity6–9. Oil-in-water NE, namely MF59 and AS03, when combined with H5N1 influenza vaccines were found to be safe, enhanced the immunogenicity and achieved significant antigen dose-sparing in adults, older adults and children10–24. AS03-adjuvanted H5N1 vaccine was approved by US FDA for national vaccine stock pile for human use in the US25. However, the precise mechanism of oil-in-water emulsions in enhancing the immunogenicity of vaccines is still unclear, although a transient release of ATP in muscle cells has been suggested to play a role in the adjuvanticity of MF5926. Furthermore, since these oil-in-water adjuvants are proprietary, developing adjuvants/formulations that enhance the immunogenicity of poorly immunogenic avian influenza vaccines and investigating their mechanisms of action is an active area of investigation27–32. Here, we report the development of an oil-in-water NE to overcome the poor immunogenicity of H5N1 monovalent influenza subvirion vaccine (A/Vietnam/1203/2004) and investigate the mechanism of its adjuvanticity. Our results demonstrate that the nanoemulsion is stable for over two years. It enhances the immunogenicity of H5N1 vaccine with significant antigen dose-sparing and protects mice from clade 1 as well as clade 2 virus challenge. The enhanced immunogenicity is due to the recruitment of DC-progenitor cells, their differentiation and maturation and the enhanced function of DC.
Methods
Preparation of NE
The NE consists of a water phase: 98% PBS and 2% Tween 80 and an oil phase: 90% Squalene and 10% α-Tocopherol. Tween 80, squalene and α-Tocopherol were purchased from Sigma (St. Louis, MO).
The aqueous phase was added at 80% (v/v) to the oil phase to yield a crude emulsion. The crude emulsion was processed through a M110-P microfluidizer (Microfluidics, Newton, MA) for 5 passes at 20,000 psi.
Determination of NE particle size
Nanoemulsion particle sizes were determined using a Wyatt Technology Corporation DynaPro Plate Reader (Santa Barbara, CA), which uses scattered light intensity fluctuations to calculate a diffusion coefficient and radius in nm for objects in solution. The instrument was equipped with an 832.5 nm laser and measurements were performed using a scattering angle of 158° at 25°C. To test NE stability, the oil-in-water NE was aliquoted and stored at four different temperatures of 4°, room temperature, 37°, or 56° C. Stability of NE was evaluated by following a phase separation visually as well as by the particle size distribution analysis.
Mice immunization
The immunogenicity of un-adjuvanted and NE-adjuvanted H5N1 vaccines were assessed using groups of 10 female BALB/c mice. On day 0, mice were immunized by intramuscular (i.m.) route with H5N1 vaccine alone at 3, 0.1 and 0.01 µg of HA or vaccine combined with NE. In some experiments, mice were immunized with H5N1 vaccine plus Imject™ Alum Adjuvant (ThermoFisher Waltham, MA) as control. In other studies addressing the impact of prior immunization with seasonal influenza vaccine on the immunogenicity of NE-adjuvanted H5N1 vaccine, mice were immunized with seasonal influenza vaccine 4 weeks before the administration of H5N1 vaccine. Intramuscular injection was performed in both quadriceps muscles with 50 µl dose per quadriceps. Both the vaccine and adjuvants were mixed prior to immunization. Control groups received either PBS solution or NE alone. A second immunization (boost) was repeated on day 28 with the same formulations. Blood samples were collected at 3 weeks following the first immunization, and then at 3 weeks, 3, 6 and 9 months following the second immunization. Four weeks after boost, 5 mice from each group were challenged with 25 µl in each nostril of 5×LD50 of viruses generated by reverse genetics (rg), containing HA and NA from A/Viet Nam/1203/2004 (H5N1) [rgA/VN/04, CDC-RGE2] or HA and NA from A/Indonesia/05/2005 [rgA/IN/05, IBCDC-RG2E2] and the remaining six gene segments from A/Puerto Rico/8/1934-(PR8). Animal were monitored daily for morbidity by measuring changes in body weight. Any mouse which lost >25% of its pre-challenge body weight was euthanized and considered succumbed to infection. For cell recruitment studies, mice were injected with 3 µg H5N1 vaccine with or without NE in one leg and the untreated, contralateral muscle served as a negative control. To examine the uptake of antigen by DCs in vivo, 50 µg FITC-conjugated ovalbumin (Life Technologies, Grand Island, NY) with or without NE were intramuscularly injected to mice. The muscle injection sites and draining lymph nodes were collected one day after injection and dissociated into single-cell suspensions to measure OVA uptake by flow cytometry. All animal research was approved by the CDC-IACUC and was conducted in an AAALAC-accredited facility.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA). The one way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was used to analyze differences among treatments for flow cytometry data and log transformed antibody (HI) titers; data were presented as mean ±SEM. The Logrank (Mantel-Cox) test was used to compare percent survival among groups of mice. All differences were considered statistically significant when the p-value was <0.05.
