Abstract
We compared the innate immune response to newly emerged swine origin H3N2 influenza A variant virus containing the M gene from A(H1N1)pdm09 virus (A(H3N2)vpm), with 2010 swine-origin A(H3N2)v and seasonal A(H3N2) viruses. Our results demonstrated that A(H3N2)vpM virus-induced myeloid dendritic cells secreted significantly lower levels of type I interferon (IFN) but produced significantly higher levels of pro-inflammatory cytokines and induced potent inflammasome activation. The reduction in antiviral immunity with increased inflammatory responses upon A(H3N2)vpM virus infection suggest that these viruses have the potential for increased disease severity in susceptible hosts.
Keywords: A(H3N2)vpM, myeloid dendritic cell, pro-inflammatory cytokines, type I IFN, ROS, inflammasome activation
Swine A(H3N2) viruses emerged in the late 1990s and spread widely in the US swine population. These viruses arose through the introduction of human H3 and N2 genes into swine influenza viruses and subsequent reassortment events resulting in an internal gene (TRIG) cassette comprised of human lineage polymerase basic 1 (PB1) gene, avian lineage polymerase basic 2 (PB2) and polymerase acidic (PA) genes, and swine lineage nucleoprotein (NP), matrix (M), and nonstructural (NS) genes[1] (the triple reassortant H3N2 virus, trH3N2). Up until 2011, human infections with swine origin trH3N2 [known as A(H3N2) variant or A(H3N2)v] viruses have been sporadic. However, during 2011–12, 321 human infections with novel A(H3N2)v viruses possessing the M gene from A(H1N1)pdm09 virus (referred as A(H3N2)vpM) were reported, predominately in children[2, 3]. The M gene from A(H1N1)pdm09 was acquired from the Eurasian swine lineage whose origins are from Eurasian avian. Although there were no reports of community-wide spread of these viruses, animal model studies suggested that the pandemic M gene contributed to improved transmission efficiency of H1N1pdm09 viruses [4]. In addition to viral determinants, host factors may also contribute to the ability of animal viruses to infect humans. The innate immune system recognizes specific pathogen-associated molecular patterns (PAMPs) in microbial pathogens using pattern recognition receptors (PRRS) expressed on innate immune cells, especially dendritic cells (DCs), which are professional antigen-presenting cells[5]. PRRs respond to PAMPs by triggering the induction of antiviral cytokines including type I interferon (IFN) and pro-inflammatory cytokines. Induction of a balanced antiviral and pro-inflammatory response is crucial to control viral replication and activate optimal adaptive immune responses. Using human PBMCs, we show that, unlike the seasonal A(H3N2) and other A(H3N2)v viruses, the newly emerging A(H3N2)vpM virus dampened antiviral cytokine production by mDCs but induced mDCs to produce significantly higher levels of pro-inflammatory cytokines to prime a mixed Th1/Th2 responses. The imbalance between antiviral and inflammatory responses was associated with increased virus replication in mDCs.
Materials and Methods
Cells
Madin-Darby canine kidney (MDCK) cells were purchased from American Type Culture Collection (ATCC). Human peripheral blood mononucleated cells (PBMCs) were isolated from citrated blood samples of healthy donors using lymphoprep (Stemcell technologies). Monocytes were isolated from PBMCs using human Monocyte Isolation Kit II (Miltenyi biotec). CDC’s institutional review board (IRB)-approved written informed consent was obtained from all donors.
Influenza Viruses
A/Wisconsin/15/2009 [WS/09, A/Perth/16/2009-like seasonal A(H3N2)]; A/Minnesota/11/2010 [MN/10, A(H3N2)v]; A/Indiana/08/2011 (IN/11, H3N2vpM) were propagated in MDCK cells. Virus titers were determined by plaque Assay on MDCK cells. Human PBMCs were infected with influenza viruses at a multiplicity of infection (MOI) of 1.0.
