Abstract
Avian H7N9 influenza virus infection with fatal outcomes continues to pose a pandemic threat and highly immunogenic vaccines are urgently needed. In this report we show that baculovirus-derived recombinant H7 hemagglutinin protein, when delivered with RIG-I ligand, induced enhanced antibody and T cell responses and conferred protection against lethal challenge with a homologous H7N9 virus. These findings indicate the potential utility of RIG-I ligands as vaccine adjuvants to increase the immunogenicity of recombinant H7 hemagglutinin.
Keywords: H7HA, RIG-I ligand, cell-mediated immunity, antibody responses, protective immunity, mouse model, influenza
1. Introduction
Since the first case of human infection with a novel avian H7N9 influenza virus in China in 2013, over 650 cases with a 35% fatality rate have been reported, raising concerns about the pandemic potential of A(H7N9) viruses [1]. The lack of protective immunity to the virus in the majority of the human population highlights the critical need to develop an effective vaccine to A(H7N9) virus. However, avian influenza vaccines are poorly immunogenic in healthy adults even at high antigen doses, unless they are adjuvanted [2]. Recently, the U.S. Food and Drug Administration (FDA) approved an ASO3 (oil-in-water emulsion)-adjuvanted A(H5N1) influenza vaccine for national stockpile [3]. However, an increased incidence of narcolepsy in children and adults after receiving an ASO3-adjuvanted A/H1N1pdm09 vaccine had been reported and the reasons for it were not clear [4, 5]. Furthermore, the precise molecular mechanisms of ASO3 in enhancing the immunogenicity of influenza vaccines is not known and many of the adjuvants that are approved with seasonal or pandemic influenza vaccines are proprietary. Effective, safe and non-proprietary adjuvants acting through well-defined mechanisms are therefore needed.
The innate immune system recognizes microbial structures through pattern recognition receptors (PRRs) [6]. Recognition of microbes by PRRs results in rapid production of antiviral cytokines such as type I interferons as well as pro-inflammatory cytokines for induction of the adaptive immune responses [7]. Retinoic-acid-inducible gene-I (RIG-I) is a key cytosolic receptor that recognizes 5’-triphosphate (5’ppp)-containing viral RNA in influenza virus infection [8] which plays a pivotal role in activating the host antiviral innate response. We have previously shown that in vitro activation of RIG-I with a synthetic 5’pppRNA inhibits drug-resistant avian H5N1 as well as 1918 and 2009 pandemic influenza virus replication [9]. In addition, we also showed that RIG-I ligand effectively enhances the adaptive immune response against 2009 A(H1N1) inactivated monovalent split virus vaccine in a mouse model [10]. In this study, we expanded our studies to explore the adjuvanticity of RIG-I ligand for the poorly immunogenic recombinant avian influenza hemagglutinin (rH7HA) protein.
2. Materials and Methods
2.1 RIG-I ligand and antigens
5' triphosphate double stranded RNA (5' ppp-dsRNA), a synthetic ligand for retinoic acid-inducible protein (RIG-I), was purchased from Invivogen (San Diego, CA). The biological activity and absence of bacterial contamination of 5' ppp-dsRNA have been confirmed by the manufacturer. GenJet™ Plus DNA In Vivo Tranfection Reagent (SignaGen, CA) was used to deliver 5'ppp-dsRNA. Recombinant HA protein from A/Shanghai/2/2013 (rH7HA) influenza virus was expressed and purified using the baculovirus expression vector system, as described previously [11].
2.2 Immunization and virus challenge
Six to eight-week old female BALB/c mice (Jackson Laboratories, Bar Harbor, ME) were immunized (5 animals/group) by the intramuscular (i.m.) route with 3 µg of rH7HA with or without 5' ppp-dsRNA or mock-immunized with PBS using 50 µl in each quadriceps muscle. 5' ppp-dsRNA was used at concentrations of 25 µg per mouse and mixed 1:1 with GenJet™ Plus DNA In Vivo Transfection Reagent before immunization. Four weeks later, mice were boosted with the same immunization regimen. Sera were obtained three weeks post-primary and post-boost to determine antibody responses. Four weeks post-boost, mice were challenged with 50 × LD50 of wild type A(H7N9) virus (A/Anhui/1/2013, AH1) and monitored for weight loss and survival for 15 days. Animal research was conducted under the guidance of the CDC’s Institutional Animal Care and Use Committee in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility. Mice that lost >25% of their pre-infection body weight were euthanized.