Results
Oil-in-water NE formulation and characterization
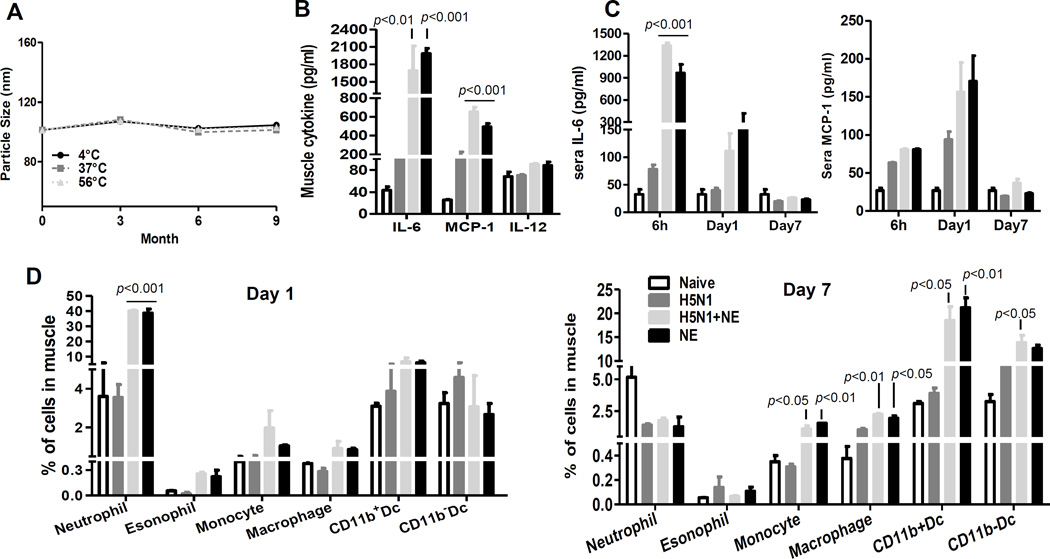
The oil-in-water NE contains Squalene and α-Tocopherol, Tween 80 and PBS. The mean particle size of oil-in-water NE shortly after preparation was 99 ± 2 nm, within the typical size range of NE (20–200 nm)33. To investigate the stability of NE formulation, NE was aliquoted and stored at 4°C, 37°C and 56°C for up to 9 months and at room temperature for up to 2 years. Samples’ particle size was analyzed at 0, 3, 6, 9 and 24 months after preparation. There was no phase separation of emulsion at any time points. Furthermore, the particle size for this formulation remained constant over 9 months post-formulation at all storage temperatures tested (4°C, 37°C and 56°C) indicating the thermostability of NE (Figure. 1A). Even after 2 years post-formulation, the particle sizes of nanoemulsion stored at room temperature were stable (101.3 ± 1.0 nm vs 100.0 ± 1.0 nm at day 0 vs 2 years respectively).
Figure 1. NE induced significantly higher level of local and systemic IL-6 and MCP-1 production with rapid recruitment of innate cells to injection site.
(A) Particle size of nanoemulsion determined by Dynamic Light Scattering using DynaPro Plate Reader (Santa Barbara, CA) indicate particle stability stored for 9 months at different temperatures (circle 4°C; square 37°C; triangle 56°C). (B–D) Female Balb/c mice (6–8 weeks old, 5 mice/group) were immunized by i.m. route with H5N1 vaccine (3 µg) with or without NE: (B) Muscle tissues from injection site were obtained 6 hour post-immunization and homogenates were prepared for Bio-plex analysis for IL-6, MCP-1 and IL-12 production (p<0.01, p<0.001 as compared to vaccine alone group). (C) IL-6 and MCP-1 level in the sera of mice at 6 h, day 1 and 7 after immunization was assessed using Bio-plex analysis (p<0.001 as compared to vaccine alone group). (D) Caudal thigh muscles were exercised on day 1 or 7 after vaccination and prepared for single cell suspension. Multicolor FACS staining was performed to analyze the infiltration of difference innate cell subsets in the muscle. The frequency of different cell subsets in the muscle was presented (p<0.05, p<0.01, p<0.001 as compared to vaccine alone group). The data are representative of at least 2 independent experiments and the error bars represent standard error of means (SEM).
NE induces high levels of IL-6 and MCP-1 locally and systemically
To study the mechanism of the adjuvanticity of NE, we immunized mice with H5N1 vaccine (3 µg) in the presence or absence of NE by i.m. injection in quadriceps muscle and measured cytokine production at the injection site. Muscle tissues from the injection sites were obtained at 6 h post-immunization and homogenates were prepared for the measurement of cytokine production by Bio-plex. 15–17 fold increase of IL-6 and 3–4 fold increase of MCP-1 were detected locally upon injection of NE, with or without vaccine (Figure 1B) compared to vaccine alone group. Next, the cytokine levels in the sera of mice at 6 h, day 1 and 7 after immunization were similarly assessed using Bio-plex analysis. As early as 6 h post-immunization, the level of pro-inflammatory cytokine, IL-6, increased 12–17 fold in the sera of mice immunized with NE or NE-adjuvanted vaccine, as compared with sera from mice immunized with vaccine alone, which were maintained up to 24 h (Figure 1C). Furthermore, NE injection also increased the release of monocyte chemoattractant protein-1 (MCP-1) into the serum on day 1 post-immunization (Figure 1C). The levels of IL-6 and MCP-1 in sera on day 7 post-immunization were much lower than those at day 1 and there were no differences in IL-6 or MCP-1 production among different treatment groups (Figure 1C). The serum levels of TNF-α, IFN-γ, IL-1β and MIP-1β were similar among groups with or without NE at all time points tested (data not shown). Taken together, our results demonstrated that NE induced higher levels of IL-6 and MCP-1 locally as well as systemically.
NE induces rapid recruitment of innate cells to injection site
The elevated pro-inflammatory and chemokine production at the local injection sites prompted us to examine the cellular recruitment into the injection sites. To characterize the cellular infiltrates, multicolor FACS analysis of the single cell suspensions from collagenase-digested muscle tissues were performed. Neutrophils were defined as Ly6G+, monocytes as Ly6Chigh, eosinophils as Ly6Glow/SSChigh, macrophages as F4/80+. At 24 h after immunization, we observed a significant increase in neutrophil recruitment (11 fold) in the NE-injected groups as compared to vaccine alone group (Figure 1D). The percentage of monocytes were also slightly increased at the injection site. One week after immunization, the frequency of neutrophils in different treatment groups was similar. Dendritic cells (DCs) are professional antigen presenting cells (APCs) which link innate and adaptive immunity. DCs were defined as CD11c+/I-A/Ehigh and can be further separated as CD11b+ and CD11b− subtype. As shown in Figure 1d, NE did not significantly affect the percentage of DC at 24 h post-immunization. However, on day 7 post-immunization, the percentage of monocytes was significantly elevated in the NE treated group with or without vaccine (3–5 fold). Consequently, the percentage of macrophages and DCs, which were differentiated from monocytes, increased 2–5 fold. The combination of vaccine and adjuvant did not further increase the adjuvant-induced cellular influx. Taken together, these findings suggest that NE induced rapid recruitment of inflammatory cells to the injection site and facilitated the development and differentiation of dendritic cells.