Flow cytometry staining
Cells were re-suspended in PBS containing 10% fetal bovine serum (FBS) and stained with following antibodies to analyze DC subsets in PBMC: CD3-PE-Cy™7, CD14-Alexa Fluor® 700, CD19-PE-Cy™7, CD56-PE-Cy™7, CD123-PerCP-Cy5.5, CD11c-APC and HLA-DR- APC-Cy7 (BD Bioscience). For detection of intracellular cytokine (ICC), Brefeldin A was added into culture in the last 6 hours of incubation. Cells were surface stained with subset markers and made permeable with Cytofix/cytoperm solution (BD Bioscience). Cells were then stained with following antibodies: IL-6-PE, TNF-α-Pacific Blue™, IL-1β-FITC, IL-10-PE (Biolegend) and IL-12p40/70-PE (Miltenyi Biotech). Samples were analyzed using LSRII Flow cytometer (BD Biosciences) and the data were analyzed using FlowJo software (Tree Star, Inc.)
Real-time PCR and cytokine detection
RNA was isolated using the RNeasy® Mini Kit (Qiagen) and cDNA was generated using the Transcriptor Reverse Transcriptase (Roche Applied Science). Resulting cDNA was diluted 1:5 and 2 µl was used in a SYBR Green (SA Bioscience) based real-time PCR reaction using a Mx3000 real-time PCR instrument (Stratagene). Cytokine and chemokine levels in cell culture supernatants were assayed using ELISA kits (IFN-α and IFN-β; PBL biomedical Laboratories) and Pro-Human cytokine 17-plex assay (Bio-Rad Laboratories, Inc.).
Immunoblotting and Co-immunoprecipitation
1 × 106 of virus-induced DCs were washed with chilled PBS and sonicated in 55 µl of 2× SDS sample buffer (20 mM dithiothreitol, 6% SDS, 0.25 M Tris, pH 6.8, 10% glycerol, 10 mM NaF and bromophenyl blue). The extracts were heated at 95°C for 5 min. Equal quantities of solubilized protein were resolved by 10% SDS-PAGE, blotted to nitrocellulose membrane and probed with following antibodies: anti-NLRP3 (EMD Millipore),, anti-ASC (Apoptosis-associated Speck-like Protein Containing a Caspase Recruitment Domain), anti-caspase-1, anti-cleaved caspase-1 (Cell Signaling) and anti-β-actin antibody (Sigma-Aldrich). Proteins were visualized with SuperSignal West Pico chemiluminescent substrate (Pierce). For co-immunoprecipitation, cells were harvested in RIPA buffer (Sigma Aldrich), and cell lysates were incubated with anti-caspase-1 overnight at 4°C followed by incubation with protein A Dynabeads (Invitrogen) for 2 h. The beads were washed three times with PBS, suspended in Laemmli buffer (62.5mM Tris-HCl pH 6.8, 25% glycerol, 2% SDS, 0.01% Bromophenol blue), boiled for 10 min and spin down. Supernatants were collected and analyzed for the expression of NLRP3 and ASC by immunoblotting.
Mixed lymphocyte reaction assay
Influenza virus-induced dendritic cells and naïve T cells were isolated by FACS sorting. 5 × 104 dendritic cells were cultured together with naïve CD4+CD45RA+CD45RO− T cells from a different donor (1 × 105) in 200 µl RPMI complete medium in 96-well round-bottomed plates. After 5 days, supernatants were collected for detection of cytokine production by Pro-human cytokine 17-plex assay (Bio-Rad Laboratories, Inc.).
Detection of ROS production
Isolated monocytes were mock or viruses infected for overnight. Cells were harvested and re-suspended at 106 cells/ml in HBSS containing Ca2+ and Mg2+. 100 µl of cells were aliquoted into wells of 96-well flat-bottomed white microtiter plates and rested for 20 min in incubator. Luminol-012 (Wako) was added at a concentration of 50µM followed by 0.3 units of horseradish peroxidase (Sigma). Plates were immediately read using a BioTek plate reader.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA). Groups were compared by one-way ANOVA followed by Tukey’s multiple comparison test. Data were presented as mean ±SD. All differences were considered statistically significant when the p-value was ≤0.05.