2.3 Cell-mediated immune responses
Single cell suspensions were prepared from spleen and bone marrow one week after the boost immunization. To detect intracellular cytokine production, 1 × 106 cells from spleen were stimulated in vitro with a reassortant virus containing HA and NA from A/Shanghai/2/2013(H7N9) and the remaining six gene segments from A/Puerto Rico/8/1934-(PR8) [IDCDC-RG32A] here after referred to as SH2/PR8, at an MOI of 1 for 16 h with GolgiPlug™ (BD Bioscience, San Jose, CA) added during the last 6 h of incubation. Cells were surface stained with either anti-CD4 or anti-CD8 antibody (BD Bioscience), followed by intracellular staining with anti-IFN-γ antibody (BD Bioscience). Samples were analyzed using an LSRII Flow cytometer (BD Biosciences), and the cytometric data were analyzed using FlowJo software version 9.3.3 (Tree Star, Inc., Ashland, OR).
The frequency of HA-specific antibody-secreting cells (ASCs) in the spleen and bone marrow was detected by an ELISPOT assay. Briefly, 1–1.5 × 106 cells were added onto antigen-coated plates and incubated overnight at 37°C in a humidified atmosphere with 5% CO2. The plates were incubated with biotinylated anti-mouse IgG (Southern Biotech, Birmingham, AL) followed by alkaline phosphatase-conjugated streptavidin and developed with Vector Blue Alkaline Phosphatase Substrate Kit III (Vector Laboratories, Burlingame, CA). Spot forming units were counted using ImmunoSpot® (Cellular Technology Ltd., Shaker Heights, OH) and expressed as a percentage of antigen-specific IgG secreting B cells out of the total IgG-secreting B cells.
2.4 Serological responses
Sera from all mice were subjected to treatment overnight with receptor-destroying enzyme (RDE) from Vibrio Cholerae (Denka Seiken, Tokyo, Japan) at 37°C to destroy non-specific serum inhibitor activity. Hemagglutination inhibition (HI) antibody titers were determined using 4 hemagglutination units of SH2/PR8 viruses and horse red blood cells.
2.5 Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA). Groups were compared by one-way ANOVA followed by Tukey’s multiple comparison test. The Mann–Whitney U test was used to determine significance of antibody (HI) titers. The Log-Rank (Mantel-Cox) test was used to compare percent survival among groups of mice. Data were presented as mean ± SEM. All differences were considered statistically significant when the p value was ≤0.05.
3. Results
3.1 RIG-I ligand enhanced antibody responses
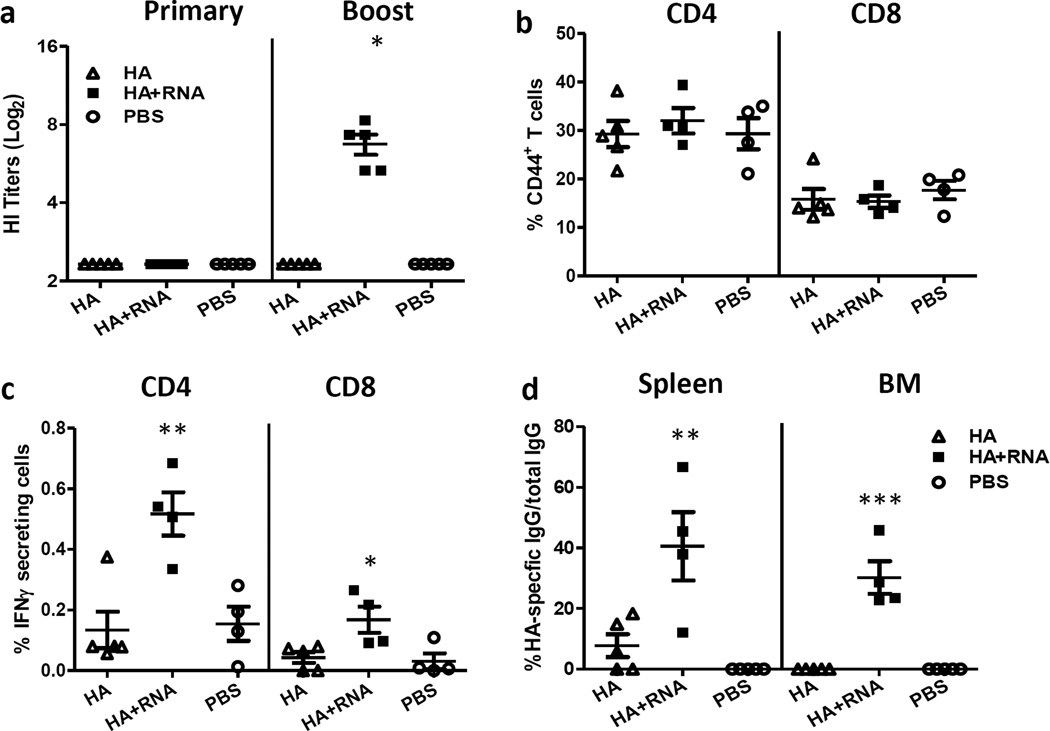