NE enhances DC antigen uptake
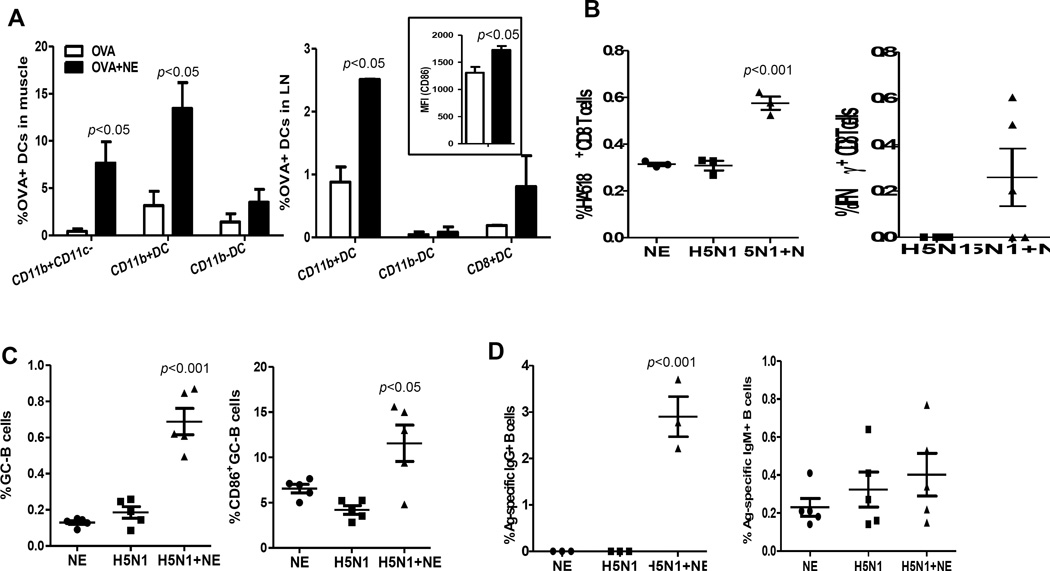
DCs are sentinels of the immune system. Upon sensing danger signals, DCs quickly capture, process and present the antigen to B and T cells in the draining lymph nodes to initiate adaptive immune responses. To examine antigen uptake by innate cells at injection site, we immunized animals with FITC-conjugated OVA with or without NE. As shown in Figure 2a, CD11b+CD11c− cells in muscle from mice immunized with OVA-FITC with NE had a 13 fold increase in the percentage of OVA+ cells at 24 h post-immunization. The influx of neutrophils and monocytes in injection site, which are CD11b+CD11c− cell, are most likely cells that took up FITC-OVA. Among DC subsets, CD11b+DC was the major DC subset that captured antigen. In order to present antigen to naïve T cells, DC must migrate from injection site to the closest draining lymph nodes. To examine DC migration in lymph nodes, inguinal lymph nodes were harvested one day after immunization with H5N1 vaccine in the presence or absence of NE. Single cell suspensions were prepared for flow cytometric analysis. As shown in Figure 2A, FITC-OVA bearing DCs were detected in inguinal lymph nodes. Among all DC subsets examined in lymph node, NE adjuvantation increased OVA uptake by CD11b+DCs 3 fold as compared to mice immunized with OVA only. Furthermore, NE increased the median fluorescence intensity (MFI) of maturation marker, CD86, expression on CD11b+DCs in lymph node. However, CD11b−DCs and lymph node resident CD8+ DCs showed comparable level of OVA uptake with or without NE. These findings suggest that, NE differentially modulate function of different innate cell types.
Figure 2. NE enhanced innate and adaptive immunity.
(A) Female Balb/c mice (6–8 weeks old, 5 mice/group) were immunized by i.m. route with 50 µg OVA-FITC with or without NE. 24 h later, single cell suspensions from muscle tissues were prepared. The frequencies of OVA-FITC+ cells in CD11c+I-A/E+SSClowCD11b+(CD11b+DC), CD11c+I-A/E+SSClowCD11b−(CD11b−DC), CD11c−/lowI-A/E+CD11b+ were presented; single cell suspensions were prepared from inguinal and the frequency of OVA-FITC+DC and the median fluorescence intensity (MFI) of CD86 of CD11b+DC were presented (p<0.05 as compared to OVA alone group). (B) Female Balb/c mice (6–8 weeks old, 5 mice/group) were immunized by i.m. route with 3 µg H5N1 vaccine with or without NE or with NE only. 14 days later, inguinal lymph node were collected and the frequency of HA518-specific CD8 T cells was presented (p<0.001 as compared to vaccine alone group). IFNγ-producing CD8 T cells from H5N1-immunized mice were also measured by intracellular cytokine staining on day 5 following in vitro restimilation with rgA/VN/04 virus and the frequency of IFNγ+CD8+ T cells was presented. (C–D) Female Balb/c mice (6–8 weeks old, 5 mice/group) were immunized with 3 µg H5N1 vaccine with or without NE or NE only by i.m. route. A week later, inguinal lymph nodes and spleens were collected and B cells (B220+ CD3−) were stained. The lymph nodes were analyzed for GC-participating B cells (B220+CD3−GL7+CD38−), plasma cells (B220−CD138+), and the frequency of B cells expressing high CD86 among GC-participating B cells (p<0.05, p<0.001 as compared to vaccine alone group) (C). The A/VN/04-specific ASCs were analyzed from spleen and shown as Ag-specific IgG+ ASCs and Ag-specific IgM+ ASCs (p<0.001 as compared to vaccine alone group) (D). The data are representative at least 3 independent experiments and the error bars represent standard error of means (SEM).