Results and discussion
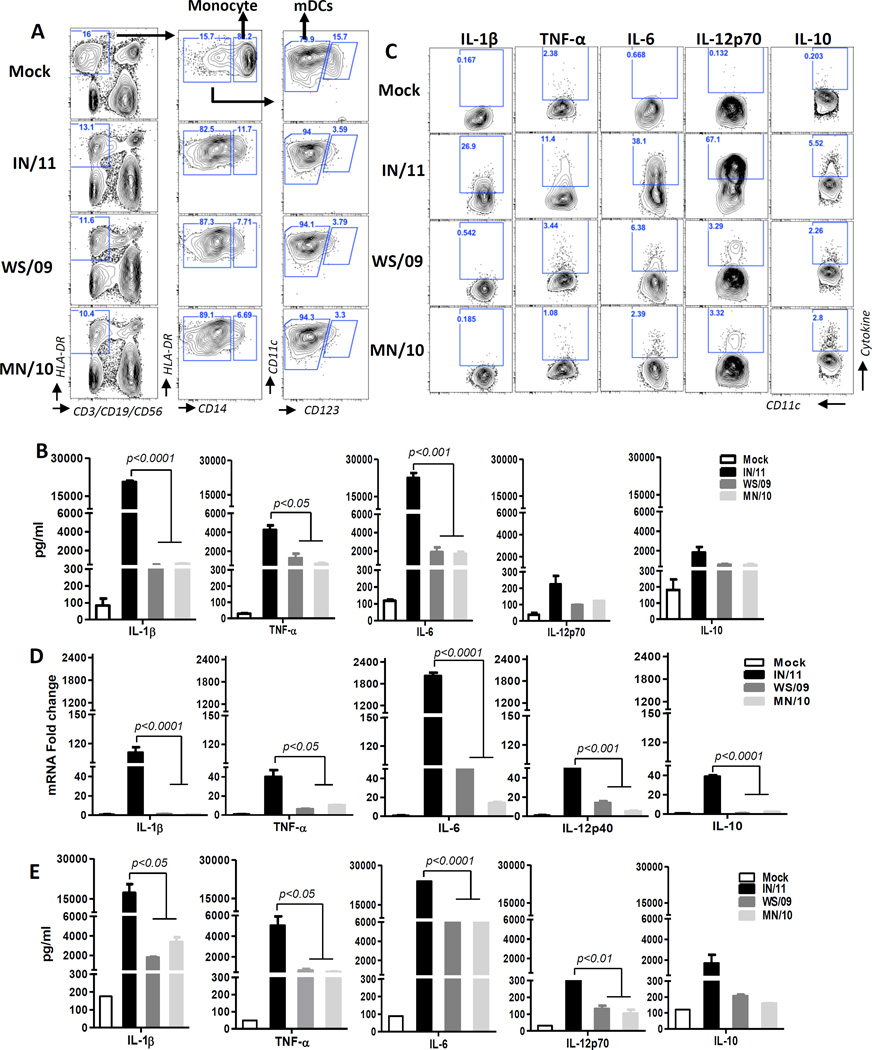
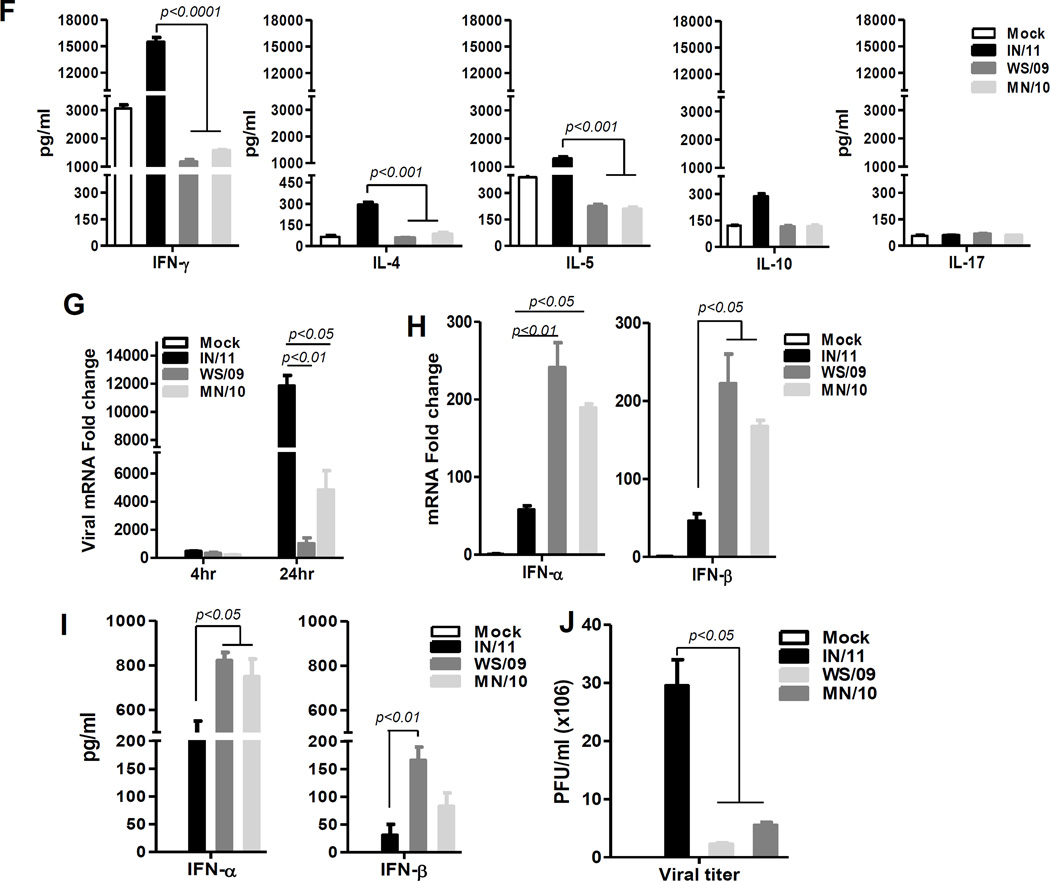
We earlier demonstrated that in response to influenza A virus infection, monocytes rapidly differentiated into mDC that predominately secreted type I IFN but low amounts of pro-inflammatory cytokines [6]. The response to newly emerging A(H3N2)v containing the M gene from pandemic H1N1 (IN/11) was similarly examined compared to 2010 A(H3N2)v virus containing a classical North American swine M gene (MN/10) and seasonal A(H3N2) (WS/09) viruses. Consistent with our earlier findings, infected monocytes (HLA-DR+CD14+) rapidly differentiated into mDCs (HLA-DR+Lin−CD11c+CD123low/−) in response to IN/11, as well as MN/10 and WS/09 viruses (Fig 1A). However, while MN/10- and WS/09-infected PBMCs secreted moderate levels of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6 and IL-12p70) and anti-inflammatory cytokine (IL-10), the amount of pro-inflammatory cytokines were remarkably higher in response to IN/11 virus infection (Fig 1B). Further, ICC characterization of the differentiated mDC population demonstrated that a higher proportion of mDCs stimulated with IN/11 virus produced IL-1, TNF-α, IL-6, IL-12p40/70 and IL-10 as compared to mDCs stimulated with MN/10 