Protection against influenza virus infection is primarily mediated by neutralizing antibodies. Recombinant influenza H7 hemagglutinins from A/Netherlands/219/2003 (H7N7) and A/New York/107/2003 (H7N2) have been shown to be poorly immunogenic and induce lower neutralizing antibody titers in mice than do seasonal hemagglutinins [12]. Therefore, we examined whether RIG-I ligand can enhance antibody responses against poorly immunogenic H7 protein. Mice were immunized using a prime-boost regimen at week 1 and 4 as described in the Materials and Methods. Sera were collected three weeks after primary and booster immunization to measure HI titers against A(H7N9) virus (SH2/PR8). No detectable HI titers were observed in any vaccine group following primary immunization (Fig. 1a). Upon booster immunization, mice immunized with unadjuvanted rH7HA still did not display detectable HI titers. In contrast, mice immunized with rH7HA with RIG-I ligand developed significantly higher HI titers (p<0.05) (Fig. 1a) indicating that RIG-I ligand enhanced antibody responses induced by rH7HA protein.
Figure 1. RIG-I activation enhanced serum antibody responses and cell-mediated immune responses.
BALB/c mice (5 animals/group) were immunized intramuscularly (i.m.) with recombinant H7HA protein (3 µg) with or without 5’pppRNA or mock-immunized with PBS as control. Mice were boosted one month later with the same immunogen formulation as they received previously. (a) Serum samples were collected three weeks post- primary and three post- booster immunizations and HI titers were measured against SH2/PR8 virus (*p<0.05 as compared to H7HA and PBS group). (b–c) Spleens were collected at 1 week after booster immunization and single cell suspensions were prepared. Cells were stimulated in vitro with SH2/PR8 virus (MOI=1) overnight with GolgiPlug™ added in the last 6 hours of incubation. The percentage of CD44+ activated CD4+ or CD8+ T cells were measured by surface staining of CD44 (b); the percentage of IFNγ-producing activated CD4+ or CD8+ T cells were then measured by intracellular cytokine staining (c, **p<0.01 or *p<0.05 as compared to H7HA and PBS group). (d) Spleen and bone marrow samples were collected at one week post-boost and the frequency of recombinant H7HA protein-specific IgG+ ASCs was measured using the ELISPOT assay. The percentage of HA-specific IgG+ ASCs in total IgG+ ASCs are presented (**p<0.01 or ***p<0.001 as compared to H7HA and PBS group). The error bars represent standard error of the means (SEM).