NE enhances adaptive immune responses
Since DCs function as professional APCs to initiate the adaptive immune responses, the enhanced DC frequency and function by NE prompted us to investigate the outcome of T as well as B cell responses in the presence of NE. For this study, mice were immunized with H5N1 vaccine with or without NE by i.m. route. Draining lymph nodes were harvested for analysis of antigen-specific B and T cell responses at day 7 and 14 post-immunization. HA518-specific CD8+ T cells were identified with H-2Kd/IYSTVASSL pentamer and the activation status of antigen-specific CD8 T cells was assessed by intracellular cytokine staining. In mice immunized with H5N1 vaccine alone, the frequencies of HA518-specific CD8 T cells were very low. In contrast, NE adjuvanted-H5N1 vaccine significantly increased the frequency of HA518-specific CD8 T cells about 2 fold (Figure 2B). Furthermore, a higher percentage of IFN-γ producing CD8 T cells were detected in mice immunized with NE-adjuvanted H5N1 vaccine, as compared to mice immunized with vaccine alone (Figure 2b). However, mice immunized with H5N1 vaccine with or without NE had similar frequency of IFN-γ producing CD4 T cells in lymph node (data not shown). We next examined B cells participating in germinal center reaction and their activation status. Compared to mice immunized with vaccine alone, mice vaccinated with NE-adjuvanted H5N1 vaccine displayed higher percentage of B cells participating in GC-reaction and higher expression levels of CD86 on B cells (Figure 2C). The overall percentage of plasma cells was very low and did not show differences among different groups (data not shown). We also assessed the frequency of Ag-specific IgG and IgM antibody secreting cells in spleen and noted that NE-adjuvanted vaccine significantly increased the IgG-secreting B cells with marginal increase in IgM-secreting B cells (Figure 2d).
NE enhances immunogenicity of H5N1 vaccine
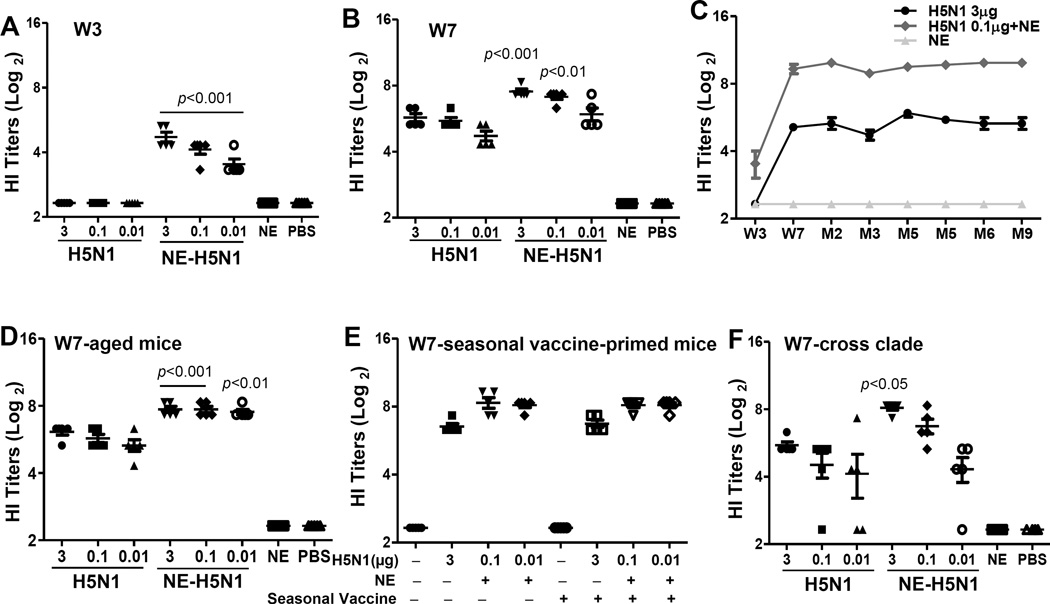
We first assessed the immunogenicity of NE-adjuvanted H5N1 monovalent influenza subvirion vaccine in young Balb/c mice (6–8 weeks old). Mice were immunized i.m. with 3, 0.1 or 0.01 µg of H5N1 vaccine alone or with NE. Four weeks following primary immunization, all mice were boosted with the respective vaccine preparations. Sera samples were collected 3 weeks after primary (week 3) and on booster (week 7) immunizations to measure HI titers against homologous virus strain, rgA/VN/04. As shown in Figure 3a, immunization with H5N1 vaccine alone did not induce detectable antibody titers and NE-adjuvanted vaccine induced low level of HI titers at week 3 in a dose dependent manner. Two out of five mice immunized with NE-adjuvanted vaccine (3 µg) showed a HI titer of 40 even after single vaccination (Figure 3A). Following boost immunization, at week 7, mice immunized with vaccine alone developed low level of antibody titer. However, NE-adjuvanted vaccine significantly increased HI antibody titer. Even the lowest dose of vaccine (0.01 µg), when combined with NE, induced comparable HI titers to those elicited by the highest dose of vaccine (3 µg) without NE (Figure 3B). These results indicate that NE significantly enhanced the immunogenicity of H5N1 vaccine with dose-sparing. The NE-adjuvanted vaccine was superior in enhancing H5N1 immunogenicity than alum-adjuvanted H5N1 vaccine (Supplementary Figure 1A).
Figure 3. NE enhanced immunogenicity of H5N1 vaccine.