and WS/09 viruses, which may have contributed to the higher amount of these cytokines in PBMC culture supernatants (Fig 1C). Representative data from one donor were presented in figure 1 and similar findings from additional four donors were observed (Fig S1). RT-PCR and Bio-plex analysis of in vitro differentiated mDCs from monocytes and their culture supernatant further confirmed that IN/11-induced mDCs were potent inducers of inflammatory mediators (Fig 1D, 1E). As professional APCs, mDCs secrete different cytokines to skew T cell response into one or more of the identified types of effector or tolerogenic T cells [7]. The robust IL-12p70 and moderate levels of IL-10 cytokine specifically induced by IN/11-induced mDCs (Fig 1C, 1D, 1E) are of importance for Th1 and Th2 polarization respectively. A mixed lymphocyte reaction (MLR) confirmed that naïve CD4 T cells secreted significantly higher levels of Th1 (IFN-γ) and Th2-type (IL-4, IL-5) cytokines when co-cultured with allogeneic IN/11-induced mDCs from monocytes compared to CD4 T cells co-cultured with WS/09- and MN/09-induced mDCs (Fig 1F). Collectively, our results demonstrated that A(H3N2)vpM virus infection led to significantly greater pro-inflammatory cytokine production to prime a mixed Th1/Th2 response.