3.2 RIG-I ligand increased the functional T cell immune responses
Cell-mediated immune responses are critical for antibody production by B cells in response to influenza virus infection or vaccination [13]. To evaluate the cell-mediated immune responses, 6 to 8-week old BALB/c mice were immunized (i.m.) with 3 µg of rH7HA with or without RIG-I ligand (5’pppRNA) or mock-immunized with PBS. Four weeks later, mice were boosted with the same formulation. Spleens were harvested one week post-boost and cells were re-stimulated in vitro with SH2/PR8 virus. Functional CD4 and CD8 T cells were analyzed by surface CD44 expression and intracellular cytokine production. The percentage of activated CD44+CD4 or CD44+CD8 T cells was similar among all groups (Fig. 1b). However, in response to in vitro A(H7N9) virus re-stimulation, the percentage of IFN-γ producing CD44+CD4 or CD44+CD8 T cells was significantly increased in mice that received rH7HA with 5’pppRNA (p<0.01 for CD4 T cells and p<0.05 for CD8 T cells) (Fig. 1c), compared to mice immunized with rH7HA alone or PBS. Therefore, addition of RIG-I ligand significantly increased the frequency of antigen-responsive functional CD4 and CD8 T cells in the spleen.
3.3 RIG-I ligand enhanced the memory B cell responses
The H7HA antigen-specific B cell responses in the spleen and bone marrow at one week post boost immunization were examined by ELISPOT assay. As shown in Fig. 1c, mice immunized with rH7HA without RIG-I ligand displayed either very low or undetectable H7HA-specific IgG secreting cells in the spleen and bone marrow. In contrast, the number of H7HA protein-specific IgG ASCs in both spleen and bone marrow was significantly increased in the presence of RIG-I ligand, suggesting that the addition of RIG-I ligand significantly enhanced memory B cell responses in both primary and secondary lymphoid tissues (p<0.01 in spleen and p<0.001 in bone marrow) (Fig. 1d).
3.4 Addition of RIG-I ligand to rH7HA protein enhanced the protective immunity against viral challenge
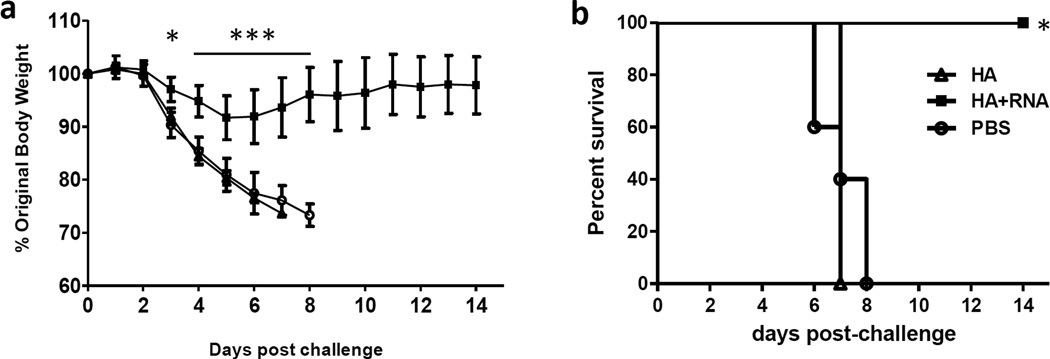
To assess protective immunity, mice were challenged with a 50 LD50 of wild-type A(H7N9) virus (A/Anhui/1/2013, AH1). All mice immunized with rH7HA alone or PBS lost body weight dramatically and succumbed to infection by day 7 post-challenge. However, mice immunized with rH7HA adjuvanted with 5’pppRNA had minimal body weight loss (p<0.05 for day 3 post challenge and p<0.001 for day 4–8 post challenge) (Fig. 2a) and survived A(H7N9) virus challenge (p<0.05) (Fig.2b). Therefore, addition of RIG-I ligand significantly enhances the protective immunity conferred by rH7HA protein.
Figure 2. Addition of RIG-ligand to rH7HA protein enhanced the protective immunity against viral challenge.