Female Balb/c mice (6–8 weeks old, 5 mice/group) were immunized with H5N1 subvirion vaccine (3, 0.1, 0.01 µg) with or without NE twice at 4 weeks apart. Control mice received NE only or PBS at both time points. (A) Sera were collected at 3 weeks following primary immunization (A) and secondary immunization (B) to measure hemagglutination inhibition (HI) titers against rgA/VN/04 (H5N1) virus (p<0.01, p<0.001 as compared to vaccine (3 µg) alone group). (C) Female Balb/c mice (6–8 weeks old, 5 mice/group) were immunized with two doses of H5N1 subvirion vaccine (3 µg) or NE-adjuvanted vaccine (0.1 µg) or NE alone 4 weeks apart. Sera were collected at 3 weeks following primary immunization, 3 weeks and again 2–9 months post-booster immunizations to measure the kinetics of antibody responses by hemagglutination inhibition (HI) assay against rgA/VN/04 (H5N1) virus (circle H5N1 3 µg; square H5N1 3 µg+NE; triangle NE alone). (D) 3 weeks post-booster immunization, sera from aged mice (18 months old) were assessed for HI titer against rgA/VN/04 virus (p<0.01, p<0.001 as compared to vaccine (3 µg) alone group). (E) Female Balb/c mice (6–8 weeks old, 5 mice/group) were immunized with seasonal influenza vaccine 4 weeks prior to receiving two doses of H5N1 vaccination. 3 weeks post-booster immunization, sera were tested for HI titer against rgA/VN/04 virus. (F) Week 7 sera were measured HI titer against clade 2 rgA/IN/05 virus (p<0.05 as compared to vaccine (3 µg) alone group).
To examine the longevity of the immune responses induced by NE-adjuvanted H5N1 vaccine, mice were immunized with 3 µg vaccine or 0.1 µg vaccine along with NE using prime-boost regimen as described in Methods. Sera were collected at weeks 3, 7 and then 2, 3, 4, 5, 6 and 9 months post-immunization to measure HI titers against rgA/VN/04. As shown in Figure 3c, vaccine alone induced low levels of HI titers at week 7 post-immunization and the antibody titer remained at similar level up to 9 months post-vaccination. Following boost immunization at week 7, NE significantly increased the antibody response as compared to vaccine alone group. Most importantly, the high antibody titers induced by NE-adjuvanted H5N1 vaccine were maintained up to 9 months.
NE overcomes age-related immune dysfunction and enhances immunogenicity of H5N1 vaccine
The adjuvanticity of NE was next assessed in aged mice, as with aging, the ability to mount effective immune responses to vaccines declines. 18 months old Balb/c mice were immunized with prime-boost regimen at week 1 and 4 as described previously. Sera were collected at 3 weeks post-primary vaccination and again at 3 weeks post-booster vaccination to test HI titers. No detectable antibody titer was observed after primary immunization with vaccine alone. In the presence of NE as an adjuvant, low level of antibody titer was detected in 3 out of 5 mice immunized with 3 µg vaccine plus NE and 1 out of 5 mice immunized with 0.1 µg vaccine plus NE (Supplementary Figure 1B). After secondary immunization, mice immunized with vaccine alone developed detectable HI titers. However, all mice immunized with NE-adjuvanted vaccine developed high level of HI titers; even the lowest vaccine dose (0.01 µg) with NE induced comparable levels of HI titers as the groups that received 3 or 0.1 µg of HA (Figure 3D). More importantly, antibody responses elicited in aged mice were comparable to those seen in young adult mice (Figure 3b). These findings indicate the potential utility of NE in older population to enhance immune responses to vaccination.
Pre-existing immunity to seasonal influenza does not impact the adjuvanticity of NE-H5N1 vaccine
To assess if preexisting immunity against influenza will impact the adjuvanticity of NE, mice were immunized seasonal vaccine which elicited high HI titers against A/Brisbane/59/2007 three weeks post-immunization (data not shown). Next, naïve or seasonal vaccine-primed mice were immunized with 3 µg of H5N1 vaccine alone or 0.1 µg or 0.01 µg of H5N1 vaccine with NE. The antibody responses were subsequently measured at three weeks after booster vaccination. As shown earlier, after two doses of vaccination, vaccine alone group displayed detectable levels of HI titers. A marked increase in HI titer was detected in NE-adjuvanted groups, even at 0.01 µg dose i.e., a 300-fold less dose. Seasonal vaccine-primed mice had similar level of HI titers as un-primed mice (Figure 3E) indicating that the adjuvanticity of NE was not impacted by preexisting immune response to seasonal influenza vaccine.
NE enhances cross-clade antibody response
Next, the effect of NE on the cross-clade immune responses was investigated. Mice were immunized with 3, 0.1 or 0.01 µg H5N1 vaccine (formulated from clade 1 H5N1 virus, A/VN/04) with or without NE following prime-boost regimen. Sera were collected at 3 weeks after primary or booster immunization and tested for HI titers against clade 2 virus, rgA/IN/05. Primary immunization with vaccine alone elicited very low level of cross-reactive HI titer. However, mice immunized with the highest dose of vaccine (3 µg) plus NE developed HI titer above 40 even after a single vaccination (data not shown). At 3 weeks post-booster immunization, all mice immunized with 3 µg vaccine and 3 out of 5 mice immunized with 0.1 µg vaccine had titer ≥40. H5N1 vaccine with NE further increased the antibody responses by 4–6 folds against clade 2 virus in a dose-dependent manner. Even two mice immunized with the lowest vaccine dose (0.01 µg) with NE had a HI titer of 40 (Figure 3F). Thus, NE enhanced cross-reactive antibody response against clade 2 virus.