Figure 1. A(H3N2)vpM virus-induces significantly higher levels of pro-inflammatory cytokines, primes mixed Th1/Th2 responses but suppresses anti-viral cytokine induction.
Human PBMCs (A–C) or purified monocytes (D–I) were mock infected with cell culture medium or infected with IN/11, MN/10 or WS/09 at MOI of 1 for 16 h. (A) Flow cytometric analysis of different cell subsets in PBMCs. Monocytes are Lineage(CD3/CD19/CD56)−HLA-DR+CD14+ and mDCs are Lineage−HLA-DR+CD14−CD11c+CD123−. (B) The production of IL-1β, TNF-α, IL-6, IL-10 and IL-12p70 in PBMC culture supernatants was examined by Bio-Plex assay. (C) The production of pro-inflammatory cytokines by virus-induced mDCs as assayed by intracellular cytokine staining. (D) The mRNA expression of inflammatory cytokines by virus-induced mDCs from purified monocytes was assessed by real-time RT-PCR. (E) The production of cytokines in culture supernatants of virus-induced DCs from purified monocytes was examined by Bio-Plex assay. (F) Cytokine production (IFN-γ, IL-4, IL-5, IL-17, IL-10) in supernatant of virus-induced DC from purified monocytes and naïve CD4 T cell co-culture was assayed by Bio-Plex. (G) The vRNA expression in mock or virus-infected mDCs from purified monocytes was examined by real-time PCR. (H) The mRNA expression of IFN-α and IFN-β in mock or virus-infected mDCs from purified monocytes was examined by real-time RT-PCR. (I) The production of IFN-α and IFN-β in mock or virus-induced mDC from purified monocytes culture supernatants were assayed by ELISA. (J) Supernatants from mock or virus-infected mDCs generated from purified monocytes were collected to measure virus titer by MDCK cell-based plaque assay. All data were analyzed using Prism software (GraphPad Software, Inc.). Data are expressed as means±SD and the significances of differences determined by one-way ANOVA followed by Tukey’s multiple comparison test. P values < 0.05 were considered significant. Data are representative of three independent experiments.
Next, we examined the production of type I IFN by influenza virus-induced mDCs. Although, viral RNA levels indicated similar infection rates (Fig 1G), in comparison to MN/10 and WS/09 viruses, IN/11 virus infection resulted in remarkably decreased levels of type I IFN mRNA (Fig 1H) and protein expression (Fig 1I) by mDCs at 16 hr post-infection. UV-inactivation diminished the virus-induced type I IFN production indicating that replication competent viruses are required (Fig S2). In addition, IN/11 virus, as compared to WS/09 and MN/10 viruses, induced lower levels of IRF3 and IRF7 activation leading to the reduced secretion of type I IFN by virus-induced DCs (Fig S3). Consequently, significantly more viruses were detected in the culture supernatant collected from IN/11-infected mDCs (Fig 1J) as well as more viral RNA in infected cells (Fig 1G). Our results suggested that, compared to other H3N2 viruses, A(H3N2)vpM virus-induced mDCs dampened innate antiviral immune responses by suppressing type I IFN production. Osteltamivir treatment before or early in the course of infection remarkably reduced the production of IFN-α, but not pro-inflammatory cytokines (IL-1β and IL-6), induced by IN/11 virus infection (Fig S4).