BALB/c mice (5 mice/group) were immunized intramuscularly (i.m.) with recombinant H7HA protein (3 µg) with or without 5’pppRNA or PBS control. Mice were boosted one month later with the same vaccine formulation they received earlier. Immunized mice were challenged with 50 LD50 of wild type A(H7N9) virus and weight loss (*p<0.05 or ***p<0.001 as compared to H7HA and PBS group) (a) and survival (*p<0.05 as compared to H7HA and PBS group) (b) were monitored for 15 days post-challenge. The error bars represent standard error of the means (SEM).
4. Discussion
To meet the global demand in the event of a pandemic with avian influenza viruses, a number of strategies (egg-dependent and egg-independent) to manufacture influenza vaccines as well as adjuvants to improve their immunogenicity and provide a dose-sparing effect are being explored [14, 15]. Cell-based vaccines, virus-like particle (VLP), viral-vector delivery and recombinant viral protein production technologies overcome the vaccine production bottleneck especially when the supply of embryonated hen eggs, the substrate for conventional vaccine production, is impacted due to avian influenza. The recent outbreak of A(H7N9), a low pathogenic avian influenza A virus with first reported human fatal outcomes, underscores the need for a highly immunogenic vaccine to prevent A(H7N9) virus infection. In this study we investigated the utility of rH7HA protein as a potential candidate vaccine. Avian influenza vaccines, including H7 vaccines, whether egg- or cell-derived, recombinant proteins or live attenuated influenza viruses (LAIV), have been shown to be poorly immunogenic and required adjuvants to enhance their immunogenicity [12, 16–18].
The innate immune system recognizes influenza virus through members of at least three distinct classes of PRRs, including Toll-like receptors, RIG-I and the NOD-like receptor family member, which are essential and sufficient for the induction of innate immune defense and type I interferon response [19]. Hence, RIG-I ligands have potential utility as vaccine adjuvants. In a previous study, we demonstrated that RIG-I ligand, a 5’ppp-dsRNA, not only enhanced the immunogenicity of a pandemic H1N1 monovalent vaccine but also achieved dose-sparing effect and conferred protective immunity by activating the RIG-I pathway [10]. This was accomplished by enhancing the frequency of follicular CD4 T helper cells and germinal center B cells. Furthermore, RIG-I activation was shown to be superior to both TLR ligands and alum adjuvant for induction of A(H1N1) influenza virus-specific antibody responses [10]. In our current study, we further extended our earlier observations on the utility of RIG-I ligand by showing that it acts as a potent molecular adjuvant even for poorly immunogenic H7 avian influenza vaccine. Our results demonstrated that the RIG-I ligand (5’pppRNA)-adjuvanted rH7HA protein is highly immunogenic and confer complete protection against homologous A(H7N9) viral challenge. Consistent with our previous report with H1N1 monovalent vaccine, we extended earlier findings and demonstrated that 5’ppp-dsRNA also increased functional CD4 T cell and B cell responses to rH7HA[10]. In addition, functional CD8 T cell responses was significantly increased in mice that received rH7HA with 5’pppRNA. CD8 T cell responses against influenza viruses are the major mediators to provide heterosubtypic immunity [20]. Thus, utility of RIG-I ligand as adjuvant will be an advantage in broadening protective immunity to viruses with new pandemic potential.
Acknowledgments
We thank members of the Immunology and Pathogenesis Branch in the Influenza Division, Centers for Disease Control and Prevention for providing reagents and constructive comments for this study. Work was supported by the Influenza Division, Centers for Disease Control and Prevention.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the funding agencies
Potential conflicts of interest: All authors report no potential conflicts.