NE enhances protective and cross-clade protective efficacy of H5N1 vaccine
Next, we examined whether the increased antibody titers elicited by NE-H5N1 vaccine lead to protection against a live virus challenge. Protection was evaluated in mice that were immunized with two doses (4 weeks apart) of 0.1 µg vaccine alone, or 0.1 or 0.01 µg of H5N1 vaccine with NE. Four weeks after boost, mice were challenged intranasally with 5 × LD50 of the homologous clade 1 strain rgA/VN/04 or clade 2 H5N1 virus, rgA/IN/05. Morbidity and mortality were monitored up to 2 weeks post-challenge. When challenged with homologous virus (Figure 4A), all mice immunized with adjuvant alone died or had to be euthanized. However, 60% mice survived when vaccinated with 0.1 µg vaccine without NE, while 100% mice immunized with same amount of antigen with NE survived. Likewise, 80% mice immunized with 10 times less antigen (0.01 µg) with NE survived challenge (Figure 4A). Upon clade 2 virus challenge, 20% of mice immunized with 0.1 µg of vaccine alone survived challenge. When mice were immunized with same amount of antigen with NE, 80% of them survived the challenge. However, 40% of mice immunized with 10 times less antigen (0.01 µg) with NE survived challenge (Figure 4B). Taken together, these data demonstrated that NE significantly enhanced protective efficacy of the H5N1 vaccine against both clade 1 and clade 2 viral challenge.
Figure 4. NE enhanced protective immunity of H5N1 vaccine.
Female Balb/c mice (6–8 weeks old, 5 mice/group) were immunized with two doses of H5N1 subvirion vaccine (0.1 µg) or NE-adjuvanted vaccine (0.1 or 0.01 µg) or NE alone 4 weeks apart. Four weeks following boost immunization, mice were challenged with 5 LD50 of homologous strain of H5N1 virus, rgA/VN/04 (A) and heterologous strain, rgA/IN/05 virus (B) and mortality was monitored (square H5N1 0.1 µg; diamond H5N1 0.1 µg+NE; circle H5N1 0.01 µg+NE; open square NE alone). The data are representative 2 independent experiments.
Discussion
Highly pathogenic avian H5N1 influenza remains a serious pandemic threat due to its continued circulation in birds and its involvement in fatal human infections. A safe and effective vaccine will be the most effective intervention for H5N1 pandemic. Stockpiling candidate pandemic vaccines based on existing H5N1 strains is currently considered to be the most attractive strategy by the WHO34. However, without an adjuvant, H5N1 vaccines generally have been observed to be poorly immunogenic, even at HA doses of 30 µg or higher6. Alum-adjuvanted H5N1 vaccines failed to show significant enhancement of the immunogenicity in several studies6,8. However, H5N1 vaccines adjuvanted with oil-in-water emulsions, such as MF59 and AS03, have not only overcome the poor immunogenicity but also provided significant antigen dose-sparing12,13,35,36. Since, all these adjuvants are proprietary, we developed an oil-in-water nanoemulsion adjuvant, which is composed of squalene, α-tocopherol and Tween 80. The particle size of NE ranged from 100–200 nm, within the typical nanoemulsion size33. The nanoemulsion was stable up to 9 months when tested under temperatures of 4°C, 37°C and 56°C suggesting a good thermal stability under a broad range of temperatures.
We also explored the mechanism of action of NE in this study. Consistent with previous findings, NE induces the local production of cytokines, including IL-6 and MCP-1, but not TNF-α, IFN-γ, IL-1β and MIP-1β and IL-1237. The influx of innate cells to the immunization site was also observed as early as 24 h post-vaccination and the infiltrating cell types include neutrophils, monocytes, macrophages and DCs, which is consistent to prior reports38. The rapid and robust production of MCP-1 at the local injection site explains the influx of monocytes and their subsequent differentiation into macrophages and DCs. While the enhanced uptake of antigen by MF59 adjuvanted vaccine was reported earlier39, we demonstrated that a specific subset of DCs, CD11b+DCs in the muscles and lymph nodes actively captured antigen. In muscle, CD11b+CD11c− cells including monocytes, macrophages and neutrophils internalized antigen in the presence of NE adjuvant. Enhanced antigen-specific CD4 T cell responses were observed with AS03-adjuvanted of trivalent influenza vaccine40. NE-adjuvanted H5N1 vaccine also induced antigen specific CD8 T cell responses. Consistent with the previous reports with MF59-adjuvanted vaccines, we also observed increases in germinal center B cell differentiation and activation with NE-adjuvanted H5N1 vaccine41. In addition, we demonstrated increased frequency of IgG secreting cells in spleen with NE-adjuvanted vaccine indicating that NE enhanced the overall frequency of memory B cells in spleen.
This stable preparation of NE mixed with the H5N1 subvirion vaccine significantly enhanced the immunogenicity of H5N1 vaccine. After a single vaccination of young mice, H5N1 vaccine alone failed to induce HI titer ≥40. However, 2 out of 5 mice immunized with NE-adjuvanted vaccine displayed a HI titer of ≥40 against homologous virus. Two doses of NE-adjuvanted H5N1 vaccine, given one month apart, significantly increased HI titers in a dose-dependent manner with significant antigen-sparing effect, as compared to non-adjuvanted vaccine. In addition, cross-clade antibody responses were significantly enhanced by NE-adjuvantation as well. Consequently, comparable protection and cross-clade protection were achieved by using 10 times less antigen when adjuvanted with NE. Antigen-sparing is very important for pandemic preparedness because worldwide influenza vaccine production capacity is limited. Alum has not been able to provide significant antigen sparing and hence, is not an effective adjuvant for H5N16,42. In our study, NE was shown to be superior in enhancing H5N1 immunogenicity as compared to alum (data not shown). Furthermore, the antibody titers elicited by NE-adjuvanted H5N1 vaccine were maintained up to 9 months in mice which could provide long-lasting protection.
The immunogenicity and efficacy of the vaccines wane with aging and increasing vaccine dose, adjuvanting vaccines, and increasing number of vaccine doses during the influenza season are some of the potential strategies to overcome the poor immunogenicity of influenza vaccines in this population. A high dose vaccine has been approved specifically for people 65 years and older43. Another strategy for improving the immunogenicity of vaccine is using effective vaccine adjuvants and MF59-adjuvanted seasonal influenza vaccine has been approved for older adults recently in the US44. Our results demonstrated that NE enhanced the immunogenicity of H5N1 vaccine in aged mice (18 months old). In older mice, even the 300 times lower dose of vaccine (0.01 µg) adjuvanted with NE gave rise to higher titers than the 3 µg of non-adjuvanted vaccine. More importantly, antibody titers elicited by NE in aged mice were comparable to those seen in younger mice. Furthermore, our results also indicate that pre-existing immunity to seasonal influenza vaccine did not impact the adjuvanticity of NE.