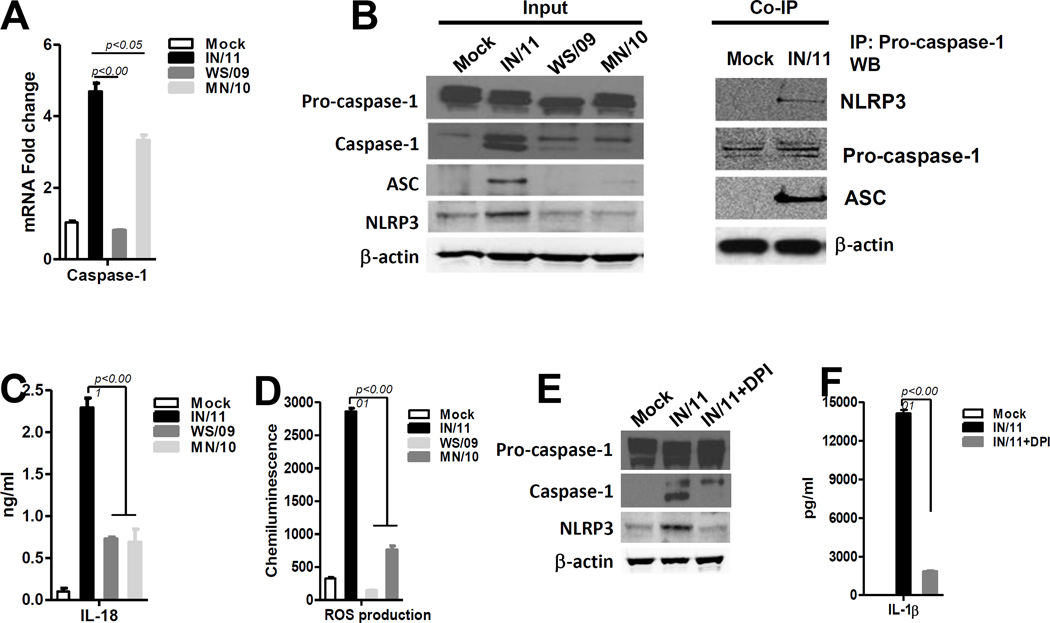
Increased levels of IL-1β in response to IN/11 infection prompted us to study the mechanism mediating IL-1β activation. IL-1β is synthesized as an inactive precursor protein that is cleaved by caspase-1 to release the mature secretory protein [8]. Caspase-1 itself is synthesized as a pro-enzyme that is cleaved during activation [9]. Interestingly, an increase in caspase-1 mRNA was detected upon infection with IN/11 and MN/10, but not by WS/09 virus (Fig 2A). In mDC cell lysates, comparable levels of pro-caspase-1 were detected upon mock or virus infection. However, substantially higher expression of caspase-1 was only detected upon IN/11 infection (Fig 2B). Activation of caspase-1 occurs in the inflammasome following assembly of three key proteins: Nod-like receptor family protein 3 (NLRP3), apoptosis associated speck-like protein containing a CARD (ASC) and pro-caspase-1[10]. Consistent with the potent activation of caspase-1, IN/11 virus infection also induced NLRP3 and ASC activation as detected by western blot (Fig 2B). Using co-immunoprecipitation assay, we further demonstrated that NLRP3 or ASC protein can be co-immunoprecipitated with pro-caspase-1 from cell lysate of IN/11-stimulated monocytes stimulated monocytes, but not mock-treated monocytes, which suggested the inflammasome ternary complex formation upon IN/11 stimulation (Fig 2B). IL-1β and caspase-1 are also involved in processing of mature IL-18 [10] which has been shown to improve early defense against influenza virus infection [11]. We detected a substantially increased level of IL-18 in culture supernatant of IN/11 virus-infected cells compared to those from MN/10 or WS/09 infected cells (Fig 2C). Reactive oxygen species (ROS) generated in response to several stimuli including influenza virus infection[12] are involved in inflammasome activation[13]. We found that IN/11 virus infection significantly increased ROS levels as detected using a chemiluminescent probe (Fig 2D).Pre-treatment for 30 min with the ROS inhibitor, diphenyleneiodonium (DPI, 10 µM, Sigma-Aldrich), substantially reduced NLRP3 and caspase-1 activation (Fig 2E) and subsequently IL-1β secretion in mDCs upon IN/11 infection (Fig 2F). Therefore, consistent with previously published observations [14], our results clearly showed that A(H3N2)vpM virus induces ROS production, which is required for potent inflammasome activation through NLRP3-ASC-pro-caspase-1 interaction leading to caspase-1 activation and culminating in the generation of IL-1β and IL-18.
Figure 2. A(H3N2)vpM virus induces potent inflammasome activation through ROS production.