References
- 1.Tanner WD, Toth DJ, Gundlapalli AV. The pandemic potential of avian influenza A(H7N9) virus: a review. Epidemiol Infect. 2015:1–16. doi: 10.1017/S0950268815001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoelscher M, Gangappa S, Zhong W, Jayashankar L, Sambhara S. Vaccines against epidemic and pandemic influenza. Expert Opin Drug Deliv. 2008;5:1139–1157. doi: 10.1517/17425247.5.10.1139. [DOI] [PubMed] [Google Scholar]
- 3.U.S.F.a.D. Administration. FDA approves first adjuvanted vaccine for prevention of H5N1 avian influenza. 2013 Nov [Google Scholar]
- 4.Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsen P, Saarenpaa-Heikkila O, Kilpi T. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dauvilliers Y, Arnulf I, Lecendreux M, Monaca Charley C, Franco P, Drouot X, d'Ortho MP, Launois S, Lignot S, Bourgin P, Nogues B, Rey M, Bayard S, Scholz S, Lavault S, Tubert-Bitter P, Saussier C, Pariente A, Narcoflu VFsg. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain. 2013;136:2486–2496. doi: 10.1093/brain/awt187. [DOI] [PubMed] [Google Scholar]
- 6.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 8.Rehwinkel J, Tan CP, Goubau D, Schulz O, Pichlmair A, Bier K, Robb N, Vreede F, Barclay W, Fodor E, Reis e Sousa C. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Ranjan P, Jayashankar L, Deyde V, Zeng H, Davis WG, Pearce MB, Bowzard JB, Hoelscher MA, Jeisy-Scott V, Wiens ME, Gangappa S, Gubareva L, Garcia-Sastre A, Katz JM, Tumpey TM, Fujita T, Sambhara S. 5'PPP-RNA induced RIG-I activation inhibits drug-resistant avian H5N1 as well as 1918 and 2009 pandemic influenza virus replication. Virol J. 2010;7:102. doi: 10.1186/1743-422X-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni RR, Rasheed MA, Bhaumik SK, Ranjan P, Cao W, Davis C, Marisetti K, Thomas S, Gangappa S, Sambhara S, Murali-Krishna K. Activation of the RIG-I pathway during influenza vaccination enhances the germinal center reaction, promotes T follicular helper cell induction, and provides a dose-sparing effect and protective immunity. J Virol. 2014;88:13990–14001. doi: 10.1128/JVI.02273-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Carney PJ, Chang JC, Villanueva JM, Stevens J. Structural analysis of the hemagglutinin from the recent 2013 H7N9 influenza virus. J Virol. 2013;87:12433–12446. doi: 10.1128/JVI.01854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchfield K, Kamal RP, Tzeng WP, Music N, Wilson JR, Stevens J, Lipatov AS, Katz JM, York IA. Recombinant influenza H7 hemagglutinins induce lower neutralizing antibody titers in mice than do seasonal hemagglutinins. Influenza Other Respir Viruses. 2014;8:628–635. doi: 10.1111/irv.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu Rev Immunol. 2004;22:711–743. doi: 10.1146/annurev.immunol.22.012703.104527. [DOI] [PubMed] [Google Scholar]
- 14.Sambhara S, Poland GA. H5N1 Avian influenza: preventive and therapeutic strategies against a pandemic. Annu Rev Med. 2010;61:187–198. doi: 10.1146/annurev.med.050908.132031. [DOI] [PubMed] [Google Scholar]
- 15.Luke CJ, Subbarao K. Improving pandemic H5N1 influenza vaccines by combining different vaccine platforms. Expert Rev Vaccines. 2014;13:873–883. doi: 10.1586/14760584.2014.922416. [DOI] [PubMed] [Google Scholar]
- 16.Rudenko L, Kiseleva I, Stukova M, Erofeeva M, Naykhin A, Donina S, Larionova N, Pisareva M, Krivitskaya V, Flores J, Russian LTSG. Clinical testing of pre-pandemic live attenuated A/H5N2 influenza candidate vaccine in adult volunteers: results from a placebo-controlled, randomized double-blind phase I study. Vaccine. 2015;33:5110–5117. doi: 10.1016/j.vaccine.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS One. 2012;7:e49704. doi: 10.1371/journal.pone.0049704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, Hoschler K, Saville M, Vogel FR, Barclay W, Donatelli I, Zambon M, Wood J, Haaheim LR. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine. 2009;27:1889–1897. doi: 10.1016/j.vaccine.2009.01.116. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altenburg AF, Rimmelzwaan GF, de Vries RD. Virus-specific T cells as correlate of (cross-)protective immunity against influenza. Vaccine. 2015;33:500–506. doi: 10.1016/j.vaccine.2014.11.054. [DOI] [PubMed] [Google Scholar]