In summary, our results demonstrated that the oil-in-water nanoemulsion preparation significantly improved the immunogenicity of H5N1 vaccine by activating innate and adaptive immune responses in young and aged mice, resulting in enhanced antibody responses and protective efficacy against both clade 1 and clade 2 H5N1 viruses.
Supplementary Material
Acknowledgments
Funding: the Influenza Division, Centers for Disease Control and Prevention.
We thank members of the Immunology and Pathogenesis Branch in the Influenza Division, Centers for Disease Control and Prevention for providing reagents and constructive comments on this research. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of Centers for Disease Control and Prevention.
Footnotes
The authors declare no conflict of interest.
References
- 1.World Health Organization. Cumulative number of confirmed human cases of avian influenza A(H5N1) reproted to WHO, 2003–2015. Published on 2015 and retrieved on Jan 12, 2016. http://www.who.int/influenza/human_animal_interface/EN_GIP_20151214cumulativeNumberH5N1cases.pdf?ua=1.
- 2.Luke CJ, Subbarao K. Improving pandemic H5N1 influenza vaccines by combining different vaccine platforms. Expert review of vaccines. 2014;13:873–883. doi: 10.1586/14760584.2014.922416. [DOI] [PubMed] [Google Scholar]
- 3.Rudenko L, Kiseleva I, Stukova M, Erofeeva M, Naykhin A, Donina S, et al. Clinical testing of pre-pandemic live attenuated A/H5N2 influenza candidate vaccine in adult volunteers: results from a placebo-controlled, randomized double-blind phase I study. Vaccine. 2015;33:5110–5117. doi: 10.1016/j.vaccine.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. The New England journal of medicine. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 5.De Gregorio E, Tritto E, Rappuoli R. Alum adjuvanticity: unraveling a century old mystery. European journal of immunology. 2008;38:2068–2071. doi: 10.1002/eji.200838648. [DOI] [PubMed] [Google Scholar]
- 6.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368:991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 8.Keitel WA, Campbell JD, Treanor JJ, Walter EB, Patel SM, He F, et al. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I–II randomized clinical trial. The Journal of infectious diseases. 2008;198:1309–1316. doi: 10.1086/592172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan TM, Richmond PC, Skeljo MV, Pearce G, Hartel G, Formica NT, et al. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine. 2008;26:4160–4167. doi: 10.1016/j.vaccine.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 10.Vesikari T, Forsten A, Herbinger KH, Cioppa GD, Beygo J, Borkowski A, et al. Safety and immunogenicity of an MF59((R))-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adults and the elderly. Vaccine. 2012;30:1388–1396. doi: 10.1016/j.vaccine.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Bihari I, Panczel G, Kovacs J, Beygo J, Fragapane E. Assessment of antigen-specific and cross-reactive antibody responses to an MF59-adjuvanted A/H5N1 prepandemic influenza vaccine in adult and elderly subjects. Clinical and vaccine immunology : CVI. 2012;19:1943–1948. doi: 10.1128/CVI.00373-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PloS one. 2009;4:e4384. doi: 10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 14.Chen WH, Jackson LA, Edwards KM, Keitel WA, Hill H, Noah DL, et al. Safety, Reactogenicity, and Immunogenicity of Inactivated Monovalent Influenza A(H5N1) Virus Vaccine Administered With or Without AS03 Adjuvant. Open Forum Infect Dis. 2014;1:ofu091. doi: 10.1093/ofid/ofu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulligan MJ, Bernstein DI, Frey S, Winokur P, Rouphael N, Dickey M, et al. Point-of-Use Mixing of Influenza H5N1 Vaccine and MF59 Adjuvant for Pandemic Vaccination Preparedness: Antibody Responses and Safety. A Phase 1 Clinical Trial. Open Forum Infect Dis. 2014;1:ofu102. doi: 10.1093/ofid/ofu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diez-Domingo J, Baldo JM, Planelles-Catarino MV, Garces-Sanchez M, Ubeda I, Jubert-Rosich A, et al. Phase II, randomized, open, controlled study of AS03-adjuvanted H5N1 pre-pandemic influenza vaccine in children aged 3 to 9 years: follow-up of safety and immunogenicity persistence at 24 months post-vaccination. Influenza Other Respir Viruses. 2015;9:68–77. doi: 10.1111/irv.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei SH, Liu MT, Tsai YC, Liao CH, Chen CM, Wang WY, et al. The safety and immunogenicity of a MF59-adjuvanted H5N1 prepandemic influenza vaccine in healthy adults primed with homologous or heterologous H5N1 vaccines: an observational study. BMC infectious diseases. 2014;14:587. doi: 10.1186/s12879-014-0587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosalaraksa P, Jeanfreau R, Frenette L, Drame M, Madariaga M, Innis BL, et al. AS03B-adjuvanted H5N1 influenza vaccine in children 6 months through 17 years of age: a phase 2/3 randomized, placebo-controlled, observer-blinded trial. The Journal of infectious diseases. 2015;211:801–810. doi: 10.1093/infdis/jiu548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czajka H, Unal S, Ulusoy S, Usluer G, Strus A, Sennaroglu E, et al. A phase II, randomised clinical trial to demonstrate the non-inferiority of low-dose MF59-adjuvanted pre-pandemic A/H5N1 influenza vaccine in adult and elderly subjects. J Prev Med Hyg. 2012;53:136–142. [PubMed] [Google Scholar]
- 20.Lasko B, Reich D, Madan A, Roman F, Li P, Vaughn D. Rapid immunization against H5N1: a randomized trial evaluating homologous and cross-reactive immune responses to AS03(A)-adjuvanted vaccination in adults. The Journal of infectious diseases. 2011;204:574–581. doi: 10.1093/infdis/jir328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langley JM, Risi G, Caldwell M, Gilderman L, Berwald B, Fogarty C, et al. Dose-sparing H5N1 A/Indonesia/05/2005 pre-pandemic influenza vaccine in adults and elderly adults: a phase III, placebo-controlled, randomized study. The Journal of infectious diseases. 2011;203:1729–1738. doi: 10.1093/infdis/jir172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vesikari T, Karvonen A, Tilman S, Borkowski A, Montomoli E, Banzhoff A, et al. Immunogenicity and safety of MF59-adjuvanted H5N1 influenza vaccine from infancy to adolescence. Pediatrics. 2010;126:e762–e770. doi: 10.1542/peds.2009-2628. [DOI] [PubMed] [Google Scholar]
- 23.Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, et al. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol. 2011;31:443–454. doi: 10.1007/s10875-010-9490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu DW, Hwang SJ, Lim FS, Oh HM, Thongcharoen P, Yang PC, et al. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine. 2009;27:7428–7435. doi: 10.1016/j.vaccine.2009.07.102. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Food and Drug Administration. FDA approves first adjuvanted vaccine for prevention of H5N1 avian influenza. Published on 2013 and retrieved on Jan 12, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm376444.htm.