(A) The mRNA expression of caspase-1 in mock infected with cell culture medium or virus-infected mDCs was examined by real-time RT-PCR. (B) The expression of –pro-caspase-1, caspase-1, ASC, NLRP3 and β-actin by virus-induced DCs were examined by immunoblotting: Pro-caspase-1 was immunoprecipitated from mock or virus-induced DCs and immunoprecipitates were analyzed for the presence of NLRP3 and ASC by immunoblotting. (C) IL-18 production in supernatants of mock or virus-infected mDCs was examined by ELISA. (D) The production of ROS by mDCs was assayed using the chemiluminescent probe L-012 (50 µM, Wako, Germany). (E) Cell lysates of virus-induced mDC with or without DPI pre-treatment were analyzed for pro-caspase-1, caspase-1, NLRP3 and β-actin expression by immunoblot (F) The production of IL-1β by virus-induced DC with or without DPI pre-treatment was examined by ELISA. All data were analyzed using Prism software (GraphPad Software, Inc.). Data are expressed as means±SD and the significances of differences determined by one-way ANOVA followed by Tukey’s multiple comparison test. P values < 0.05 were considered significant. Data are representative of three independent experiments.
M2 protein derived from A/PR/8/34 (H1N1), highly pathogenic influenza virus strains, A/Viet/1194/2004 (H5N1) and 1918 Spanish flu A/Brevig Mission/1/1918 (H1N1) have been shown to be sufficient to trigger signal 2 for NLRP3 inflammasome activation[15], However, introducing M gene of pdmH1N1 along with HA and NA from pdmH1N1 into swine H3N2 virus (sw/TX/98) did not elevate pro-inflammatory cytokine production and inflammasome activation (Fig S5) suggesting that HA and NA from H3N2 virus together with pdmM2 are needed for inflammasome activation.
In summary, A(H3N2)vpM virus infection skewed mDCs function from innate antiviral to inflammatory responses by suppressing type I IFN production yet promoting pro-inflammatory cytokine secretion. The altered balance between host antiviral and inflammatory response induced by A(H3N2)vpM virus-stimulated mDCs suggests that these viruses have the potential for increased disease severity in susceptible hosts.
Supplementary Material
Acknowledgments
JMK received funds from Juvaris Bio-Therapeutics, Inc. and Glaxo Smith-Kline as cooperative research agreement;
Financial support: This work was supported by the Influenza Division, Centers for Disease Control and Prevention.
We thank members of the Immunology and Pathogenesis Branch in the Influenza Division, Centers for Disease Control and Prevention for providing reagents and constructive comments on this research.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the funding agencies
Potential conflicts of interest: Other authors declared no potential conflicts of interest.
References
- 1.Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology. 2012;422:151–160. doi: 10.1016/j.virol.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Bowman AS, Sreevatsan S, Killian ML, et al. Molecular evidence for interspecies transmission of H3N2pM/H3N2v influenza A viruses at an Ohio agricultural fair, July 2012. Emerg Microbes Infect. 2012;1:e33. doi: 10.1038/emi.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skowronski DM, Janjua NZ, De Serres G, et al. Cross-reactive and vaccine-induced antibody to an emerging swine-origin variant of influenza A virus subtype H3N2 (H3N2v) J Infect Dis. 2012;206:1852–1861. doi: 10.1093/infdis/jis500. [DOI] [PubMed] [Google Scholar]
- 4.Pearce MB, Jayaraman A, Pappas C, et al. Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets. Proc Natl Acad Sci U S A. 2012;109:3944–3949. doi: 10.1073/pnas.1119945109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 6.Cao W, Taylor AK, Biber RE, et al. Rapid differentiation of monocytes into type I IFN-producing myeloid dendritic cells as an antiviral strategy against influenza virus infection. J Immunol. 2012;189:2257–2265. doi: 10.4049/jimmunol.1200168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 8.Thornberry NA, Bull HG, Calaycay JR, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 9.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Kanneganti TD, Body-Malapel M, Amer A, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Mori I, Hossain MJ, Dong L, Takeda K, Kimura Y. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J Gen Virol. 2004;85:423–428. doi: 10.1099/vir.0.19596-0. [DOI] [PubMed] [Google Scholar]
- 12.Akaike T, Noguchi Y, Ijiri S, et al. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci U S A. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen IC, Scull MA, Moore CB, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.