- 26.Vono M, Taccone M, Caccin P, Gallotta M, Donvito G, Falzoni S, et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci U S A. 2013;110:21095–21100. doi: 10.1073/pnas.1319784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galliher-Beckley A, Pappan LK, Madera R, Burakova Y, Waters A, Nickles M, et al. Characterization of a novel oil-in-water emulsion adjuvant for swine influenza virus and Mycoplasma hyopneumoniae vaccines. Vaccine. 2015;33:2903–2908. doi: 10.1016/j.vaccine.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 28.Clegg CH, Roque R, Perrone LA, Rininger JA, Bowen R, Reed SG. GLA-AF, an emulsion-free vaccine adjuvant for pandemic influenza. PloS one. 2014;9:e88979. doi: 10.1371/journal.pone.0088979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stavaru C, Onu A, Lupulescu E, Tucureanu C, Rasid O, Vlase E, et al. Technology Transfer of Oil-in-Water Emulsion Adjuvant Manufacturing for Pandemic Influenza Vaccine Production in Romania: Preclinical Evaluation of Split Virion Inactivated H5N1 Vaccine with Adjuvant. Hum Vaccin Immunother. 2015;0 doi: 10.1080/21645515.2015.1111495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong L, Liu F, Fairman J, Hong DK, Lewis DB, Monath T, et al. Cationic liposome-DNA complexes (CLDC) adjuvant enhances the immunogenicity and cross-protective efficacy of a pre-pandemic influenza A H5N1 vaccine in mice. Vaccine. 2012;30:254–264. doi: 10.1016/j.vaccine.2011.10.103. [DOI] [PubMed] [Google Scholar]
- 31.Andrianov AK, Decollibus DP, Marin A, Webb A, Griffin Y, Webby RJ. PCPP-formulated H5N1 influenza vaccine displays improved stability and dose-sparing effect in lethal challenge studies. J Pharm Sci. 2011;100:1436–1443. doi: 10.1002/jps.22367. [DOI] [PubMed] [Google Scholar]
- 32.Rimmelzwaan GF, Claas EC, van Amerongen G, de Jong JC, Osterhaus AD. ISCOM vaccine induced protection against a lethal challenge with a human H5N1 influenza virus. Vaccine. 1999;17:1355–1358. doi: 10.1016/s0264-410x(98)00390-9. [DOI] [PubMed] [Google Scholar]
- 33.Shah PBD, Shelat P. Nanoemulsion: A Pharmaceutical Review. Sys Rev Pharm. 2010;1:24–32. [Google Scholar]
- 34.Osterhaus AD. Pre- or post-pandemic influenza vaccine? Vaccine. 2007;25:4983–4984. doi: 10.1016/j.vaccine.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 35.O'Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59((R)) adjuvant: a phoenix that arose from the ashes. Expert review of vaccines. 2013;12:13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- 36.Garcon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert review of vaccines. 2012;11:349–366. doi: 10.1586/erv.11.192. [DOI] [PubMed] [Google Scholar]
- 37.Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Seubert A, Monaci E, Pizza M, O'Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. Journal of immunology. 2008;180:5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 39.Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, et al. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cellular immunology. 1998;186:18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- 40.Couch RB, Bayas JM, Caso C, Mbawuike IN, Lopez CN, Claeys C, et al. Superior antigen-specific CD4+ T-cell response with AS03-adjuvantation of a trivalent influenza vaccine in a randomised trial of adults aged 65 and older. BMC infectious diseases. 2014;14:425. doi: 10.1186/1471-2334-14-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lofano G, Mancini F, Salvatore G, Cantisani R, Monaci E, Carrisi C, et al. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. Journal of immunology. 2015;195:1617–1627. doi: 10.4049/jimmunol.1402604. [DOI] [PubMed] [Google Scholar]
- 42.Ehrlich HJ, Muller M, Oh HM, Tambyah PA, Joukhadar C, Montomoli E, et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. The New England journal of medicine. 2008;358:2573–2584. doi: 10.1056/NEJMoa073121. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Food and Drug Administration. FDA Approves A High Dose Seasonal Influenza Vaccine Specifically Intended for People Ages 65 and Older. Published on 2009 and retrieved on Jan 12, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm195483.htm.
- 44.U.S. Food and Drug Administration. FDA approves first seasonal influenza vaccine containing an adjuvant. Published on 2015 and retrieved on Jan 12, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm474295.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.